Abstract
Background
Calorie restriction increases longevity in many organisms, and calorie restriction or its mimetic might increase longevity in humans. It is unclear if calorie restriction/dieting contributes to cognitive impairment. During this randomized controlled trial, the effect of 6 months of calorie restriction on cognitive functioning was tested.
Methods
Participants (n = 48) were randomized to one of four groups: (1) control (weight maintenance), (2) calorie restriction (CR; 25% restriction), (3) CR plus structured exercise (CR + EX, 12.5% restriction plus 12.5% increased energy expenditure via exercise), or (4) low-calorie diet (LCD; 890 kcal/d diet until 15% weight loss, followed by weight maintenance). Cognitive tests (verbal memory, visual memory, attention/concentration) were conducted at baseline and months 3 and 6. Mixed linear models tested if cognitive function changed significantly from baseline to months 3 and 6, and if this change differed by group. Correlation analysis was used to determine if average daily energy deficit (quantified from change in body energy stores) was associated with change in cognitive test performance for the three dieting groups combined.
Results
No consistent pattern of verbal memory, visual retention/memory, or attention/concentration deficits emerged during the trial. Daily energy deficit was not significantly associated with change in cognitive test performance.
Conclusions
This randomized controlled trial suggests that calorie restriction/dieting was not associated with a consistent pattern of cognitive impairment. These conclusions must be interpreted in the context of study limitations, namely small sample size and limited statistical power. Previous reports of cognitive impairment might reflect sampling biases or information processing biases.
INTRODUCTION
CALORIE RESTRICTION EXTENDS the lifespan of a number of organisms1 and, in one of the first trials of calorie restriction in humans, 6 months of calorie restriction improved two biomarkers of longevity (lowered fasting insulin levels and lowered 24-hour body temperature).2 As calorie restriction research moves from animal models to human trials, it is important to measure the full effect of calorie restriction on cognition, including learning and memory.3
Calorie restriction inhibits age-related cognitive decline in rats,4 yet a number of human studies report that self-reported dieting or calorie restriction is associated with cognitive impairment, e.g., memory, attention, processing speed, and concentration deficits.5-9 However, cognitive impairment is frequently mediated by self-reported dieters’ preoccupying thoughts about food and body weight/shape.6-8,10,11 Additionally, when calorie restriction is initiated through experimental manipulations or documented by weight loss, the association between dieting and cognitive deficits is absent or diminished. For example, Green et al.12 report that 24-hour food deprivation only affected one test, a low processing load tapping task, and did not affect sustained attention, attention focus, reaction time, or immediate memory. Similarly, a 12-week diet that produced mean weight loss of 7.9 kg among obese women had little effect on cognitive performance compared to controls,13 and 50% calorie restriction among obese women did not result in long-term impairment of immediate memory or attention, rather, slower reaction time was the only consequence of calorie restriction.14 Finally, no signs of cognitive impairment were found in a group of wrestlers who lost 5% of their body weight in 5 to 10 days prior to the competition, compared to wrestlers who lost less than 1% of body weight.15
The reasons for the aforementioned discrepant findings are not clear, but it is possible that the selection of self-reported dieters affected the results. Many of the studies relied on samples of young females who reported dieting, and their performance on cognitive or neuropsychological tests were compared to people who did not report dieting. Such designs do not allow the researcher to conclude whether differences in cognitive function are due to dieting or to other characteristics of the self-reported dieters. Randomized controlled trials in which dieting or calorie restriction can be quantified are needed to clarify the relation between dieting and cognitive function.
The purpose of this study was to examine the effects of 6 months of calorie restriction on cognitive functioning during a randomized controlled trial in a sample of healthy men and women who did not report dieting upon entry into the study. Participants were randomized to one of three calorie restriction/dieting conditions or a control condition for 6 months and neuropsychological/cognitive tests, including verbal memory, visual memory, and attention/concentration, were performed at baseline and after 3 and 6 months of intervention. Change in performance on the neuropsychological tests was examined between groups, and the associations between daily energy deficit, measured by change in body energy stores, and changes in cognitive function were examined.
METHODS
Participants
Participants were 48 overweight (25 ≤ body mass index < 30) adult (25 years ≤ age < 45 years for females; 25 years ≤ age < 50 years for males) participants enrolled in a multisite calorie restriction study entitled Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE). Data were collected at the Pennington Biomedical Research Center and a description of this Center’s CALERIE trial is reported elsewhere.2 Briefly, the primary aim of CALERIE was to test for metabolic adaptation, or a reduction in metabolic rate beyond that predicted by change in body composition, during calorie restriction. Therefore, the sample size and statistical power were determined based on measures of 24-hour energy expenditure, and cognitive function was measured to test if calorie restriction negatively impacted cognitive performance. Participants provided written informed consent and the study was approved by the Institutional Review Board. Participants were provided with monetary compensation for participation.
Twelve participants were randomized to each of the following treatment groups for the 6-month study: (1) control (weight maintenance diet), (2) calorie restriction (CR; 25% CR based on baseline energy requirements), (3) CR plus structured exercise (CR + EX; 12.5% CR plus 12.5% increase in energy expenditure via structured exercise), and (4) low-calorie diet (LCD; 890 kcal/d liquid formula diet until 15% of body weight is lost, followed by weight maintenance). The randomization procedure was stratified by sex and body mass index (BMI; below or above BMI = 27.5). Participants in the CR + EX group gradually increased structured exercise over 6 weeks until a 12.5% increase above baseline energy expenditure was achieved. Energy intake was modified during this period to produce a 25% total energy deficit. Energy expenditure during exercise was closely monitored by measuring the energy costs of different levels of exercise (Vmax Sensormedics, Yorba Linda, CA) to determine the required exercise duration. Heart rate was monitored (Polar S-610, Polar Beat, Port Washington, NY) also during all exercise sessions.
The methods of the study are described elsewhere.2 Briefly, the energy required for weight maintenance at baseline was calculated from two 14-day doubly labeled water (DLW) assessments and 2 weeks of providing a weight maintenance diet at the center. During the intervention, participants were provided with meals from the Center’s Metabolic Kitchen from weeks 1 to 12 and 22 to 24 to tightly control the level of calorie restriction. During weeks 13 to 22, participants ate a self-selected diet based on their individual calorie prescriptions. Participants attended weekly group meetings led by psychologists throughout the 24-week trial where they learned techniques and a calorie counting system to adhere to their calorie target.
Daily energy deficit calculated by change in energy stores
The relation between change in cognitive function and average daily energy deficit was evaluated. As an index of calorie restriction, the energy balance of participants was quantified by calculating the change in body energy stores (fat mass and fat-free mass, measured by the mean of two consecutive measurements using dual x-ray absorptiometry at baseline and weeks 12 and 24) versus the energy intake required for weight maintenance at baseline. To convert the changes in fat mass (FM) and fat-free mass (FFM) to energy, the following energy coefficients were used: for weight loss, 1 g of FM = 9.3 kcal and 1 g of FFM = 1.1 kcal; for weight gain, 1 g FM = 13.1 kcal and 1 g FFM = 2.2 kcal.16 Therefore, the mean daily change in body energy stores from baseline to week 12 and baseline to week 24 is equal to the change in energy stored in FM plus the change in energy stored in FFM, divided by the number of days between body composition assessments. Average daily energy balance is then determined by dividing the daily change in energy stores by the daily energy intake required for weight maintenance and expressed as a percentage of baseline energy intake. Note that the above calculations did not consider the change in energy expenditure induced by weight changes.
Tests of cognitive function
Rey Auditory and Verbal Learning Test (RAVLT)
The RAVLT17 is considered a reliable and valid measure of verbal memory.17-19 When completing the RAVLT, the experimenter reads a list of 15 words and the participant recalls as many words as possible. This is repeated for five trials (Trial I through V). A distracter trial (Trial B) is then administered and memory for 15 new words is tested. Trial VI is then administered, and the participant is asked to recall words from the original list. After a 30-minute delay, the participant is asked to recall as many words as possible from the original list (Delayed Recall). Finally, a Recognition test is administered, during which participants are presented with a list that contains words that were and were not on the original list. Performance on the RAVLT is expressed in z scores (mean = 0, standard deviation [SD] = 1) based on normative data. The RAVLT has alternate forms; therefore, it can be used in serial evaluations. The RAVLT was used to quantify: (1) short-term verbal memory (Trial I-V Total score), (2) memory for stimuli presented during a distracter task (Trial B), (3) memory following the distracter task (Trial VI), (4) Delayed Recall, and (5) Recognition memory. Alternate forms were used at months 0 (baseline), 3, and 6.
Auditory Consonant Trigram (ACT)
The ACT20,21 is a test of short-term memory and retention. Three consonants, e.g., Q-D-N, are read by the examiner and the participant is asked to count backwards from a given number to prevent rehearsal of the consonants. After 9, 18, or 36 seconds, the participant recalls the three consonants. Responses are translated into z scores based on normative data. The ACT is useful for identifying short-term memory deficits22 and is a valid measure of memory functioning across age and education level, and is appropriate for serial evaluations.23
Benton Visual Retention Test (BVRT)
The BVRT24 is a reliable and valid measure of visual perception and memory.24-26 Participants are exposed to a figure for 10 seconds and are asked to reproduce the figure as accurately as possible after removal of the figure. Correct and Error scores are converted to Correct Deviation and Error Deviation scores by subtracting the participant’s score from the expected score, which is based on age and intelligence quotient. Intelligence quotient was estimated from the formula of Barona and Chastain.27 The BVRT has alternate forms and serial testing is appropriate. Different forms were used at months 0, 3, and 6. Correct Deviation and Error Deviation scores are reported. Correct Deviation scores that are 4 points less than expected (-4) are indicative of impairment. Error Deviation scores 5 or more points above expected indicate impairment.24
Conners’ Continuous Performance Test-II (CPT-II)
Attention/concentration, inattentiveness, and impulsivity were measured with the CPT-II,28 a 15-minute computer-based test that measures an individual’s ability to attend to and concentrate on prompts of visual stimuli. The CPT-II has been found to be reliable and valid and is appropriate for serial evaluations.28 Performance on the CPT-II is expressed in t scores (mean = 50, SD = 10) generated from normative data. Higher t scores on all scales indicate worse performance; t scores 60 to 64 are considered moderately atypical and t scores = 65 are markedly atypical. Low t scores on only two scales, Beta (Response Style) and Reaction Time (RT), are indicative of possible deficits, as outlined below. In the present study, the following scores were examined.
Performance profile score
Beta (Response Style) is the trade-off between speed and accuracy, and t scores > 60 suggest a cautious response style and t-scores < 40 indicate that the participant is responding to all targets and is less concerned if he/she is correct.
Inattentiveness
Omissions are the number of targets to which the participant did not respond. Higher t scores indicate sluggish responses and inattention.
Detectability is the ability to discriminate between target and nontargets.
Reaction Time (RT) is the mean reaction time for all target responses. High t scores indicate slow reaction time and inattentiveness.
Reaction Time Standard Error (RT Std. Error) is the consistency of reaction times to targets. High t scores indicate variability in reaction times due to inattentiveness.
Impulsivity
Commissions are the number of nontarget items to which the participant responds. Higher t scores indicate impulsivity.
Reaction Time (RT) is described above. Low t scores reflect a fast reaction time and, in conjunction with a large percentage of commission errors, indicate impulsivity.
Perseverations are physiologically improbable responses (< 100 msec) following stimulus presentation. High t scores indicate anticipatory responding or random responding.
Vigilance
Reaction Time (RT) Block Change is the slope of change in reaction times over the time blocks. Higher t scores suggest a slower reaction time and a loss of vigilance.
Procedure
Participants completed tests of cognitive function during a 5-day inpatient stay during baseline, and months 3, and 6. Data on a number of end points were collected, including psychological variables, neuroendocrine function, metabolism, and muscle and fat biopsies. With few exceptions, the order of procedures was maintained for each inpatient stay. Neuropsychological tests were conducted in the early afternoon.
Data analysis
Change scores for each outcome variable from baseline to month 3 and 6 were calculated (change scores = performance at follow-up minus baseline). A mixed linear model was used to test for change in performance over time, and differences among the four groups on change over time. Baseline values were entered as covariates and α was set at 0.05 for these analyses. Because the sample sizes were relatively small among the four groups (n = 12), the results were expressed also in terms of effects sizes (ES). Generalized η2, an effect size measure, was used to express the proportion of variance in change scores accounted for by treatment.29 This ES was selected since it is less affected by study design, can be compared across studies, is preferred when analysis of variance is used, and adheres to Cohen’s30 guidelines on interpreting the size of ES.29,31 Cohen’s guidelines suggest that ES of 0.02, 0.13, and 0.26 represent small, medium, and large ES, respectively.30
Correlation analysis was used to examine the association between mean daily energy deficit at month 6 and change in cognitive function. This correlation analyses included the combined data of participants in the three dieting groups. α was set at 0.01 for the correlation analysis.
RESULTS
Demographic characteristics of the study sample
The descriptive characteristics of the study sample at baseline and weight loss at month 6 are summarized in Table 1. The three dieting groups (CR, CR + EX, LCD) lost weight compared to the control group. One person dropped out of the control group for personal reasons and one LCD participant was lost to follow-up.
Table 1.
Descriptive Characteristics of the Study Sample at Baseline and Percent of Body Weight Lost at Month 6
| Control | CR | CR + EX | LCD | |
|---|---|---|---|---|
| Gender (male/female) | 5/7 | 6/6 | 5/7 | 5/7 |
| Race (W/AA/Asian or Latino) | 8/4/0 | 7/4/1 | 7/4/1 | 8/4/0 |
| Age (year) | 37 (2.1) | 39 (1.5) | 36 (1.6) | 38 (2.2) |
| Weight (kg) | 81.7 (2.6) | 80.9 (3.3) | 81.9 (3.0) | 82.0 (3.1) |
| Body mass index (kg/m2) | 27.8 (0.6) | 27.8 (0.4) | 27.5 (0.5) | 27.7 (0.5) |
| Weight loss (%) at month 6 | -1.0 (1.1) | -10.4 (0.9) | -10.0 (0.8) | -13.9 (0.7) |
| RAVLT (z-scores) | ||||
| Trial I-V | -0.34 (0.28) | -0.22 (0.28) | 0.14 (0.29) | 0.03 (0.28) |
| Trial B (Distracter) | -0.26 (0.29) | 0.03 (0.23) | 0.54 (0.34) | 0.21 (0.32) |
| Trial VI | -0.16 (0.26) | -0.02 (0.33) | -0.16 (0.33) | 0.05 (0.22) |
| Delayed Recall | 0.01 (0.25) | -0.18 (0.35) | -0.03 (0.33) | 0.10 (0.29) |
| Recognition | -0.34 (0.26) | -0.57 (0.38) | -0.74 (0.38) | -0.42 (0.37) |
| ACT (z-scores) | ||||
| 9-second delay | -0.42 (0.33) | -0.20 (0.30) | 0.32 (0.27) | 0.55 (0.20) |
| 18-second delay | -0.74 (0.29) | -0.03 (0.18) | 0.15 (0.18) | 0.08 (0.25) |
| 36-second delay | -0.01 (0.26) | 0.41 (0.30) | 0.37 (0.27) | 0.67 (0.22) |
| BVRT | ||||
| Correct Deviation | -1.42 (0.36) | -0.75 (0.43) | -1.33 (0.50) | -0.17 (0.42) |
| Error Deviation | 1.67 (0.63) | -0.08 (0.66) | 2.58 (0.97) | -0.75 (0.52) |
| CPT-II (t scores) | ||||
| Response Style (Beta) | 52.5 (4.5) | 54.7 (3.2) | 48.6 (1.1) | 55 (4.7) |
| Omissions | 48.6 (1.9) | 51.2 (2.8) | 49 (1.3) | 49.4 (3.6) |
| Detectability | 51.1 (2.4) | 46.7 (3.4) | 50.7 (1.9) | 46.1 (2.8) |
| Reaction Time (RT) | 43.9 (3.9) | 47.3 (5.1) | 38.6 (2.2) | 41.9 (2.9) |
| RT Standard Error | 51.6 (3.8) | 51.1 (3.7) | 44.8 (2.3) | 45.9 (2.9) |
| Commissions | 50.4 (2.5) | 45.4 (3.0) | 49.5 (2.3) | 45.3 (2.6) |
| Perseverations | 57 (3.9) | 49.6 (3.3) | 53.2 (7.0) | 53.3 (3.9) |
| RT Block Changes | 52.5 (4.8) | 53.7 (3.8) | 50.6 (2.7) | 57.8 (4.0) |
W, white; AA, African American; RAVLT, Rey Auditory and Verbal Learning Test; ACT, Auditory Consonant Trigram; BVRT, Benton Visual Retention Test; CPT-II, Conner’s Continuous Performance Test.
Weight loss is expressed as a percent of original body weight.
Mean performance on the cognitive tests at baseline are also represented.
Standard errors of the mean are in parentheses.
Tests of cognitive function
RAVLT
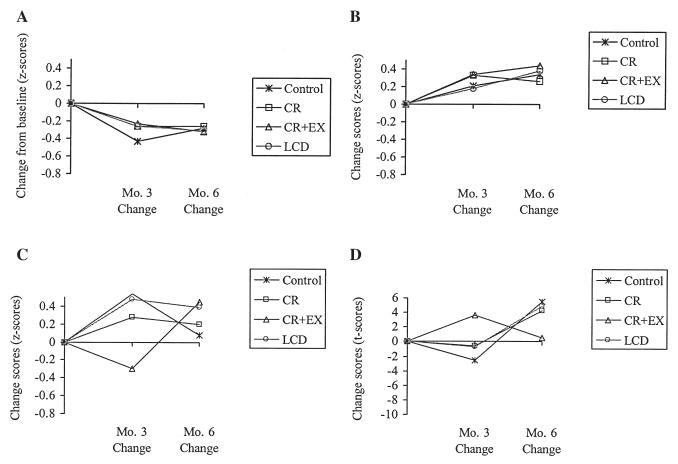
Mean performance on the RAVLT at baseline for the four groups of participants was within normal limits (mean performance at baseline is depicted in Table 1). Subsequent performance on the RAVLT indicated no consistent verbal memory performance decrements during the calorie restriction trial. Short-term verbal memory (Trial I-V) did not change significantly at month 3 (p = 0.06) or month 6 (p = 0.88), and change in performance on Trial I-IV did not differ among the groups. Table 2 contains p values for the mixed linear models that tested if change in performance at months 3 and 6 differed by group. Memory performance on the Distracter task (Trial B) decreased at months 3 and 6, indicated by marginally significant time effects (p = 0.05), although change in performance on Trial B did not differ among the groups at month 3 or 6 (Table 2) and the control group experienced a decrease in performance that was commensurate with the three dieting groups (Fig. 1A). Memory performance on Trial VI improved significantly at month 3 (p < 0.01), but not at month 6 (p = 0.89). Change in performance on Trial VI did not differ among the groups at month 3 or month 6. Memory performance on the Delayed Recall and Recognition tasks did not change during the trial (p > 0.09), and change in performance on these tasks did not differ among the groups at month 3 or 6.
Table 2.
P-Values for the Mixed Linear Models that Tested if the Groups Differed Significantly on Change Scores at Month 3 or 6. Effect Sizes, which Represent the Proportion of Variance in Change Scores Accounted for by Treatment, are also Provided
|
Mo. 3 Group Effect (p value) |
Mo. 6 Group Effect (p value) |
ES (Generalized η2) |
|
|---|---|---|---|
| Verbal Memory—RAVLT | |||
| Trial I-V | 0.32 | 0.35 | 0.04 |
| Trial B (Dist.) | 0.97 | 0.99 | 0.00 |
| Trial VI | 0.21 | 0.06 | 0.07 |
| Delayed Recall | 0.27 | 0.89 | 0.04 |
| Recognition | 0.64 | 0.36 | 0.00 |
| Verbal Memory—ACT | |||
| 9-second Delay | 0.96 | 0.97 | 0.00 |
| 18-second Delay | 0.98 | 0.34 | 0.03 |
| 36-second Delay | 0.69 | 0.21 | 0.06 |
| Visual Memory—BVRT | |||
| Correct Deviation | 0.53 | 0.93 | 0.01 |
| Error Deviation | 0.40 | 0.84 | 0.01 |
| Attention/concentration—CPT-II | |||
| Beta (Response Style) | 0.72 | 0.81 | 0.00 |
| Omissions | 0.86 | 0.30 | 0.02 |
| Detectability | 0.51 | 0.54 | 0.01 |
| Reaction time | 0.14 | 0.73 | 0.02 |
| RT Std. Error | 0.25 | 0.55 | 0.02 |
| Commissions | 0.56 | 0.27 | 0.01 |
| Perseverations | 0.47 | 0.24 | 0.04 |
| RT Block Changes | 0.70 | 0.85 | 0.02 |
RAVLT, Rey Auditory and Verbal Learning Test; ACT, Auditory Consonant Trigram; BVRT, Benton Visual Retention Test; CPT-II, Conner’s Continuous Performance Test; RT, Reaction Time.
FIG. 1.
Mean change scores by group for one subtest from each of the neuropsychological tests administered in the study.
Effect sizes (generalized η2) for the RAVLT are depicted in Table 2. The proportion of variance in change scores accounted for by treatment is small (0.00 to 0.07), indicating that treatment condition had little effect on change in memory performance over time.
ACT
Participants’ mean performance on the ACT at baseline was within normal limits (see Table 1). Performance on the ACT 9-second and 18-second Delay conditions improved significantly at months 3 (p < 0.05), and 6 (p < 0.01), but this effect was not significant for the 36-second Delay condition (p > 0.05). Change in performance on the 9-second, 18-second, and 36-second Delay conditions did not differ among the groups at month 3 or 6 (Table 2). An illustration of change in performance on the ACT 9-second Delay condition over time for each group is provided in Figure 1B. Table 2 includes effect sizes that demonstrate that the proportion of variance in change scores accounted for by treatment is small (0.00 to 0.06).
BVRT
At baseline, participants’ visual perception and visual memory performance were not impaired, as indicated by Correct Deviation and Error Deviation scores that were within normal limits (Table 1). Visual perception and memory was not negatively affected during the trial. The Correct Deviation and Error Deviation scores did not change significantly at month 3 (p > 0.25) or month 6 (p > 0.20), and change in performance did not differ among the groups at month 3 or 6. Additionally, ES calculations indicated that only 1% of the variance in change in visual memory performance over time was attributable to group (Table 2). An illustration of change in performance on the BVRT over time for each group is provided in Figure 1C.
CPT-I
Attention and concentration at baseline was within normal limits; none of the t scores exceeded the cut-score for “moderately atypical” (t score ≥ 60; see Table 1). The results of the CPT-II at month 3 and 6 indicate that attention and concentration were not impaired during the calorie restriction trial, and ES calculations indicated that only a small proportion (0.00 to 0.04) of the variance in change scores was attributable to group (Table 2).
CPT-II, Performance Profile
Participants Response Style (Beta) did not change significantly at month 3 (p = 0.99) or month 6 (p = 0.06). Change in Beta did not differ among the groups at months 3 or 6 (Table 2), and Figure 1D indicates that the change scores were similar among groups.
CPT-II, Inattentiveness
The t score for Omissions decreased significantly at month 3 (p < 0.01), indicating improved performance (fewer Omissions). This effect was not significant at month 6 (p = 0.81), and change in Omission scores did not differ among the groups at months 3 or 6 (Table 2). Detectability t scores decreased (improved) significantly at months 3 and 6 (p < 0.01), and change in Detectability scores did not differ among the groups at months 3 or 6. Reaction Time (RT) scores increased significantly at month 3 and month 6 (p values < 0.05). Change in RT scores did not differ significantly among the groups at months 3 or 6. Despite the increase in RT, the scores were within normal limits and were between t-scores of 41 and 53. Reaction Time Standard Error (RT Std. Error) t scores did not change at months 3 or 6 (p > 0.36), and the change scores did not differ among the groups at month 3 or 6.
CPT-II, Impulsivity
The Commissions t score decreased (improved) significantly at month 3 and 6 (p < 0.001), but these change scores did not differ significantly among groups. As noted earlier, Reaction Time (RT) scores increased at months 3 and 6, but change in RT scores did not differ among the groups and the scores were within normal limits. Perseveration t scores decreased (improved) significantly at month 3 (p < 0.01) and month 6 (p = 0.05), and change in Perseveration t scores did not differ among the groups at months 3 or 6.
CPT-II, Vigilance
The Reaction Time (RT) Block Change t scores did not change significantly at months 3 or 6 (p > 0.80). The change in RT Block Change scores did not differ among groups. These results suggest that there was no change in vigilance performance.
Correlation analyses
The correlation analysis indicated that daily energy deficit was not significantly associated with change in cognitive function at month 6 (α = 0.01). With the exception of an r value of 0.39 (p = 0.02) between change on Trial VI (RAVLT) and average daily energy deficit, all other r values were between -0.25 and 0.23 (p > 0.15).
DISCUSSION
The results of this randomized controlled trial indicate that calorie restriction or dieting, marked by significant weight loss and quantification of average daily energy deficit from change in energy stores, is not associated with a consistent pattern of deficits in verbal memory, visual memory, or attention/concentration performance. Only one of the verbal memory subtests, Trial B on the RAVLT, demonstrated a decrease in performance during the trial, but change in verbal memory was not a function of group assignment and the control group experienced a decrease commensurate with the three dieting groups (see Fig. 1A). Verbal memory performance, measured with the ACT, improved during the trial and change scores did not differ among the groups. Performance on the BVRT indicated that visual perception and memory was not negatively affected by calorie restriction/dieting. Additionally, attention/concentration was not negatively affected by calorie restriction, as demonstrated by improved performance on four of the CPT-II subtests. Change in performance on the CPT-II was not associated with group assignment. Lastly, effect size calculations (generalized eta squared) indicated that the proportion of variance in change scores accounted for by treatment was small across cognitive tests (0.00 to 0.07), and daily energy deficit was not associated with change scores on any test.
The results of this randomized controlled trial do not support previous studies reporting that self-reported dieting was associated with cognitive functioning deficits.5-9 Rather, this study supports research that found no relation or small inconsistent relations between dieting/calorie restriction and cognitive impairment.12-15
Daily energy deficit or calorie restriction in this study was documented through weight loss and change in body energy stores. Interpretation of the results of this study in the context of previous findings suggests that overt calorie restriction is not the reason for diminished cognitive function among self-reported dieters. Rather, self-reported dieters appear to have greater preoccupation with food and their body size/shape, and this preoccupation frequently mediates the association between dieting and cognitive impairment.6-11 Therefore, cognitive deficits among self-reported dieters could be due to the allocation of mental resources to task irrelevant cognitions, which limits mental resources for other cognitive tasks.10 If this is true, it suggests that cognitive deficits of self-reported dieters are similar to information processing biases that have been demonstrated in eating disorder samples and people who are overly concerned about their body weight/shape,32 many of whom score high on measures of dietary restraint. Indeed, self-reported restraint has been found to influence performance on cognitive tests. Green et al.33 found that high restraint dieters had the worst performance on cognitive tests, with a high restraint/nondieting group performing intermediately between nondieters and the high-restraint dieters. These results demonstrate the effect of restraint on cognitive function, even in the absence of self-reported dieting.
This study represents an important step toward examining the full effect of calorie restriction on cognition, which is necessary when studying calorie restriction in humans.3 The results of the present study add to the literature by addressing some of the limitations of previous studies. First, a group of nondieting middle-age men and women were recruited for this randomized controlled trial. Second, participants were randomized to one of three dieting conditions or a control condition, and cognitive function was measured at baseline (before dieting) and follow-up with validated neuropsychological instruments that are appropriate for serial evaluations. Third, daily energy deficit was quantified by change in body energy stores and the association between average daily energy deficit and change in cognitive function was examined.
The results of the present study must be interpreted in the context of its limitations. The most significant limitation includes the small sample size of each group (n = 12) and limited statistical power to detect group differences. This weakness affects the confidence with which the conclusions of the study are supported by the results. Effect sizes were calculated for each cognitive test to obtain a quantitative measure of the proportion of variance in change scores accounted for by treatment. The effect sizes are independent of sample size and consider variability, and they indicated that the proportion of variance in change scores accounted for by treatment was small. To further explore the extent to which the conclusions of the study were limited by small sample size, the number of participants per group needed to detect significant (α = 0.05) differences between groups was calculated based on the observed effect size.
The calculations for the verbal memory tests indicated that 32 participants per group were needed for the RAVLT Trial VI subtest, which had the largest effect size (0.07). For the RAVLT Trial I-V score and the ACT 36 second delay subtests, 51 and 72 participants per group were needed to detect a significant difference between groups. Nevertheless, the ACT 36-sec delay data indicated that every group except the CR group experienced increased performance. All other verbal memory tests required 87 (RAVLT, Delayed Recall) to more than 167 participants per group to detect significant differences. These analyses suggest that group differences could be detected with relatively large group sample sizes for two or three of the verbal memory subtests, but the other five to six subtests require quite large sample sizes that would likely be considered impractical in randomized controlled trials.
Furthermore, the small effect sizes for these tests (effect sizes range from 0.00 to 0.07) suggest that the effect of CR on cognitive function may not be clinically meaningful. Consequently, the conclusion that no consistent pattern of verbal memory performance deficits was apparent during the trial appears valid, although this conclusion is qualified by low statistical power and additional research with larger sample sizes is needed.
The visual memory measures had very small effect sizes (0.01) and required more than 492 participants per group to detect changes. Similarly, the attention/concentration subtests of the CTP-II had small effect sizes (0.00 to 0.04) and the number of participants per group required to detect significant differences ranged from 90 for the CPT-II Perseverations subtest to more than 190 for the other seven subtests. Therefore, it appears that the conclusion that no consistent patterns of visual memory or attention/concentration deficits are present is valid, although this conclusion is qualified by the small sample size of the study. Future research is needed that includes larger samples and additional tests of visual memory.
The small sample size also limited the ability to examine potential differences in change in cognitive function between dieting groups (CR and LCD) and participants who achieved similar energy deficit through exercise. Exercise has been found improve psychological well-being, particularly among men,34 but differential effects of energy deficit from exercise and dieting on cognitive function have not been thoroughly examined.
In conclusion, this study found no consistent evidence of deficits in verbal memory, visual memory, or attention/concentration associated with calorie restriction or dieting. Further research is needed with larger sample sizes to determine if self-reported dieters display information processing biases that occupy mental resources and negatively impact performance on certain cognitive tests.
ACKNOWLEDGMENTS
Emily York-Crowe was affiliated with the Pennington Biomedical Research Center at the time that the research was conducted. The authors want to thank the CALERIE participants and the remaining members of the Pennington CALERIE Research Team including: Leonie Heilbronn, James P. DeLany, Lilian de Jonge, D. Enette Larson Meyer, Steven R. Smith, Tuong Nguyen, Marlene M. Most, Anthony Alfonso, Catherine Champagne, Brenda Dahmer, Andy Deutsch, Paula Geiselman, Jennifer Howard, Jana Ihrig, Michael Lefevre, Darlene Marquis, Connie Murla, Jennifer Rood, Aimee Stewart and Vanessa Tarver. We also want to thank Health and Nutrition Technology, Carmel, CA for providing us with the HealthOne formula used in the study, and Health Management Resources (HMR™; Boston, MA) for permission to use the HMR Calorie System©.
This work was supported by the following grants: U01 AG20478 (PI: Eric Ravussin, Ph.D.) and 1 K23 DK068052-01A2 (PI: Corby Martin, Ph.D.). Leanne M. Redman, Ph.D. is supported by a Training Fellowship awarded by the NHMRC of Australia (ID 349553).
REFERENCES
- 1.Walford RL, Harris SB, Weindruch R. Dietary restriction and aging: historical phases, mechanisms and current directions. J Nutr. 1987;117:1650–1654. doi: 10.1093/jn/117.10.1650. [DOI] [PubMed] [Google Scholar]
- 2.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vitousek KM, Manke FP, Gray JA, Vitousek MN. Calorie restriction for longevity: II-The systematic neglect of behavioural and psychological outcomes in animal research. Eur Eating Disord Rev. 2004;12:338–360. [Google Scholar]
- 4.Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiol Aging. 1992;13:369–373. doi: 10.1016/0197-4580(92)90110-j. [DOI] [PubMed] [Google Scholar]
- 5.Green MW, Rogers PJ. Impaired cognitive functioning during spontaneous dieting. Psychol Med. 1995;25:1003–1010. doi: 10.1017/s0033291700037491. [DOI] [PubMed] [Google Scholar]
- 6.Green MW, Rogers PJ. Impairments in working memory associated with spontaneous dieting behaviour. Psychol Med. 1998;28:1063–1070. doi: 10.1017/s0033291798007016. [DOI] [PubMed] [Google Scholar]
- 7.Kemps E, Tiggemann M. Working memory performance and preoccupying thoughts in female dieters: evidence for a selective central executive impairment. Br J Clin Psychol. 2005;44:357–366. doi: 10.1348/014466505X35272. [DOI] [PubMed] [Google Scholar]
- 8.Kemps E, Tiggemann M, Marshall K. Relationship between dieting to lose weight and the functioning of the central executive. Appetite. 2005;45:287–294. doi: 10.1016/j.appet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Vreugdenburg L, Bryan J, Kemps E. The effect of self-initiated weight-loss dieting on working memory: the role of preoccupying cognitions. Appetite. 2003;41:291–300. doi: 10.1016/s0195-6663(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 10.Green MW, Elliman NA, Rogers PJ. Impaired cognitive processing in dieters: failure of attention focus or resource capacity limitations? Br J Health Psychol. 1997;2:259–267. [Google Scholar]
- 11.Shaw J, Tiggemann M. Dieting and working memory: preoccupying cognitions and the role of the articulatory control process. Br J Health Psychol. 2004;9:175–185. doi: 10.1348/135910704773891032. [DOI] [PubMed] [Google Scholar]
- 12.Green MW, Elliman NA, Rogers PJ. Lack of effect of short-term fasting on cognitive function. J Psychiatr Res. 1995;29:245–253. doi: 10.1016/0022-3956(95)00009-t. [DOI] [PubMed] [Google Scholar]
- 13.Bryan J, Tiggemann M. The effect of weight-loss dieting on cognitive performance and psychological well-being in overweight women. Appetite. 2001;36:147–156. doi: 10.1006/appe.2000.0389. [DOI] [PubMed] [Google Scholar]
- 14.Kretsch MJ, Green MW, Fong AK, Elliman NA, Johnson HL. Cognitive effects of a long-term weight reducing diet. Int J Obes Relat Metab Disord. 1997;21:14–21. doi: 10.1038/sj.ijo.0800353. [DOI] [PubMed] [Google Scholar]
- 15.Landers DM, Arent SM, Lutz RS. Affect and cognitive performance in high school wrestlers undergoing rapid weight loss. J Sport Exerc Psychol. 2001;23:307–316. doi: 10.1123/jsep.23.4.307. [DOI] [PubMed] [Google Scholar]
- 16.Tataranni PA, Harper IT, Snitker S, Del Parigi A, Vozarova B, Bunt J, Bogardus C, Ravussin E. Body weight gain in free-living Pima Indians: effect of energy intake vs expenditure. Int J Obes Relat Metab Disord. 2003;27:1578–1583. doi: 10.1038/sj.ijo.0802469. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M. Rey Auditory and Verbal Learning Test: A Handbook. Western Psychological Services; Los Angeles: 1996. [Google Scholar]
- 18.Geffen GM, Butterworth P, Geffen LB. Test-retest reliability of a new form of the auditory verbal learning test (AVLT) Arch Clin Neuropsychol. 1994;9:303–316. [PubMed] [Google Scholar]
- 19.Ryan JJ, Rosenberg SJ, Mittenberg W. Factor analysis of the Rey auditory verbal learning test. Int J Clin Neuropsychol. 1984;6:239–241. [Google Scholar]
- 20.Peterson LR. Short-term memory. Sci Am. 1966;215:90–95. doi: 10.1038/scientificamerican0766-90. [DOI] [PubMed] [Google Scholar]
- 21.Peterson LR, Peterson MJ. Short-term retention of individual verbal items. J Exp Psychol. 1959;58:193–198. doi: 10.1037/h0049234. [DOI] [PubMed] [Google Scholar]
- 22.Lezak MD. Neuropsychological Assessment. 3rd ed Oxford University Press; New York: 1995. [Google Scholar]
- 23.Stuss DT, Stehem LL, Poirier CA. Comparison of three tests of attention and rapid information processing across six age groups. Clin Neuropsychol. 1987;1:139–152. [Google Scholar]
- 24.Sivan AB. Benton Visual Retention Test. 5th ed The Psychological Corporation, Harcourt Brace & Company; San Antonio, TX: 1992. [Google Scholar]
- 25.Larrabee GJ, Kane RL, Schuck JR, Francis DJ. Construct validity of various memory testing procedures. J Clin Exp Neuropsychol. 1985;7:239–250. doi: 10.1080/01688638508401257. [DOI] [PubMed] [Google Scholar]
- 26.Swan GE, Morrison E, Eslinger PJ. Interator agreement on the Benton Visual Retention Test. Clin Neuropsychol. 1990;4:37–44. doi: 10.1080/13854049008401495. [DOI] [PubMed] [Google Scholar]
- 27.Barona A, Chastain RL. An improved estimate of premorbid IQ for Blacks and Whites on the WAIS-R. Int J Clin Neuropsychol. 1986;8:169–173. [Google Scholar]
- 28.Conners CK. Conners’ Continuous Performance Test (CPT II) Multi-Health Systems Inc.; Toronto: 2000. [Google Scholar]
- 29.Olejnik S, Algina J. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods. 2003;8:434–447. doi: 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- 30.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Lawrence Erlbaum; Mahwah, NJ: 1988. [Google Scholar]
- 31.Bakeman R. Recommended effect size statistics for repeated measures designs. Behav Res Methods. 2005;37:379–384. doi: 10.3758/bf03192707. [DOI] [PubMed] [Google Scholar]
- 32.Sebastian SB, Williamson DA, Blouin DC. Memory bias for fatness stimuli in the eating disorders. Cogn Ther Res. 1996;20:275–286. [Google Scholar]
- 33.Green MW, Rogers PJ, Elliman NA, Gatenby SJ. Impairment of cognitive performance associated with dieting and high levels of dietary restraint. Physiol Behav. 1994;55:447–452. doi: 10.1016/0031-9384(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 34.Kiernan M, King AC, Stefanick ML, Killen JD. Men gain additional psychological benefits by adding exercise to a weight-loss program. Obes Res. 2001;9:770–777. doi: 10.1038/oby.2001.106. [DOI] [PubMed] [Google Scholar]