Abstract
Pictures from the International Affective Picture System (IAPS) were selected to manipulate affective valence (unpleasant, neutral, pleasant) while keeping arousal level the same. The pictures were presented in an oddball paradigm, with a visual pattern used as the standard stimulus. Subjects pressed a button whenever a target was detected. Experiment 1 presented normal pictures in color and black/white. Control stimuli were constructed for both the color and black/white conditions by randomly rearranging 1 cm square fragments of each original picture to produce a “scrambled” image. Experiment 2 presented the same normal color pictures with large, medium, and small scrambled condition (2, 1, and 0.5 cm squares). The P300 event-related brain potential demonstrated larger amplitudes over frontal areas for positive compared to negative or neutral images for normal color pictures in both experiments. Attenuated and nonsignificant valence effects were obtained for black/white images. Scrambled stimuli in each study yielded no valence effects but demonstrated typical P300 topography that increased from frontal to parietal areas. The findings suggest that P300 amplitude is sensitive to affective picture valence in the absence of stimulus arousal differences, and that stimulus color contributes to ERP valence effects.
Keywords: International Affective Picture System, IAPS, event-related potentials (ERPs), P300, arousal, valence, scrambled image, color
1.0 Introduction
1.1. Valence and arousal
The affective components of arousal (calm to exciting) and valence (unpleasant to pleasant) contribute to the quantity and quality of emotional experience (Bradley and Lang, 1994). For example, during free viewing, participants spend more time on arousing images compared to those rated as less arousing (Lang et al., 1993). Indeed, event-related potential (ERP) studies have suggested that highly arousing affective pictures elicit larger positive-going waveforms than less intense images (Maratos et al., 2000; Schupp et al., 2000). Differences in valence dimensions also can modulate ERP outcomes (Bernat et al., 2001), with positive and negative affective pictures producing overall larger positive-going ERPs than neutral control pictures (Cuthbert et al., 2000; Dolcos and Cabeza, 2002; Schupp et al., 2004). Furthermore, negative valence stimuli generate higher emotional intensity than those with positive valence—the so called “negative bias” effect (Ito et al., 1998; Lang et al., 1990). In most ERP studies, however, arousal and valence stimulus characteristics are not independent, so that valence differences may originate more from arousal than stimulus valence content (Olofsson et al., 2008).
1.2. Color and picture features
Color may be a contributing factor in the affective picture processing since the manipulation of hue, saturation, and brightness can influence emotional responses (Hupka et al., 1997; Kaya and Epps, 2004). Subjects report feelings of pleasantness in conjunction with the brightening of an image, while darkening it produces unpleasant affect (Marston, 1927). Moreover, different colors elicit differential arousal levels: subjective ratings of green-yellow, blue-green, and green were reported to be the most arousing colors, whereas purple-blue and yellow-red were the least arousing (Valdez and Mehrabian, 1994). As arousal level contributes to the P300 and other ERP component amplitude, stimulus color may contribute to affective findings (cf. Cuthbert et al., 2000; Polich and Kok, 1995).
In addition to color, variables such as featural size can alter ERP measures (e.g., Covington and Polich, 1996; Polich et al., 1996). Indeed, the physical attributes such as overall complexity and featural composition that define affective content appear to help determine emotional reactivity (Bradley et al., 2007; Codispoti et al., 2006a, 2006b). Exactly how stimulus attributes interact with affective processes is uncertain, but evaluation of fundamental properties such as color and featural construction are likely to be involved (Dolcos and Cabeza, 2002; LeDoux, 1995). Physical stimulus characteristics may therefore contribute to the automatic and attentional processes that underlie early and late affective ERP responsivity (cf. Conroy and Polich, 2007; Öhman and Mineka, 2001; Rozenkrants et al., 2008; Schupp et al., 2003).
1.3. Present study
The present study was designed to address these issues by assessing affective processing of visual images that varied systematically in valence while keeping arousal level constant. Experiment 1 evaluated affective pictures presented in color and black/white. Control stimuli for each condition were constructed by rearranging square sections of each picture so that their visual image characteristics were distorted. Experiment 2 presented the same normal color pictures and three scrambled stimulus conditions that varied in section size to assess ERP valence effects and how the degree of distortion contributes to affective stimulus processing.
2.0 Method
2.1. Participants
Each experiment employed different groups of 16 right-handed female undergraduates (Experiment 1: M=19.8, SD=1.4 years; Experiment 2: M=20.5, SD=2.3 years). All subjects reported an absence of neurological disorders, normal or corrected-to-normal vision, and provided written informed consent. Subjects were compensated with course credit or $10/hour.
2.2. Stimuli and procedure
Figure 1 illustrates stimuli similar to those used in each study. The same were employed in each experiment and were selected from the International Affective Picture System (IAPS), with the IAPS image numbers specified in the Appendix. Each picture was a visual image that had been rated on arousal and valence using a 1-9 point scale by female and male undergraduates (Lang et al., 1995). A total of 24 pictures were selected for each stimulus category to define unpleasant (M=2.5, SD=0.3), neutral (M=4.9, SD=0.6), and pleasant (M=7.4, SD=0.4) valence levels while maintaining the same arousal level for the unpleasant (M=5.1, SD=0.3), neutral (M=5.0, SD=0.4), and pleasant (M=5.0, SD=0.3) categories. Both valence and arousal ratings were highly similar across rater gender. Each image was a target stimulus in an oddball task, with the red/white pattern as the standard stimulus.
Figure 1.

Upper Panel: Experiment 1 sample stimuli for the normal color, normal black/white, scrambled color, scrambled black/white from each condition. Lower Panel: Experiment 2 sample stimuli from the normal pictures, large scrambled, medium scrambled, and small scrambled images used in each condition. These images are very similar to the IAPS pictures and are used here to avoid copyright infringement.
Experiment 1 consisted of four experimental conditions: normal color pictures, normal black/white pictures, scrambled color pictures, and scrambled black/white pictures. IAPS images are in color and were transformed into black/white by means of a MATLAB routine. The scrambled images were constructed by randomly rearranging 1 cm2 areas of the image. The scrambled pictures also were transformed into black/white images. In the black/white presentation conditions, the standard stimulus image was black/white. Experiment 2 employed the same normal color images. The scrambled conditions varied the size of square from large to small with 2.0 cm2, 1.0 cm2, and 0.5 cm2 squares presented in different conditions. This procedure maintains visual characteristics (color, spatial attributes, intensity) but systematically varies the available visual featural content of the overall image.
Each of the four experimental conditions in both studies contained 72 target pictures among 168 standard stimulus presentations, resulting in a total of 240 randomly ordered trials with a target probability of 0.30 and standard stimulus probability of 0.70. The condition order was counterbalanced in each experiment across subjects, who sat in a chair that was 75 cm from the computer screen. All stimuli were 9 × 12 cm, displayed centrally for 1000 ms on a light grey background at normal viewing luminance. A 2000 ms inter-stimulus interval was used. Participants were instructed to respond with a mouse-click when a target stimulus was presented and to refrain from responding to the standard stimulus. Rest periods were provided.
2.3. Recording conditions
Electroencephalographic activity was recorded from 21 electrode sites including Fz, Cz, Pz, Fp1/2, F3/4, F7/8, C3/4, T7/8, P3/4, P7/8, O1/2, referenced to linked earlobes, with a forehead ground and impedances of 10 KΩ or less. Additional electrodes were placed at the outer canthi as well as above and below the left eye to measure electro-ocular (EOG) activity with a bipolar recording. The bandpass was 0.02-50 Hz (3dB/octave), and the EEG was sampled at 256 Hz for 1000 ms, with a 100 ms pre-stimulus baseline. Waveforms were averaged off-line, and trials on which the EEG or EOG exceeded ±100 μV were rejected.
3.0 Results
3.1. Behavioral data
Error rate (ER) was defined as the percent of incorrect responses and was less than 1% for all conditions in both experiments; it will not be considered further. Response time (RT) was defined as the time from stimulus onset to the button press response, and the mean was computed over trials for each subject from each valence category and stimulus condition.
Experiment 1
RT was assessed with a three-factor (3 valence × 2 color × 2 scrambled) analysis of variance. RT did not differ among valence conditions (unpleasant=509, neutral=502, pleasant=504 ms). RT was marginally longer for the color compared to black/white (513 vs. 495 ms) stimuli, F(1,15)=3.90, p<.10, and appreciably longer for normal compared to scrambled (523 vs. 486 ms) stimuli, F(1,15)=14.5, p<.002. No reliable interactions were obtained. Task performance was therefore comparable across valence categories but affected by the color and scrambled conditions.
Experiment 2
RT was assessed with a two-factor (3 valence × 4 stimulus types) analysis of variance. RT did not differ among valence conditions (unpleasant=505, neutral=504, pleasant=512 ms) but was longest for normal picture targets and decreased as scramble size decreased (558, 509, 484, 477 ms, respectively), F(3,45)=27.5, p<.0001. Task performance again was comparable across valence, but RT decreased from the normal as scrambled size decreased.
Comment on Behavioral Data
The absence of errors and the highly consistent mean RT among valence conditions for both experiments indicate that task performance was virtually perfect and that valence did not influence the simple target detection task employed. The longer RT observed for the normal picture images compared to scrambled stimuli suggests that the pictures were processed for content, whereas the scrambled stimuli were not. As demonstrated in Experiment 2, RT decreased with decreases in scrambled block size, which implies that as content accessibility decreased with the smaller sizes target identification occurred more readily and quickly. These results are consistent with the P300 ERP outcomes discussed next.
3.2. ERP analyses
For each experiment, preliminary analyses indicated that the major valence outcomes were localized around the P300 component, and the mean amplitude within the latency window of 300-500 ms relative to the pre-stimulus baseline was analyzed. Experiment 1 was designed to extend previous work using the oddball task, which included assessing the medial and adjacent coronal electrode arrays (Conroy and Polich, 2007). Experiment 2 was designed to determine how perceivable features in the scrambled condition might contribute to valence outcomes. As no valence-related coronal effects were obtained in Experiment 1, only data from the midline electrodes were assessed in Experiment 2. Greenhouse-Geisser corrections to the df were applied as needed, with the corrected probabilities reported.
Experiment 1
Figure 2 illustrates the grand averages with each valence category overlapped for normal (upper) and scrambled (lower) stimuli from the medial including the midline electrodes for each condition. Figure 3 illustrates the mean amplitude from the frontal (F3, Fz, F4), central (C3, Cz, C4), and parietal (P3, Pz, P4) electrodes for each valence condition. Preliminary analyses indicated that the normal pictures compared to scrambled pictures produced two distinct patterns. Hence, four-factor repeated measures analyses of variance (3 valence × 2 color × 3 coronal × 3 anterior-to-posterior electrodes) were applied separately to the P300 measures from the normal and scrambled conditions. This approach defines the electrode factors as crossed so that the coronal and frontal-parietal directions are assessed as separate main effects along with the resulting interactions.
Figure 2.
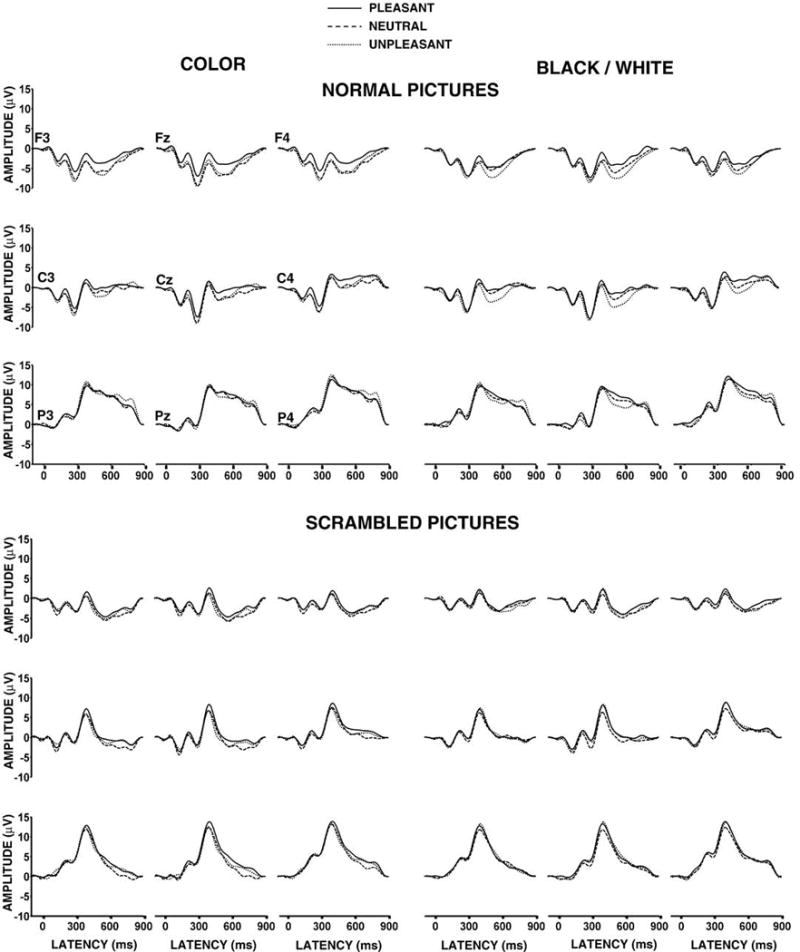
Grand ERP averages from the pleasant, neutral, and unpleasant stimuli for the normal (upper) and scrambled (lower) picture color and black/white conditions from the left medial, medial, and right medial electrode positions over the frontal, central, and parietal areas. Electrode locations are indicated in the upper left panel.
Figure 3.
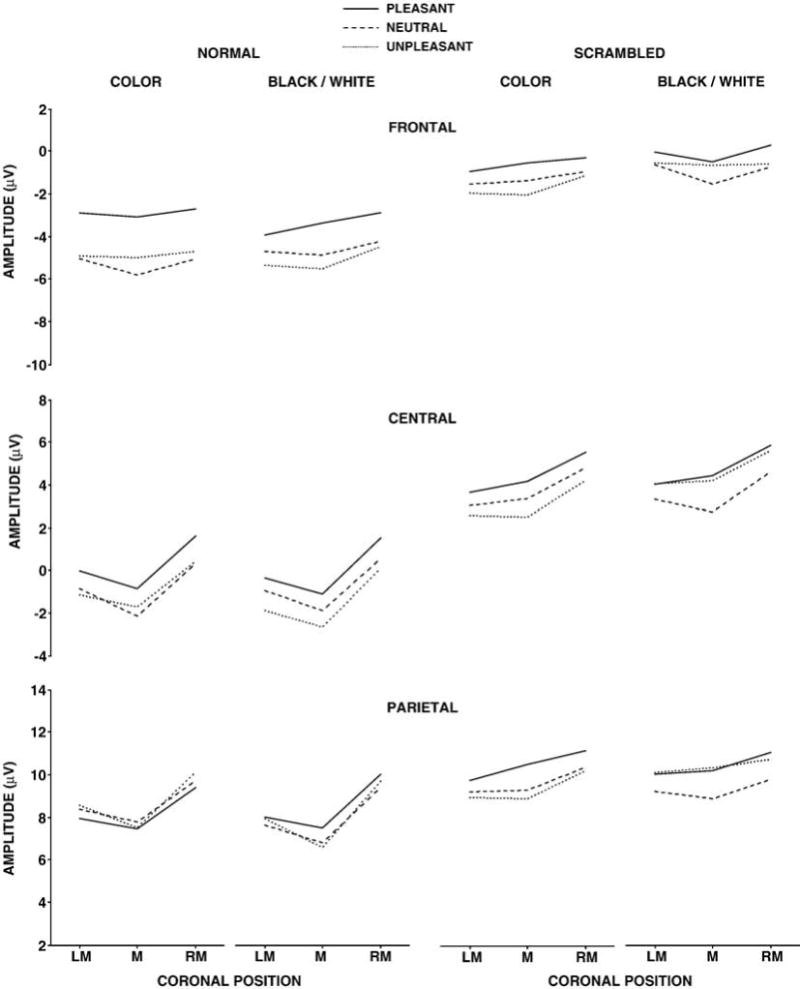
Mean P300 amplitude from the pleasant, neutral, and unpleasant stimuli for the normal (left) and scrambled (right) color and black/white stimulus conditions as a function of the left medial (LM), medial (M), and right medial (RM) electrode positions over the frontal, central, and parietal areas. Note that the scales differ for the frontal, central, and parietal electrodes but that the ranges are the same across scales.
The normal picture stimuli yielded no main effects for valence or color. However, P300 mean amplitude demonstrated a greater difference among the valence categories over the frontal compared to parietal sites to produce a significant two-factor interaction between valence and anterior-to-posterior electrode location, F(4,60)=6.64, p<.005. Amplitude increased from the frontal to parietal electrodes, F(2,30)=93.79, p<.0001, and amplitude varied across the coronal electrode locations overall, F(2,30)=20.38, p<.0001. These patterns changed over the scalp, with amplitude minimal over the midline frontally and maximal over the midline parietally to produce a significant two-factor interaction between the frontal-to-parietal and coronal electrode positions, F(4,60)=6.11, p<.005. Taken together, these outcomes suggest that stimulus valence differences affected component amplitude primarily over the frontal electrodes for both color and black/white pictures.
The scrambled picture stimuli yielded no main effects or interactions for valence or color. Amplitude increased from the frontal-to-parietal electrodes, F(2,30)=89.57, p<.0001, with an amplitude change obtained across the coronal electrode locations, F(2,30)=7.16, p<.005. Only the usual P300 scalp topography amplitude changes were found for the scrambled stimuli.
The mean amplitude data from each condition were then each analyzed with separate three-factor repeated measures analyses of variance (3 valences × 3 coronal × 3 anterior-to-parietal electrode). For the normal color stimuli, P300 mean amplitude was larger for the pleasant relative to the unpleasant and neutral stimuli over the frontal electrodes, with no valence effects observed over the parietal electrodes to yield a reliable interaction between valence and anterior-to-parietal electrode location, F(2,30)=7.38, p<.001. The normal color pictures also demonstrated amplitude differences for coronal location, F(2,30)=13.39, p<.001, anterior-to-posterior electrode position, F(2,30)=97.31, p<.0001, and an interaction between the two electrode factors, F(4,60)=5.21, p<.01. For the normal black/white stimuli, no reliable valence differences were obtained, but the P300 mean amplitude varied across coronal locations, F(2,30)=19.37, p<.001, increased from frontal to parietal, F(2,30)=78.46, p<.001, with an interaction between the electrode factors also obtained, F(4,60)=4.44, p<.01.
The scrambled pictures yielded reliable frontal-to-parietal and coronal electrode effects for both color, F(2,30)=88.46, p<.0001 and F(2,30)=6.74, p<.01, and black/white, F(2,30)=78.52, p<.0001 and F(2,30)=6.43, p<.01, stimulus conditions. Thus, only the normal color pictures demonstrated reliable valence differences for P300 mean amplitude.
Experiment 2
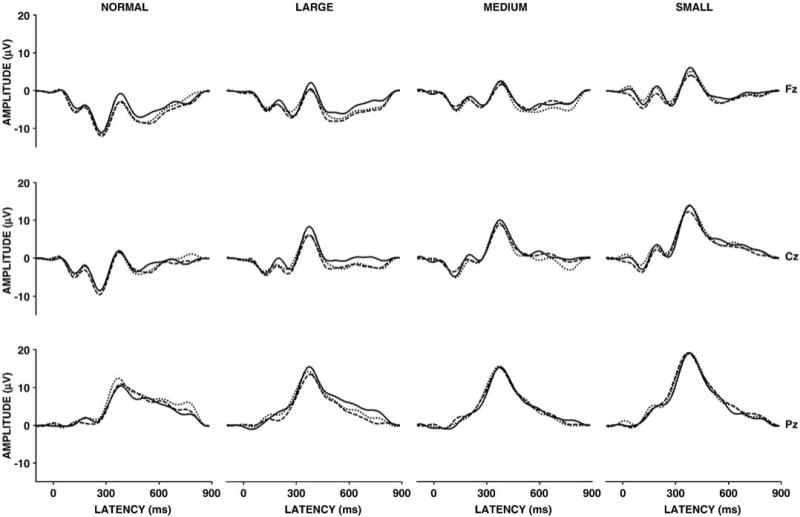
Figure 4 illustrates the grand averages with each valence category overlapped for normal (left) and scrambled (large, medium, small) stimuli from the midline electrodes for each condition. Figure 5 illustrates the mean amplitude for each valence in each condition as a function of the midline electrodes (Fz, Cz, Pz) for each stimulus condition. A three-factor repeated measures analysis of variance (3 valence × 4 stimulus type conditions × 3 midline electrodes) was applied to the P300 measures from all conditions.
Figure 4.
Grand ERP averages from the pleasant, neutral, and unpleasant stimulus images for the normal pictures, large scrambled, medium scrambled, and small scrambled stimulus conditions from the midline electrodes.
Figure 5.
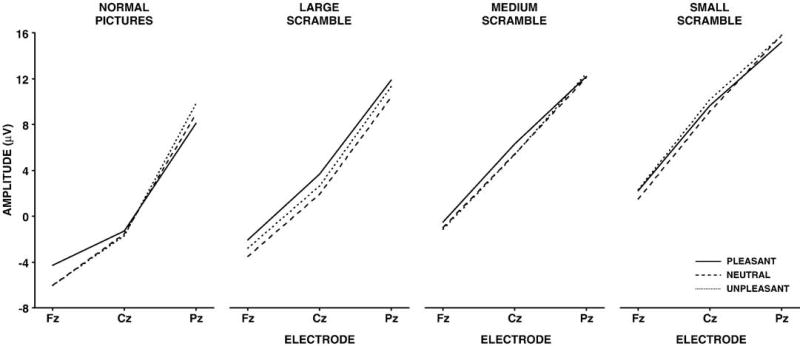
Mean P300 amplitude from the pleasant, neutral, and unpleasant stimuli for the normal pictures, large scrambled, medium scrambled, and small scrambled stimulus conditions as a function of midline electrode location.
No overall main effects for valence were found. P300 mean amplitude increased from the normal to small scrambled condition, F(3,45)=31.87, p<.0001. Amplitude increased from the frontal to parietal electrodes, F(2,30)=58.79, p<.0001. Valence and the electrode factor produced a two-way interaction, such that amplitudes were larger for positive compared to neutral and negative stimuli over the frontal electrode, whereas amplitudes were larger for negative and neutral stimuli over the parietal electrode, F(4,60)=4.20, p<.03. A two-way interaction between stimulus type and electrode was found, which suggested that the amplitude increase across stimulus type differed from frontal to parietal locations, F(6,90)=5.75, p<.0001. In sum, valence altered component amplitude primarily over the frontal electrodes.
The mean amplitude data from each stimulus condition were then analyzed separately with two-factor repeated measures analyses of variance (3 valences × 3 midline electrodes). For the normal stimulus condition a reliable interaction between valence and electrode was obtained, such that larger amplitudes for the positive stimuli were found over the frontal electrode and larger amplitudes for the negative stimuli were found over the parietal electrode, F(4,60)=8.28, p<.001. No other stimulus type yielded any main effect for valence or any interaction between valence and electrode. All stimulus conditions demonstrated highly significant main effects for the electrode factor, which were produced by the consistent increase in amplitude from the frontal to parietal electrodes, F(2,30)>40.0+, p<0.0001 (all cases). Thus, only the normal pictures demonstrated reliable valence differences for P300 mean amplitude.
4.0 Discussion
The major finding was that normal color affective picture stimuli that varied in valence in the absence of variation in arousal rating produced reliable P300 mean amplitude valence differences that were strongest over the frontal recording sites. Normal black/white pictures did not yield statistically reliable valence effects. In Experiment 1, the scrambled color and black/white images demonstrated no influence of valence and yielded topographically different ERP patterns from the normal images. In Experiment 2, even when scrambled picture segments depicted recognizable features, no valence effects were obtained. For both studies, the normal pictures demonstrated smaller P300 amplitudes than the scrambled pictures. This outcome likely reflects the differences in stimulus processing between the two conditions as indicated by the RT data: Pictures engage perceptual and cognitive processing mechanisms more strongly than the non-pictures made from the scrambled images. Taken together, the findings suggest that normal color pictures produce the P300 valence effects when arousal level is controlled, and that altering the image features across scrambled square sizes eliminated the influence of picture affect to produce a stimulus rather than perceptual content driven P300 (Polich, 2007).
The obtained amplitude differences for stimulus valence categories were notably largest over the frontal electrodes (Conroy and Polich, 2007; Delplanque et al., 2004, 2005, 2006). In particular, ERP amplitudes from pleasant stimuli were more positive than those from unpleasant stimuli, with waveforms from neutral stimuli somewhat variable (Bernat et al., 2001; Cuthbert et al., 2000; Schupp et al., 2000). As this outcome was obtained in the absence of arousal variation, valence rating level determined P300 amplitude differences for the color pictures. Relative to the pleasant images, the neutral and negative pictures produced smaller frontal components, which may reflect a lack of affective impact when arousal level is constant (Rozenkrants et al., 2008; Olofsson and Polich, 2007). However, the pattern of valence differences appears to vary with how stimuli are rated, arousal, and gender of the participant as sometimes negative valence stimuli demonstrate larger P300 components than neutral or positive (cf. Conroy and Polich, 2007; Olofsson et al., 2008). The consistency of frontal vs. parietal differences for valence categories does appear to be reliable and is likely the genesis of most ERP valence effects.
The influence of color on emotional processing implies that visual attributes contribute to affective ERP amplitude changes (Hupka et al., 1997; Kaya and Epps, 2004; Valdez and Mehrabian, 1994). These effects may be related to affective influences that originate during early stimulus processing (Bernat et al., 2001; Smith et al., 2003). That black/white normal images did not produce reliable valence differences implies that visual affect stems in part from image color. It may be that object color helps to define perceptual integrity and modulate affective processing related to memory activation (Dolcos and Cabeza, 2002; LeDoux, 1995; Schupp et al., 2006). That physical features of a visual image affects affective perception is a topic that is receiving considerable recent attention (Bradley et al., 2007; Codispoti et al., 2007; Delplanque et al., 2007; Rozenkrants et al., 2008). Whether specific colors are directly related to the valence of affective reactivity remains to be determined, but it appears likely that physical attributes of emotional stimuli are associated with both arousal and valence image characteristics in important ways (Olofsson et al., 2008).
The scrambled images in both experiments eliminated valence modulations of ERP amplitude and produced the frontal-to-parietal topography distributions typically obtained for non-picture visual stimuli. The scrambled images were composed of the same featural attributes as the regular pictures, although the feature combination did not produce a recognizable whole (cf. Codispoti et al., 2006a, 2006b). Indeed, in Experiment 2, as the size of the scrambled squares became smaller and features less distinct, P300 amplitude increased proportionately. For both studies, no reliable amplitude differences for valence or any interaction between valence and topography were found for the scrambled stimuli (cf. Bradley et al., 2007; Delplanque et al., 2007). The resulting ERPs from scrambled stimuli therefore suggest that the features of the normal pictures directly determine the valence-related outcomes obtained.
Acknowledgments
The order of the first two authors is alphabetical, and each received an Undergraduate Research Fellowship from University of California, San Diego. M. Cano is now at the Neurosciences Program, University of California, Berkeley; Q. Class is at the Clinical Psychology program, University of Indiana. This study was supported by NIDA grant RO1-DA018262 and NIAAA 3 P50-AA06420. This paper is publication number 19041 from The Scripps Research Institute.
Appendix
| Valence Category | IAPS Image Numbers |
|---|---|
| Unpleasant: | 2053, 2205, 2276, 2375.1, 2900, 3160, 3181, 3230, 3300, 3301, 6242, 7359, 9041, 9140, 9180, 9181, 9301, 9340, 9415, 9421, 9435, 9520, 9561, 9830 |
| Neutral: | 1030, 1112, 1121, 1230, 1303, 1390, 1945, 2220, 2635, 2780, 3210, 3550.2, 5455, 6900, 7496, 7560, 7620, 8211, 8232, 9411, 9582, 9635.2, 9913 |
| Pleasant: | 1440, 1463, 1590, 1710, 1722, 1731, 1999, 2058, 2340, 2345, 2352.1, 2391, 2550, 4641, 5480, 5660, 5830, 7260, 7350, 7470, 8120, 8461, 8510, 8540 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernat E, Bunce S, Shevrin H. Event-related brain potentials differentiate positive and negative mood adjectives during both supraliminal and subliminal visual processing. Int J Psychophysiol. 2001;42:11–34. doi: 10.1016/s0167-8760(01)00133-7. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Löw A, Lang PJ. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology. 2007;44:364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, De Cesarei A, Cardinale R. Implicit and explicit categorization of natural scenes. Prog Brain Res. 2006a;156:53–65. doi: 10.1016/S0079-6123(06)56003-0. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Junghöfer M, Schupp HT. The categorization of natural scenes: Brain attention networks revealed by dense sensor ERPs. Neuroimage. 2006b;32:583–591. doi: 10.1016/j.neuroimage.2006.04.180. [DOI] [PubMed] [Google Scholar]
- Conroy MA, Polich J. Affective valence and P300 when stimulus arousal level is controlled. Cogn Emot. 2007;21:891–901. [Google Scholar]
- Covington JW, Polich J. P300, stimulus intensity, and modality. Electroencephalogr Clin Neurophysiol. 1996;100:579–584. doi: 10.1016/s0168-5597(96)96013-x. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol Psychol. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Lavoie ME, Hot P, Silvert L, Sequeira H. Modulation of cognitive processing by emotional valence studied through event-related potentials in humans. Neurosci Lett. 2004;356:1–4. doi: 10.1016/j.neulet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Rigoulot S, Sequeira H. Arousal and valence effects on event-related P3a and P3b during emotional categorization. Int J Psychophysiol. 2006;60:315–322. doi: 10.1016/j.ijpsycho.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Delplanque S, Silvert L, Hot P, Sequeira H. Event-related P3a and P3b in response to unpredictable emotional stimuli. Biol Psychol. 2005;68:107–120. doi: 10.1016/j.biopsycho.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Delplanque S, N'diaye K, Scherer K, Grandjean D. Systematic measure of spatial frequencies for IAPS pictures by a discrete wavelet analysis. J Neurosci Methods. 2007;165:144–150. doi: 10.1016/j.jneumeth.2007.05.030. [DOI] [PubMed] [Google Scholar]
- Dolcos F, Cabeza R. Event-related potentials of emotional memory: Encoding pleasant, unpleasant, and neutral pictures. Cogn Affect Behav Neurosci. 2002;2:252–263. doi: 10.3758/cabn.2.3.252. [DOI] [PubMed] [Google Scholar]
- Hupka RB, Zaleski Z, Otto J, Reidl L, Tarabrina NV. The colors of anger, envy, fear, and jealousy: A cross-cultural study. J Cross Cult Psychol. 1997;28:156–171. [Google Scholar]
- Ito TA, Larsen JT, Smith NK, Cacioppo JT. Negative information weighs more heavily on the brain: the negativity bias in evaluative categorizations. J Pers Soc Psychol. 1998;75:887–900. doi: 10.1037//0022-3514.75.4.887. [DOI] [PubMed] [Google Scholar]
- Kaya N, Epps HH. Relationship between color and emotion: A study of college students. Coll Stud J. 2004;38:396–405. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical manual and affective ratings. Gainesville: University of Florida, Center for Research in Psychophysiology; 1995. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang SF, Nelson CA, Collins PF. Event-related potentials to emotional and neutral stimuli. J Clin Exp Neuropsychol. 1990;12:946–958. doi: 10.1080/01688639008401033. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Annu Rev Psychol. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Allan K, Rugg MD. Recognition memory for emotionally negative and neutral words: An ERP study. Neuropsychology. 2000;38:1452–1465. doi: 10.1016/s0028-3932(00)00061-0. [DOI] [PubMed] [Google Scholar]
- Marston WM. Primary colors and primary emotions. Psyche. 1927;30:4–33. [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biol Psychol. 2008;77:247–65. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Polich J. Affective visual event-related potentials: Arousal, repetition, and time-on-task effects. Biol Psychol. 2007;75:101–108. doi: 10.1016/j.biopsycho.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Ellerson PC, Cohen J. P300, stimulus intensity, modality, and probability. Int J Psychophysiol. 1996;23:55–62. doi: 10.1016/0167-8760(96)00028-1. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: An integrative review. Biol Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenkrants B, Olofsson JK, Polich J. Affective visual event-related potentials: Arousal, valence, and repetition effects for normal and distorted pictures. Int J Psychophysiol. 2008;67:114–23. doi: 10.1016/j.ijpsycho.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: Event-related brain potential studies. Prog Brain Res. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. Attention and emotion: An ERP analysis of facilitated emotional stimulus processing. Neuroreport. 2003;14:1107–1110. doi: 10.1097/00001756-200306110-00002. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Junghöfer M, Weike AI, Hamm AO. The selective processing of briefly presented affective pictures: An ERP analysis. Psychophysiology. 2004;41:441–449. doi: 10.1111/j.1469-8986.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention, please: Electrocortical responses to positive and negative stimuli. Neuropsychologia Special Issue: The cognitive neuroscience of social behavior. 2003;41:171–183. doi: 10.1016/s0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Valdez P, Mehrabian A. Effects of color on emotions. J Exp Psychol Gen. 1994;123:394–409. doi: 10.1037//0096-3445.123.4.394. [DOI] [PubMed] [Google Scholar]


