Abstract
An unusual variant of Meloidogyne arenaria was discovered on roots of a traveler's tree (Ravenala madagascariensis) intended for display at a public arboretum in Pennsylvania. The population aroused curiosity by the lack of visible galling on the roots of the infected plant, and the female vulval region was typically surrounded by egg sacs. Most morphometrics of the population fit within the ranges reported for M. arenaria, with a mosaic of features in common with either M. platani or other tropical Meloidogyne spp. Molecular characterization included analysis of four loci. The mitochondrial sequence, extending from cytochrome oxidase II (COII) to the 16S (1RNA) gene, was nearly identical to another M. arenaria population and closely related to sequences from M. morocciensis and M. thailandica. The 28S D2-D3 expansion segment was most similar to those from M. arenaria, M. incognita and M. paranaensis, and the IGS-2 was most related to those from M. thailandica, M. arenaria and M. incognita. Analysis of partial Hsp90 genomic sequences revealed the greatest similarity to M. arenaria, M. thailandica and an Hsp90 haplotype from M. floridensis, and a composite sequence comprised of EST from M. arenaria. No morphological or molecular features clearly distinguished this population as a new species, and, when considered as a whole, the evidence points to its identification as M. arenaria.
Keywords: gall, Hsp90, intergenic spacer, mitochondrial DNA, molecular biology, Ravenala madagascariensis, ribosomal DNA, root-knot nematode, taxonomy, traveler's tree, variation
Root-knot nematodes (Meloidogyne spp.) are economically important plant parasites affecting a broad range of host plants, and thus far 95 nominal species have been described. In June 2007, a root-knot nematode (RKN) was discovered on roots of a traveler's tree (Ravenala madagascariensis Sonnerat) that was intended for exhibit at a public garden in Pennsylvania. Growing in an artificial soil mixture, the affected palm-like tree was obtained by the arboretum from a grower in Florida. Information regarding the precise origin of the traveler's tree was not available. Diseased root material was sent via a Delaware extension specialist to the USDA Nematology Laboratory in Beltsville, MD, for identification.
The development of molecular methods to identify the four major RKN (M. incognita (Kofoid and White, 1919) Chitwood, 1949; M. arenaria (Neal, 1889); M. javanica (Treub, 1885) Chitwood, 1949; and M. hapla Chitwood, 1949) has been the goal of numerous studies. While isozyme analysis continues to be an effective way to discriminate many RKN species (Esbenshade and Triantaphyllou, 1990; Carneiro, 2000), DNA sequencing is straightforward and has the added benefit of providing data useful for phylogenetic analysis. DNA markers that have aided identification of Meloidogyne species include the ribosomal DNA small subunit (SSU) 18S (Powers, 2004), large subunit (LSU) 28S D2-D3 expansion segments (Chen et al., 2003; Palomares Ruis et al., 2007), intergenic spacer (IGS) (Blok et al., 1997; Wishart et al., 2002), internal transcribed spacer (Powers and Harris, 1993) and mitochondrial DNA (Powers and Harris, 1993; Stanton et al., 1997; Blok et al., 2002; Xu et al., 2004; Jeyaprakash et al., 2006). Random amplified polymorphic DNA (RAPD) (Cenis, 1993; Williamson et al., 1997; Dong et al., 2001; Cofcewicz et al., 2004; Adam et al., 2007) and sequence characterized amplified regions (SCAR) markers (Zijlstra et al., 2000; Randig et al., 2002) have also been developed. One study combined IGS PCR, SCAR markers and RAPD analysis into a diagnostic key for discrimination of seven RKN species (Adam et al., 2007). Recently, a partial sequence from the protein-coding gene Hsp90 was used to confirm the identity of an unusually aggressive population of M. hapla affecting coffee in Maui, HI (Handoo et al., 2005b). This locus also showed potential for establishing phylogenetic relationships among plant-parasitic nematode species (Skantar and Carta, 2004).
This work describes the morphological features of an unusual population of M. arenaria found on traveler's tree, highlighting differences from the values expected for the species and points of overlap with related species. Also, a study of its relationship to other tropical RKN, based upon analysis of mitochondrial sequences (the interval from cytochrome oxidase COII to the 16S rRNA gene, 1RNA), the 28S D2-D3 expansion segment, the ribosomal intergenic spacer regions (IGS) and Hsp90, is presented.
Materials and Methods
Morphological Characterization: Soil and root material from the infected traveler's tree were sent to the U.S. Department of Agriculture (USDA) Nematology Laboratory in Beltsville, MD, for species identification. Egg masses were kept in petri dishes at room temperature in a small amount of water. J2 were extracted from soil by sieving and Baermann funnel methods. After fixation overnight in 3% formaldehyde at room temperature, females, males and J2 were later dissected from infected roots. J2 and males were fixed in 3% formaldehyde and processed to glycerin by the formalin-glycerin method (Hooper, 1970; Golden, 1990). Procedures used in measuring and preparing specimens were essentially those of Golden and Birchfield (1972), except some fixed females were cut and mounted in clear lactophenol solution. Measurements of all stages were made with an ocular micrometer with measurements in micrometers, unless otherwise stated. Images were taken on a Zeiss Ultraphot III (Carl Zeiss, Inc., Jena, Germany, and Baltimore Instrument Company, Baltimore, MD, USA) with a Toshiba IKTU CCD camera (Toshiba Corp., Japan) or a Zeiss Axiophot 100 Inverted microscope (Zeiss, Inc., Thornwood, NY) with a Q Imaging RTV Micropublisher 5 CCD camera (QImaging, Surrey, BC, Canada). Partial body images were stitched using Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA).
Molecular Characterization: Single J2 of the Ravenala population and others (M. arenaria from Maryland; M. floridensi, from Florida) were mechanically disrupted in 20 μl of extraction buffer as described by Thomas et al. (1997) then stored in PCR tubes at –80°C until needed. Meloidogyne thailandica from Thailand consisted of a pool of nine J2 in 50 μl, treated as above. Extracts were prepared by incubating the tubes at 60°C for 60 min, followed by 95°C for 15 min to deactivate the proteinase K and were centrifuged briefly prior to use in PCR. Typically, 2.5 μl of each nematode extract was used per 25 μl PCR reaction. Primers used in this study are listed in Table 1. Reactions also contained 1 unit Eppendorf MasterTaq (Brinkmann, Westbury, NY) and the buffer supplied by the manufacturer; all other components were added as described in the specific protocols for each gene. Ribosomal PCR products were amplified from the intergenic spacer (IGS-2) according to Blok et al. (2002) and from the 28S D2-D3 expansion segment, as described by De Ley et al. (2005). Partial Hsp90 sequences were amplified as previously described (Skantar and Carta, 2004). Amplification of the mitochondrial region between the COII and lRNA genes was a modification of Powers and Harris (1993) and Tigano et al. (2005), as follows: 1 cycle at 94°C for 2 min, followed by 45 cycles of 94°C for 30 sec, 48°C for 30 sec and 68°C for 2 min, ending with 1 cycle of 68°C for 5 min.
Table 1.
List of Primers used for DNA analysis
PCR products were visualized with UV illumination after ethidium bromide staining. DNA was excised from the gels and purified with the QIAquick Gel Extraction Kit (Qiagen, Valencia, CA). Cleaned PCR products were either sequenced directly or cloned into vector pCR2.1 and transformed into Escherichia coli TOP10 cells according to the TOPO TA cloning kit instructions (Invitrogen, Carlsbad, CA). Plasmid DNA was prepared with a Qiagen miniprep kit (Qiagen, Valencia, CA) and digested with Eco RI to confirm the correct insert identity. Sequencing was performed at the University of Maryland Center for Biosystems Research. For each locus, 2 to 4 clones or PCR products representing two or more individuals were analyzed. New sequences were submitted to GenBank under accession numbers EU364878-EU364890 and FJ238508.
DNA sequences were assembled using Sequencher 4.7 (Genecodes, Ann Arbor, MI) and analyzed using the BLASTN megablast program optimized for highly similar sequences (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Similar sequences were downloaded from GenBank, aligned with ClustalW2 with default parameters (Larkin et al., 2007), and, if necessary, alignments were trimmed in GeneDoc (Nicholas et al., 1997). For each locus alignment, distance-based neighbor-joining (NJ) methods were used to infer the phylogenetic relationships among species (Saitou and Nei, 1987).
Results and Discussion
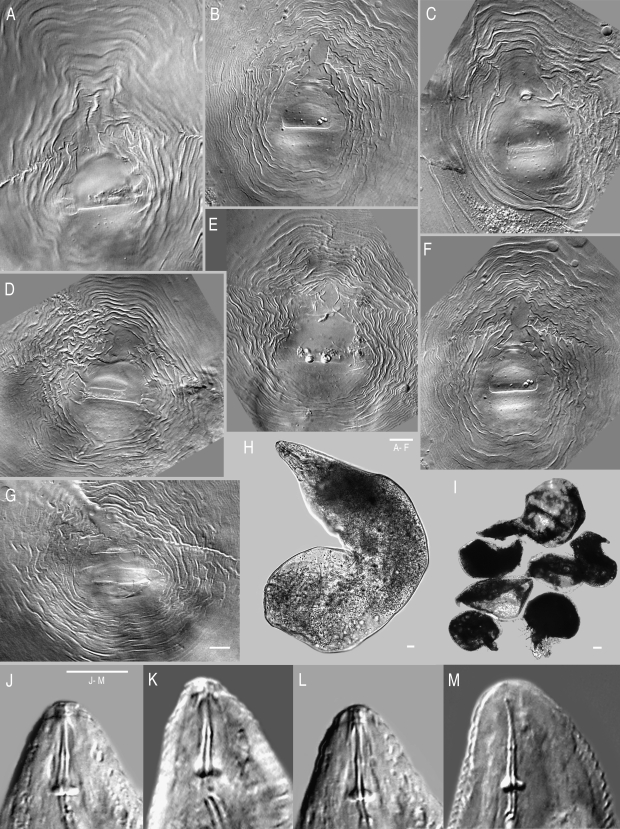
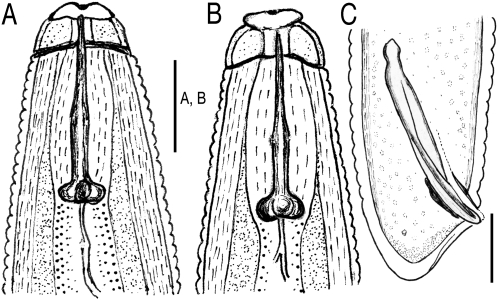
Morphological analysis: The population did not produce any galls on traveler's tree, and female vulval region was typically surrounded by egg sacs. Morphological variation made this population particularly difficult to identify, and it was initially thought to represent a new species closely related to M. arenaria, M. incognita and M. javanica. While this population consisted of a mosaic of morphological similarities to several species, most measurements fit within the ranges previously reported for M. arenaria. Perineal patterns in females were quite variable (Fig. 1A—F), with a round to high pattern showing occasional wings or shoulders with a slight lateral line. In several specimens, the central perineum was surrounded by alternating coarse and fine striae, with higher frequency, more interrupted waves than seen in typical M. arenaria (Fig. 1G). Perineal patterns lacked the laterally elongated perivulval region usually found in M. arenaria (Cliff and Hirschmann, 1985; Hirschmann, 1985; Eisenback and Triantaphyllou, 1991), but instead had a round to dorso-ventral elongation. Vulval openings in this population exhibited prominent lateral seam-like depressions.
Fig. 1.
Light micrographs of Meloidogyne arenaria females. (A – F) Perineal patterns from traveler's tree population. (G) Example of typical Meloidogyne arenaria perineal pattern, imaged from slide G9957, USDA Nematode Collection. Specimen from Blacksburg, VA; host = tomato. (H – I) Female body, traveler's tree population, high and low power images, respectively. J – M) Traveler's tree population, anterior ends with stylets.
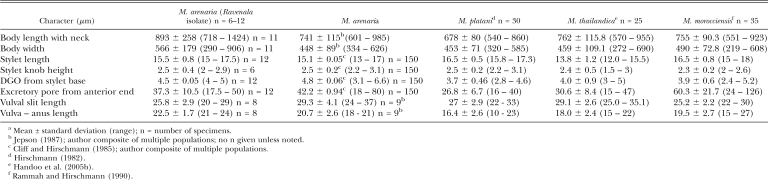
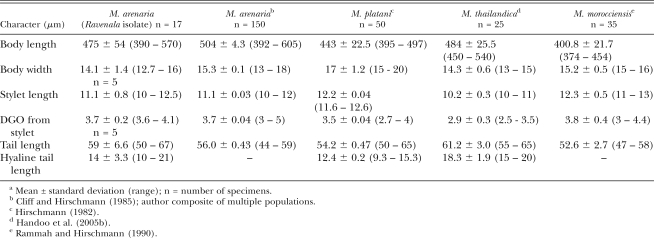
The stylet cone in females was slightly curved (Fig. 1J-M). Female stylet knobs were rounded to broad, sloping posteriorly, and did not consistently have the gradual sloping of the shaft found in typical M. arenaria (Eisenback et al., 1980, 1981; Eisenback and Triantaphyllou, 1991), but instead showed a lateral aspect more like that of M. incognita. Female stylet length (Table 2) was consistent with M. arenaria and distinguished the population from M. thailandica Handoo, Skantar, Carta and Erbe, 2005, a species found on ginger (Zingiber spp.) from Thailand (Handoo et al., 2005a), and M. morocciensis Rammah and Hirschmann, 1990 from peach (Prunus persica L. Batsch) in Morocco (Rammah and Hirschmann, 1990). The dorsal gland outlet (DGO), excretory pore position and vulva to anus length were consistent with the ranges for M. arenaria (Table 2). Female shapes were typical of M. arenaria but many were bent into L-shapes (Fig. 1H,I). Most females exhibited a posterior protuberance (Fig. 1H) that was absent in M. thailandica. The ranges for female body length and width were considerably larger than for other M. arenaria populations, M. thailandica and M. morocciensis.
Table 2.
Morphometric comparisona of females from the Ravenala isolate compared with other M. arenaria populations, M. platani, M. thailandica and M. morocciensis
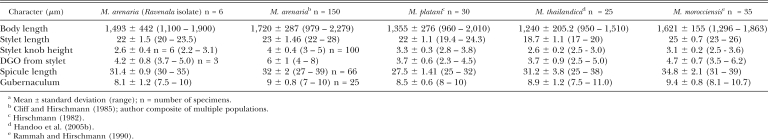
Male stylet length and spicule length were consistent with typical M. arenaria, but body length and gubernaculum were shorter (Table 3). Mean male stylet length distinguishes the current population (22 μm) and previously reported M. arenaria value (23 μm) from M. thailandica (18.7 μm) and M. morocciensis (25 μm). Also, mean body length (1,493 μm) was longer than in M. thailandica (1,240 μm) and shorter than M. morocciensis (1,621 μm). The Ravenala population also lacked the bidentate terminus in the spicules (Fig. 2C) found in M. thailandica.
Table 3.
Morphometric comparisona of males from the Ravenala isolate with other M. arenaria populations, M. platani, M. thailandica and M. morocciensis
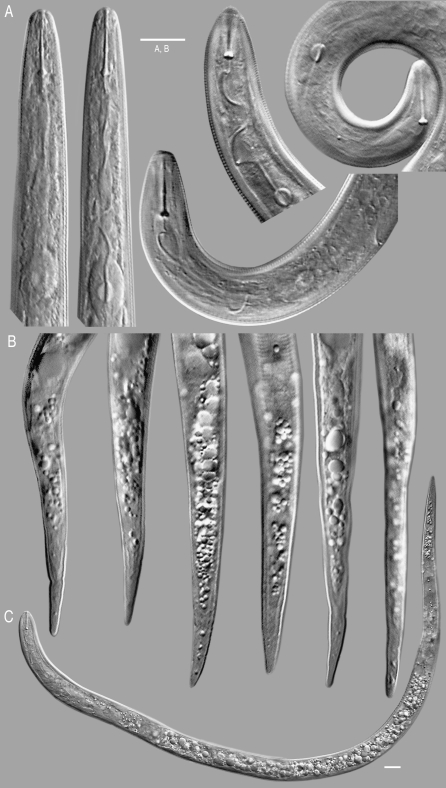
Fig. 2.
Drawings of Meloidogyne arenaria males from traveler's tree. (A) Lateral view of the anterior end. (B) Dorso-ventral view, anterior end. C) Tail with spicule and gubernaculum.
Second-stage juvenile stylets (Fig. 3A) were very similar to other M. arenaria, with mean length (11.1 μm) distinct from the shorter stylet of M. thailandica (10.2 μm) and the longer one of M. morocciensis (12.3 μm) (Table 4). Juvenile tails were long, tapering to a rounded to finer point with 1 to 4 refractive bodies consistently noticed in the hyaline portion (Fig. 3B). Juvenile tail length was on the high end of the range of other M. arenaria populations and almost as large as that of M. thailandica (Table 4). However, the Ravenala population was easily distinguished by a much shorter hyaline tail length (14 μm) than reported for M. thailandica (18.3 μm).
Fig. 3.
Light micrographs of Meloidogyne arenaria second-stage juveniles from traveler's tree. (A) Anterior ends with stylet and pharynx. (B) Tails. C) Whole specimen.
Table 4.
Morphometric comparisona of second-stage juveniles from the Ravenala isolate with other M. arenaria populations, M. thailandica and M. morocciensis
Considering characters previously shown to exemplify M. arenaria, the Ravenala population instead shared some qualitative characters with M. platani Hirschmann, 1982 (Rammah and Hirschmann, 1990). Most notably among them were a wavy female perineal pattern, less sloping male and female stylet knob shape, and shorter female knob height. However, the Ravenala population differs markedly from M. platani in having longer female body and longer vulval-anal distance (Table 2); the perineal pattern lines are not as uniformly fine, and lateral field and tail tip are generally distinct. While stylet knob heights in both M. platani and the Ravenala population were lower than the range reported by Cliff and Hirschmann (1985), they were consistent with measurements (range 2-4 μm; average 2.8 μm) from other triploid populations (Rammah and Hirschmann, 1990).
The male DGO was also found to be useful for characterizing M. arenaria populations (Rammah and Hirschmann, 1990). The M. arenaria population from Ravenala had an intermediate range and average DGO (Table 3), falling between M. platani (Hirschmann, 1982) and the values reported for multiple M. arenaria populations. However, the Ravenala population and M. platani both lacked bifid spicule termini and areolation in the male lateral field and had an unusually high head region below the similarly shaped cap (Fig. 2A,B). The shape of the male tail was relatively acute in both M. platani and the Ravenala population (Fig. 2C) as compared to typical M. arenaria (Jepson, 1987). The hyaline tail length was indistinct in typical M. arenaria (Cliff and Hirschmann, 1985), but was apparent in the M. arenaria population and in M. platani. Although the Ravenala population showed features intermediate between typical M. arenaria and M. platani, the latter induced large galls on sycamore, a non-host of M. arenaria, and failed to reproduce on peanut, corn or sweet potato (Hirschmann, 1982).
Molecular analysis: Results of the molecular studies mirrored the morphological analysis, revealing a mosaic of molecular similarities to several RKN species. Unfortunately, no DNA sequences were available for M. platani, so it was not possible to complement those morphological comparisons with molecular data.
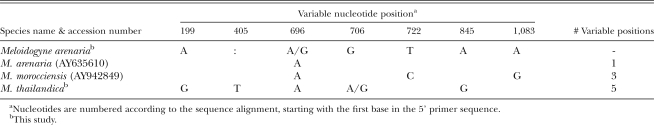
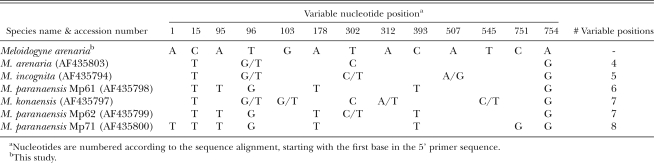
The 1.1 kb size of the mitochondrial COII-1RNA PCR product from the Ravenala population is shared in common with race 1 and race 2 of M. arenaria populations from Georgia, North and South Carolina, and Florida (Powers and Harris, 1993), populations from Ivory Coast, Portugal and the French West Indies (Blok et al., 2002), and one from Brazil (Tigano et al., 2005). Other species that presented similarly sized products include M. morocciensis (1,113 bp), and M. floridensis (1,110 bp) (Tigano et al., 2005), isolated from peach (Prunus persicus L.) in Morocco and Florida, respectively; M. thailandica (1,113 bp) from ginger (Zingiber spp.) in Thailand (Handoo et al., 2005a); and M. paranaensis (1,255 bp) from coffee (Coffea arabica L.) (Tigano et al., 2005). Mitochondrial COII-1RNA PCR products from M. javanica and M. incognita are larger (Powers and Harris, 1993; Tigano et al., 2005), thus distinguishing these species from the Ravenala population. Products of 1.7 kb have been reported for some M. arenaria populations (Powers and Harris, 1993). These most likely correspond to previously described haplotype C/H, which can be distinguished from the smaller type A by virtue of a 529 bp deletion in the intergenic region (Hugall et al., 1994; Stanton et al., 1997). The mitochondrial COII-1RNA sequence (EU364879) was identical to M. arenaria (Table 5), with one ambiguous position (A/G) found in the Ravenala population. Neighbor-joining (NJ) analysis (not shown) grouped the Ravenala isolate with another M. arenaria population (AY635610) and with M. morocciensis (AY942849), in agreement with previous results (Tigano et al., 2005), and with M. thailandica (EU364879). Despite the similarly sized amplification product, the M. floridensis mitochondrial sequence contained large indels relative to the M. arenaria sequence and was more similar to one from M. incognita (Tigano et al., 2005).
Table 5.
Variable nucleotide positions in mitochondrial DNA
The D2-D3 expansion segment of large subunit (LSU) 28S rDNA was 754 bp for the Ravenala population (EU364889). An alignment with six of the most closely related sequences from GenBank revealed 13 variable nucleotide positions (Table 6). The most similar sequence (AF435803) was from an M. arenaria race 2 population from soybean (Tenente et al., 2004), which differed at 4 bp, including one ambiguous position. Other highly similar sequences included M. incognita (AF435794) also from soybean, M. konaensis (AF435797) from coffee in Hawaii, and Brazilian M. paranaensis populations Mp61 (AF435798), Mp62 (AF435799) and Mp71 (AF435800), also isolated from coffee. Because some previously reported 28S D2-D3 sequences (Tenente et al., 2004) contained ambiguous bases (most likely due to direct sequencing of PCR products, not clones), it is unclear how many separate haplotypes occur within these species. A separate comparison of D3 sequences including several more from GenBank (not shown) provides some clues. Previous D3 analyses showed that M. arenaria, M. incognita and M. hapla contained seven haplotypes among them, with two (haplotypes 5 and 7) found in all three species (Chen et al., 2003), and examination of M. thailandica cloned D3 PCR products revealed five haplotypes (A – E) (Handoo et al., 2005a). Here we found that the M. arenaria population from Ravenala shared the D3 haplotype 5 in common with populations M. arenaria MA, M. incognita COA and MUL and M. javanica JNC, J811 and VW4 (Chen et al., 2003), which was also identical to the B haplotype of M. thailandica (Handoo et al., 2005a). Since most of the distinguishing polymorphisms in our comparisons were located in D2 (Table 6), it appears this region may provide better phylogenetic resolution within the apomictic group, particularly when multiple clones from a population are examined.
Table 6.
Variable nuclotide positions in 28S rDNA D2-D3 expansion segment of various root-knot nematodes
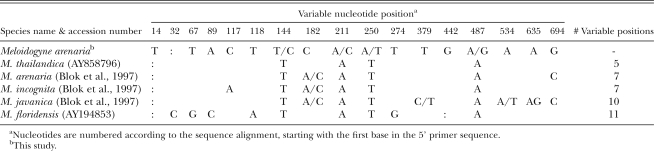
The amplified IGS-2 region, between the 5S and 18S rDNA, was 716 bp for the Ravenala population (EU364878), consistent with the size previously reported for the tropical RKN species and distinct from larger products found in M. mayaguensis and smaller in M. hapla (Blok et al., 1997). Out of 717 bp aligned, 17 positions were variable among the RKN compared (Table 7). The most similar sequences were from M. thailandica, M. arenaria, M. incognita, M. javanica and M. floridensis. No polymorphisms set M. arenaria clearly apart, and, except for the distinctive M. floridensis sequence, nucleotide ambiguities in this marker obscured the boundaries between species.
Table 7.
Variable nucleotide positions in the ribosomal intergenic space region IGS-2 of various root-knot nematodes
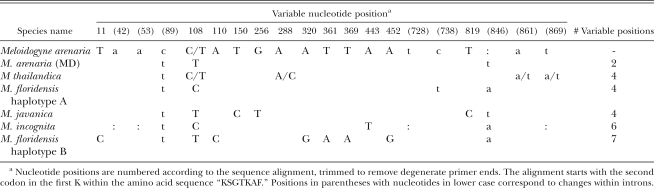
Hsp90 PCR products of ∼1 kb were sequenced for the Ravenala population (EU364880), M. arenaria from Maryland (FJ238508) and several other RKN species, including M. thailandica (EU364882) from Thailand (Handoo et al., 2005a), M. floridensis (EU364884 - EU364888) from Florida and Georgia (Handoo et al., 2004), M. javanica (AF201338) from Maryland and M. incognita (EU364881). All partial Hsp90 sequences consisted of five exons and four introns. The three Florida and one Georgia M. floridensis populations were represented by two distinct Hsp90 haplotypes. The rest of the Meloidogyne populations yielded single sequences, although a few polymorphic positions were detected among them (Table 8). The alignment was trimmed to 972 bases to eliminate the degenerate primer end sequences, which were assumed to be unreliable. Among the sequences compared, there were 20 polymorphic positions, with eight base changes within introns, two changes at first codon positions, five at second positions and four at third positions. Meloidogyne arenaria from Maryland was nearly identical to the Ravenala population, with one C-T transition and one single base insertion. Sequence divergences for M. thailandica, M. floridensis haplotypes A and B, M. javanica and M. incognita were <1%, similar to what was found for the other loci examined. In contrast to the low level of divergence among the apomictic species of RKN, two M. hapla sequences determined previously (Handoo et al., 2005b) were significantly different (17%) from the Ravenala population. This separation of M. hapla from the clade of the apomictic species is consistent with findings involving other loci (Hugall et al., 1994, 1999; Chen et al., 2003; Tigano et al., 2005; Castagnone-Sereno, 2006).
Table 8.
Variable nuclotide positions in an alignment of partial Hsp90 genomic sequences of various root-knot nematodes
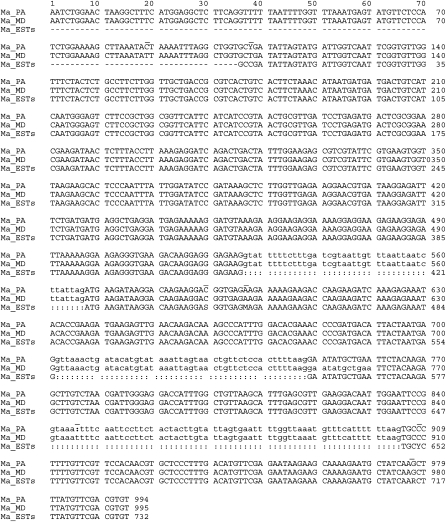
An existing sequence dataset from an M. arenaria cDNA library contained several overlapping EST corresponding to Hsp90, so a composite sequence was constructed from GenBank accessions BI747178, BI747494 and BI745855. The EST assembly sequence contained a number of ambiguous bases and differed from the Ravenala population coding sequence at six positions (Fig. 4). Some of the ambiguities appeared towards the ends of the individual EST (not shown) and so may be due to unreliable base calls from the single-pass reads or they may represent genuine Hsp90 sequence variants. Examination of Hsp90 clones from multiple populations of M. arenaria will be necessary to establish the extent of intraspecific variability within the gene, but it appears that Hsp90 is neither better nor worse at resolving these species than the mitochondrial or ribosomal markers examined.
Fig. 4.
Alignment of partial Hsp90 genomic sequence from variant M. arenaria population from traveler's tree compared with sequence from morphologically typical M. arenaria from MD and composite cDNA sequence assembled from M. arenaria EST. The genomic sequence was trimmed of degenerate primer end bases, and the 3’ end of the EST composite extending beyond the genomic amplicons was trimmed. Genomic intron sequences from the M. arenaria population are in lower case. Abbreviations: R = A or G; Y = C or T; W = A or T; M = A or C; S = C or G; dash (-) indicates missing data; colon (:) indicates intron gaps not present in EST. Positions that vary in at least one of the sequences are indicated by overline.
While most populations of M. arenaria induce robust galls, some cause smaller, bead-like galls (Eisenback and Triantaphylou, 1991). However, none have been reported to fail to induce galls as this one did. The plant order Zingiberales contains eight families, including Strelitziaceae, Zingiberaceae and Heliconiae. Strelitziaceae (Quénéhervé et al., 1997) contains one species within the genus Ravenala (including traveler's tree, R. madagascariensis), one in Phenakospermum and five within Strelitzia (including bird-of-paradise). Very little has been reported on Meloidogyne species incidence specifically within Ravenala. However, all three major parthenogenetic species attack ginger (Zingiber, within the Zingiberaceae) (Baptista dos Santos and Lazaro Lozano, 1993), and M. thailandica (Handoo et al., 2005a) also attacks Heliconia spp. (false bird-of-paradise), a genus within Heliconiae. Host preferences represent convergent phenotypes, since members of the same host race show substantial genetic variability (Baum et al., 1994). The paradoxical genetic diversity of the four major parthenogenic RKN species (Castagnone-Sereno, 2006) is most prominent in M. arenaria populations (Powers, 2004). Perhaps then, it is not surprising that such a rare host response phenotype should accompany such morphological and molecular variation.
Specimens of the appropriate stage or quantity desirable for replicates of isozyme analysis (Esbenshade and Triantaphyllou, 1990), RAPD (Cenis, 1993; Randig et al., 2002) or host range tests were lacking and unfortunately could not be included in the diagnosis. SCAR PCR assays using species-specific primers (Zijlstra et al., 2000) were not performed, as the choice of molecular markers examined for limited specimens was influenced by future utility of the sequences for broader phylogenetic studies. These assays would likely have affirmed the morphological and molecular results presented here. Positive scoring for M. arenaria through isozymes, RAPD or SCAR markers would have added further support for the diagnosis; however, confusing, new or negative results with these tests would have affirmed our conclusion that this population was an atypical variant of M. arenaria.
This study highlights the fact that the lines for species demarcation seem to be fading as new Meloidogyne species and populations are characterized. Accurate diagnosis requires a wide range of morphological and molecular characters such as those presented here. The degree of intraspecific variation relative to interspecific variation observed for the tropical RKN further highlights the need for additional characters and molecular markers that will clearly discriminate the these species as well as inform their evolutionary relationships.
Footnotes
The authors thank Bob Mulrooney, Extension Specialist, University of Delaware, for supplying the population from traveler's tree and Janete A. Brito, Florida Dept. of Agriculture and Consumer Services, for populations of M. floridensis. We thank Maria Hult, Sharon Ochs and Donna Ellington for excellent technical assistance.
Mention of a trade name or commercial product in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
This paper was edited by Paula Agudelo.
Literature Cited
- Adam MAM, Phillips MS, Blok VC. Molecular diagnostic key for identification of single juveniles of seven common and economically important species of root-knot nematode (Meloidogyne spp) Plant Pathology. 2007;56:190–197. [Google Scholar]
- Baptista dos Santos B, Lazaro Lozano LA. Occurrence of root-knot nematodes of the genus Meloidogyne in ginger from Goiás and Paraná states, Brazil. Revista de Agricultura (Piracicaba) 1993;68:269–270. [Google Scholar]
- Baum TJ, Lewis SA, Dean RA. Isolation, characterization, and application of DNA probes specific to Meloidogyne arenaria . Phytopathology. 1994;84:489–494. [Google Scholar]
- Blok VC, Phillips MS, Fargette M. Comparison of sequences from the ribosomal DNA intergenic region of Meloidogyne mayaguensis and other major root-knot nematodes. Journal of Nematology. 1997;29:16–22. [PMC free article] [PubMed] [Google Scholar]
- Blok VC, Wishart J, Fargette M, Berthier K, Phillips MS. Mitochondrial DNA differences distinguishing Meloidogyne mayaguensis from the major species of tropical root-knot nematodes. Nematology. 2002;4:773–781. [Google Scholar]
- Carneiro RMDG. Enzyme phenotypes of Meloidogyne spp. populations. Nematology. 2000;2:645–654. [Google Scholar]
- Castagnone-Sereno P. Genetic variability and adaptive evolution in parthenogenetic root-knot nematodes. Heredity. 2006;96:282–289. doi: 10.1038/sj.hdy.6800794. [DOI] [PubMed] [Google Scholar]
- Cenis JL. Identification of four major Meloidogyne spp. by random amplified polymorphic DNA (RAPD-PCR) Phytopathology. 1993;83:76–78. [Google Scholar]
- Chen P, Roberts PA, Metcalf AE, Hyman BC. Nucleotide substitution patterning within the Meloidogyne rDNA D3 region and its evolutionary implications. Journal of Nematology. 2003;35:404–410. [PMC free article] [PubMed] [Google Scholar]
- Chitwood BG. Root-knot nematodes, part I. A revision of the genus Meloidogyne Goeldi, 1887. Proceedings of the Helminthological Society of Washington. 1949;16:90–104. [Google Scholar]
- Cliff GM, Hirschmann H. Evaluation of morphological variability in Meloidogyne arenaria . Journal of Nematology. 1985;17:445–459. [PMC free article] [PubMed] [Google Scholar]
- Cofcewicz ET, Carneiro RMDG, Castagnone-Sereno P, Quénéhervé P. Enzyme phenotypes and genetic diversity of root-knot nematodes parasitising Musa in Brazil. Nematology. 2004;6:85–95. [Google Scholar]
- De Ley P, Tandingan De Ley I, Morris K, Abebe E, Mundo-Ocampo M, Yoder M, Heras J, Waumann D, Rocha-Olivares A, Burr AHJ, Baldwin JG, Thomas WK. An integrated approach to fast and informative morphological vouchering of nematodes for applications in molecular barcoding. Philosophical Transactions of the Royal Society B. 2005;360:1945–1958. doi: 10.1098/rstb.2005.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K, Dean RA, Fortnum BA, Lewis SA. Development of PCR primers to identify species of root-knot nematodes: Meloidogyne arenaria, M. hapla, M. incognita, and M. javanica . Nematropica. 2001;31:271–280. [Google Scholar]
- Eisenback JD, Hirschmann H, Sasser JN, Triantaphyllou AC. A Guide to the Four Most Common Species of Root-Knot Nematodes, (Meloidogyne species) with a pictorial key. Raleigh, NC: North Carolina State University; 1981. [Google Scholar]
- Eisenback JD, Hirschmann H, Triantaphyllou AC. Morphological comparison of Meloidogyne female head structures, perineal patterns, and stylets. Journal of Nematology. 1980;12:300–313. [PMC free article] [PubMed] [Google Scholar]
- Eisenback JD, Triantaphyllou HH. Root-knot nematodes: Meloidogyne species and races. In: Nickle WR, editor. Manuel of Agricultural Nematology. New York: Marcel Dekker; 1991. pp. 191–274. [Google Scholar]
- Esbenshade PR, Triantaphyllou AC. Isozyme phenotypes for the identification of Meloidogyne species. Journal of Nematology. 1990;22:10–15. [PMC free article] [PubMed] [Google Scholar]
- Golden AM. Preparation and mounting nematodes for microscopic observation. In: Zuckerman BM, Mai WF, Krusberg LR, editors. Plant Nematology Laboratory Manual. Amherst, MA: University of Massachusetts Agricultural Experiment Station; 1990. pp. 197–205. [Google Scholar]
- Golden AM, Birchfield W. Heterodera graminophila n. sp. (Nematoda: Heteroderidae) from grass with key to closely related species. Journal of Nematology. 1972;4:147–154. [PMC free article] [PubMed] [Google Scholar]
- Handoo ZA, Nyczepir AP, Esmenjaud D, van der Beek JG, Castagnone-Sereno P, Carta LK, Skantar AM, Higgins JA. Morphological and molecular characterization of Meloidogyne floridensis n. sp. (Nematoda: Meloidogynidae), a root-knot nematode parasitizing peach in Florida. Journal of Nematology. 2004;36:20–35. [PMC free article] [PubMed] [Google Scholar]
- Handoo ZA, Skantar AM, Carta LK, Erbe EF. Morphological and molecular characterization of a new root-knot nematode, Meloidogyne thailandica n. sp. (Nematoda: Meloidogynidae) parasitizing ginger (Zingiber spp.) in Thailand. Journal of Nematology. 2005a;37:343–353. [PMC free article] [PubMed] [Google Scholar]
- Handoo ZA, Skantar AM, Carta LK, Schmitt DP. Morphological, molecular and host-range evaluation of a Meloidogyne hapla population damaging coffee (Coffea arabica) in Maui, Hawaii. Journal of Nematology. 2005b;37:136–145. [PMC free article] [PubMed] [Google Scholar]
- Hirschmann H. Meloidogyne platani n. sp. (Meloidogynidae), a root-knot nematode parasitizing American sycamore. Journal of Nematology. 1982;14:84–95. [PMC free article] [PubMed] [Google Scholar]
- Hirschmann H. The genus Meloidogyne and morphological characters differentiating its species. In: Sasser JN, Carter CC, editors. An Advanced Treastise on Meloidogyne Volume I, Biology and Control. Raleigh, NC: North Carolina State University; 1985. pp. 79–93. [Google Scholar]
- Hooper DJ. Handling, fixing, staining and mounting nematodes. In: Southey JF, editor. Laboratory methods for work with plant and soil nematodes. 5th edition. London: Her Majesty's Stationery Office; 1970. pp. 39–54. [Google Scholar]
- Hugall A, Moritz C, Stanton J, Wolstenholme DR. Low, but strongly structured mitochondrial DNA diversity in root-knot nematodes (Meloidogyne) Genetics. 1994;136:903–912. doi: 10.1093/genetics/136.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugall A, Stanton J, Moritz C. Reticulate evolution and the origins of ribosomal internal transcribed spacer diversity in apomictic Meloidogyne . Molecular Biology and Evolution. 1999;16:157–164. doi: 10.1093/oxfordjournals.molbev.a026098. [DOI] [PubMed] [Google Scholar]
- Jepson SB. Identification of root-knot nematode (Meloidogyne species) Wallingford, UK: CAB International; 1987. [Google Scholar]
- Jeyaprakash A, Tigano MS, Brito J, Carneiro RMDG, Dickson DW. Differentiation of Meloidogyne floridensis from M. arenaria using high-fidelity PCR amplified mitochondrial AT-rich sequences. Nematropica. 2006;36:1–12. [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HB, Jr, Deerfield DW., II GeneDoc: Analysis and visualization of genetic variation. EMBNEW.NEWS. 1997;4:1–4. http://www.psc.edu/biomed/genedoc. [Google Scholar]
- Palomares Ruis JE, Vovlas N, Troccoli A, LieBanas G, Landa BB, Castillo P. A new root-knot nematode parasitizing sea rocket from Spanish Mediterranean coastal sand dunes: Meloidogyne dunesis n. sp. (Nematoda: Meloidogynidae) Journal of Nematology. 2007;39:190–202. [PMC free article] [PubMed] [Google Scholar]
- Powers T. Nematode molecular diagnostics: From bands to barcodes. Annual Review of Phytopathology. 2004;42:367–383. doi: 10.1146/annurev.phyto.42.040803.140348. [DOI] [PubMed] [Google Scholar]
- Powers TO, Harris TS. A polymerase chain reaction method for identification of five major Meloidogyne species. Journal of Nematology. 1993;25:1–6. [PMC free article] [PubMed] [Google Scholar]
- Quénéhervé P, van den Berg E, Topart P, Hostachy B. Ecological analysis of the host specificity of plant-parasitic nematodes associated with some cultivated ornamentals in Martinique (French West Indies) Nematologica. 1997;43:214–227. [Google Scholar]
- Rammah A, Hirschmann H. Meloidogyne morocciensis n. sp. (Meloidogyninae), a root-knot nematode from Morocco. Journal of Nematology. 1990;22:279–291. [PMC free article] [PubMed] [Google Scholar]
- Randig O, Bongiovanni M, Carneiro RMDG, Castagnone-Sereno P. Genetic diversity of root-knot nematodes from Brazil and development of SCAR marker specific for the coffee-damaging species. Genome. 2002;45:862–870. doi: 10.1139/g02-054. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Skantar AM, Carta LK. Molecular characterization and phylogenetic evaluation of the Hsp90 gene from selected nematodes. Journal of Nematology. 2004;36:466–480. [PMC free article] [PubMed] [Google Scholar]
- Stanton J, Hugall A, Moritz C. Nucleotide polymorphisms and an improved PCR-based mtDNA diagnostic for pathenogenetic root-knot nematodes (Meloidogyne spp.) Fundamental and Applied Nematology. 1997;20:261–268. [Google Scholar]
- Tenente GCMV, De Ley P, Tandingan De Ley I, Karssen G, Vanfleteren JR. Sequence analysis of the D2/D3 region of the large subunit rDNA from different Meloidogyne isolates. Nematropica. 2004;34:1–12. [Google Scholar]
- Thomas WK, Vida JT, Frisse LM, Mundo M, Baldwin JG. DNA sequences from formalin-fixed nematodes: Integrating molecular and morphological approaches to taxonomy. Journal of Nematology. 1997;29:250–254. [PMC free article] [PubMed] [Google Scholar]
- Tigano MS, Carneiro RMDG, Jeyaprakash A, Dickson DW, Adams BJ. Phylogeny of Meloidogyne spp. based on 18S rDNA and the intergenic region of motochondrial DNA sequences. Nematology. 2005;7:851–862. [Google Scholar]
- Williamson VM, Caswell-Chen EP, Westerdahl BB, Wu FF, Caryl G. A PCR assay to identify and distinguish single juveniles of Meloidogyne hapla and M. chitwoodi . Journal of Nematology. 1997;29:9–15. [PMC free article] [PubMed] [Google Scholar]
- Wishart J, Phillips MS, Blok VC. Ribosomal intergenic spacer: A polymerase chain reaction diagnostic for Meloidogyne chitwoodi, M. fallax, and M. hapla . Phytopathology. 2002;92:884–892. doi: 10.1094/PHYTO.2002.92.8.884. [DOI] [PubMed] [Google Scholar]
- Xu J, Liu P, Meng Q, Long H. Characterisation of Meloidogyne species from China using isozyme phenotypes and amplified mitochondrial DNA restriction fragment length polymorphism. European Journal of Plant Pathology. 2004;110:309–315. [Google Scholar]
- Ye W, Giblin-Davis RM, Davies KA, Purcell MF, Scheffer SJ, Taylor GS, Center TD, Morris K, Thomas Molecular phylogenetics and the evolution of host plant associations in the nematode genus Fergusobia (Tylenchida: Fergusobiinae) Molecular Phylogenetics and Evolution. 2007;45:123–141. doi: 10.1016/j.ympev.2007.02.027. [DOI] [PubMed] [Google Scholar]
- Zijlstra C, Donkers-Venne DTHM, Fargette M. Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterized amplified region (SCAR) based PCR assays. Nematology. 2000;2:847–853. [Google Scholar]