Abstract
BACKGROUND: Cardiorespiratory fitness (CRF) and physical activity (PA) are inversely related to the occurrence of type 2 diabetes (T2D). Both play an important role in reducing serum ferritin (SF) concentration. Increased SF concentration is considered a contributing factor for developing T2D. METHODS: The present cohort study investigated 5,512 adult participants enrolled in the Aerobics Center Longitudinal Study (ACLS) between 1995 and 2001. The subjects completed a comprehensive medical examination and a SF evaluation, and had been followed up until either diabetes onset, death, or the cut-off date of November 2007. Three CRF levels were categorized. SF quartile levels were defined by gender and menopausal status. The incidence of T2D was calculated for 10,000 person-years, and hazard ratios (HR) were computed to predict the incidence of T2D based on SF quartiles and CRF levels. RESULTS: SF concentration was significantly higher in males than in females (148.5 ± 104.7 ng/ml vs. 52.2 ± 45.9 ng/ml) and was inversely associated with CRF levels. In the high CRF group, 32.7% of participants had a low SF concentration whereas only 16.8% of participants had a high SF concentration level. After adjusting for potential confounders, male participants in the highest SF quartile level had a 1.7 times (HR: 1.67, 95% CI: 1.05, 2.66; p-trend = 0.027) increased risk for developing T2D compared with those in the lowest SF quartile group. CONCLUSION: Lower SF concentration was associated with lower risk of developing T2D in those regularly participating in CRF. The findings from this study suggest that SF concentration could be used as a diabetic predictor. Based on these results clinicians and public health professionals should promote regular physical activity or fitness to reduce the incidence of T2D.
Keywords: type 2 diabetes, ferritin, cardiorespiratory fitness, physical activity, insulin
Introduction
Serum ferritin (SF) concentration, a biomarker of iron stores, is found to be higher in diabetic individuals, in particular those with type 2 diabetes (T2D). [1-3]. A number of studies indicate that increased SF concentrations are associated with an increased incidence of T2D development. SF may affect the glucose metabolic process that impairs the insulin effect and results in T2D [4]. It may also lead to impaired hepatic insulin extraction [5-6]. Tissue and organ damage occurs when the SF titer is high [7]. If untreated, elevated SF may cause damage to pancreatic beta-cells that in turn induce abnormalities in insulin synthesis and insulin secretion, and as such eventually result in diabetes [5].
Recently, the potential role of SF in T2D has gained attention. A number of studies suggest that SF is an important contributor to inducing the onset of T2D [1, 4, 8-13]. A study conducted in Finland found that males with high body iron stores were 2.4 times more likely to have diabetes than those with lower iron stores [11]. However, little is known about the potential relationship between CRF and SF concentration levels and their combined effect on the development of T2D [14]. SF concentration has been found to be positively associated with body mass index (BMI), alcoholic intake, triglyceride levels, and elevated diastolic and systolic blood pressure [15, 16]. Some studies suggested that SF has positive associations with only plasma oxidized LDL, and not with LDL-cholesterol [7], while others found a significant association between SF concentration and cholesterol [15, 17].
Some studies reported that physical activity (PA) plays an important role in reducing SF concentration [18, 19], but others reported contradicting outcomes [16, 20]. A decrease in SF concentration was also shown to depend on the duration and frequency of PA [18, 21]. Moderate PA, defined as activity burns of 3 to 5.9 metabolic equivalents (METs) or 3.5 to 7 kcal/minute [22], was found to be more effective in lowering SF concentration than vigorous activity [20], defined as activity burns of more than 6.0 METs or more than 7 kcal/minute [22]. Others found that regular exercise could also decrease SF concentrations [23]. Another study, assessing subjects after a 24 week period, reported a significant decrease in mean SF in subjects who walked five days per week, but not in those who walked only three days per week [21]. A Finnish Study [18] showed that mean SF concentration was 16.8% lower in individuals within the highest quartile of PA (>2.6 hours per week) compared to those within the lowest quartile of activity (<0.4 hours per week), and was 19.9% lower in individuals within the highest category of PA frequency (>3 sessions per week) compared to those within the lowest activity frequency (<1 session per week). Nevertheless, other studies have proven to be inconclusive with respect to detecting significant associations between PA or CRF levels and SF concentration [16, 24].
The objectives of this study were to evaluate 1. the association between SF concentration and incidence of T2D, and 2. the effects of CRF levels on SF and its potential contribution to the development of T2D. We hypothesize that elevated SF concentration is an independent predictor of a subject's potential for developing an increased risk of T2D, and that CRF is inversely correlated to SF levels, ultimately resulting in a decreased risk of T2D.
Methods
Sample
The Aerobics Center Longitudinal Study (ACLS) is an ongoing epidemiologic study being conducted by the Cooper Institute since 1970. In this study, we analyzed 5,512 study participants enrolled into the ACLS between 1995 and 2001, who had completed 1) a comprehensive medical examination, 2) a medical survey questionnaire, and 3) who participated in follow up sessions until diabetes onset, death, or the cut-off date of November 2007. This analysis was restricted to participants who had a SF evaluation and were free from diabetes, cardiovascular diseases, and other medical conditions at baseline. The study protocol was approved by the Institutional Review Board (IRB) of the University of North Texas Health Science Center at Fort Worth, Texas, and the Cooper Institute in Dallas, Texas.
Measurements
Variables used in this study included the incidence of T2D (outcome variable), SF concentration, CRF (independent variables), demographic characteristics, family history of diabetes, and other possible identified confounders (covariates). Diabetes was diagnosed by physicians of the Cooper Institute by following the American Diabetes Association guidelines, using one of two criteria. Firstly, if fasting-plasma glucose ≥7 mmol/l (126 mg/dl) was reported at a clinical follow-up evaluation or, secondly, if a response to a health survey stated that the subject was either currently taking hypoglycemic medication or was diagnosed with T2D by their personal physician. BMI was classified into three levels: 1. normal: 18.5- 24.9 kg/m2, 2. overweight: 25.0-29.9 kg/m2, and 3. obese ≥ 30.0 kg/m2. Smoking status was categorized as having never smoked, being a former smoker, or being a current smoker. Alcohol consumption was dichotomized as light (less than 5 drinks per week) or moderate (equal to or greater than 5 drinks per week).
Cardiorespiratory fitness was measured using a maximal treadmill exercise test according to the Balke protocol [25]. CRF was categorized from the CRF quintiles across sex-age-specific groups developed by Blair et al. [26]. For this study three levels of CRF were determined: low was defined by the CRF quintile level I or the lowest 20% of the distribution; moderate was defined by the CRF quintiles II and III or the middle 40% of the distribution; and high was defined by CRF quintiles IV and V or the upper 40% of the distribution [26-28].
According to the 2000 U.S. Census, most females in the U.S. reach menopause at the age of 55 years [29]. We therefore defined pre-menopausal female as age 54 years or younger and postmenopausal females were defined as age 55 years or older at the baseline. All subjects were classified across SF quartiles as follows: SF quartile I: <80 ng/ml in males, <21 ng/ml in premenopausal females, and <37 ng/ml in postmenopausal females; SF quartile II: 80-124 ng/ml in males, 21-35 ng/ml in premenopausal females, and 37-58 ng/ml in postmenopausal females; SF quartile III: 124-188 ng/ml in males, 35-60 ng/ml in premenopausal females, and 58-90 ng/ml in postmenopausal females; SF quartile IV: <188 ng/ml in males, >60 ng/ml in premenopausal females, and <90 ng/ml in postmenopausal females. Other serum parameters, including cholesterol, HDL, glucose, fibrinogen, and hematocrit, were classified into normal, high, or low, based on Mosby's Diagnostic and Laboratory Test Reference (3rd ed.), as established by Pagana and Pagana [30].
Statistical Analysis
A chi-square test was used to define the relationship between SF categories, CRF quintile levels, and other risk factors for T2D. Person-time was calculated from the baseline examination to new-onset diabetes, the last examination or the last follow-up. Incidence rate of T2D was calculated for 10,000 person-years. Cox proportional hazards models were used to study the effect of baseline SF concentration on developing T2D. All proportionality assumptions were assessed by checking the log cumulative survival plots for exposure categories. The Wald test is used to prove that either the single or joint effect of different levels of CRF and SF concentrations in the proportional hazard model is fitted. We also checked the interaction between SF and CRF on diabetes incidence. The model with interaction does not fit the data better than a model without interaction. So the interaction is not a significant predictor and not included in the model.
Hazard ratios were computed with 95% confidence intervals (CI) to predict the incidence of T2D based on SF quartiles and CRF level after adjustment for possible confounders. Analysis of variance was used to compare the mean SF quartile among the CRF levels, the BMI levels, and other serum parametric factors across the SF quartile levels. The statistical significance level was set at α = 0.05. SAS version 9.1.3 for Windows was used to analyze all data [31].
Results
Table 1 shows the baseline characteristics of 3,748 men and 1,764 women. The participants were predominantly white (93.2%) with ages ranging from 20 to 83 years. Men had a higher mean BMI than women (29.2 ± 11.9 vs. 25.6 ± 10.0). The percentage of moderate drinkers and current smokers was higher in males (15.9% and 15.3%, respectively) than in females (2.4% and 5.7%, respectively). More than 68.5% of the women were in the high CRF level compared to approximately 52.9% of men. SF concentration levels were significantly higher in men than women. Male participants were more likely to have high cholesterol than females (14.3% and 12.0%, respectively), and to have low HDL than females (23.5% and 3.0%, respectively).
Table 1. Baseline characteristics of study subjects by gender and serum ferritin levels.
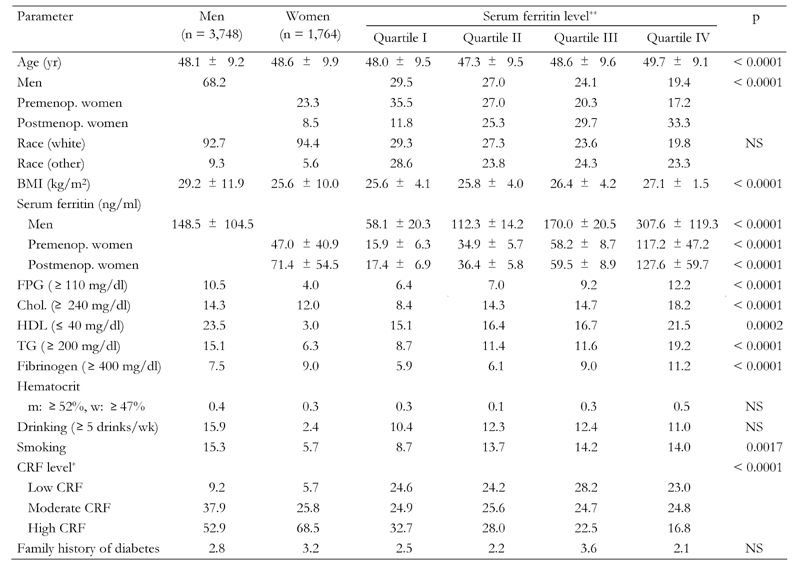
Data are percentages unless indicated as means ± SD. p-value by chi- square or analysis of variance (ANOVA). Premenop.: premenopausal. Postmenop.: postmenopausal. BMI: body mass index. FPG: fasting plasma glucose. Chol.: cholesterol. HDL: high density lipoprotein. CRF: cardiorespiratory fitness. NS: not significant. * Cardiorespiratory fitness levels classified based on age-sex-specific fitness quintiles (I: lowest; V: highest); CRF low = quintile I, CRF moderate = quintile II and III, CRF high = the two highest quintiles IV and V. ** Serum ferritin levels classified as follows: SF quartile I: <80 ng/ml in males, <21 ng/ml in premenopausal females, and <37 ng/ml in postmenopausal females; SF quartile II: 80-124 ng/ml in males, 21-35 ng/ml in premenopausal females, and 37-58 ng/ml in postmenopau-sal females; SF quartile III: 124-188 ng/ml in males, 35-60 ng/ml in premenopausal females, and 58-90 ng/ml in postmenopausal females; SF quartile IV: <188 ng/ml in males, <60 ng/ml in premenopausal females, and <90 ng/ml in postmenopausal females.
A significant relationship between CRF levels and SF concentration levels could be observed (Table 1). A higher median SF existed among participants with a low CRF level. The characteristics of serum parameters across the SF quartile levels are displayed in Table 1. The means of SF concentration were significantly higher in males, 148.0 ± 104 ng/ml, than in premenopausal females or postmenopausal females, 47.0 ± 40.9 ng/ml and 71.4 ± 54.5 ng/ml, respectively. The proportion of abnormal serum parameters including low HDL, high blood glucose, cholesterol, triglyceride levels and fibrinogen significantly increased with elevated SF concentration levels, with the exception of hematocrit.
Age- and race-adjusted hazard ratios (Table 2) were used to quantify the strength of the association between SF concentration and the risk of developing T2D. The results showed that men in the highest ferritin quartile, i.e. obese men, and men with high plasma TG all had an increased T2D risk (HR: 2.07; 95% CI: 1.33, 3.23; HR: 2.11; 95% CI: 1.33, 2.35; and HR: 2.11; 95% CI: 1.33, 2.35, respectively). Also, women with high cholesterol had an increased T2D risk (HR: 2.16; 95% CI: 1.12, 4.18). Surprisingly, former female smokers had a decreased T2D risk compared with those who had never smoked (HR: 0.20; 95% CI: 0.05, 0.84).
Table 2. Hazard ratios for development of type 2 diabetes (adjusted for age and ethnicity) among Aerobics Center Longitudinal Study participants (1995-2001).
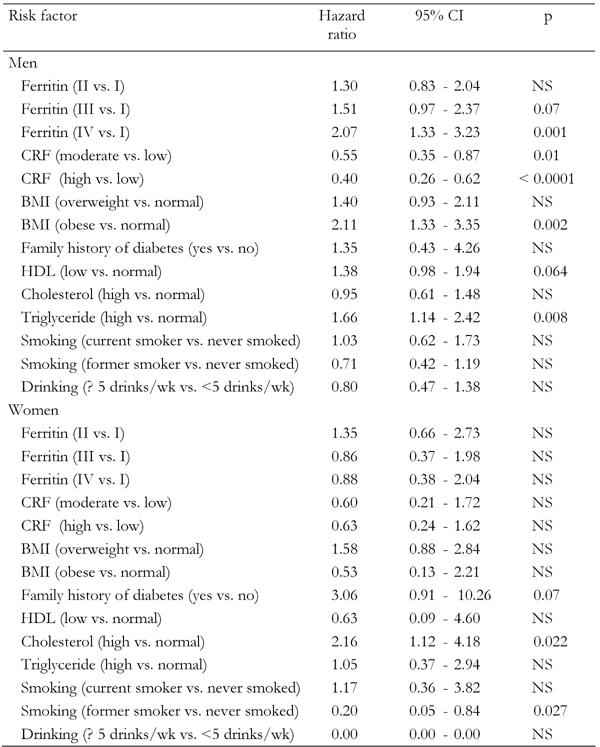
HDL: high density lipoprotein. BMI: body mass index. CRF: cardiorespiratory fitness.
Table 3 lists the age-adjusted incidence rates of T2D by the SF quartile levels. The association of high SF levels with incidence of T2D was significant only in men. The number of male new-onset diabetes cases increased monotonically from 36 (SF quartile I) to 45 (SF quartile IV). The cumulative incidence rate of T2D across SF concentrations increased from 3.4% (SF quartile I) to 6.4% (SF quartile IV) in men (Figure 1). The incidence rates increased from 72.4 new-onset cases of T2D per 10,000 person-years (SF quartile I) to 148.2 new-onset cases of diabetes per 10,000 person-years (SF quartile IV) (p = 0.0228) in men (Table 3).
Table 3. Incidence of type 2 diabetes across serum ferritin quartile levels by gender.
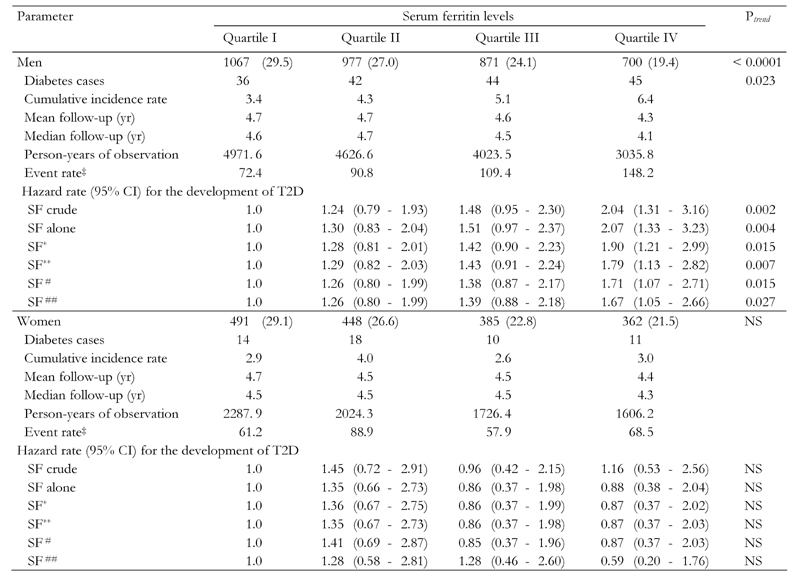
Serum ferritin (SF) levels classified as follows: SF quartile I: <80 ng/ml in males, <21 ng/ml in premenopausal females, and <37 ng/ml in postmenopausal females; SF quartile II: 80-124 ng/ml in males, 21-35 ng/ml in premenopausal females, and 37-58 ng/ml in postmenopausal females; SF quartile III: 124-188 ng/ml in males, 35-60 ng/ml in premenopausal females, and 58-90 ng/ml in postmeno-pausal females; SF quartile IV: <188 ng/ml in males, <60 ng/ml in premenopausal females, and <90 ng/ml in postmenopausal females.
Figure 1. Cumulative incidence rate of type 2 diabetes for men.
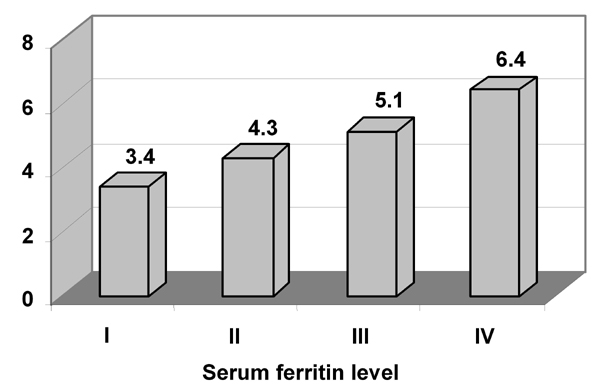
The figure shows the incidence rate of diabetes for male study participants in the four serum ferritin (SF) level quartiles. Higher SF levels were significantly associated with a higher diabetes rate. p = 0.023.
Hazard ratios for the development of T2D in different models are also shown in Table 3. The relationship between the risk of developing T2D and SF levels was significant in males. Compared to the SF quartile I category, male participants who had the highest SF quartile had a 1.67 to 2.07-fold increased T2D risk. The results per model were: model I: SF without adjustment for possible confounding factors (Ptrend = 0.002); model II: SF after adjustment for age and race (Ptrend = 0.004); model III: SF and CRF level adjusted for age, and race (Ptrend = 0.015); model IV: SF adjustment for BMI, age, and race (Ptrend = 0.007); model V: SF, CRF adjusted for BMI, age and race (Ptrend = 0.015); model VI: SF and CRF adjusted for BMI, age, race and other possible serum risk factors (Ptrend = 0.027), respectively.
Discussion
Elevated SF concentration can be one of several pathological metabolic abnormalities leading to the development of T2D. This led us to explore the relationship between SF levels and the incidence of T2D. We found that males with an elevated SF concentration (fourth quartile) were more likely to develop T2D than those who had lower SF concentration levels. These findings were consistent with those reported in the literature [13]. Females had a much lower SF concentration compared to males in the same quartile, especially premenopausal females. This may be due to losing iron during menstrual cycles [23]. In this regard, Jiang et al. reported that an elevated SF concentration is associated with an increased risk of T2D in healthy women [3].
Male subjects who were obese or overweight had a significant increased risk of developing T2D, even after adjustments for age and race. Sui et al. reported overweight and obese females had a higher T2D risk [33]. However, in our sample, females were only marginally overweight (mean BMI 25.6 ± 10.0), so the association with T2D was less apparent than in the male participants' group.
A hazard ratio of 1.67 (SF IV vs. SF I), the cumulative diabetes incidence rate, and the 10,000 person-years diabetes rate all supported the hypothesis that SF concentration directly correlated with the incidence of T2D (Table 3). These findings were consistent with those reported in the literature [3, 4, 13]. Our results relating to CRF levels included a 44% risk reduction for developing T2D among males with moderate CRF levels (HR: 0.56; 95% CI: 0.37,0.85) and 58% (HR: 0.42; 95% CI: 0.29,0.63) among males with high CRF when compared with those in the low CRF level group. These findings support other reports indicating that CRF is inversely associated with the incidence of T2D [33, 34]. Other investigators found that PA or CRF was a protective factor of T2D [7, 13].
Although no statistically significant interaction between CRF and SF concentration was identified in the model, using interaction terms, the results of this study demonstrated a higher risk of developing T2D among males associated with low fitness and high SF concentration levels. Consistent with the literature, other authors found that PA or CRF were associated with reduced levels of SF concentration or stored iron [18, 20, 21, 23, 35]. In our study, among males, the adjusted hazard ratios for the development of T2D increased by 67% for those within the highest SF quartile. This is consistent with finding from Jehn et al. demonstrating that an elevated SF level might be a cause for new onset of T2D [4].
The limitations of the study are related to the sample. Firstly, although the ACLS data has been collected since 1970, SF concentration was only measured from 1995 to 2001; this limited the number of participants and follow-up time. Secondly, our sample was comprised primarily of white, well-educated participants of a higher socio-economic class who may have a healthier nutrition than individuals from a lower socio-economic class. Thirdly, high iron-intake nutrition may influence SF concentration and the study did not address the issue of diet when assembling the participant sample. Therefore, future studies are required to further explore the effects of CRF on SF concentration and T2D among females from a larger sample population and with a longer follow-up period.
Conclusions
The number of individuals participating in PA has increased. However, the prevalence and incidence of T2D have increased sharply, possibly due to the fact that more than 60% U.S. adults who participate in PA are doing below the recommended levels. PA or CRF was found to be inversely associated with the prevalence and incidence of T2D. Increased body weight was positively associated with an increased risk of T2D. The risk of developing T2D increased substantially when both low CRF and high BMI occurred in the same individual, especially in females [33]. SF concentration was found to be an independent predictor of the development of T2D, particularly in males with high SF concentration.
Based on our study, among those with high body weight and high SF levels, CRF was found to be inversely associated with both T2D incidence and SF concentrations. The ultimate outcome was a decreased incidence of T2D. Therefore, physicians should measure SF concentrations so as to assess the individual’s potential for developing T2D and also to encourage patients to participate in PA programs in order to reduce the risk of developing T2D.
Acknowledgments
This study was supported by the National Institutes of Health grant #AG06945 and #HL62508, and by the Communities Foundation of Texas on recommendation of Nancy Ann and Ray L. Hunt. We thank Dr. Kenneth H. Cooper for establishing the Aerobics Center Longitudinal Study, and the Cooper Clinic physicians and technicians for collecting baseline data. We also thank Melba Morrow and Beth Wright for data support.
References
- 1.Acton RT, Barton JC, Passmore LV, Adams PC, Speechley MR, Dawkins FW, Sholinsky P, Reboussin DM, McLaren GD, Harris EL et al. Relationships of serum ferritin, transferrin saturation, and HFE mutations and self-reported diabetes in the hemochromatosis and iron overload screening (HEIRS) study. Diabetes Care. 2006;29(9):2084–2089. doi: 10.2337/dc05-1592. [DOI] [PubMed] [Google Scholar]
- 2.Canturk Z, Cetinarslan B, Tarkun I, Canturk NZ. Serum ferritin levels in poorly- and well-controlled diabetes mellitus. Endocr Res. 2003;29:299–306. doi: 10.1081/erc-120025037. [DOI] [PubMed] [Google Scholar]
- 3.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA. 2004;291:711–717. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- 4.Jehn ML, Guallar E, Clark JM, Couper D, Duncan BB, Ballantyne CM, Hoogeveen RC, Harris ZL, Pankow JS. A prospective study of plasma ferritin level and incident diabetes: The atherosclerosis risk in communities (ARIC) study. Am J Epidemiol. 2007;165(9):1047–1054. doi: 10.1093/aje/kwk093. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Cross-talk between iron metabolism and diabetes. Diabetes. 2002;51:2348–2354. doi: 10.2337/diabetes.51.8.2348. [DOI] [PubMed] [Google Scholar]
- 6.Niederau C, Berger M, Stremmel W, Starke A, Strohmeyer G, Ebert R, Siegel E, Creutzfeldt W. Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia. 1984;26(6):441–444. doi: 10.1007/BF00262217. [DOI] [PubMed] [Google Scholar]
- 7.Ikeda Y, Suehiro T, Yamanaka S, Kumon Y, Takata H, Inada S, Ogami N, Osaki F, Inoue M, Arii K, Hashimoto K. Association between serum ferritin and circulating oxidized low-density lipoprotein levels in patients with type 2 diabetes. Endocr J. 2006;53(5):665–670. doi: 10.1507/endocrj.k06-010. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Hernandez-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: Effects on insulin sensitivity and beta-cell function. Diabetes. 2002;51:1000–1004. doi: 10.2337/diabetes.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among U.S. adults. Diabetes Care. 1999;22:1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 10.Haap M, Fritsche A, Mensing HJ, Haring HU, Stumvoll M. Association of high serum ferritin concentration with glucose intolerance and insulin resistance in healthy people. Ann Intern Med. 2003;139:869–871. doi: 10.7326/0003-4819-139-10-200311180-00029. [DOI] [PubMed] [Google Scholar]
- 11.Salonen JT, Tuomainen TP, Nyyssonen K, Lakka HM, Punnonen K. Relation between iron stores and non-insulin dependent diabetes in men: Case-control study. BMJ. 1998;317:727. doi: 10.1136/bmj.317.7160.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Z, Hu X, Yuan B, Pan X, Meyer HE, Holmboe-Ottesen G. Association between serum ferritin, hemoglobin, iron intake, and diabetes in adults in jiangsu, China. Diabetes Care. 2006;29:1878–1883. doi: 10.2337/dc06-0327. [DOI] [PubMed] [Google Scholar]
- 13.Forouhi NG, Harding AH, Allison M, Sandhu MS, Welch A, Luben R, Bingham S, Khaw KT, Wareham NJ. Elevated serum ferritin levels predict new-onset type 2 diabetes: results from the EPIC-norfolk prospective study. Diabetologia. 2007;50(5):949–956. doi: 10.1007/s00125-007-0604-5. [DOI] [PubMed] [Google Scholar]
- 14.Lecube A, Hernandez C, Genesca J, Esteban JI, Jardi R, Garcia L, Simo R. Diabetes is the main factor accounting for the high ferritin levels detected in chronic hepatitis C virus infection. Diabetes Care. 2004;27(11):2669–2675. doi: 10.2337/diacare.27.11.2669. [DOI] [PubMed] [Google Scholar]
- 15.Galan P, Noisette N, Estaquio C, Czernichow S, Mennen L, Renversez JC, Briançon S, Favier A, Hercberg S. Serum ferritin, cardiovascular risk factors and ischaemic heart diseases: A prospective analysis in the SU.VI.MAX (SUpplementation en Vitamines et mineraux AntioXydants) cohort. Public Health Nutr. 2006;9(1):70–74. doi: 10.1079/phn2005826. [DOI] [PubMed] [Google Scholar]
- 16.Milman N, Kirchhoff M. Relationship between serum ferritin and risk factors for ischaemic heart disease in 2235 danes aged 30-60 years. J Intern Med. 1999;245:423–433. doi: 10.1046/j.1365-2796.1999.00465.x. [DOI] [PubMed] [Google Scholar]
- 17.Peach HG, Barnett NE. Relationship between serum ferritin concentration and established risk factors among men in a population with a high mortality from cardiovascular disease. [Accessed February 5, 2009];Nutrition and Dietetics: The Journal of the Dieticians Association of Australia. 2002 Available online at http://www.highbeam.com/doc/1G1-89430552.html. [Google Scholar]
- 18.Lakka TA, Nyyssonen K, Salonen JT. Higher levels of conditioning leisure time physical activity are associated with reduced levels of stored iron in Finnish men. Am J Epidemiol. 1994;140:148–160. doi: 10.1093/oxfordjournals.aje.a117225. [DOI] [PubMed] [Google Scholar]
- 19.Liu JM, Hankinson SE, Stampfer MJ, Rifai N, Willett WC, Ma J. Body iron stores and their determinants in healthy postmenopausal US women. Am J Clin Nutr. 2003;78:1160–1167. doi: 10.1093/ajcn/78.6.1160. [DOI] [PubMed] [Google Scholar]
- 20.Furqan M, Nafees M, Jilani T, Hijazi M. Relationship between physical activity and serum ferritin levels. Annals of Abbasi Shaheed Hospital and Karachi Medical and Dental College. 2002;7:306–309. [Google Scholar]
- 21.Naimark BJ, Ready AE, Sawatzky JA et al. Serum ferritin and heart disease: The effect of moderate exercise on stored iron levels in postmenopausal women. Can J Cardiol. 1996;12:1253–1257. [PubMed] [Google Scholar]
- 22.CDC. [Accessed January 7, 2009];General physical activities defined by level of intensity. 2006 Available online at http://www.cdc.gov/nccdphp/dnpa/physical/pdf/PA_Intensity_table_2_1.pdf.
- 23.Bartfay WJ, Bartfay E, Axelsson J, Sigurdsson SB, Naimark B. The relationship of serum ferritin with sex and exercise in canadians of icelandic descent: Implications for prevention of coronary artery disease. Can J Cardiol. 1995;11:305–310. [PubMed] [Google Scholar]
- 24.Bergstrom E, Hernell O, Persson LA. Endurance running performance in relation to cardiovascular risk indicators in adolescents. Int J Sports Med. 1997;18:300–307. doi: 10.1055/s-2007-972638. [DOI] [PubMed] [Google Scholar]
- 25.Balke B, Ware RW. An experimental study of physical fitness of air force personnel. U S Armed Forces Med J. 1959;10:675–688. [PubMed] [Google Scholar]
- 26.Blair SN, Kampert JB, Kohl HW 3rd, Barlow CE, Macera CA, Paffenbarger RS Jr, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- 27.Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 28.Kampert JB, Blair SN, Barlow CE, Kohl HW 3rd. Physical activity, physical fitness, and all-cause and cancer mortality: A prospective study of men and women. Ann Epidemiol. 1996;6:452–457. doi: 10.1016/s1047-2797(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 29.CDC. [February 26, 2008];Women's reproductive health: Menopause. 2006 Available online at http://www.cdc.gov/ReproductiveHealth/WomenRH/Menopause.htm.
- 30.Pagana KD, Pagana TJ. Mosby's diagnostic and laboratory test reference. 3rd ed. Mosby; 1997. [Google Scholar]
- 31.SAS Institute I. SAS version 9.1.3 for windows. Carry, NC: 2006. [Google Scholar]
- 32.Wilson JG, Lindquist JH, Grambow SC, Crook ED, Maher JF. Potential role of increased iron stores in diabetes. Am J Med Sci. 2003;325:332–339. doi: 10.1097/00000441-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Sui X, Hooker SP, Lee IM, Church TS, Colabianchi N, Lee CD, Blair SN. A prospective study of cardiorespiratory fitness and risk of type 2 diabetes in women. Diabetes Care. 2008;31:550–555. doi: 10.2337/dc07-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LaMonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol. 2005;99:1205–1213. doi: 10.1152/japplphysiol.00193.2005. [DOI] [PubMed] [Google Scholar]
- 35.Crouter SE, Fitzhugh E, Booth J, DellaValle DM, Haas JD. Relationship between iron deficiency, physical activity, and BMI in US women; NHANES 99-02. FASEB J. 2007;21:858. [Google Scholar]


