Abstract
The mechanisms by which resin based materials induce adverse effects in patients have not been completely elucidated. Here we show that 2-hydroxyethyl methacrylate (HEMA) induces apoptotic cell death in oral keratinocytes. Functional loss and cell death induced by HEMA was significantly inhibited in the presence of N-acetyl cysteine (NAC) treatment. NAC also prevented HEMA mediated decrease in vascular endothelial growth factor secretion. The protective effect of NAC was partly related to its ability to induce NF-κB in the cells, since HEMA mediated inhibition of nuclear NF-κB expression and function was significantly blocked in the presence of NAC treatment. Moreover, blocking of nuclear translocation of NF-κB in oral keratinocytes sensitized these cells to HEMA mediated apoptosis. In addition, since NAC was capable of rescuing close to 50% of NF-κB knockdown cells from HEMA mediated cell death, there is, therefore, an NF-κB independent pathway of protection from HEMA mediated cell death by NAC. NAC mediated prevention of HEMA induced cell death in NF-κB knockdown cells was correlated with a decreased induction of c-Jun N-terminal kinase (JNK) activity since NAC inhibited HEMA mediated increase in JNK levels. Furthermore, the addition of a pharmacologic JNK inhibitor to HEMA treated cells prevented cell death and restored NF-κB knockdown cell function significantly. Therefore, NAC protects oral keratinocytes from the toxic effects of HEMA through NF-κB dependent and independent pathways. Moreover, our data suggest the potential involvement of JNK pathway in NAC mediated protection.
Keywords: HEMA, NF-κB, dental pulp stromal cells, apoptosis, VEGF
Resin-containing materials are now used widely in dental practice and are found in direct filling materials, in fissure sealing agents, and in bonding resins or resin cements for metal, porcelain and resin inlays, veneers, crowns and bridges. They are part of “bonded” amalgam restorations, “bonded” posts and “bonded” orthodontic brackets. Most dentin bonding technologies use a primer containing the hydrophilic resin HEMA (2-hydroxyethyl methacrylate) (molecular weight 130) in combination with acid treatment to create a “hybrid layer” or “interdiffusion zone.” HEMA is also found in many medical devices and materials such as soft contact lenses, electrosurgical grounding plates and drug delivery systems (Karlgard et al., 2003). However, HEMA in such materials are polymerized. In contrast, in dentistry such materials require polymerization intraorally, thus they may at least contain 30% unpolymerized monomers which is shown to leach out to the surrounding tooth area, and in the oral environment causing significant adverse effects (Gerzina and Hume, 1994; Hamid and Hume, 1997; Hamid et al., 1998). The potential risks are direct damage to the cells and immune-mediated hypersensitivity reactions (Auzerie et al., 2003; Kanerva et al., 2002).
To find novel strategies to minimize or significantly prevent adverse effects of dental materials, studies should be designed to (1) understand the exact mechanisms by which these materials induce cell death and (2) find strategies to decrease or eliminate their toxicities while preserving their beneficial effects.
We have previously shown that cell death induced by HEMA is apoptotic and it occurs in a variety of cell types (Paranjpe et al., 2005). Apoptosis or programmed cell death is a genetically controlled and evolutionary conserved process which ensures disposal of damaged or altered cells in diverse organisms.
N-acetyl cysteine (NAC) is shown to inhibit cell death mediated by a variety of compounds including that induced by HEMA, through its anti-oxidant activity (Schweikl et al., 2007), since the involvement of reactive oxygen species (ROS) and oxidative stress in HEMA mediated apoptotic cell death has previously been reported (Schweikl et al., 2006; Spagnuolo et al., 2006). However, recent reports from our lab and those from others, suggested different mechanisms for the inhibitory function of NAC on cell death (Gustafsson et al., 2005; Paranjpe et al., 2008a; Parasassi et al., 2005). In addition, even though the involvement of NF-κB (nuclear factor kappa B) in HEMA mediated cell death was suggested previously, contrary to our findings, treatment with HEMA was found to activate rather than inhibit NF-κB activity in fibroblasts (Spagnuolo et al. 2004). Therefore, the exact mechanism(s) by which HEMA induces cell death, and NAC protects the cells from HEMA mediated cell death requires further investigation. We show in this paper that NAC resists HEMA mediated cell death via NF-κB dependent and independent and c-Jun N-terminal kinase (JNK) dependent pathways.
NF-κB is the major transcription factor which is involved in the regulation of a variety of genes responsible for survival of the cells (Beg and Baltimore, 1996; Jewett et al., 2006). The activity of NF-κB is tightly regulated by cytokines and other external regulators (Baeuerle and Henkel, 1994; Verma et al., 1995). In most cell types, NF-κB is present as a cytoplasmic heterodimer consisting of 50-kDa (p50) and 65-kDa (p65) subunits.
JNK belongs to the family of mitogen-activated protein kinases (MAPKs) that comprise of a group of serine/threonine kinases, which are responsible for phosphorylation and mediation of signal transduction from extracellular stimuli. It is believed that activation of JNK inhibits cell growth and induces cell death (Kyriakis and Avruch, 1996a,b; Kyriakis et al., 1994; Xia et al., 1995). JNK was first identified as a protein kinase that binds and phosphorylates its nuclear target c-Jun (Hibi et al., 1993). Several isoforms of JNK have been isolated to date (Gupta et al., 1996), of which JNK1 and JNK2 have been studied extensively. Extracellular stimuli which activate JNK1 and JNK2 include ultraviolet irradiation (Liu et al., 1995), mechanical forces (Hu et al., 1999), tumor necrosis factor-α (TNF-α) (Kyriakis et al., 1994), epidermal growth factor, and nerve growth factor (Minden et al., 1994) among many others.
The race to elucidate the role of NF-κB and JNK in the regulation of cell death has generated conflicting reports (Arsura et al., 2003; Jewett, 2001; Tang et al., 2002). Although many reports demonstrated an apoptotic role for the function of JNK (Arsura et al., 2003; Castrillo et al., 2003; Jewett, 2001; Tang et al., 2002), others have attributed an anti-apoptotic role for JNK when NF-κB is suppressed (Reuther-Madrid et al., 2002).
In the present study we demonstrate that HEMA induces apoptotic cell death in oral keratinocytes through the inhibition of NF-κB and increased activation of JNK. Moreover, the key role of nuclear NF-κB and JNK modulation by NAC in protection against HEMA mediated effects was investigated. In addition, we have also identified an NF-κB independent and JNK dependent pathway of protection for NAC. Overall, the results reported in this paper indicate that NAC is an effective chemo-protectant which can safely be used to protect the pulp and the surrounding tissues from adverse effects of dental restorative materials.
MATERIALS AND METHODS
Cells and reagents.
293T and HEp2 cells were cultured either in RPMI or DMEM (Cellgro, VA) supplemented with 10% fetal bovine serum (FBS), 1% nonessential amino-acids, 1% sodium pyruvate, 1% antibiotics-antimycotic, and 1% L-glutamine (Invitrogen, Carlsbad, CA). HOK16B (human oral keratinocytes) were cultured in KGM medium supplemented with 4% bovine pituitary extract, 1% hydrocortisone, 1% gentamycin-sulfate, 1% bovine insulin, 1% epidermal growth factor obtained from Cambrex-Bio (Walkersville, MD). Propidium iodide (PI), NAC, HEMA, Phorbol 12-myristate 13-acetate (PMA), and HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) were purchased from Sigma (St Louis, MO). PMSF (phenylmethylsulphonyl fluoride) was purchased from Roche Applied Science (Indianapolis, IN). The antibodies used for the western blotting were obtained from Cell Signaling (San Diego, CA). The fluorescein isothiocyanate (FITC) conjugated Annexin V/PI kit was purchased from Coulter Immunotech (Miami, FL).
DNA staining and apoptosis.
Staining was performed by labeling the cells with PI and Annexin V as described previously (Jewett et al., 1997). Cells were cultured and treated as stated in the Results section, and they were washed twice and resuspended in binding buffer containing FITC-Annexin V/PI as suggested by the manufacturer. After 15 min of incubation on ice, Annexin V/PI stained cells were analyzed by flow cytometry. EGFP transfected HOK-16B cells were only stained with PI and analyzed using Forward angle side scatter and PI as indicated in the result section. Flow cytometric analysis was performed using EPICs-ELITE flow cytometer (Coulter, Miami, FL). Dead cell fragments and debris were gated out by forward and side scatter analysis.
Preparation of the nuclear extracts.
Treated and untreated samples were incubated for fifteen minutes at 37°C, after which they were washed in buffer A (l0mM HEPES, 1.5mM MgCl2, 10mM KCl, 0.5mM dithiotreitol, and 0.5mM PMSF) without NP-40. The pellets were resuspended in 300 μl of Buffer A with 0.1% NP-40. The incubation was continued for 5 min on ice. The insoluble fraction was washed in Buffer A without NP-40 once and Buffer C (25% Glycerol, 20mM HEPES, 0.6M KC1, 1.5mM MgCl2, and 0.2mM ethylenediaminetetraacetic acid [EDTA]) was added to the pellets for 30 min. The pellets were spun down at 13,000g for 10 min in cold and the supernatants recovered were frozen in -80°C until used.
Cytoplasmic extracts and Western blotting.
Western blotting was performed as described previously (Cacalano et al., 2008). Briefly, treated and untreated cells were lysed in the lysis buffer containing 50mM Tris-HCl (pH 7.4), 150mM NaCl, 1% Nonidet P-40 (vol/vol), 1mM sodium orthovanadate, 0.5mM EDTA, 10mM NaF, 2mM PMSF, 10 μg/ml leupeptin, and 2 U/ml aprotinin for 15 min on ice. The samples were then sonicated for 3 s. The cell lysates were centrifuged at 13,000g for 10 min and the supernatants were removed and the levels of protein were quantified by the Bradford method. The cell lysates were denatured by boiling in 5× sodium dodecyl sulfate (SDS) sample buffer. Equal amounts of cell lysates were loaded onto 10% SDS-polyacrlamide gel electrophoresis and transferred onto Immobilon-P membranes (Millipore, Billerica, MA). The membranes were blocked with 5% nonfat milk in PBS plus 0.1% Tween-20 for 1 h. Primary antibodies at the predetermined dilution were added for 1 h at room temperature. Membranes were then incubated with 1:1000 dilution of horseradish peroxidase-conjugated secondary antibody. Blots were developed by enhanced chemiluminescence (purchased from Pierce Biotechnology, Rockford, IL).
Luciferase reporter assay.
293T cells and HEp2 oral keratinocytes were plated and maintained in RPMI and DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin before transfection. Transfections were done using an NF-κB Luciferase reporter vector (Doyle et al., 2002) and Lipofectamine 2000 reagent (Invitrogen, CA) in Opti-MEM media (Invitrogen, CA) for 18 h after which they were treated with HEMA, NAC, and TNF-α as indicated in the Result section. The cells were then lysed with lysis buffer and the relative Luciferase activity was measured using the Luciferase assay reagent kit obtained from Promega (Madison, WI)
Enzyme-linked immunosorbent assay.
Enzyme-linked immunosorbent assays (ELISAs) for measuring VEGF (vascular endothelial growth factor) and bFGF (basic-fibroblastic growth factor) were purchased from R&D systems (Minneapolis, MN) and used according to the manufacturer's recommendations.
Multiplex cytokine protein arrays.
The fluorokine MAP cytokine multiplex array kits for measuring VEGF and bFGF (please see the results section) were purchased from R&D Systems (Minneapolis, MN) and the procedures were conducted as suggested by the manufacturer. Briefly, the microparticle concentrates containing monoclonal antibodies against different cytokines were diluted with the microparticle diluent (provided in the kit). Fifty microliters of each of microparticle concentrate was mixed with 5 ml of the diluent in a mixing bottle. Fifty microliters of this mixture was then added to 50 μl of the culture supernatants in each well of a microplate. The microparticle mixture contained desired combination of microparticle cytokine beads for a given protein array. The plate was incubated on a horizontal orbital microplate shaker at room temperature. After 3 h of incubation the liquid was removed from the wells using a vacuum manifold. One hundred microliters of wash buffer was then added to each well and the wash step was repeated three times. Fifty microliters of the secondary Biotin antibody cocktail specific for each analyte was then added to each well and the plate was incubated for an additional hour on a shaker at room temperature. The secondary antibody was prepared in the same way as the microparticle mixture/primary antibody. This was followed by the wash step as described above. The PE conjugated Streptavidin provided in a 100× concentration was diluted to a 1× concentration with wash buffer just before use. Fifty microliters of the Streptavidin-PE was then added to the wells for 30 min, after which the wash step was repeated. The microparticles were resuspended in 100 μl of Wash Buffer, incubated for an additional 2 min on a shaker and subsequently read using the Luminex 100 Analyzer (Austin, TX). To analyze and obtain the cytokine and chemokine concentrations, a standard curve was generated by a threefold dilution of recombinant cytokines provided by the manufacturer. The analysis was performed by the software provided by Luminex.
Retroviral transduction.
HOK-16B cells were infected with culture supernatants of NIH 3T3 packaging cells infected with either GFP expressing transdominant negative allele of IκB (Van Antwerp et al., 1996) or GFP alone. The mutant IκB-alpha (IκBαM) cDNA was excised from pCMX by digesting with BamHI and EcoRV, and cloned into the pMX-IRES-EGFP retroviral vector and cut with NotI (Klenow-filled) and BamHI. Forty-eight hours after infection the HOK-16B cells were sorted and high GFP expressing cells were grown and used in the experiments.
Statistical analysis.
An unpaired, two-tailed Student t-test was performed for the statistical analysis. One-way ANOVA with a Bonferroni post-test was used to compare the different groups.
RESULTS
NAC Inhibits HEMA-Mediated Apoptosis through the Induction of NF-κB
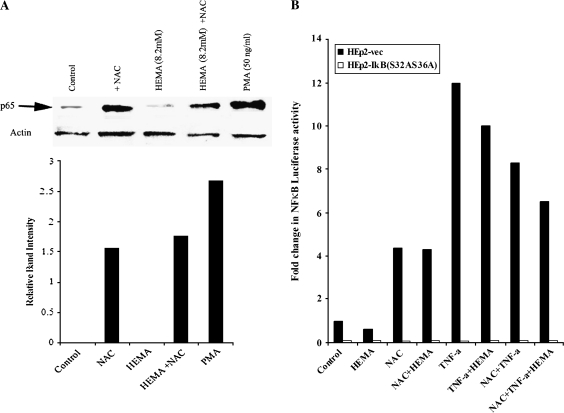
We have previously shown that HEMA induces apoptotic cell death in a variety of cell types (Paranjpe et al., 2005). The cell death induced by HEMA was shown to be dose and time dependent (Paranjpe et al., 2005, 2007). In addition, our previous studies clearly demonstrated that NAC prevented HEMA mediated cell death in a number of cell types including oral epithelial cells (Paranjpe et al., 2007). Since a correlation between suppression of NF-κB by HEMA and increased induction of cell death was observed in our previous studies, we determined whether NAC was capable of increasing NF-κB in oral epithelial cells. In our previous studies (Paranjpe et al., 2008a) we established the dose dependent protection of NAC against cell death, and determined that NAC at 20mM concentration was the optimal dose for protection of oral keratinocytes against HEMA mediated cell death. Thus we used this dose for the following experiments. Treatment with 20mM of NAC increased p65 subunit of NF-κB in nuclear extracts prepared from HEp2 cells (Fig. 1A). HEMA mediated decrease in p65 subunit of NF-κB was prevented significantly when NAC was added to HEMA treated HEp2 cells (Fig. 1A). To assess whether modulation of p65 subunit of NF-κB reflected on NF-κB activity we measured NF-κB activity using luciferase reporter assay in HEp2 cells (Fig. 1B). HEp2 cells transfected with NF-κB luciferase reporter vector were treated with TNF-α, HEMA, and NAC alone or in combinations as shown in Figure 1B, and the levels of NFκB activity were determined. NAC was able to significantly upregulate NF-κB activity in HEp2 cells, whereas HEMA inhibited NFκB activity compared to untreated controls (Fig. 1B). Indeed, at the 4 and 8 h of treatments 20–43% reduction in the NFκB activity could be observed when cells were treated with HEMA whereas 70% reduction could be seen at the 24 h of treatment when compared to untreated control cells (data not shown). NAC prevented the decrease in NF-κB activity in HEMA-treated cells (Fig. 1). As expected, TNF-α was also able to induce significant NF-κB activity (Fig. 1B). Although HEMA decreased NF-κB activity in TNF-α–treated cells moderately, the levels remained higher when compared to cells treated with HEMA alone (Fig. 1B). NAC, however, when added in combination with TNF-α had an inhibitory effect on NF-κB activity. More importantly, as expected, no increase in NF-κB activity could be observed with any of the treatments in NF-κB knock down HEp2 cells transfected with the NF-κB luciferase vector (Fig. 1B). Effect of NAC on the induction of NF-κB activity was less than TNF-α. These data, therefore, may suggest that NAC can inhibit HEMA mediated cell death partly due to its ability to increase NF-κB in HEp2 cells. Following experiments were then performed to study the extent and the magnitude of NFκB contribution in NAC mediated protection from cell death, and to establish the potential contribution of JNK in this process.
FIG. 1.
NAC increased the NF-κB levels and the activity in the cells: (A) HEp2 oral epithelial cells were treated with HEMA (8.2mM) and/or NAC (20mM) and PMA (50 ng/ml) as indicated in the figure. After an overnight incubation the nuclear extracts from each sample were prepared, and equal amounts of protein were loaded in each lane. The levels of p65 subunit of NF-κB in each lane were determined by the addition of monoclonal antibodies to p65 subunit of NF-κB. β-Actin levels were used to control for the protein loading amounts in each sample. The band densities for each lane were determined based on the β-actin loading levels and are shown in the lower panel (B) Vector-alone and IκB(S32AS36A) transfected HEp2 cells were transfected with the NF-κB Luciferase reporter vector as described in the materials and methods section, and then treated with HEMA (8.2mM), NAC (20mM), and TNF-α (20 ng/ml) and in combinations as shown in the figure for 18 h. The relative units of luciferase activity were then determined in the lysates according to the manufacturer's recommendation. Fold induction of NF-κB luciferase activity was assessed based on its comparison with untreated cells. Based on one-way ANOVA, the differences between the control group and those treated with HEMA and NAC and TNF-α are significant at p ≤0.001.
HEMA-Induced Significant Cell Death in NF-κB Knockdown Oral Keratinocytes
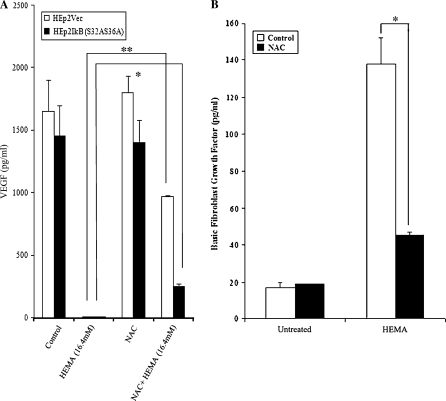
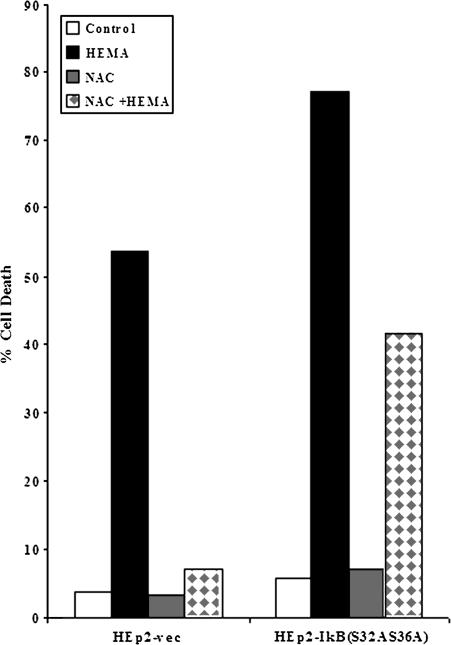
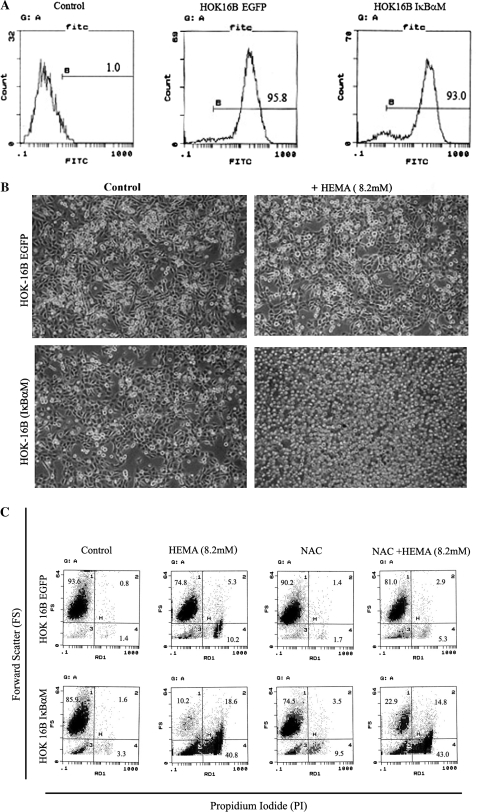
To elucidate the role of NF-κB in rescuing cells from apoptosis, we measured the effect of HEMA on oral keratinocytes with impaired NF-κB function. Two sets of oral epithelial cells were employed in these experiments to demonstrate applicability to other oral keratinocyte models. One was the established HEp2 cell line and the other was the transformed human oral keratinocytes (HOK-16B). Vector-alone and IκB(S32AS36A) transfected HEp2 cells (Jewett et al., 2003) were treated with HEMA, NAC and the combination of NAC and HEMA and cell viability and function were determined by staining with Annexin V and PI and measuring VEGF and bFGF release, respectively. Since we had established a close relationship between the induction of cell death and loss of VEGF secretion by a number of different cell types in our previous studies, we chose to use the modulation of this growth factor as a measure of functional competency (Paranjpe et al., 2007). Moreover, a significant increase in the release of bFGF was also observed when the cells were induced to undergo cell death under a number of different experimental conditions (Figs. 3B, 6B and data not shown). Therefore, when Dental Pulp Stromal Cells (DPSCs) (data not shown) or HEp2 cells (Figs. 3B and 6B) were treated with HEMA, a significant release of bFGF in the presence of decreased secretion of VEGF could clearly be seen (Figs. 3A and 3B). Addition of NAC to HEMA treated cells resulted in an increased survival of the cells and the prevention of a drop in VEGF secretion and inhibition of bFGF release by the cells (Figs. 3A and 3B). Moreover, as shown in Figure 2 HEMA induced cell death in both transfectants, albeit the levels were higher in IκB(S32AS36A) transfected HEp2 cells. NAC was protective and it inhibited HEMA mediated cell death in both transfectants, although the effect was more potent against vector-alone than IκB(S32AS36A) transfected HEp2 cells (> 90% decrease in cell death for vector-alone versus ∼50% decrease for IκB(S32AS36A) transfected HEp2 cells) (Fig. 2). Similarly, NAC prevented HEMA mediated decrease in VEGF secretion more in vector-alone than in IκB(S32AS36A) transfected HEp2 cells (Fig. 3). It is, however, important to note that NAC reproducibly inhibited HEMA mediated effects in IκB(S32AS36A) transfected HEp2 cells. These results were also confirmed when HOK-16B oral epithelial cells were used. Human oral keratinocytes (HOK-16B) were transduced with EGFP alone or trans-dominant negative allele of IκB (EGFP/IκBαM) retroviral constructs and sorted for high EGFP expressing cells using flow cytometry (Fig. 4A). IκBαM transduced HOK-16B cells secreted significantly lower amounts of interleukin-6 (IL-6) when compared to EGFP-transduced cells (data not shown). Thus, transduction of HOK-16B cells with IκBαM construct exhibited the same functional profiles as those observed in HEp2 cell transfectants (Jewett et al. 2003, 2006). HEMA treatment also inhibited IL-6 secretion by HOK-16B cells (data not shown). Untreated HOK-16B cells transduced with IκBαM construct did not exhibit elevated levels of cell death when assessed morphologically or by flow cytometric analysis of FITC-Annexin V and PI stained cells (Figs. 4B and 4C). However, as expected when treated with HEMA almost all of the IκBαM transduced HOK-16B cells underwent cell death (Figs. 4B and 4C). In contrast, very few cells in EGFP alone transduced HOK-16B cells underwent cell death when they were treated with HEMA (Figs. 4B and 4C). Addition of NAC in combination with HEMA to IκBαM transduced HOK-16B cells exhibited 22.9% cell survival as compared to 10.2% in the absence of NAC which is a 50% increase in the number of surviving cells. Thus, the extent of cell survival between HEMA or HEMA and NAC treated cells is similar between NF-κB knock down HOK-16B and HEp2 cells (Fig. 2). Therefore, the levels of NF-κB independent protection from cell death by NAC is similar in HEMA treated HEp2 cell and HOK-16B cell transfectants excluding involvement and differences in media composition or the levels of the supplementation of the serum in the effect of NAC. Indeed, we did not observe differences in either HEMA mediated cell death or the levels of NAC protection when either serum free or complete medium was used in the experiments (data not shown). Thus, these results indicated that NAC protects the cells via both NF-κB dependent and independent pathways since NAC was also capable of decreasing cell death and loss of function in NF-κB knock down cells.
FIG. 3.
HEMA mediated decrease in VEGF secretion and increase in bFGF was prevented by NAC in HEp2 cells. (A) Vector alone and IκB super-repressor transfected HEp2 cells were incubated with and without HEMA (16.4mM) and NAC (20mM) overnight, and the supernatants were removed and assayed for VEGF secretion using bead based cytokine array kit (**p ≤0.0001 and *p ≤0.001, unpaired, two-tailed t-test). The results were confirmed using a sensitive and specific single ELISA assay for VEGF (data not shown). (B) Vector alone were incubated with and without HEMA (16.4mM) and NAC (20mM) overnight, and the supernatants were removed and assayed for bFGF secretion using the bead based cytokine array kit (*p ≤0.001, unpaired, two-tailed t-test).
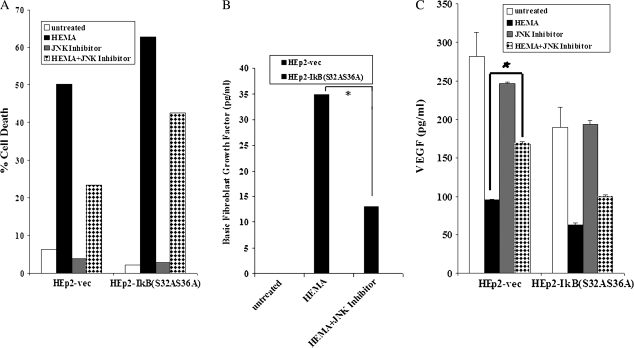
FIG. 6.
JNK Inhibitor (SP600125) prevented HEMA-mediated death of HEp2 cells. (A) pRcCMV vector alone (HEp2 Vec) and IκB(S32AS36A) transfected HEp2 cells were cultured in DMEM at a concentration of 2 × 105 cells per well and treated with HEMA (16.4mM), and the JNK inhibitor (25μM) for a period of 18 h. The cells were then stained with FITC-Annexin V and PI and analyzed using the EPICs ELITE flow cytometer. pRcCMV vector alone (HEp2-Vec) and IκB(S32AS36A) transfected HEp2 cells were cultured, and treated as described in Figure 6A, and the supernatants were removed and assayed for bFGF (B) and VEGF secretion (C) using bead based cytokine array kit (*p ≤0.01, unpaired, two-tailed t-test).
FIG. 2.
NAC inhibits HEMA mediated cell death. Vector-alone and IκB(S32AS36A) transfected HEp2 cells were treated with HEMA (16.4mM) in the presence and absence of NAC (20mM). After an overnight incubation the levels of cell death were determined using PI and FITC-Annexin V staining.
FIG. 4.
HEMA-mediated cell death in NF-κB knockdown HOK-16B. (A) To determine the efficiency of transduction, retrovirally infected HOKs (HOK-16B EGFP and HOK-16B IκBαM) were analyzed for GFP expression by flow cytometry. Similar levels of GFP expression were observed in EGFP and IκBαM transduced HOK-16B cells. (B) Retrovirally transduced HOKs (HOK-16B EGFP and HOK-16B IκBαM) were incubated with and without HEMA (8.2mM) overnight after which photographs were taken using an inverted microscope (20×). (C) Retrovirally transduced HOKs (HOK-16B EGFP and HOK-16B IκBαM) were incubated with and without HEMA (8.2mM) and NAC (20mM) overnight, and cell death were determined using forward angle light scatter and staining with PI. The numbers in each quadrant represent the percentages of cells positive for that quadrant. Ten thousand events were analyzed for each sample.
NAC Prevents HEMA-Mediated Increase in JNK Expression
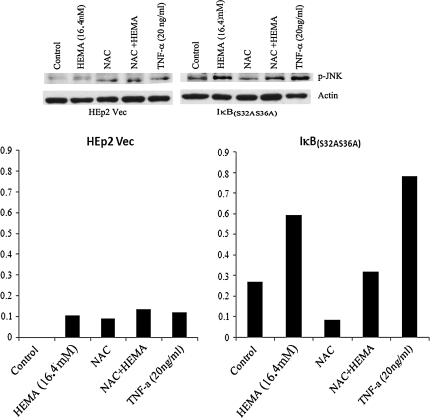
To characterize the potential involvement of other signaling components such as JNK in NF-κB independent rescue from cell death by NAC we first measured the levels of JNK induction in NAC and HEMA treated samples. IκB(S32AS36A) transfected HEp2 cells exhibited higher induction of JNK when compared to vector alone transfected cells (Fig. 5A). IκB(S32AS36A) transfected HEp2 cells were treated with HEMA (8.2mM) and NAC (20mM) for 6-12 h after which the cytoplasmic extracts were analyzed for the levels of phosphorylated c-jun N terminal kinase (p-JNK). As shown in Figure 5A in the presence of HEMA the levels of p-JNK were upregulated and NAC lowered the levels of p-JNK in control and HEMA treated IκB(S32AS36A) transfected HEp2 cells. Therefore, NAC is capable of preventing HEMA mediated increase in p-JNK in IκB(S32AS36A) transfected HEp2 cells. Similar results were also obtained when c-jun was measured in NAC treated HEp2- IκB(S32AS36A) cells (data not shown).
FIG. 5.
NAC decreases the protein levels of p-JNK in treated cells. (A) pRcCMV vector alone (HEp2-Vec) and IκB(S32AS36A) transfected HEp2 cells (5 × 106) were cultured in DMEM and subsequently treated with HEMA (16.4mM) and NAC (20mM) for 6 h after which the cells were lysed and the cytoplasmic extracts analyzed for p-JNK protein using Western blot analysis. β-Actin levels were used to control for the protein loading amounts in each sample. The band densities for each lane were determined based on the β-actin loading levels and are shown in the lower panel.
JNK Inhibitor Decreases the Levels of Cell Death in HEMA-Treated Cells
Since HEMA causes an increase in the levels of JNK, and an increase in JNK may be responsible for HEMA mediated cell death, we opted to block HEMA-mediated increase in JNK using a previously characterized JNK inhibitor with a broader spectrum of action (SP600125) (Calbiochem, CA). As seen in Figure 6A the levels of cell death in HEMA treated HEp2 cells was lower in the presence of JNK inhibitor. Moreover, the addition of JNK inhibitor to HEMA treated HEp2 cells also prevented the loss of VEGF secretion, and decreased bFGF secretion in HEp2 cell transfectants (Figs. 6B and 6C).
DISCUSSION
Local cell damage and increased inflammation are found to contribute to the complex range of responses occurring clinically in the dental pulp after tooth restoration with resin containing materials (Hebling et al., 1999). Micrograms to milligrams of resin based monomers such as HEMA is released into the adjacent aqueous phase from a broad range of resin-based bonding, cementing and direct filling materials from clinically used amounts during the first days after placement (Gerzina and Hume, 1996; Hume and Gerzia, 1996). We have found that loss of mitochondrial membrane potential and activation of effector caspases were two major hallmarks of the intrinsic death pathway induced by HEMA (Paranjpe et al., 2008a). Moreover, the analysis of the genes involved in apoptotic pathways by microarray technology indicated that several critical genes related to cell cycle checkpoints, signaling proteins and cell death mediators were also elevated in the presence of HEMA treatment (refer to supplemental data of Paranjpe et al., 2005; http://www.dentalresearch.org). Therefore, many of the inhibitors which have been shown to block apoptotic cell death should in practice block HEMA mediated cell death. However, inhibition of cell death should also parallel gain of functional competency of the cells if effective strategies are sought to reverse HEMA mediated adverse effects. In this regard, the cell death inhibitor NAC fulfills these criteria since both the death and loss of cellular function were inhibited by NAC.
Blockade of cell death by NAC was suggested to relate to its inhibitory effect on the generation of ROS and oxidative stress in HEMA treated cells since NAC was previously shown to have anti-oxidant effect (Chang et al., 2005; Spagnuolo et al., 2006). However, since well known anti-oxidants such as Trolox and Ascorbates not only did not change the course of HEMA mediated cell death but also they contributed to it (Paranjpe et al., 2007), it is therefore believed that NAC may function via mechanisms which are distinct from those reported for Trolox and Ascorbates (Walther et al., 2004). Indeed, we report here that NAC is an important inducer of NF-κB in oral epithelial cells. Increased activation of NF-κB in other epithelial cells by NAC was also reported previously (Das et al., 1995). However, in contrast to the previous reports our data clearly demonstrate an NF-κB dependent and independent protective effect of NAC on the cells. It is important to note that the addition of a number of potent activators of NF-κB such as TNF-α (Fig. 1B) and PMA/Ionomycin (data not shown) to NFκB knock down HEp2 cells were unable to increase NFκB in NF-κB knockdown HEp2 cells. Thus, the NF-κB independent protection of HEp2 cells by NAC is clearly not due to an increase in NF-κB in NF-κB knockdown HEp2 cells (Fig. 1).
NAC may provide the protective mechanism in part by increasing the anti-apoptotic proteins regulated by NF-κB. In this respect MnSOD (Mn superoxide dismutase) may be implicated in this process since NF-κB is an important modulator of MnSOD (Murley et al., 2001). In our experiments, the partial role of NF-κB in survival and function of the cells is evident by the following observations. First, treatment of HEp2 cells with HEMA significantly blocked nuclear NF-κB expression and function. This is in contrast to the previously published data where the addition of HEMA was reported to increase rather than decrease the nuclear NF-κB expression in fibroblasts (Spagnuolo et al. 2004). This discrepancy could be due to the type of cells employed or the timing of the treatment. However, we did not observe any induction of NF-κB activity by HEMA from 4 to 24 h of treatment in the cells tested. Second, the addition of NAC significantly increased NF-κB expression in oral epithelial cells, and restored NF-κB activity in HEMA-treated cells, with concomitant rescue of cell viability and function. NAC had an effect similar to TNF-α in this system. Third, when nuclear NF-κB was inhibited in oral keratinocytes both HEMA (Figs. 4B and 4C) and TNF-α (Jewett et al., 2003) exacerbated death of the cells, and the protective effect of NAC on cell death was partially lost (Paranjpe et al., 2007). Collectively, these results are consistent with a model in which NAC blocks HEMA mediated cell death partially through the induction of NFκB.
Even though a portion of NAC's protective effect on HEMA induced cell death is regulated by NFκB, a significant portion of its effect is NF-κB independent. In this regard we demonstrate that NAC prevents HEMA mediated induction of JNK. Strong and sustained activation of JNK by a variety of stresses such as UV light and hydroxy peroxides results in an induction of significant apoptosis in the cells (Butterfield et al., 1997; Chen et al., 1996). As determined by a cDNA array analysis (data not shown) expression of JNK1 in NF-κB knockdown HEp2 cells augmented MAPKK1/MKK1 gene expression in HEp2 cells (data not shown). Thus, increased activation of MAPKK1 could in turn increase the levels of JNK expression in NF-κB knockdown HEp2 cells resulting in a sustained increase in the levels and function of JNK (Reuther-Madrid et al., 2002). JNK is also an important regulator of p53 tumor suppressor protein (Fuchs et al., 1998). Activation of p53 in many different tumor cells induces growth arrest and apoptosis (Eliyahu et al., 1984; Lane, 1984). JNK prolongs the half-life of p53 (Fuchs et al., 1998). In accordance, we have observed significant induction of p53 associated protein, proline dehydrogenase (proline oxidase 1) gene when JNK was expressed in NF-κB knock down HEp2 cells (manuscript submitted). HEp2 cells do not express p53 tumor suppressor protein and thus loss of p53 expression is believed to be one of the contributory mechanisms to their transformation and survival. Thus, increased JNK activity may serve to increase the levels of p53 and its target protein proline dehydrogenase (proline oxidase 1) gene resulting in the augmentation of cell death seen in this study. These possibilities are currently under investigation in our laboratory.
We have also found that the inverse increase in JNK and NF-κB pathways in HEp2 cells is responsible for the synergistic upregulation of IgFbp6 as determined by the gene and protein analysis (Cacalano et al., 2008). Both the IgFbp6 and proline dehydrogenase gene (proline oxidase 1) were previously shown to play significant roles in programming for death of a variety of cells (Hu et al., 2007; Pandhare et al., 2006). Whether synergistic induction of abovementioned genes in combination by HEMA are responsible for cell death of HEp2 cells should await future investigations.
Although the pharmacologic JNK inhibitor SP600125 was used to selectively target JNK1, 2, and 3 in previous studies, in some recent studies the specificity of this inhibitor has been challenged, pointing to perhaps the limitation of such an approach in delineating the mechanisms involving the JNK pathway (Bain et al., 2003). Therefore, although it is likely that JNK is one of the important mediators of HEMA induced cell death in HEp2 cells, further genetic as well as pharmacological studies are warranted and will be performed in our future studies to establish the extent and the level of the contribution of this pathway in HEMA mediated cell death.
To determine the levels of functional competency under different experimental conditions we opted to use the status of VEGF and bFGF release by the cells. These two growth factors exhibit different release profiles depending on the levels of cell viability and can be used to determine the functional competency of the cells under different experimental conditions. Whereas viable HEp2 cells are capable of secreting high levels of VEGF, cells which are signaled to undergo cell death loose this ability and decrease the levels of VEGF secretion (Figs. 3A and 6C). In contrast, those that are signaled to undergo cell death release high levels of bFGF (Figs. 3B and 6B). Therefore, by using these two parameters we were able to demonstrate that JNK inhibitor was able to prevent cell death and maintain the functional competency of the HEp2 cell transfectants. Since blocking NF-κB in HEp2 cells abolishes the ability of the cells to release bFGF, we could only determine bFGF release in vector-alone transfected HEp2 cells (Fig. 3B). Thus, NAC mediated decrease in bFGF release from DPSCs or HEp2 cells is another piece of supporting evidence regarding the role of NAC in increase functional activation of NF-κB (data not shown and Fig. 3B).
The results reported in this paper indicated that the protective effect of NAC on HEMA mediated cell death maybe induced via mechanisms other than or in addition to an anti-oxidant effect which was reported previously (Schweikl et al., 2006). We have reported that protection of NAC is partly due to its capacity to induce differentiation of the cells (Paranjpe et al., 2007) due to the increased induction of important transcription factors such as NF-κB and an increase in a number of important differentiation genes reported previously (Paranjpe et al., 2007). The full spectrum of signals responsible for the induction of differentiation in the cells by NAC is not established yet, but it clearly involves both the NF-κB and JNK signaling pathways (Figs. 1A and 5). Overall, studies reported in this paper and those which were performed in vivo (Paranjpe et al., 2008b) indicated that NAC is an important protective agent which could be used in dental restorations to protect the pulp cells from toxic effects of restorative materials. In addition, they indicated that there may be mutually inclusive and exclusive effects of NF-κB and JNK in NAC mediated protection from HEMA mediated cell death.
FUNDING
National Institutes of Health, National Institute of Dental and Craniofacial Research (NIH-NIDCR) grant (# RO1-10331).
References
- Arsura M, Panta GR, Bilyeu JD, Cavin LG, Sovak MA, Oliver AA, Factor V, Heuchel R, Mercurio F, Thorgeirsson SS, et al. Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: Implications in liver tumor formation. Oncogene. 2003;22:412–425. doi: 10.1038/sj.onc.1206132. [DOI] [PubMed] [Google Scholar]
- Auzerie V, Chiali A, Bussel A, Brouet JC, Fermand JP, Dubertret L, Senet P. Leg ulcers associated with cryoglobulinemia: Clinical study of 15 patients and response to treatment. Arch. Dermatol. 2003;139:391–393. doi: 10.1001/archderm.139.3.391. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: An update. Biochem. J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Butterfield L, Storey B, Maas L, Heasley LE. c-Jun NH2-terminal kinase regulation of the apoptotic response of small cell lung cancer cells to ultraviolet radiation. J. Biol. Chem. 1997;272:10110–10116. doi: 10.1074/jbc.272.15.10110. [DOI] [PubMed] [Google Scholar]
- Cacalano NA, Le D, Paranjpe A, Wang MY, Fernandez A, Evazyan T, Park NH, Jewett A. Regulation of IGFBP6 gene and protein is mediated by the inverse expression and function of c-jun N-terminal kinase (JNK) and NFkappaB in a model of oral tumor cells. Apoptosis. 2008;13:1439–1449. doi: 10.1007/s10495-008-0270-1. [DOI] [PubMed] [Google Scholar]
- Castrillo A, Traves PG, Martin-Sanz P, Parkinson S, Parker PJ, Bosca L. Potentiation of protein kinase C zeta activity by 15-deoxy-delta(12,14)-prostaglandin J(2) induces an imbalance between mitogen-activated protein kinases and NF-kappa B that promotes apoptosis in macrophages. Mol. Cell. Biol. 2003;23:1196–1208. doi: 10.1128/MCB.23.4.1196-1208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HH, Guo MK, Kasten FH, Chang MC, Huang GF, Wang YL, Wang RS, Jeng JH. Stimulation of glutathione depletion, ROS production and cell cycle arrest of dental pulp cells and gingival epithelial cells by HEMA. Biomaterials. 2005;26:745–753. doi: 10.1016/j.biomaterials.2004.03.021. [DOI] [PubMed] [Google Scholar]
- Chen YR, Wang X, Templeton D, Davis RJ, Tan TH. The role of c-Jun N-terminal kinase (JNK) in apoptosis induced by ultraviolet C and gamma radiation. Duration of JNK activation may determine cell death and proliferation. J. Biol. Chem. 1996;271:31929–31936. doi: 10.1074/jbc.271.50.31929. [DOI] [PubMed] [Google Scholar]
- Das KC, Lewis-Molock Y, White CW. Activation of NF-kappa B and elevation of MnSOD gene expression by thiol reducing agents in lung adenocarcinoma (A549) cells. Am. J. Physiol. 1995;269:L588–L602. doi: 10.1152/ajplung.1995.269.5.L588. [DOI] [PubMed] [Google Scholar]
- Doyle S, Vaidya S, O'Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- Eliyahu D, Raz A, Gruss P, Givol D, Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984;312:646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Fuchs SY, Adler V, Buschmann T, Yin Z, Wu X, Jones SN, Ronai Z. JNK targets p53 ubiquitination and degradation in nonstressed cells. Genes Dev. 1998;12:2658–2663. doi: 10.1101/gad.12.17.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerzina TM, Hume WR. Effect of dentine on release of TEGDMA from resin composite in vitro. J. Oral Rehabil. 1994;21:463–468. doi: 10.1111/j.1365-2842.1994.tb01160.x. [DOI] [PubMed] [Google Scholar]
- Gerzina TM, Hume WR. Diffusion of monomers from bonding resin-resin composite combinations through dentine in vitro. J. Dent. 1996;24:125–128. doi: 10.1016/0300-5712(95)00036-4. [DOI] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Gustafsson AC, Kupershmidt I, Edlundh-Rose E, Greco G, Serafino A, Krasnowska EK, Lundeberg T, Bracci-Laudiero L, Romano MC, Parasassi T, et al. Global gene expression analysis in time series following N-acetyl L-cysteine induced epithelial differentiation of human normal and cancer cells in vitro. BMC Cancer. 2005;5:75. doi: 10.1186/1471-2407-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid A, Hume WR. The effect of dentine thickness on diffusion of resin monomers in vitro. J. Oral Rehabil. 1997;24:20–25. doi: 10.1046/j.1365-2842.1997.00490.x. [DOI] [PubMed] [Google Scholar]
- Hamid A, Okamoto A, Iwaku M, Hume WR. Component release from light-activated glass ionomer and compomer cements. J. Oral Rehabil. 1998;25:94–99. doi: 10.1046/j.1365-2842.1998.00247.x. [DOI] [PubMed] [Google Scholar]
- Hebling J, Giro EM, Costa CA. Human pulp response after an adhesive system application in deep cavities. J. Dent. 1999;27:557–564. doi: 10.1016/s0300-5712(99)00034-2. [DOI] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Hu CA, Donald SP, Yu J, Lin WW, Liu Z, Steel G, Obie C, Valle D, Phang JM. Overexpression of proline oxidase induces proline-dependent and mitochondria-mediated apoptosis. Mol. Cell. Biochem. 2007;295:85–92. doi: 10.1007/s11010-006-9276-6. [DOI] [PubMed] [Google Scholar]
- Hu YL, Li S, Shyy JY, Chien S. Sustained JNK activation induces endothelial apoptosis: Studies with colchicine and shear stress. Am. J. Physiol. 1999;277:H1593–H1599. doi: 10.1152/ajpheart.1999.277.4.H1593. [DOI] [PubMed] [Google Scholar]
- Hume WR, Gerzia TM. Bioavailability of components of resin-based materials which are applied to teeth. Crit. Rev. Oral Biol. Med. 1996;7:172–179. doi: 10.1177/10454411960070020501. [DOI] [PubMed] [Google Scholar]
- Jewett A. Activation of c-Jun N-terminal kinase in the absence of NFkappaB function prior to induction of NK cell death triggered by a combination of anti-class I and anti-CD16 antibodies. Hum. Immunol. 2001;62:320–331. doi: 10.1016/s0198-8859(01)00218-x. [DOI] [PubMed] [Google Scholar]
- Jewett A, Cacalano NA, Teruel A, Romero M, Rashedi M, Wang M, Nakamura H. Inhibition of nuclear factor kappa B (NFkappaB) activity in oral tumor cells prevents depletion of NK cells and increases their functional activation. Cancer Immunol. Immunother. 2006;55:1052–1063. doi: 10.1007/s00262-005-0093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewett A, Cavalcanti M, Bonavida B. Pivotal role of endogenous TNF-alpha in the induction of functional inactivation and apoptosis in NK cells. J. Immunol. 1997;159:4815–4822. [PubMed] [Google Scholar]
- Jewett A, Wang MY, Teruel A, Poupak Z, Bostanian Z, Park NH. Cytokine dependent inverse regulation of CD54 (ICAM1) and major histocompatibility complex class I antigens by nuclear factor kappaB in HEp2 tumor cell line: Effect on the function of natural killer cells. Hum. Immunol. 2003;64:505–520. doi: 10.1016/s0198-8859(03)00039-9. [DOI] [PubMed] [Google Scholar]
- Kanerva L, Pelttari M, Jolanki R, Alanko K, Estlander T, Suhonen R. Occupational contact urticaria from diglycidyl ether of bisphenol A epoxy resin. Allergy. 2002;57:1205–1207. doi: 10.1034/j.1398-9995.2002.13118.x. [DOI] [PubMed] [Google Scholar]
- Karlgard CC, Wong NS, Jones LW, Moresoli C. In vitro uptake and release studies of ocular pharmaceutical agents by silicon-containing and p-HEMA hydrogel contact lens materials. Int. J. Pharm. 2003;257:141–151. doi: 10.1016/s0378-5173(03)00124-8. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996a;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Sounding the alarm: Protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 1996b;271:24313–24316. doi: 10.1074/jbc.271.40.24313. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lane DP. Cell immortalization and transformation by the p53 gene. Nature. 1984;312:596–597. doi: 10.1038/312596a0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gorospe M, Yang C, Holbrook NJ. Role of mitogen-activated protein kinase phosphatase during the cellular response to genotoxic stress. Inhibition of c-Jun N-terminal kinase activity and AP-1-dependent gene activation. J. Biol. Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis RJ, Johnson GL, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science (New York, N. Y.) 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- Murley JS, Kataoka Y, Hallahan DE, Roberts JC, Grdina DJ. Activation of NFkappaB and MnSOD gene expression by free radical scavengers in human microvascular endothelial cells. Free Radic. Biol. Med. 2001;30:1426–1439. doi: 10.1016/s0891-5849(01)00554-8. [DOI] [PubMed] [Google Scholar]
- Pandhare J, Cooper SK, Phang JM. Proline oxidase, a proapoptotic gene, is induced by troglitazone: Evidence for both peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms. J. Biol. Chem. 2006;281:2044–2052. doi: 10.1074/jbc.M507867200. [DOI] [PubMed] [Google Scholar]
- Paranjpe A, Bordador LC, Wang MY, Hume WR, Jewett A. Resin monomer 2-hydroxyethyl methacrylate (HEMA) is a potent inducer of apoptotic cell death in human and mouse cells. J. Dent. Res. 2005;84:172–177. doi: 10.1177/154405910508400212. [DOI] [PubMed] [Google Scholar]
- Paranjpe A, Cacalano NA, Hume WR, Jewett A. N-acetylcysteine protects dental pulp stromal cells from HEMA-induced apoptosis by inducing differentiation of the cells. Free Radic. Biol. Med. 2007;43:1394–1408. doi: 10.1016/j.freeradbiomed.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjpe A, Cacalano NA, Hume WR, Jewett A. Mechanisms of N-acetyl cysteine-mediated protection from 2-hydroxyethyl methacrylate-induced apoptosis. J. Endodontics. 2008a;34:1191–1197. doi: 10.1016/j.joen.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Paranjpe A, Sung EC, Cacalano NA, Hume WR, Jewett A. N-acetyl cysteine protects pulp cells from resin toxins in vivo. J. Dent. Res. 2008b;87:537–541. doi: 10.1177/154405910808700603. [DOI] [PubMed] [Google Scholar]
- Parasassi T, Brunelli R, Bracci-Laudiero L, Greco G, Gustafsson AC, Krasnowska EK, Lundeberg J, Lundeberg T, Pittaluga E, Romano MC, Serafino A. Differentiation of normal and cancer cells induced by sulfhydryl reduction: Biochemical and molecular mechanisms. Cell Death Differ. 2005;12:1285–1296. doi: 10.1038/sj.cdd.4401663. [DOI] [PubMed] [Google Scholar]
- Reuther-Madrid JY, Kashatus D, Chen S, Li X, Westwick J, Davis RJ, Earp HS, Wang CY, Jr, Baldwin AS., Jr The p65/RelA subunit of NF-kappaB suppresses the sustained, antiapoptotic activity of Jun kinase induced by tumor necrosis factor. Mol. Cell. Biol. 2002;22:8175–8183. doi: 10.1128/MCB.22.23.8175-8183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweikl H, Hartmann A, Hiller KA, Spagnuolo G, Bolay C, Brockhoff G, Schmalz G. Inhibition of TEGDMA and HEMA-induced genotoxicity and cell cycle arrest by N-acetylcysteine. Dent Mater. 2007;23:688–695. doi: 10.1016/j.dental.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Schweikl H, Spagnuolo G, Schmalz G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006;85:870–877. doi: 10.1177/154405910608501001. [DOI] [PubMed] [Google Scholar]
- Spagnuolo G, D'Anto V, Cosentino C, Schmalz G, Schweikl H, Rengo S. Effect of N-acetyl-L-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials. 2006;27:1803–1809. doi: 10.1016/j.biomaterials.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Spagnuolo G, Mauro C, Leonardi A, Santillo M, Paterno R, Schweikl H, Avvedimento EV, Rengo S. NF-kappaB protection against apoptosis induced by HEMA. J. Dent. Res. 2004;83:837–842. doi: 10.1177/154405910408301103. [DOI] [PubMed] [Google Scholar]
- Tang F, Tang G, Xiang J, Dai Q, Rosner MR, Lin A. The absence of NF-kappaB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol. Cell. Biol. 2002;22:8571–8579. doi: 10.1128/MCB.22.24.8571-8579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: Intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Walther UI, Siagian II, Walther SC, Reichl FX, Hickel R. Antioxidative vitamins decrease cytotoxicity of HEMA and TEGDMA in cultured cell lines. Arch. Oral Biol. 2004;49:125–131. doi: 10.1016/j.archoralbio.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science (New York, N. Y.) 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]