Abstract
Resolution of inflammation is essential. While supplementation of ω-3 fatty acids is widely used, their availability at sites of inflammation is not known. To this end, a multidisciplinary approach was taken to determine the relationship of circulating ω-3 to inflammatory exudates and the generation of resolution signals. Here, we monitored resolvin precursors in evolving exudates, which initially paralleled increases in edema and infiltrating neutrophils. We also prepared novel microfluidic chambers to capture neutrophils from a drop of blood within minutes that permitted single cell monitoring. In these, DHA-derived Resolvin D1 rapidly stopped neutrophil migration while precursor DHA did not. In second organ injury via ischemia-reperfusion, resolvin metabolically stable analogs were potent organ protectors reducing neutrophils. Together, these results indicate that circulating ω-3 fatty acids rapidly appear in inflammatory sites that require conversion to resolvins that control excessive neutrophil infiltration, protect organs, and foster resolution.
Keywords: inflammation, lipid mediators, neutrophils
Introduction
Mounting evidence indicates that the resolution of acute inflammation is a highly coordinated and biochemically active process that was once thought to be a passive event (for recent review, see ref. (1)). During inflammatory responses to infections, neutrophils first migrate into tissues to participate in host defense, and then, if successful, these tissues return to their homeostatic functions (2). Tissue level resolution programs are actively initiated and the number of tissue associated PMN is reduced (1). Thus, it is possible that excessive inflammatory responses and their progression to chronic inflammation can result from a local failure to resolve (1). The active processes by which acute inflammation is resolved to tissue homeostasis are of considerable interest since many widely occurring human diseases are associated with chronic inflammation. These include arthritis (3) and periodontal disease (4) as well as diseases that are recognized relatively recently to have aberrant inflammation as a component of the disease including asthma, cardiovascular disease and Alzheimer's disease (5-7).
The process of resolution is actively controlled in part by formation of newly described endogenous chemical mediators which as local autacoids stimulate pro-resolving mechanisms (for recent reviews, see refs. (1, 8)). These pro-resolving mediators are derived from essential fatty acids that include lipoxins from arachidonic acid (AA, C20:4) and resolvins and protectins from eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) (8) that are biosynthesized in inflammatory exudates during spontaneous resolution. When specific resolvins and/or protectins are administered in vivo, each stimulates multi-cellular tissue level responses geared to bring tissues back to homeostasis in a process coined cellular and molecular catabasis (9).
The impact of ω-3 fatty acids (EPA and DHA) themselves has been evaluated in numerous clinical studies (10-14). These reports and many others emphasize the potential benefits of dietary supplementation with ω-3 fatty acids. Along these lines, i.v. administration of ω-3 fatty acids leads to clinical improvements in patients with rheumatoid arthritis (12). In healthy subjects, ω-3 fatty acids reduce LPS-induced fever as well as inflammatory responses (13). Soy nuts, enriched with ω-3 fatty acids, improve systolic blood pressure and low-density lipoprotein cholesterol levels in hypertensive postmenopausal women (14). In children at risk of type 1 diabetes, ω-3 fatty acids reduce risk (15). DHA is widely appreciated for its neurotrophic and neuroprotection roles that require esterification into phospholipids (16, 17). DHA tissue levels also appear to be critical since in diseases such as cystic fibrosis the DHA tissue-stores appear to be depleted (18). As noted above, ω-3 fatty acids are precursors to potent families of mediators, namely resolvins and protectins that are biosynthesized in spontaneously resolving exudates and are stereoselective in their formation and actions (1). Recently, the original structural elucidation of resolvin D1 (RvD1) (19) was confirmed by total organic synthesis, and its complete stereochemistry was established as 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid (20). Thus, the relationship between circulating EPA and DHA and inflammation-resolution is of interest and a focus of this report.
The ω-3 fatty acids have been measured in humans in many studies and show a wide range. In healthy subjects, EPA levels range from ~0.5-2.8% of total fatty acids and DHA ranges from ~1.3-5.0% (21-25). For the purpose of direct comparisons see Supplementary Table 1 (online and references within). This wide range of values for humans appears to reflect diet (25). Since ω-3 fatty acids are now recognized as biosynthetic precursors to potent local mediators, their relationship between circulation and exudate formation may be relevant in the resolution of acute inflammation. Given the importance of this question, a multidisciplinary effort was undertaken to address the availability of ω-3 fatty acids to acute inflammatory exudates and to assess the direct actions on cells and tissues compared to their potent bioactive product(s), namely a resolvin.
Here, using a multi-pronged and multidisciplinary approach, we report a) that both labeled EPA and DHA from circulation rapidly appear at sites of evolving inflammatory exudates in vivo, b) in microfluidic chambers, RvD1 and not equal concentrations of its precursor directly act on single human PMN and c) metabolically stable resolvin mimetics have potent organ protective actions in murine ischemiareperfusion injury. These findings emphasize the importance of circulating levels of ω-3 fatty acids and their conversion by exudates to resolvins that directly regulate both tissue inflammation and organ injury.
Materials and Methods
Animals
All animals used in the present study were male FVB mice (Charles River Laboratories, Wilmington, MA) that were 6- to 8-weeks-old (weighing 20-25 g). They were maintained in a temperature and light-controlled environment, and had unlimited access to food (Laboratory standard rodent diet 5001 (Lab Diet, St. Louis, MO), containing EPA 1.5 %, DHA 1.9 % of total fatty acids, and tap water. Experiments were performed in accordance with the Harvard Medical School Standing Committee on Animals guidelines for animal care (Protocol no. 02570).
Chemicals
RvD1 and 17-(R/S)-methyl RvD1 were each synthesized by total organic synthesis for these experiments from starting materials of known chirality in enantiomerically and geometrically pure form* by the Organic Synthesis Core, NIH P50-DE-016191. RvE1 and the 19-p-fluorophenoxy-RvE1 methyl ester were prepared in an enantiomerically and geometrically pure form as in (26).** Physical and spectroscopic properties matching biogenic and synthetic RvD1 and RvE1were as reported (20, 27). The integrity of each synthetic resolvin and their analogs was assured by monitoring the physical properties with LC-UV-tandem mass spectrometry just prior to evaluating their biological activities.
Murine peritonitis
Male FVB mice were anesthetized with isoflurane (Hospira Inc, Lake Forest, IL). Both 1 μg d5-DHA (4Z,7Z,10Z,13Z,16Z,19Z-docosahexaenoic-21,21,22,22,22-d5 acid: Cayman Chemical, Ann Arbor, MI) and 1 μg d5-EPA (5Z,8Z,11Z,14Z,17Z-eicosapentaenoic-19, 19,20,20,20-d5 acid: Cayman Chemical, Ann Arbor, MI) in 100 μl of 2% ethanol (v/v) in saline or vehicle alone were administered by bolus tail vein injection. Approximately five minutes later, peritonitis was initiated with intraperitoneal administration of 1 mg of zymosan A (Sigma Aldrich, St. Louis, MO). For control experiments, 1 ml of saline was injected intraperitoneally. At indicated time intervals, mice were euthanized with an overdose of isoflurane, and peritoneal exudates were collected by lavage with 5 ml of DPBS without either Ca2+ or Mg2+. Exudate cells and supernatants were separated by centrifugation (10 min, 1000 rpm, 4°C), aliquots of supernatants were collected, and 4 volumes of cold acetone were added to precipitate proteins. Serum albumin and total protein levels were determined (28).
For tracer experiments, mice fasted overnight (~18h) before 500 nCi of [1-14C]-DHA (docosahexaenoic acid 4,7,10,13,16,19-[1-14C], American Radiolabeled Chemicals Inc, St. Louis, MO) was injected via the tail vein. At indicated time intervals, exudates were collected (vide supra) and the radioactivity was determined using a scintillation counter (Multi-purpose scintillation counter LS6500, Beckman Coulter, Fullerton, CA).
Differential counts and FACS analysis
Aliquots of lavage cells were assessed for total and differential leukocyte counts via light microscopy to identify individual cell types (i.e., neutrophil, monocyte, etc.). For flow cytometry analysis, aliquots of 0.5 × 106 cells were stained with 0.25 μg FITC-conjugated anti-mouse F4/80, 0.1 μg PE-conjugated anti-mouse Ly-6G, and 0.1 μg PerCP-Cy5.5-conjugated anti-mouse CD11b, or 0.1μg PE-conjugated Rat IgG2c,κ isotype control as a background stain. Cells were washed and analyzed using FACSort (BD Biosciences, San Jose, CA) (29).
Mass spectrometric analysis of deuterium labeled EPA and DHA
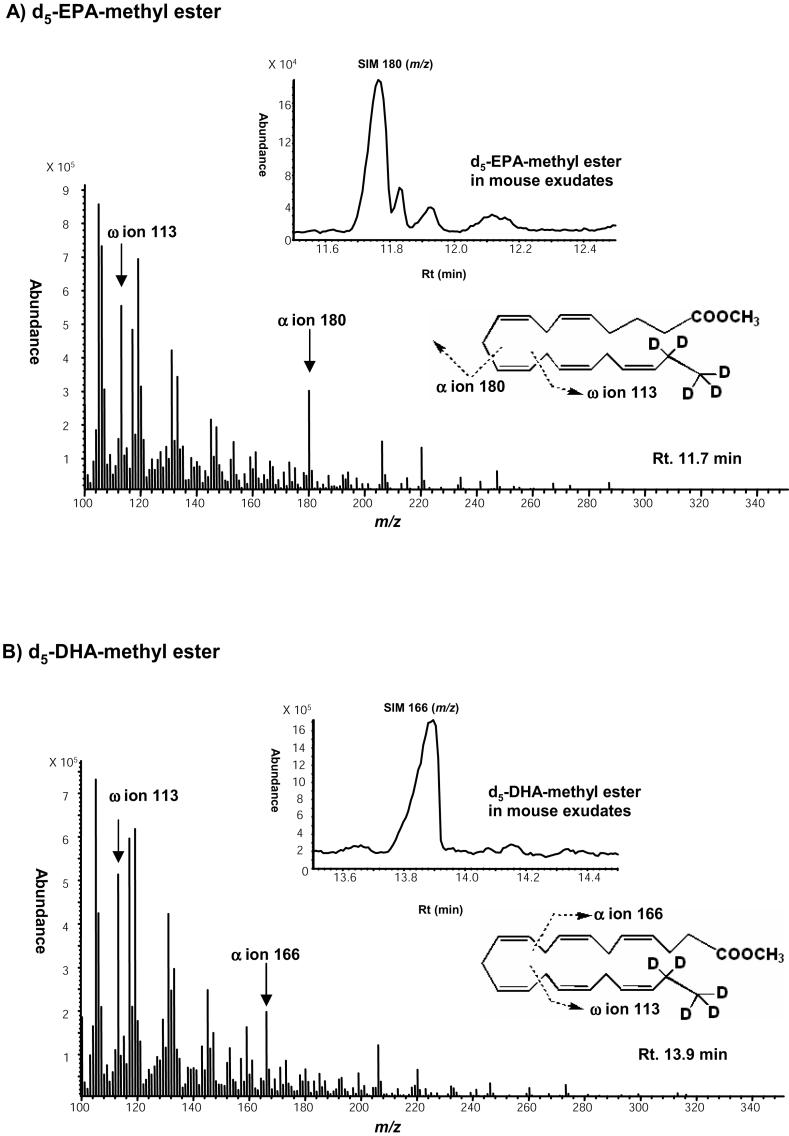
Upon collection, 2 volumes of cold methanol containing internal standard (500 ng/sample, trans-parinaric acid, Molecular Probe, Eugene, OR) were added to exudates, which were stored at -20 °C for 1 hr. After centrifugation, samples were diluted and applied to C18 cartridge columns (Extract-Clean EV SPE, Alltech, Deerfield, IL). Lipids were extracted using hexane and methyl formate and each fraction was collected and its unesterified fatty acids converted to corresponding methyl ester using diazomethane treatment (30). The esters were suspended in hexane, and then injected in GC-MS. Electron-impact GC-MS was carried out using an HP 6890 GC system with HP5973 Mass Selective Detector (Hewlett-Packard) equipped with an HP-5MS capillary column (0.25 mm ID × 30 m, 0.25 μm, Agilent Technologies Inc., Wilmington, DE) operating at a mass range from m/z 70 to 800. The ionization voltage was 70 eV and the ion source temperature was 230°C. Chromatography was carried out using a column temperature maintained at 150°C for 2 min and then programmed to increase 10°C/min up to 230°C, 5°C/min up to 280°C, and then maintained at 280°C for 10 min with a helium flow rate of 1.0 ml/min. EPA and DHA were separated and the area beneath each chromatographic peak of their deuterium labels was obtained by integration using a Chemstation integrator Version D.02.00.275 (Agilent Technologies Inc). For an internal standard, parinaric acid was selected since it exists in plants and its spectrum does not overlap with those of endogenous fatty acids for diagnostic ions. We selected the ω-3-derived ion +CH(CH=CH)CH2(CH=CH)CD2CD3=113 for both d5-EPA and d5-DHA methyl ester and M+(290) for methyl parinarate. Identification was conducted by examining and retention times as in Fig. 1 (d5-EPA-methyl ester: 11.7 min, methyl-parinarate; 13.1 min, d5-DHA; 13.9 min). Calibration curves were obtained for each: d5-EPA; y=0.0016x+0.0313 (r2=0.9954, 50-500ng/ml), d5-DHA; y=0.0015x-0.0524 (r2=0.9809, 50-500ng/ml).
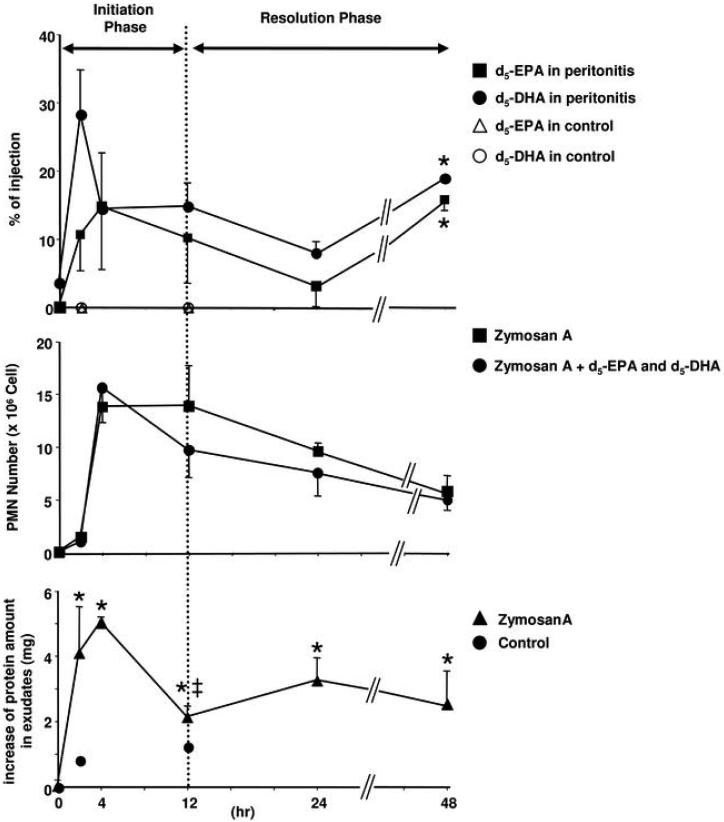
Figure 1. Circulating deuterium-labeled ω-3 fatty acids (d5-EPA and d5-DHA) appear in inflammatory exudates.
FVB mice (6-8 wk) received d5-EPA and d5-DHA i.v. (1 μg/mouse) just before i.p. challenge with zymosan A (1 μg/ml/mouse). Peritoneal exudates were collected at 0, 2, 4, 12, 24 and 48 h and taken to solid phase extraction. Eluate fractions were treated with diazomethane and taken to GC-MS. Results represent the mean ± SEM from n=3, separate mice.
Mass spectra of d5-EPA methyl ester and d5-DHA methyl ester. d5-EPA and d5-DHA were derivatized into methyl esters and injected into GC-MS.
A) Mass spectrum of d5-EPA methyl ester and SIM chromatogram of d5-EPA in mouse exudate. The ω-3 derived ion (+CH(CH=CH)CH2(CH=CH)CD2CD3=113) and α ion 180 were selected as diagnostic ions. The retention time was 11.7 min.
B) Mass spectrum of d5-DHA methyl ester and SIM chromatogram of d5-DHA in mouse exudate. The ω-3 ion 113 and a ion 160 were selected as diagnostic ions. The retention time was 13.9 min.
C) Time course of d5-EPA and d5-DHA in peritoneal exudates. Upper panel: Results shown as % of i.v. injection; closed squares: d5-EPA; closed circles: d5-DHA; open triangles: d5-EPA in control (absence of zymosan A); open circles: d5-DHA in control (absence of zymosan A). Results represent the mean ± SEM from n=3, *, Significantly different from 24 h, p< 0.05. Control: each two separate experiments.
Middle panel: Time course of PMN infiltration. Closed squares: zymosan A treatment; closed circles: zymosan A + d5-EPA and d5-DHA. Results represent the mean ± SEM from n=3. Lower panel: Closed triangle: Increase of total protein amount in peritoneal exudates without d5-EPA and d5-DHA. Results represent the mean ± SEM from n=7~20 (35). *, Significantly different from 0 h, p<0.05. ‡, significantly different from 4 h, p<0.05. Closed circle: Increase of total protein amount in peritoneal exudates of control mice. Mice were injected i.v. with d5-DHA and d5-EPA, and 5 min later 1 ml of saline was administered i.p. T0= total protein was 1.67 mg with zymosan A, and in the absence of zymosan A was 1.17 mg. Control experiment: each two separate experiments.
Ischemia-reperfusion-induced second-organ injury
Mice were anesthetized by intraperitoneal injection of pentobarbital (80 mg kg-1, Nembutal sodium solution NDC 0074-3778-04). Hind-limb ischemia was initiated using tourniquets consisting of a rubber band placed on each hind limb as in ref. (31). Mice were subjected to hind limb ischemia for 1 h, after which the tourniquets were removed to initiate reperfusion. Resolvins and their analogs were each administered at 1 μg/mouse (i.e., DHA, RvD1, RvD1 methyl ester, 17-(R/S)-methyl-RvD1 methyl ester, RvE1, or 19-p-fluorophenoxy-RvE1 methyl ester) in vehicle (5 μl ethanol in 120 μl sterile saline) and compared to vehicle alone. They were administered intravenously to the tail vein ~5 min before the start of the reperfusion period. For post-reperfusion challenge, at 60 min and 90 min after tourniquet release, mice were administered 100 ng of 17-(R/S)-methyl-RvD1-methyl ester prepared as in (20) or vehicle alone intravenously. At the end of this reperfusion period (2 h), the mice were euthanized with an overdose of anesthetic and the lungs were quickly harvested, frozen in liquid nitrogen, and stored at -80°C. The right lungs were homogenized from individual mice and centrifuged, and the tissue levels of myeloperoxidase (MPO) in the resulting supernatants were determined using a mouse MPO enzyme-linked immunosorbent assay (Hycult biotechnology, Cell Sciences, Uden, The Netherlands).
Microfluidic chambers and leukocyte motility
A new microfluidic chamber was designed for testing the actions of putative novel lipid mediators on the chemotactic function(s) of neutrophils. These analyses were performed immediately after neutrophils were separated from components of whole blood within the chamber (vide infra), which routinely averaged less than 5 min, and lipid mediator (test compounds) adsorption to the chamber surfaces was prevented through the use of polyethylene glycol (PEG) coatings. The microfluidic chamber with microstructured valves was fabricated in elastomeric materials using standard microfabrication technologies (32). Briefly, two layers of poly (dimethylsiloxane) (PDMS), molded on two silicon wafers, were bonded together and on top of a glass slide. A network of channels was formed between the glass and the first layer, and control channels for the microstructured valves were formed between the first and second layers of PDMS. For details of the fabrication process, see Supplementary Materials.
After assembly, different sections of the chamber were isolated through the actuation of the valves and each section chemically modified for specific functionalities. The surfaces of the network channels in the gradient generator section were modified with PEG to prevent absorption of the lipid mediators to the PDMS surfaces (see Results section; Fig. 3 illustration). Briefly, the PDMS surface of the gradient generation networks was treated with 5% (v/v) 3-(trimethoxysilyl)-propyl acrylate (92%, Aldrich, Milwaukee, WI) in acetone and followed by rinsing the chamber with acetone. Next, poly(ethylene glycol) diacrylate (PEGDA, Aldrich) with 1% photo initiator (2,2-dimethoxy-2-phenyl-acetophenone, Aldrich) was introduced to the chamber. After 15 min, the chamber was washed with 5% H2O in acetone and with pure acetone subsequently, and then dried with air. After this surface modification procedure, the chambers were stored in the refrigerator for up to a week. The surfaces of the chemotaxis chambers, where neutrophils were captured from whole blood, were modified by physical absorption of P-selectin (10 ng/mL for 30 minutes, R&D Systems, Minneapolis, MN) followed by blocking the entire channel network with 2% human serum albumin (HSA, Sigma-Aldrich) in Hanks' balanced salt buffer (HBSS, Sigma-Aldrich) immediately before use. The separation between the different sections of the chamber during the surface modification was achieved by keeping the valves between the main channels and the gradient generators closed.
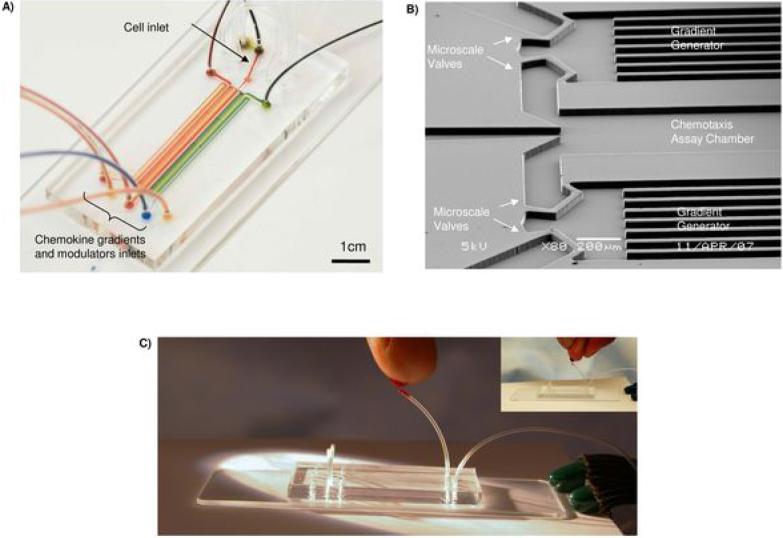
Figure 3. Microfluidic chamber for PMN chemotaxis from whole blood.
A) Overview of the microfluidic chamber for isolating neutrophils from whole blood and successive exposure of the cells to pre-formed chemokine gradients and lipid mediators.
B) Rapid switching between chemotaxis conditions was achieved by using microfluidic valves integrated on the chip, and pneumatically controlled from the outside.
C) One drop of blood from a finger stick illustrates the loading of the chamber with whole blood.
For neutrophil chemotaxis experiments, the chambers were purged with HBSS containing 0.1% HSA, with care to remove all bubbles. Four syringes, two with buffer, one with IL-8 chemoattractant (R&D Systems) and one with the lipid mediator, i.e., RvD1 or other related compounds tested in HBSS were placed in syringes (1 mL) with a syringe pump set for 0.1 μL/min. The flow was allowed to stabilize for 3 min, with the microscale valves between the chemotaxis chamber and the two gradient generators closed (see Fig. 3). Two steady-state gradients of IL-8 (0 to 10 nM) were formed in the two gradient generators, one alone and one with an overlaying uniform concentration of the lipid mediator, and were ready to be delivered to the chemotaxis chambers. Approximately 5-10 μL of capillary blood was collected from healthy volunteers by finger-prick using a BD genie lancet (Becton, Dickinson and Company, Franklin Lakes, NJ) using Protocol No. 1999-P-001297 approved by the Partners Human Research Committee. The whole blood was then quickly mixed with heparin (10 μL) in a syringe tip (30G, Small Parts). After opening the cell inlet valve, the suspension was slowly pushed through the tubing (Tygon, Small Parts) into the microfluidic chamber. The blood stayed in the main channel for 3 minutes and then the valve was opened for the two chemokine gradient generators. The flow removed the majority of red blood cells and other cells that were not tethered with the chamber attached P-selectin, thus allowing direct observation of the neutrophils captured on the surface of the chamber. After 10-15 minutes, the gradient was switched to the gradient containing the chemokine alone or either native DHA or RvD1. The migration of neutrophils in the chemokine gradient and their individual response(s) to addition of RvD1 or DHA were recorded with a video and/or CCD camera and cell migration was analyzed using the cell tracking function in Metamorph (Molecular Devices, Sunnyvale, CA, USA). At least a dozen cells per condition were tracked and analyzed for displacement in the direction of the gradient and along the flow.
Statistical analysis
Results from both in vitro and in vivo experiments were analyzed by Student's t test with p values ≤ 0.05 taken as statistically significant. The migration of neutrophils in the microfluidic chambers is presented as average and standard error of the mean displacement from the original position at the time of the gradient switching.
Results
From circulation to inflammatory exudates
To determine whether circulating ω-3 fatty acids in their unesterified form are available to evolving inflammatory exudates, we administered intravenous deuterium labeled ω-3 fatty acids, e.g., d5-EPA and d5-DHA, to mice simultaneously experiencing localized inflammatory responses, namely peritonitis. The magnitude of the inflammatory insult was selected to be a self-limited spontaneous resolving peritonitis (33) in order to monitor the potential presence of deuterium labeled fatty acids in the inflammatory exudates (Fig. 1A-B). Both deuterium labeled EPA and DHA were identified within the local inflammatory exudates within 2 h. As shown in Fig. 1C (upper panel), both d5-EPA and d5-DHA rapidly appeared in exudates during the time course of initiation of inflammation. The levels of both unesterified d5-EPA and d5-DHA gradually declined at 24 h. In this spontaneous resolution system, maximum inflammation as defined by maximum PMN infiltrates within the exudates was at ~4h and the resolution phase ranged between 12-24 h.
As depicted in Fig. 1C (middle panel), PMN infiltration into the exudates was monitored. By definition, these exudates contain serum proteins (34) that were determined within the peritoneal exudates throughout the time course (Fig. 1 lower panel, cf. ref. (35)). Of interest, the time course of both d5-EPA and d5-DHA paralleled the initial appearance of edema and increases in protein levels in these inflammatory exudates. Their presence also coincided with PMN infiltration (Fig. 1C). At 48 h, both d5-EPA and d5-DHA levels were significantly greater than at 24 h. It is noteworthy that, without inflammation (i.e., peritonitis), neither d5-EPA nor d5-DHA appeared in peritoneal lavages (Fig. 1). Thus, EPA and DHA, the precursors for resolvins and protectins, rapidly appeared within these developing inflammatory exudates from peripheral circulation and are delivered to exudates during both the initiation and resolution phases.
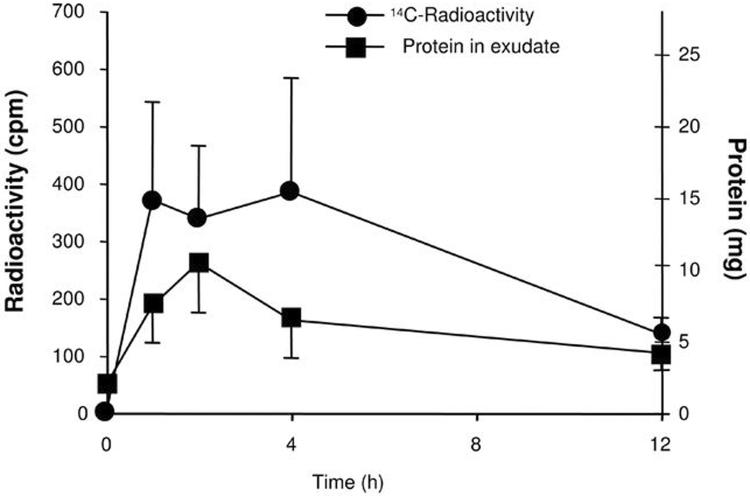
Next, we used a second approach to verify that DHA, i.e., a representative ω-3 fatty acid, indeed appears in exudates directly from peripheral circulation (Figure 2). To this end, radiolabeled ω-3 fatty acid tracer 14C-DHA was administered i.v., and we monitored recovery of 14C from DHA and the total protein amount in murine exudates. In order to reduce potential and immediate influences of diet, these experiments were performed with mice that fasted overnight before receiving intravenous 14C-DHA. Exudates were collected at 1, 2, 4 and 12 h. At 1 h, 14C label had already reached maximal exudate levels and protein levels appeared to parallel the 14C radioactivity profile. These results suggest that 14C-DHA and its products rapidly appear coincident with increases in protein levels and increases in PMN infiltration within the exudate site of inflammation. Thus, they confirm that tracer levels of 14C-DHA and its initial transformation products rapidly appeared within the exudates during early initiation of the inflammatory response in vivo (Fig. 2). Of note, these tracer levels of DHA did not reduce leukocyte trafficking as monitored by FACS analysis of the exudates (see Supplement, Fig. 2).
Figure 2. Rapid exudate appearance of 14C-DHA from circulation.
Each exudate was mixed with scintillation fluid and radioactivity was counted with a scintillation counter. Extracellular protein levels were determined by the Bradford method and expressed as the total amount in each sample. Three different groups of animals and experiments were performed.
Single-cell monitoring of real-time responses demonstrate the direct impact of RvD1 but not its precursor DHA
The original isolation and structure elucidation of RvD1 demonstrated its presence and potent anti-inflammatory and pro-resolution actions in spontaneous resolving exudates and disease models in vivo (19, 20). Here, we questioned whether RvD1 or its precursor DHA has direct actions with human neutrophils and specifically whether RvD1 can alter the directed movements of single cells along chemotactic gradients. This is a central point because excessive infiltration of PMN and release of pro-inflammatory mediators is a well-appreciated key event in causing tissue damage and uncontrolled inflammation. To address this, we used a 1-μl microfluidic chamber that was engineered with microstructure membranes (see Fig. 3). The chamber permitted the isolation of individual PMNs from one drop of whole blood (see Methods and Fig. 3C) following a simple finger prick. After isolation, the microstructured valves in the chamber (Fig. 3B) were used to control the timing of exposure of the captured PMNs to the two distinct chemotactic gradients (Fig. 3A) (see Methods). Images of the PMN before and during exposure to the two gradients were continuously monitored using a camera and recorded (Fig. 4A).
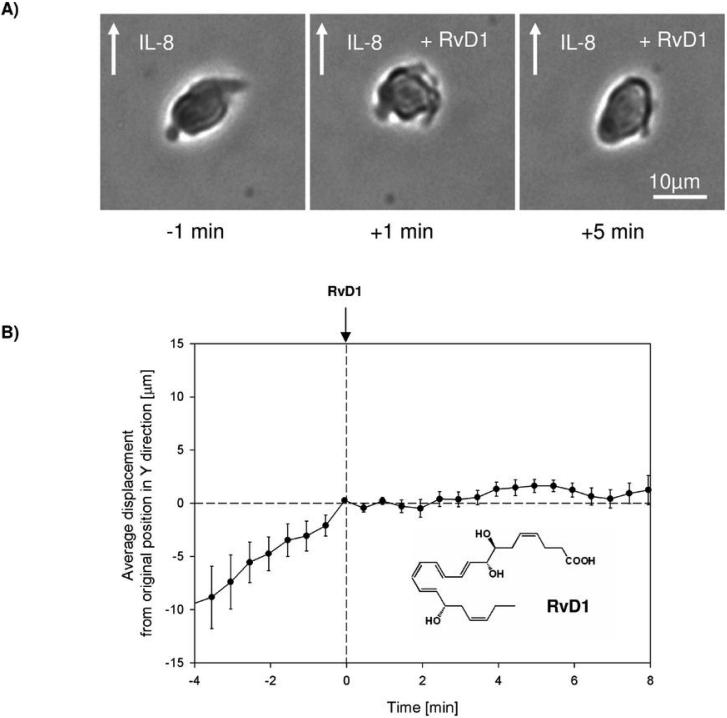
Figure 4. RvD1 stops neutrophil chemotaxis.
A) Neutrophils having polarized morphology during exposure to IL-8 gradient (- 1 min), quickly become rounded (+ 1 min) and were unable to regain their polarized morphology (+ 5 min) after exposure to RvD1.
B) Population analysis shows that normal neutrophil migration in an IL-8 gradient is immediately and effectively blocked after exposure to RvD1. Results represent the mean ± SEM, n=12.
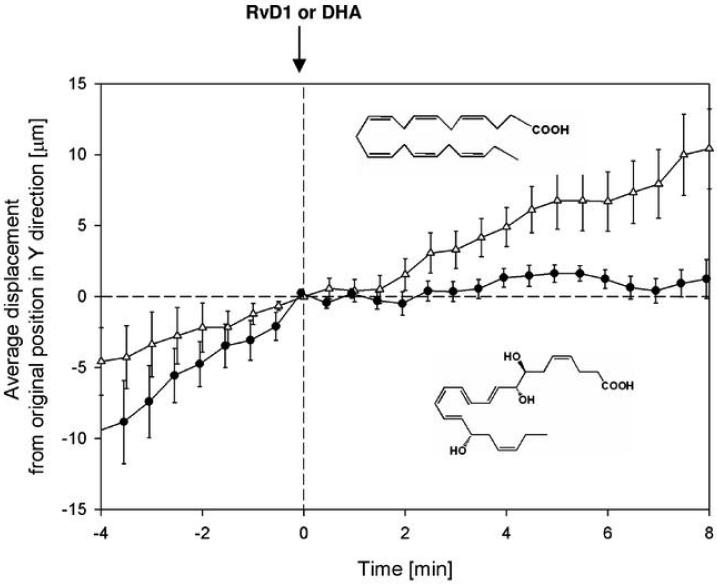
The PMN trafficked along the IL-8 gradient (see Supplement, Fig. 3) and displayed the typical shape change and morphology of PMN during chemotaxis in a linear gradient (cf. ref. (36), Fig. 4A left panel). Between 0 and 8 min, RvD1 at 10 nM was uniformly infused in the microfluidic chamber. Before exposure to RvD1, individual PMN movements and distances were proportional to time and totaled ~30-40 μm. Almost immediately on exposure to RvD1, PMN dramatically changed shape (see Fig. 4A middle panel) and ceased directed chemotactic movements (Fig. 4AB and see Supplementary Video online). Figure 4B shows the average displacement, demonstrating a highly reproducible stopping of PMN migration when exposed to RvD1 (n=12). In sharp contrast, DHA, the biosynthetic precursor to RvD1, at an equal molar dose did not stop PMN migration (Fig. 5A). Thus, by tracing the displacement of single cells in the direction of an IL-8 chemotactic gradient before and after RvD1 was introduced into the chamber, we were able to record the direct actions of RvD1 on PMN. Thus, these results establish the ability of RvD1 to essentially completely stop PMN movements, almost immediately upon exposure, as well as RvD1's ability to stimulate rapid shape changes of PMN. These single-cell-recorded PMN responses were not shared by DHA, the RvD1 metabolic precursor in exudates, when introduced at equal molar concentrations in these microfluidic chambers.
Figure 5. DHA, precursor of RvD1, failed to stop neutrophil chemotaxis.
Direct comparisons of DHA and RvD1 in an IL-8 gradient with PMN isolated from a drop of whole blood. Closed circle, RvD1; open triangle, DHA. Results represent the mean ± SEM, n=12, for each.
Resolvins are organ protective in vivo
Ischemia followed by reperfusion is a well-appreciated pathophysiologic mechanism of organ injury and local tissue damage that can be initiated by excessively activated PMN. This system in mice (Figure 6) models the second organ injury and tissue damage observed in humans following tourniquet release of vessels in surgery involving, for example, extremities (37, 38). The occlusion of blood vessels creates local ischemia and remote organ injury that has many features of rapid, acute inflammation and tissue damage (31). On release of the tourniquet occlusion, reflow is initiated and aberrantly activated PMN rapidly perfuse and infiltrate secondary organs, causing local damage (31). Here, we evaluated RvD1 in a remote organ injury system, i.e., hind-limb ischemia/reperfusion, to assess whether RvD1's ability to stop PMN migration in microchambers can in turn reduce PMN mediated tissue and organ damage in vivo. Using the time line illustrated in Figure 6 (upper panel), PMN accumulation in the lung was assessed by monitoring increases in both tissue histology and tissue MPO, a leukocyte marker enzyme. The extent of lung tissue injury is associated with PMN activation and organ infiltration (31).
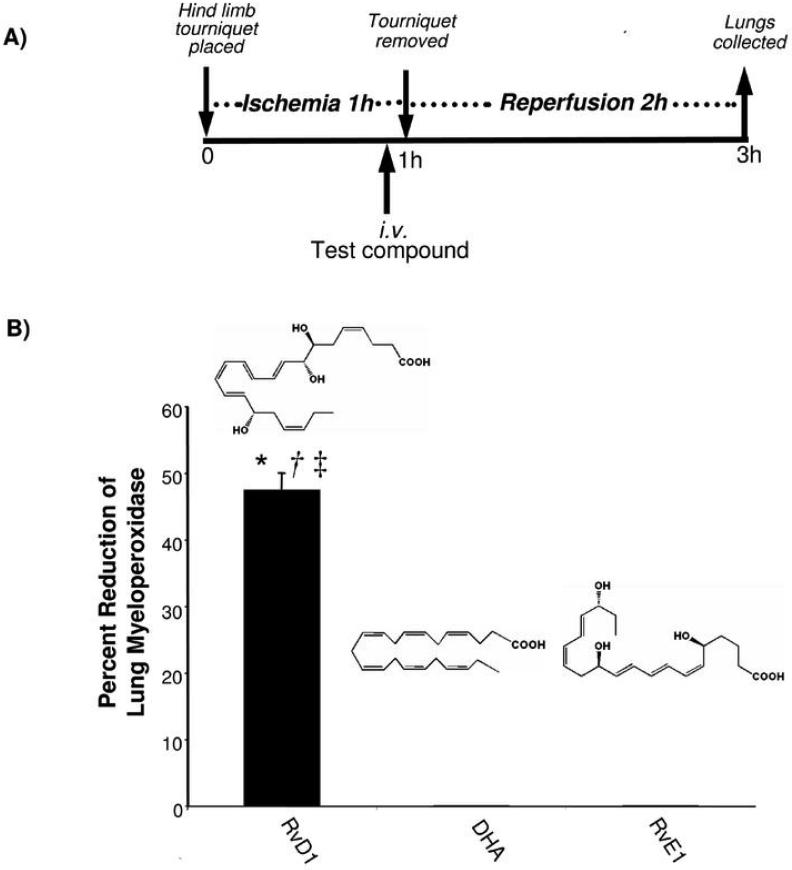
Figure 6. RvD1 protects from second organ lung injury following ischemia/reperfusion.
A) Time course of experiment.
B) RvD1 protects lung from ischemia/reperfusion injury.
Hind-limb ischemia was induced in mice by creating tourniquets using a rubber band on each hind limb. After 1 h, the tourniquets were removed and reperfusion ensued. Test compounds (1 μg of DHA, RvD1, RvE1) in vehicle were administered intravenously 5 min before the start of the reperfusion period. At the end of the reperfusion period (2 h), lungs were collected and MPO levels were determined. Results indicate % reduction of control, mean ± SEM (n=4~6, control; n=12). RvD1, solid bar; DHA, white bar; RvE1, striped bar. *, significantly different from values obtained with vehicle, p<0.02. †, significantly different from values obtained with DHA, p<0.01. ‡, significantly different from values obtained with RvE1, p<0.002.
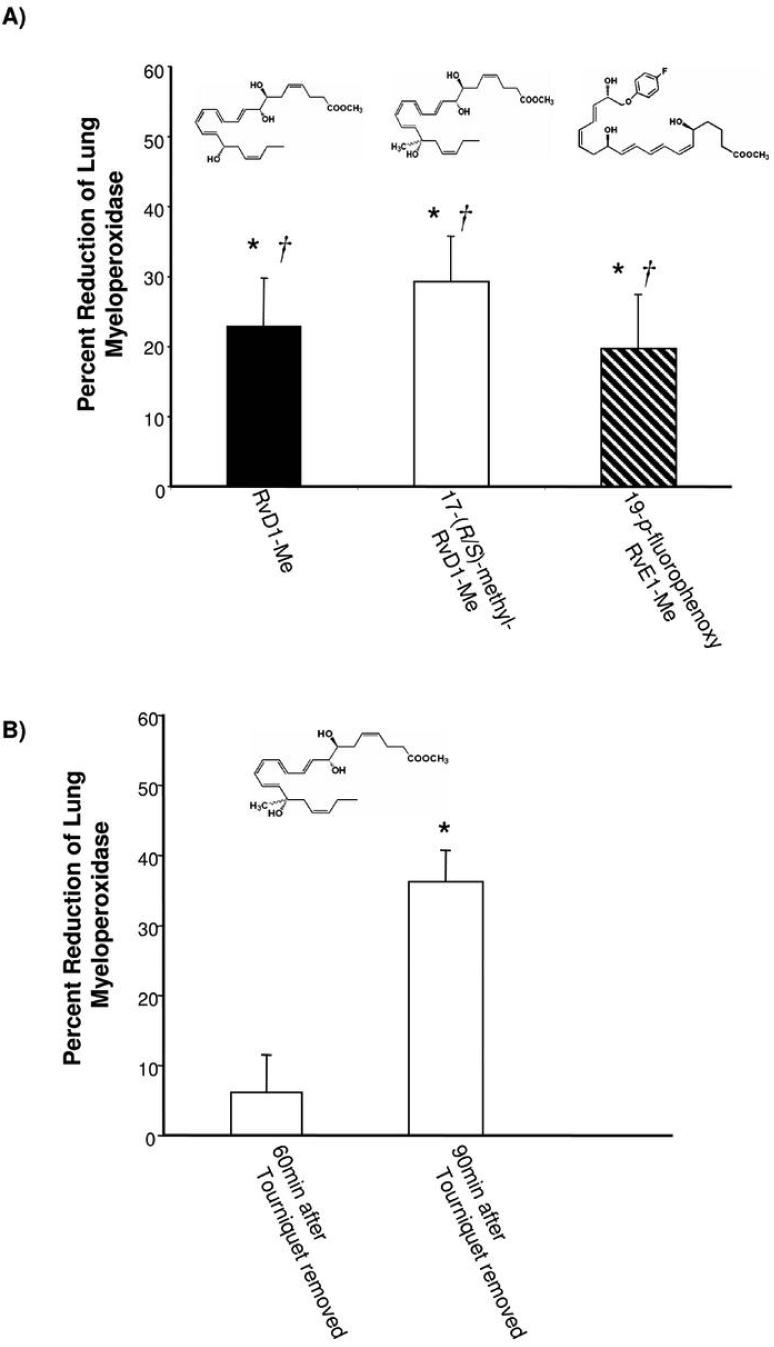
In this system, we also directly compared the actions of RvD1 to its biosynthetic precursor DHA as well as to another resolvin, namely, RvE1 which is a potent product of EPA that is anti-inflammatory in both oral inflammation (39) and colitis (reviewed in ref. (1)). RvD1, but neither RvE1 nor DHA, was able to protect the lung tissues from excessive leukocyte infiltration (Fig. 6). The histology and reduction in leukocyte infiltration (not shown) was confirmed by the reduction in the lung associated MPO values. At 1 μg/mouse, RvD1 sharply reduced ~50% leukocyte infiltration in the reperfusion tissues. In contrast at equal doses, neither DHA nor native RvE1 gave significant reduction in PMN tissue infiltration. Since RvD1 undergoes local metabolic inactivation (20) as does RvE1 (26, 40), blocking of their respective metabolic sites of inactivation within each molecule de novo was undertaken using metabolically stable analog mimetics. To this end, both 17-(R/S)-methyl RvD1 and RvD1 were prepared by total organic synthesis for in vivo administration. Both RvD1 and the related analogs (structures shown in Fig. 7) sharply reduced MPO levels in lung tissues. Of interest, the metabolically stable analog of RvE1 namely, 19-p-fluorophenoxy RvE1 at 1 μg/mouse, also significantly reduced leukocyte infiltration while native RvE1 was unable to protect lung from PMN-induced damage in this system. It is noteworthy that mouse lung tissue enzymatically converts RvE1 to 18-oxo-RvE1 (26), which is devoid of bioactivity. The 19-p-fluorophenoxy RvE1 prevents this metabolic inactivation (Fig. 7) and as documented here displayed potent protective actions dramatically reducing PMN infiltration and second organ injury of the lung. The actions of resolvins during reperfusion were investigated. Ninety minutes after tourniquet release, 100 ng of 17-(R/S)-methyl-RvD1 methyl ester was administered. The RvD1 analog lowered the increases in MPO levels in lung while, at 60 min after ischemia-reperfusion, administration of this RvD1 analog (100 ng i.v.) did not protect from second organ injury. Thus, by preventing the local metabolic inactivation of resolvins their ability to reduce PMN infiltration into distal organs was sustained for an enhanced beneficial action when given before reflow and at 90 min after reflow compared to controls.
Figure 7. Resolvin and related analogs are protective in vivo.
A) Structures and actions of compounds used in this experiment. Test compounds [1 μg of RvD1 carboxymethyl ester (RvD1-Me), 17-(R/S)-methyl-RvD1 carboxymethyl ester (17-(R/S)-methyl-RvD1-Me), and 19-p-fluorophenoxy-RvE1 methyl ester (19-pfluorophenoxy-RvE1-Me)] in vehicle were administered intravenously. Results indicate % reduction compared to control, mean ± SEM (n=3~5, control; n=12). RvD1-Me, solid bar; 17-(R/S)-methyl-RvD1-Me, white bar; 19-p-fluorophenoxy-RvE1-Me, striped bar. *, significantly different from values obtained with vehicle, p<0.02. †, significantly different from values obtained with DHA, p<0.01.
B) Post-reperfusion challenge with Resolvin D1. At 60 min and 90 min after tourniquet release, 100 ng of 17-(R/S)-methyl-RvD1-Me or vehicle was administered intravenously. Results indicate % reduction compared to parallel controls, mean ± SEM (n=3). *, significantly different from values obtained with vehicle, p<0.02.
Discussion
During the course of spontaneous resolution of acute inflammation, ω-3 fatty acids are precursors for the biosynthesis of newly uncovered anti-inflammatory and pro-resolving lipid mediators (1, 8). These new families of mediators, coined resolvins and protectins, were identified in vivo in resolving exudates and serve in molecular circuits that actively promote endogenous anti-inflammation and resolution of local inflammation (35). However, the mechanism of ω-3 fatty acid mobilization in vivo during inflammation-resolution has not been addressed. In this present report, evidence for new mechanisms is presented which indicates that unesterified ω-3 fatty acids also known as “free” fatty acids rapidly appear within the inflammatory exudates moving directly from circulation into the site of inflammation. The movement of EPA and DHA parallels those of both albumin and trafficking leukocytes. Also, single cell analyses of human PMN using a newly engineered microfluidics chamber provide direct evidence that DHA-derived resolvin D1 at nanomolar concentrations, and not its precursor DHA at equimolar levels, stops PMN chemotactic responses to spatial gradients of the chemokine IL-8.
Once formed, resolvins are active on target cells in their immediate milieu and are then inactivated by site-specific metabolism (26, 40). To enhance and prolong their actions, analogs of both RvD1 and RvE1 were prepared that delay their local inactivation in tissues, which proved to protect organs in vivo from ischemia-reperfusion injury by reducing neutrophil tissue infiltration. These results demonstrate for the first time that ω-3 levels in circulating blood are rapidly made available to sites of inflammation in vivo for their local utilization by exudates to generate potent bioactive local mediators, i.e., resolvins in situ. The resolvins in turn act directly on target cells to stimulate endogenous anti-inflammation and protect organs from excessive neutrophil infiltration and subsequent local tissue damage.
After ingestion, EPA and DHA are distributed throughout the human body (41). DHA is predominantly distributed in retina, sperm, cerebral cortex, spleen and red blood cells, whereas EPA is found in muscle, liver, spleen and red blood cells (42). For example, DHA is esterified in phospholipids of microglial cells in culture and on activation of these cell, DHA is released from the phospholipids for enzymatic processing (43, 44). DHA is also, from recent results, the precursor for two separate families of mediators that are structurally distinct, namely D series resolvins and protectins. The protectins possess potent biological actions and a conjugated triene structure as distinguishing features (19). EPA is the precursor for E series resolvins that show potent actions in several complex disease models, including inflammatory bowel disease, periodontal diseases and asthma (reviewed in ref. (45)). However, the availability of unesterified or “free” ω-3 fatty acids EPA and DHA for processing during inflammation-resolution was of interest in the present studies and required a multidisciplinary approach and new tools to address these key points.
The level of total fatty acids in human blood is ~343 mg/100 ml plasma (46). Based on this value and Suppl. Table 1, between 48 to 490 mg of EPA and DHA exist in human blood as basal levels. The contribution of de novo ω-3 fatty acids to the total amount in healthy human subjects, however, appears to be quite low. The proportion of a-linolenic acid converted to EPA is likely on the order of 0.20 to 8.0%, and the extent of conversion of α-linolenic acid to DHA is 0.05 to 4.0 % (47, 48). Thus, humans require ω-3 fatty acid intake via diet and/or supplementation. Currently, the FDA states that the dietary intake of EPA and DHA should not exceed 3 g/day (49) since excess supplementation on the order of a gram/day was found to reduce the already low endogenous EPA and DHA biosynthesis (47).
Although DHA and EPA are widely believed to possess anti-inflammatory properties themselves, the specific mechanisms responsible for these actions are still evolving. The ω-3 fatty acids are generally thought to replace the sn-2 position in phospholipid stores that is usually the positional site of esterified ω-6 fatty acids such as arachidonic acid (41). It is well appreciated that, upon activation, cells release arachidonic acid from the sn-2 position of phospholipids via cytosolic phospholipase A2 for conversion to eicosanoids. For example, among the potent bioactive eicosanoids produced by leukocytes, the prostaglandins and leukotrienes are broadly considered pro-inflammatory mediators (50). The sn-2 position of phospholipids that can become substituted with ω-3 fatty acids (DHA, EPA) is currently thought to simply compete for these enzymatic reactions, thus blocking the utilization of arachidonate and production of specific eicosanoids that are pro-inflammatory and pro-thrombotic mediators. This view is consistent with results from both cultured and isolated cells in vitro when ω-3 fatty acids are supplied to isolated cells (41). To address these points in a pathophysiologic setting in the present investigations, we determined the appearance of EPA and DHA at local sites of inflammation in exudates from circulation.
By monitoring both deuterium labeled d5-EPA and d5-DHA levels from circulation as well as increases in protein levels within exudates, we found that they appear coincident in the forming exudates. Of interest, both d5-EPA and d5-DHA were identified in exudates within 1 h of initiation of inflammation and maintained levels up to 48 h. At 48 h, both d5-EPA and d5-DHA levels were significantly greater within the exudates than their levels at 24 h. The first peak of D5 fatty acids was directly delivered from the circulation. Meanwhile, the second peak at 48 h likely reflects recirculation and PLA2 expression in the resolution of inflammation. Moreover, cPLA2 and sPLA2 were highly expressed during the resolution phase (51). The second peak at 48 h could be from esterified d5-EPA and d5-DHA and released by PLA2 mechanisms. Since in human plasma the half-life of EPA is 67 h and that of DHA is 20 h (48), the clearance of EPA and DHA is relatively slow compared to xenobiotic small molecules. Taken together, the present results suggest that circulating ω-3 fatty acids are available for sites of acute inflammation, initially, directly from circulation. The presence of albumin at sites of inflammation, by definition, determines whether the inflammatory site is considered an inflammatory exudate or transudate (37). The main protein component in the inflammatory exudates generated in the current zymosan initiated peritonitis is, indeed, serum albumin demonstrated by 2D-gel electrophoresis and proteomics (35). Albumin is a well appreciated carrier protein of unesterified fatty acids and particularly DHA (52).
Hence the present results indicate that circulating DHA and EPA are directly utilized by developing inflammatory exudates and do not require specialized mobilization from complex lipids or specific phospholipase activation to initially impact inflammation and its timely resolution. Both EPA and DHA appeared rapidly in exudates in their unesterified form coincident with edema generation and movement of circulating albumin and leukocyte trafficking into the evolving inflammatory exudates. Lundy et al. also indicated that edema formation and time course of arachidonic acid in mouse peritonitis were parallel (53). These findings in mice suggest that EPA and DHA are directly mobilized for resolvin production from the circulation via albumin as the most abundant and likely main carrier into the inflammatory sites. In humans, [13C]-DHA in phosphatidylcholine was rapidly hydrolyzed and available as a free fatty acid in plasma (54). Furthermore, after ingestion of supplement, non-esterified EPA is detected in plasma (55). Taken together, these findings imply that in humans the circulating levels of EPA and DHA do not require storage and subsequent release from complex lipid precursors in order to have an important contribution to controlling inflammation and its resolution.
Next, we questioned the relationship between circulating ω-3 fatty acids and their anti-inflammatory properties in vivo. Using a new microfluidic chamber approach to rapidly isolate human neutrophils directly from circulating whole blood via capture on P-selectin coated surface, we assessed the direct actions of both precursor DHA and RvD1 on single neutrophil chemotaxis responses. Earlier procedures required time-consuming isolation of neutrophils from whole blood prior to in vitro analyses. These protocols involved several steps of centrifugation and red blood cell lysis that usually take several hours (56) to perform that could lead to changes in the responsiveness and characteristics of the isolated neutrophils. By contrast, the P-selectin based capture of neutrophils from a single drop of whole blood (~5-10 μl) is capable of performing the PMN separation in less than 5 minutes. This short time interval is ideal for assessing the activation and /or inhibition status of neutrophils from the blood of healthy donors as well as patients. The combination of separation and assessment of shape and migration responses in the same chamber is closely akin to in vivo scenarios where neutrophils roll on the endothelial surfaces (37), stick to the endothelium in regions of higher selectin expression, and respond via chemotaxis in the gradient of a chemokine, e.g., IL-8, and migrate into tissues (see ref. (37)). The small amount of blood used in the microfluidic chamber is an additional advantage for performing these analyses with human PMN because they circumvent the need for venous phlebotomy and associated risks.
Another key feature of this microfluidic chamber system is the ability to record real-time changes in morphology of PMN upon exposure to chemokines, DHA and lipid mediators such as RvD1, as well as to track migration through switches. The fast gradient switches in the chamber allowed visual assessment and recording of the earliest events after exposure of cells to RvD1 or native DHA as well as precise measurement of these change in migration direction and velocity. Currently, no other chemotaxis systems are available that allow this level of precision or time resolution. For instance, in Boyden chambers (57), one cannot directly observe the cells, the information obtained is indirect, and the system requires a large number of cells and separate controls. The Boyden chamber is an “end point assay” and one would not be able to assess whether neutrophils migrated before or after the addition of the inhibitor, or by adding the inhibitor before cells start moving if the effect would extrapolate to chemotaxis. In the Dunn and Zigmond chambers (58, 59), although one could visualize the cells in real time, it is not possible to swiftly switch between cell incubation and exposure conditions as with the present microfluidics chambers. The engineered microstructured valves for switching between independent gradients allowed the same neutrophils to serve as positive controls for migration in a chemoattractant gradient and then probed with either RvD1 or its precursor native DHA. The preservation of chemoattractant gradient before and after the switch is important for avoiding neutrophil responses to sudden changes of chemoattractant gradient. Of interest, sudden decrements in the concentration of chemokines alone have the potential to stop neutrophil migration for 3-5 minutes (60). Thus, the present direct assessment of DHA with PMN indicates that DHA itself is not a potent bioactive `stop signal' for PMN but rather requires exudate conversion to RvD1 to evoke its signaling properties on these cells. Hence, following their actions, local tissues inactivate resolvins and permit organs to return to homeostasis.
Along these lines, ischemia-reperfusion is an event of significant clinical importance. Reperfusion related tissue injury occurs during surgical procedures, particularly those involving extremities, causing both local and remote organ injury as well as increasing costs associated with prolonged post-operative recovery (38). Briefly, when vessels are surgically clamped or occlude, stasis of blood at the occlusion site leads to local ischemia and the neutrophils in that blood become activated. Upon release of the occlusion, activated leukocytes give rise to second/or remote organ injury. For example, when neutrophils from the occluded vessels of the hind limb reach the lung they cause local tissue damage (31). In many respects, the aberrant activation of neutrophils in this scenario exhibits features similar to those in uncontrolled acute inflammation and neutrophil mediated tissue damage (31, 37, 38). Given the clinical importance and patho-physiology of this type of organ injury, we investigated the direct actions of DHA, resolvins and related stable analogs (i.e., directly comparing the actions of RvD1, its 17-(R/S)-methyl analog, RvE1, and its 19-p-fluorophenoxy analog) in ischemia-reperfusion second organ injury. At equivalent doses, DHA was not protective while RvD1 and its analog as well as the stable analog of RvE1 showed potent anti-leukocyte actions each reducing infiltration into lung tissues.
Native RvE1 itself was not able to protect the lung at these low doses likely because of local inactivation. Of interest, RvE1 itself is both anti-inflammatory and pro-resolving in several inflammatory disease models (45). Recently, RvE1 was also shown to have potent actions in preventing joint damage and cartilage destruction in collagen-induced rodent arthritis* and protects from inflammation induced bone loss in periodontal disease (39). Both RvD1 and RvE1 undergo site specific metabolic inactivation (26, 40). Thus, the RvD1 and RvE1 analogs that display potent organ protective actions as demonstrated herein (Figure 7) may provide new approaches to reduce organ damage characterized by excessive PMN infiltration. PMN are now appreciated to play an important role in the pathogenesis of many life-long chronic inflammatory diseases such as arthritis (61). Recurring bouts of unresolved local acute inflammation may underlie many chronic inflammatory diseases. Thus, new means of monitoring neutrophils from peripheral whole blood within minutes, as demonstrated here with these newly designed microfluidic chambers may have implications in selecting appropriate therapies for acute and chronic inflammatory diseases.
In summation, we tracked the movements of labeled EPA and DHA from circulation to inflammatory exudates and recorded in real time the direct actions of RvD1 on human PMN migration using a newly engineered microfluidic chamber (32, 60). Using this approach, we visualized individual cellular responses with neutrophils obtained from a single drop of peripheral blood in less than 5 min. This new system provided a unique opportunity to investigate the actions of RvD1 at the single cell level. As shown in the supplementary video (online), PMN moving along the chemotactic gradient stopped movement essentially immediately after exposure to RvD1 but not with equimolar amounts of its exudate precursor DHA.
We also demonstrate new organ protective actions of resolvins in a murine model of second organ lung injury following hind limb ischemia-reperfusion. These results emphasize the substantial contribution of unesterified ω-3 fatty acids levels in circulation and their rapid availability via edema formation in exudates to mount resolution and assist in the return of local tissues to homeostasis. Recent review of the evidence for beneficial effects of fatty acids in human inflammation indicates a poor relationship and predictive value suggesting specific dose response relationships for dietary ω-3 fatty acids, and fatty acid profiles of immune cells differ between species (62). These are consistent with our results and suggest that circulating ω-3 DHA and EPA levels and their transfer by edema rather than phospholipid esterified precursors are an initial direct source for exudate biosynthesis of resolvins, and thus should be taken into account in evaluating the impact of nutrition in humans. The new pathways uncovered here are likely to be relevant in maintaining health as well as in the many diseases characterized by excessive uncontrolled inflammation.
Supplementary Material
Acknowledgments
We thank Dr. Birgitta Schmidt (Dept. of Pathology, Children's Hospital Boston) for microscopic evaluation of tissue histology, Dr. Jasim Uddin (USC) for preparation of some of the synthetic resolvins and their analogs, and Mary Halm Small for expert assistance with manuscript preparation.
This work was supported in part by National Institute of Health Grants R37-GM38765 (C.N.S), the Specialized Center for Oral Inflammation and Resolution, P50-DE016191 (C.N.S., N.A.P.), and the BioMEMS Resource Center, P41-EB002503 (M.T., D.I.).
Abbreviations
- AA
arachidonic acid [5Z,8Z,11Z,14Z]-eicosa-5,8,11,14-tetraenoic acid
- DHA
docosahexaenoic acid, [4Z,7Z,10Z,13Z,16Z,19Z]-docosa-4,7,10,13,16,19-hexaenoic acid
- EPA
eicosapentaenoic acid, [5Z,8Z,11Z,14Z,17Z]-icosa-5,8,11,14,17-pentaenoic acid
- HBSS
Hanks' balanced salt buffer
- LX
lipoxin
- MPO
myeloperoxidase
- RvE1
resolvin E1, 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid
- RvD1
resolvin D1, 7S,8R,17S-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid
- PD1/NPD1
protectin D1/neuroprotectin D1, 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid
- PMN
polymorphonuclear neutrophils
- PDMS
poly(dimethylsiloxane)
Footnotes
K.K. is the recipient of a Postdoctoral Fellowship Award from the Arthritis Foundation.
N.A.P., J. Uddin, C.N.S., manuscript in submission.
N.A.P., R.Y., G. Bernasconi, J. Uddin, C.N.S., manuscript in submission.
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
Carillo-Rivas, B., Qin, S., Savinainen, A., Chen, C., Crandall, T., Dodge, D., Dubrovskiy, A., Lau, D., Lin, M., Shumway, M., Trocha, M., Wu, Y., Schwartz, E., Gjorstrup, P., and Wu, L. 10th International Conference on Bioactive Lipids in Cancer, Inflammation and Related Diseases (Abstract #82) (2007).
References
- 1.Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu. Rev. Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 2.Majno G, Joris I. Cells, Tissues, and Disease: Principles of General Pathology. Oxford University Press; New York: 2004. [Google Scholar]
- 3.Weissmann G. Pathogenesis of rheumatoid arthritis. J. Clin. Rheumatol. 2004;10(3 Suppl):S26–S31. doi: 10.1097/01.rhu.0000130687.75646.44. [DOI] [PubMed] [Google Scholar]
- 4.Kantarci A, Van Dyke TE. Resolution of inflammation in periodontitis. J. Periodontol. 2005;76(11 Suppl):2168–2174. doi: 10.1902/jop.2005.76.11-S.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 6.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–878. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 7.Bazan NG, Colangelo V, Lukiw WJ. Prostaglandins and other lipid mediators in Alzheimer's disease. Prostaglandins Other Lipid Mediat. 2002;68-69:197–210. doi: 10.1016/s0090-6980(02)00031-x. [DOI] [PubMed] [Google Scholar]
- 8.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat. Rev. Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 9.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GISSI-Prevenzione Investigators Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 11.Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: timecourse analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 12.Leeb BF, Sautner J, Andel I, Rintelen B. Intravenous application of omega-3 fatty acids in patients with active rheumatoid arthritis. The ORA-1 trial. An open pilot study. Lipids. 2006;41:29–34. doi: 10.1007/11745-006-5066-x. [DOI] [PubMed] [Google Scholar]
- 13.Pluess TT, Hayoz D, Berger MM, Tappy L, Revelly JP, Michaeli B, Carpentier YA, Chioléro RL. Intravenous fish oil blunts the physiological response to endotoxin in health subjects. Intensive Care Med. 2007;33:789–797. doi: 10.1007/s00134-007-0591-5. [DOI] [PubMed] [Google Scholar]
- 14.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch. Intern. Med. 2007;167:1060–1067. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 15.Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, Orton HD, Barón AE, Clare-Salzler M, Chase HP, Szabo NJ, Erlich H, Eisenbarth GS, Rewers M. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. JAMA. 2007;298:1420–1428. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 16.Lefkowitz W, Lim SY, Lin Y, Salem NN., Jr. Where does the developing brain obtain its docosahexaenoic acid: relative contributions of dietary alpha-linoleic acid, docosahexaenoic acid, and body stores in the developing rat. Pediatr. Res. 2005;57:157–165. doi: 10.1203/01.PDR.0000147572.57627.AE. [DOI] [PubMed] [Google Scholar]
- 17.Bazan NG. Omega-3 fatty acids, pro-inflammatory signaling and neuroprotection. Curr. Opin. Nutr. Metab. Care. 2007;10:136–141. doi: 10.1097/MCO.0b013e32802b7030. [DOI] [PubMed] [Google Scholar]
- 18.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, Weed DA, Gelrud A, Regan MM, Laposata M, Alvarez JG, O'Sullivan BP. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 2004;350:560–569. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 19.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac R-L. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter pro-inflammation signals. J. Exp. Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y-P, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer: stereochemical assignments, anti-inflammatory properties and enzymatic inactivation. J. Biol. Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 21.Gong J, Rosner B, Rees DG, Berson EL, Weigel-DiFranco CA, Schaefer EJ. Plasma docosahexaenoic acid levels in various genetic forms of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 1992;33:2596–2602. [PubMed] [Google Scholar]
- 22.Newcomer LM, King IB, Wicklund KG, Stanford JL. The association of fatty acids with prostate cancer risk. Prostate. 2001;47:262–268. doi: 10.1002/pros.1070. [DOI] [PubMed] [Google Scholar]
- 23.Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, Willett WC, Ma J. Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002;346:1113–1118. doi: 10.1056/NEJMoa012918. [DOI] [PubMed] [Google Scholar]
- 24.Kew S, Mesa MD, Tricon S, Buckley R, Minihane AM, Yaqoob P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am. J. Clin. Nutr. 2004;79:674–681. doi: 10.1093/ajcn/79.4.674. [DOI] [PubMed] [Google Scholar]
- 25.Wakai K, Ito Y, Kojima M, Tokudome S, Ozasa K, Inaba Y, Yagyu K, Tamakoshi A, JACC Study Group Intake frequency of fish and serum levels of long-chain n-3 fatty acids: a cross-sectional study within the Japan Collaborative Cohort Study. J. Epidemiol. 2005;15:211–218. doi: 10.2188/jea.15.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arita M, Oh S, Chonan T, Hong S, Elangovan S, Sun Y-P, Uddin J, Petasis NA, Serhan CN. Metabolic inactivation of resolvin E1 and stabilization of its anti-inflammatory actions. J. Biol. Chem. 2006;281:22847–22854. doi: 10.1074/jbc.M603766200. [DOI] [PubMed] [Google Scholar]
- 27.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN. Stereochemical assignment, anti-inflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J. Exp. Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Ariel A, Fredman G, Sun Y-P, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution via modulation of CCR5 expression. Nat. Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.deBoer TJ, Backer HJ. A new method for the preparation of diazomethane. Recl. Trav. Chim. Pays. Bas. 1954;73:229–234. [Google Scholar]
- 31.Qiu F-H, Wada K, Stahl GL, Serhan CN. IMP and AMP deaminase in reperfusion injury down-regulates neutrophil recruitment. Proc. Natl. Acad. Sci. USA. 2000;97:4267–4272. doi: 10.1073/pnas.97.8.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irimia D, Toner M. Cell handling using microstructured membranes. Lab Chip. 2006;6:345–352. doi: 10.1039/b515983k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winyard PG, Willoughby DA. Inflammation Protocols. In: Walker JM, editor. Methods in Molecular Biology. Humana; Totowa, NJ: 2003. p. 378. [Google Scholar]
- 34.Meyers DG, Meyers RE, Prendergast TW. The usefulness of diagnostic tests on pericardial fluid. Chest. 1997;111:1213–1221. doi: 10.1378/chest.111.5.1213. [DOI] [PubMed] [Google Scholar]
- 35.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, Hong S, Serhan CN. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 36.Geiser T, Dewald B, Ehrengruber MU, Clark-Lewis I, Baggiolini M. The interleukin-8-related chemotactic cytokines CROalpha, GRObeta, and GROgamma activate human neutrophil and basophil leukocytes. J. Biol. Chem. 1993;268:15419–15424. [PubMed] [Google Scholar]
- 37.Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. W.B. Saunders Co.; Philadelphia: 1999. p. 1425. [Google Scholar]
- 38.Gelman S. The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology. 1995;82:1026–1060. doi: 10.1097/00000542-199504000-00027. [DOI] [PubMed] [Google Scholar]
- 39.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, Van Dyke TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J. Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 40.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J. Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 41.Lands B. A critique of paradoxes in current advice on dietary lipids. Prog. Lipid Res. 2008;47:77–106. doi: 10.1016/j.plipres.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 43.Hong S, Gronert K, Devchand P, Moussignac R-L, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells: autacoids in anti-inflammation. J. Biol. Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 44.Marcheselli VL, Hong S, Lukiw WJ, Hua Tian X, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J. Biol. Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 45.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br. J. Pharmacol. 2008;153:S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patil VS, Magar NG. Fatty acids of human blood. Biochem. J. 1960;74:427–429. doi: 10.1042/bj0740427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 48.Pawlosky RJ, Hibbeln JR, Novotny JA, Salem NN., Jr. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J. Lipid Res. 2001;42:1257–1265. [PubMed] [Google Scholar]
- 49.U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Nutritional Products Labeling and Dietary Supplements (FDA/CSFAN) Letter regarding dietary supplement health claim for omega-3 fatty acids and coronary heart disease. 2000 Docket No. 91N-0103.
- 50.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 51.Gilroy DW, Newson J, Sawmynaden P, Willoughby DA, Croxtall JD. A novel role for phospholipase A2 isoforms in the checkpoint control of acute inflammation. FASEB J. 2004;18:489–498. doi: 10.1096/fj.03-0837com. [DOI] [PubMed] [Google Scholar]
- 52.Huang BX, Dass C, Kim HY. Probing conformational changes of human serum albumin due to unsaturated fatty acid binding by chemical cross-linking and mass spectrometry. Biochem. J. 2005;387:695–702. doi: 10.1042/BJ20041624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundy SR, Dowling RL, Stevens TM, Kerr JS, Mackin WM, Gans KR. Kinetics of phospholipase A2, arachidonic acid, and eicosanoid appearance in mouse zymosan peritonitis. J. Immunol. 1990;144:2671–2677. [PubMed] [Google Scholar]
- 54.Lemaitre-Delaunay D, Pachiaudi C, Laville M, Pousin J, Armstrong M, Lagarde M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [13C]DHA in phosphatidylcholine. J. Lipid Res. 1999;40:1867–1874. [PubMed] [Google Scholar]
- 55.Surette ME, Koumenis IL, Edens MB, Tramposch KM, Chilton FH. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin. Ther. 2003;25:948–971. doi: 10.1016/s0149-2918(03)80116-9. [DOI] [PubMed] [Google Scholar]
- 56.Böyum A. Isolation of leucocytes from human blood. Further observations. Methylcellulose, detran, and ficoll as erythrocyteaggregating agents. Scand. J. Clin. Lab. Invest. Suppl. 1968;97:31–50. [PubMed] [Google Scholar]
- 57.Boyden S. The chemoactic effect of mixtures of antibody and antigen on polymorphonuclear leucocytes. J. Exp. Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zicha D, Dunn GA, Brown AF. A new direct-viewing chemotaxis chamber. J. Cell Sci. 1991;99:769–775. doi: 10.1242/jcs.99.4.769. [DOI] [PubMed] [Google Scholar]
- 59.Zigmond SH, Hirsch JG. Leukocyte locomotion and chemotaxis. J. Exp. Med. 1973;137:387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Irimia D, Liu S-Y, Tharp WG, Samadani A, Toner M, Poznansky MC. Microfluidic system for measuring neutrophil migratory responses to fast switches of chemical gradients. Lab Chip. 2006;6:191–198. doi: 10.1039/b511877h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka D, Kagari T, Doi H, Shimozato T. Essential role of neutrophils in anti-type II collagen antibody and lipopolysaccharide-induced arthritis. Immunology. 2006;119:195–202. doi: 10.1111/j.1365-2567.2006.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fritsche K. Important differences exist in the dose-response relationship between diet and immune cell fatty acids in humans and rodents. Lipids. 2007;42:961–979. doi: 10.1007/s11745-007-3106-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.