Abstract
Methoxychlor (MXC) is an organochlorine pesticide whose mono- and bis-demethylated metabolites, 2-(4-hydroxyphenyl)-2-(4-methoxyphenyl)-1,1,1-trichloroethane (OH-MXC) and 2,2-bis(4-hydroxyphenyl)-1,1,1-trichloroethane (HPTE), respectively, are estrogenic and antiandrogenic. Studies in vitro showed that treatment of channel catfish with a polycyclic aromatic hydrocarbon increased phase I and phase II metabolism of MXC. To determine the in vivo significance, groups of four channel catfish were treated by gavage for 6 days with 2 mg/kg 14C-MXC alone or 2 mg/kg 14C-MXC and 2 mg/kg benzo(a)pyrene (BaP). On day 7, blood and tissue samples were taken for analysis. Hepatic ethoxyresorufin O-deethylase activity was 10-fold higher in the BaP-treated catfish, indicating CYP1A induction. More MXC-derived radioactivity remained in control (42.8 ± 4.1%) than BaP-induced catfish (28.5 ± 3.2%), mean percent total dose ± SE. Bile, muscle and fat contained approximately 90% of the radioactivity remaining in control and induced catfish. Extraction and chromatographic analysis showed that liver contained MXC, OH-MXC, HPTE, and glucuronide but not sulfate conjugates of OH-MXC and HPTE. Liver mitochondria contained more MXC, OH-MXC, and HPTE than other subcellular fractions. Bile contained glucuronides of OH-MXC and HPTE, and hydrolysis of bile gave HPTE and both enantiomers of OH-MXC. The muscle, visceral fat, brain and gonads contained MXC, OH-MXC, and HPTE in varying proportions, but no conjugates. This study showed that catfish coexposed to BaP and MXC retained less MXC and metabolites in tissues than those exposed to MXC alone, suggesting that induction enhanced the elimination of MXC, and further showed that potentially toxic metabolites of MXC were present in the edible tissues.
Keywords: channel catfish methoxychlor residues, in vivo methoxychlor metabolism, induction of methoxychlor metabolism by benzo(a)pyrene
Methoxychlor (MXC) is the common name for 1,1,1-trichloro-2,2-bis(4-methoxyphenyl)ethane, an organochlorine pesticide that is a structural analogue of 1,1,1-trichloro-2,2-bis(chlorophenyl)ethane (DDT). MXC exhibits lower toxicity toward mammals and lower persistence in the environment than DDT and has been used widely on pets, livestock, field crops, and gardens (ATSDR, 1994). Although it is not as persistent as DDT, MXC has been found in soils and sediments as well as animal tissue (Guo et al., 2007; Wan et al., 2005), its global distribution being evident in the presence of MXC residues in tissues samples of several arctic wildlife species (Vorkamp et al., 2004). There is growing evidence to suggest that MXC exposure results in endocrine disruption in mammals, birds, amphibians, fish and invertebrates (Golub et al., 2003; Gray et al., 1985; Krisfalusi et al., 1998; Ottinger et al., 2005). There are reports that MXC causes mitochondrial dysfunction, through generation of reactive oxygen species (Bal, 1984; Gupta et al., 2006). In recognition of these adverse effects, use of MXC was restricted in California in the mid-1990,s. In 2004, the re-registration of MXC was denied by the U.S. Environmental Protection Agency, however, the pesticide is still used in other countries around the world.
The endocrine disrupting effects of MXC are due in large part to the formation of metabolites. Cytochrome P450-dependent biotransformation of MXC gives rise to the mono- and bis-demethylated primary metabolites 2-(4-hydroxyphenyl)-2-(4-methoxyphenyl)-1,1,1-trichloroethane (OH-MXC) and 2,2-bis(4-hydroxyphenyl)-1,1,1-trichloroethane (HPTE), respectively. Both metabolites exhibit significant estrogen receptor-binding activity, with HPTE being more potent than OH-MXC in rodent and fish species (Blum et al., 2008a; Ousterhout et al., 1981; Schlenk et al., 1998). The two enantiomers of OH-MXC interact differently with human estrogen receptor alpha, such that (S)-OH-MXC is three times more potent than (R)-OH-MXC (Miyashita et al., 2004). It is not known if fish estrogen receptors respond differently to either enantiomer. The hydroxyl groups in both OH-MXC and HPTE have been shown through in vitro studies to be glucuronidated and sulfonated (Hazai et al., 2004; James et al., 2008; Ohyama et al., 2004), and it is assumed that these conjugates are not biologically active. Thus, the balance between activation and detoxication pathways in a particular species will determine the duration of exposure to the estrogenic metabolites.
Due to pesticide run off, chemical waste and sewage effluents, the aquatic environment can be a sink for xenobiotics (Guo et al., 2007; Hinck et al., 2007; James and Pritchard, 1991). As well as pesticides, combustion products such as polycyclic aromatic hydrocarbons (PAHs) and industrial chemicals such as polychlorinated biphenyls are frequent contaminants of the aquatic environment (James and Kleinow, 1994). PAH of particular concern include those that are carcinogenic and widespread in the environment, such as benzo(a)pyrene (BaP) (Lijinsky, 1991; Tsai et al., 2007). Channel catfish (Ictalurus punctatus) farming is an important industry in the USA and elsewhere and farmed fish are often grown in ponds adjacent to crop-land that may be contaminated with a variety of pesticides and other xenobiotics, some of which may have adverse effects both on the fish and consumers of the fish. In order to assess the risk of exposure to anthropogenic chemicals like MXC, there is therefore a need to study their fate in food-producing species such as the catfish, which are likely to be environmentally exposed to such chemicals.
Studies in vitro of MXC phase I metabolism in the channel catfish showed that mono- and bis-demethylation was a relatively efficient process, and that exposure of the catfish to the PAH, 3-methylcholanthrene, resulted in increased hepatic and intestinal biotransformation of MXC to OH-MXC and HPTE, although the P450 isoforms responsible were not identified (Stuchal et al., 2006). The phase II glucuronidation and sulfonation of OH-MXC and HPTE proceeded more slowly, but were also increased in 3-methylcholanthrene-treated catfish compared with controls (James et al., 2008). Sulfonation of OH-MXC and HPTE was approximately ten times less efficient than their glucuronidation, both in the liver and intestine. Based on in vitro findings, it is predicted that the combined rates of glucuronidation and sulfonation of OHMXC and HPTE will be much lower than the cytochrome P450–dependent rates of OH-MXC or HPTE formation, and could lead to accumulation of these potentially harmful metabolites in MXC-exposed catfish, but that overall metabolism of MXC will be greater in catfish exposed to PAH inducing agents. The present study was designed to complement the previous in vitro studies and to address questions about the in vivo fate of MXC and its estrogenic metabolites in the food-producing channel catfish. The objectives were to investigate the tissue distribution of MXC and metabolites following oral exposure of channel catfish to low daily doses of 14C-MXC, to examine the effects of concurrent exposure to the PAH BaP on the fate of MXC, and to determine if catfish enantioselectively formed or metabolized OH-MXC.
MATERIALS AND METHODS
Chemicals
The [14C]-MXC (9.6 mCi/mmol, > 97% pure) was purchased from Sigma-Aldrich Chemical Company (St Louis, MO) and unlabelled MXC, > 99% pure, from ICN (Aurora, OH). The OH-MXC and HPTE were prepared by published methods (Hu and Kupfer, 2002; Stuchal et al., 2006) and used to biosynthesize glucuronide and sulfate conjugate standards (James et al., 2008). Standards of the (R)- and (S)-enantiomers of OH-MXC, respectively, were prepared by incubating MXC with expressed human CYP1A2 and CYP2C19 bactosomes containing nicotinamide adenine dinucleotide phosphate hydrogen (NADPH)-cytochrome P450 reductase (Xenotech LLC, Lenexa, KS) in the presence of NADPH (Hu and Kupfer, 2002). High-performance liquid chromatography (HPLC) grade solvents were purchased from Fisher Scientific and all other chemicals used were of the purest grade available and were purchased from commercial suppliers.
Animals
Male and female adult catfish (Ictalurus punctatus) weighing between 585 and 700 g were used. They were housed in flow-through conditioned water at 22°C and maintained on a purified diet as previously described (James et al., 1997) for two weeks before use.
Indwelling gavage tube.
To facilitate accurate delivery of the correct amount of treatment diet to each fish on a per weight basis and to dose the animals repetitively without handling, catfish were individually fitted and dosed via indwelling stomach tube (Kleinow, 1991). To place the tubes the fish were anesthetized with buffered tricaine methane sulfonate (MS-222) at induction and maintenance doses of 106 and 86 mg/l. Following tube installation, fish were individually housed in flow-through tanks that facilitated access to the floating end of the stomach tube without removing the fish from the water. Dosing was accomplished after a 24-h recovery period by first removing the air from the collapsible latex tubing and placing a syringe with the predetermined dosed diet on the opened end. The dose was slowly advanced followed by a control diet slurry (2.5 ml) chaser and then a small amount of air, which was used to clear the tube of food. After dosing the air-filled tube was closed until the next administration.
Dosing.
Male and female catfish were randomly assigned to either a control group dosed with 14C-MXC alone (N = 4; 667 g ± 19, mean ± SE) or a 14C-MXC-BaP treatment (N = 4; 643 g ± 23) receiving the identical MXC treatment with a BaP supplement. The total daily MXC dose for both the control and treatment groups including stable and radiolabeled compound (3 μCi/kg body weight) was set at 2 mg/kg fish weight. For the 14C-MXC-BaP treatment a separate, but identical 14C-MXC diet was formulated with BaP so that the diet amount which delivered 2 mg MXC/kg body weight also delivered 2 mg BaP. Diets were formulated with appropriate compounds (radiolabeled and stable) in batch from a powdered form of catfish semisynthetic diet brought to a pudding like consistency with 40% water (James et al., 1997). Once the compounds were added to the diet the carrier solvent was evaporated under nitrogen gas, and diets mixed to attain a homogeneous preparation. The doses for individual animals were calculated proportionally according to body weight based on a 1000 × g animal receiving the dose in 3 ml of semisynthetic food slurry. All animals were dosed daily based on body weight for six days and harvested on the seventh.
Sampling.
On the day of sampling animals were harvested in order of dosing such that tissues were collected 24 h after the last of 6 consecutive daily dosages. Following euthanasia with an overdose of tricaine methane sulfonate, catfish were weighed and sexed. The total weight of each discrete tissue (liver, brain, gonads, intestinal mucosa) and of bile was recorded. Representative aliquots of blood, bile, liver, muscle, lipid, brain, skin, and gonads were collected, weighed, vials purged with nitrogen gas, frozen at −70°C and wrapped with foil for later HPLC analysis. For the intestine the contents were drained, the intestine cut open and profusely washed with cold saline and blotted dry. The mucosa was then harvested by scraping with a glass slide and the mucosa treated as described above for other tissues. A second smaller weighed sample of each fluid or organ was taken for quantitation of radioactivity. For the liver a third sample was placed in buffer (0.25M sucrose, 0.05M Tris HCl pH 7.4, 0.1mM ethylenediaminetetraacetic acid [EDTA], 0.1mM phenylmethylsulfonyl fluoride [PMSF]) and rapidly frozen for subsequent preparation of subcellular fractions.
Subcellular Fractionation
Frozen liver samples were thawed on ice, rinsed and homogenized in a Potter-Elvehjem apparatus in four times their weight of buffer 1, comprising 0.15M potassium chloride, 0.05M potassium phosphate (pH 7.4), 0.2mM PMSF. The homogenate was centrifuged at 600 × g for 10 min to sediment nuclei and cell debris. The supernatant was then centrifuged at 13,000 × g for 20 min to sediment the mitochondria. The pellet was suspended in buffer 1 and re-centrifuged at 13,000 × g for 20 min. The washed mitochondrial pellet was resuspended in buffer 2 containing 0.25M sucrose, 0.01M N-(2-hydroxyethyl)piperazine-N'-(2-ethanesulfonic acid) pH 7.4, 5% glycerol, 0.1mM dithiothreitol, 0.1mM EDTA, and 0.1mM PMSF. The supernatant fraction from the 13,000 × g spin was centrifuged at 150,000 × g for 45 min to give the cytosolic fraction (supernatant) and microsomal fraction (pellet). The microsomal pellet was resuspended in buffer 1 and centrifuged at 150,000 × g for 30 min to yield washed microsomes (pellet) which were resuspended in buffer 2. The resuspension volumes for the mitochondrial and microsomal pellets equaled the weight of the liver sample. Aliquots were taken at each step to monitor the subcellular distribution of radioactivity.
Tissue Distribution of Radiolabeled MXC
Each tissue or hepatic subcellular fraction was measured (weight or volume) then treated as described for subsequent liquid scintillation counting. The liver cytosol (0.1 ml) and microsomal wash (0.5 ml) were added into 5 ml of Ecolume scintillation cocktail (ICN, Aurora, OH) and counted directly. Bile (0.01 ml) was bleached with hydrogen peroxide before counting. Hepatic nuclei and cell debris (0.1 ml), hepatic mitochondria (0.1 ml), hepatic microsomes (0.05 ml), lipid, muscle, brain and gonad were digested for 2 h or until dissolved in 0.5 ml of 2 N sodium hydroxide, treated with 0.3 ml of 5 N hydrochloric acid, then mixed with the scintillation cocktail, 5 ml, after being allowed to cool. Pieces of whole liver, skin, and blood were digested as above and after cooling were bleached with hydrogen peroxide before counting. To calculate % dose in each tissue, counts in each sample were corrected for the whole organ, tissue or body fluid weight and divided by the cumulative total 6-day dose of radioactivity. Muscle, skin and blood were assumed to be 60, 6, and 5% of body weight respectively (Michel et al., 1990). The visceral fat deposits were assumed to be 1% of body weight based on the lean condition of the experimental fish. For all other tissues the actual measured weight was used.
Extraction of MXC and its Metabolites from Tissues
Duplicate samples of approximately 0.5 g liver, intestinal mucosa, muscle, visceral fat or gonad were accurately weighed and homogenized with three times volume of 10mM ammonium acetate pH 4.6 buffer. A mixture of acetonitrile/ethanol (2/1), 2 ml, was added and the mixture vortex-mixed then sonicated for 2 min. The mixture was centrifuged for 20 min at 2000 × g and the supernatant transferred to clean vials. The remaining pellet was again extracted with a further 1 ml of organic solvent and the resulting supernatant added to the first and evaporated to dryness. The pellet left after extraction was digested as described for tissue samples and the residual radioactivity measured by scintillation counting. This process was also used to extract portions of the liver mitochondrial and cytosolic fractions for analysis. For liver, intestinal mucosa and muscle the extracted residue was made up in 200 μl of mobile phase before HPLC analysis as described below. The residues from extraction of visceral fat and gonad, as well as liver mitochondrial and cytosolic fractions, were reconstituted with 50 μl of ethanol and aliquots were applied to LK5DF thin layer chromatography plates along with authentic standards for MXC, OH-MXC, HPTE, and a mixture of OH-MXC and HPTE-glucuronides. The plates were developed in a mobile phase comprising diethyl ether:n-heptane 1:1 (fat and gonads) or chloroform:toluene:ethyl acetate:acetone, 3:3:2:1 (liver fractions) and the dried plates imaged for 14C in an InstantImager (Packard, Chicago, IL). Radiolabeled bands were matched with the standards as visualized by UV and quantified as a percentage of the total amount of radioactivity present. To assess the efficiency of extraction, 0.5 g pieces of liver from untreated catfish were homogenized as above, spiked with 14C-labeled MXC, OH-MXC or a mixture of HPTE-glucuronide and OH-MXC-glucuronide, and taken through the extraction procedure.
Bile Analysis and Hydrolysis
Bile samples were diluted fivefold with mobile phase, centrifuged, and analyzed by HPLC (see section on HPLC analysis). To confirm the presence of glucuronide conjugates, aliquots of bile (50 μl) were added to 450 μl of 50mM Tris-Cl buffer pH 5.0 or to 450 μl of 50mM Tris-Cl buffer pH 5.0 containing 340 units of β-glucuronidase. The tubes were incubated at 37°C for 24 h and the reaction terminated by the addition of 2 ml of ice-cold methanol. The glucuronidase protein was allowed to flocculate for 20 min and then precipitated by centrifugation. The supernatant fractions were evaporated to dryness, dissolved in 100 μl of mobile phase and analyzed by reverse-phase and chiral HPLC.
Lipid Quantitation
A modification of the Folch method (Folch et al., 1957) was used to determine the lipid content of visceral fat, gonads and liver. There was not enough brain tissue to measure the lipid content of brain by this method. About 1 g of tissue was accurately weighed and homogenized in 10 ml of chloroform/methanol (2/1). The homogenate was transferred to clean tubes and the original tubes rinsed out with a further 10 ml of solvent. The tubes were stoppered, vortex-mixed and sonicated for 5 min then centrifuged. The resulting supernatant was filtered through Whatman #1 filter paper into preweighed tubes. To the filtrate, 4 ml of 0.01M sodium chloride was added then tubes were vortex-mixed and centrifuged to separate the phases. The upper aqueous phase was pipetted out and discarded. The exposed interface was rinsed twice with 1 ml of methanol:water (1:1) without mixing the whole preparation. The lower chloroform phase was then evaporated under nitrogen and the remaining lipid weight recorded.
Glucuronidation of OH-MXC
To examine the possibility that (R)- and (S)-OH-MXC may be glucuronidated with different efficiencies in channel catfish, hepatic microsomes from control channel catfish were incubated with 500μM racemic OH-MXC and 5mM UDPGA as described previously (James et al., 2008). The incubation conditions were set so that about 10% of the OH-MXC would be converted to the glucuronide. The unreacted OH-MXC was extracted into ethyl acetate (Stuchal et al., 2006). The extracts were evaporated to dryness and the residue taken up in 0.25 ml of mobile phase (14% acetonitrile in 10mM ammonium acetate/acetic acid buffer system pH 7) and analyzed by chiral HPLC as described below.
Ethoxyresorufin O-Deethylation
To confirm that treatment with BaP-induced CYP1A activity, microsomal ethoxyresorufin-O-deethylase (EROD) activity was measured in washed hepatic microsomes as described previously (James et al., 1997).
HPLC Analysis
HPLC analysis of MXC and its metabolites was done on a Beckman Gold System (Beckman Coulter, Fullerton, CA) equipped with UV and radioactivity detectors (Flo-one beta, IN/US, Tampa, FL). MXC and metabolites were detected at 245 nm or by radiochemistry and identified by comparison of retention times with those of authentic standards or by area increase after isolation of products by appropriate assays.
Reverse-phase (C18) separation was carried out at 40°C, using a 25 cm × 4.6 mm Discovery column with a particle size of 5 μm fitted with a 2 cm × 4.0 mm guard column (Supelco, Bellefonte, PA). A gradient program operated at 1 ml/min flow rate started with 30% acetonitrile in 10mM ammonium acetate/acetic acid buffer pH 4.6, was held for 0.5 min then linearly increased to 90% acetonitrile over 20 min and to 100% acetonitrile over 5 min. The mobile phase was returned to the starting conditions over 10 min and held at 30% acetonitrile for 10 min before the next sample.
Chiral separation of (R)- and (S)-OHMXC was achieved at room temperature using a 10 cm × 4.0 mm α-glycoprotein (AGP) column with a particle size of 5 μm fitted with a 2 cm × 4 mm guard column (ChromTech Inc, Apple Valley, MN), a mobile phase of 14% acetonitrile in 10mM ammonium acetate/acetic acid buffer system (pH 7) and a flow rate of 0.9 ml/min.
Data Analysis
Treatment and organ effects were analyzed by ANOVA, followed by the Tukey's (groups with equal variance) or Dunnett's T3 (groups with unequal variance) multiple comparison tests, or by Student's t-tests as were considered appropriate. All statistical analyses were carried out using SPSS (SPSS, Inc., Chicago, IL) and SAS 9.1 (SAS Institute Inc., Cary, NC). All data is presented here as mean ± SE, N = 4 unless otherwise stated.
RESULTS
Tissue Distribution of MXC Residues
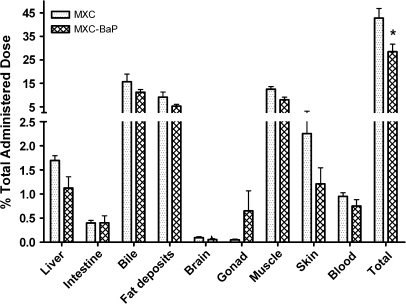
Adult channel catfish were treated daily by gavage for six days with 2 mg/kg 14C-MXC alone or 2 mg/kg 14C-MXC and 2 mg/kg BaP in groups of four. Twenty-four hours after the last dose, the catfish were sacrificed and the tissue distribution of radioactivity was determined. Figure 1 shows that the percentage of total administered dose recovered in tissues and bile was significantly greater (p < 0.001) in catfish that received MXC alone (42.8 ± 4.1%) than in those that received MXC and BaP (28.6 ± 3.2%). The percentage of total dose retained in only tissues, excluding bile, of catfish dosed with MXC alone was 27.5 ± 2.5% and 17.3 ± 2.5% in catfish dosed with MXC and BaP, values that are significantly different, p < 0.01. For each tissue or fluid, the mean % dose recovered in catfish that received MXC and BaP was lower than in catfish receiving MXC alone, but the difference reached statistical significance only when recovery in all tissues was considered. The pattern of distribution of radioactivity remaining in catfish was similar in both groups. The tissues contained 64.0 ± 4.6% of the radioactivity remaining in catfish that received MXC alone, and 66.3 ± 3.5% for catfish that received MXC and BaP. Muscle and visceral fat, accounted at 60 and 1% of catfish body weight respectively, were the tissues where most of the remaining radioactivity was found in both treatment groups.
FIG. 1.
Distribution of MXC and metabolites in channel catfish tissues and fluids 24 h after the final administered dose. One group (MXC) received 6 × 2 mg/kg MXC alone by oral gavage. The other group (MXC-BaP) received 6 × 2 mg/kg MXC and 6 × 2 mg/kg BaP by oral gavage. Values shown are mean % of the total dose administered over 6 days and bars indicate the standard error. A significant difference (p < 0.001) between the groups is indicated by an asterisk.
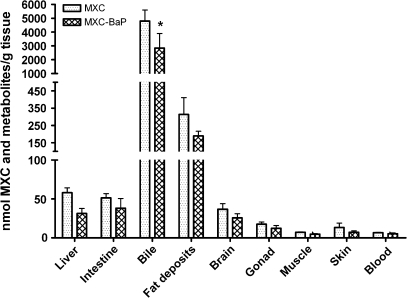
The mean concentration, nmol/g, of total 14C-MXC–derived radioactivity in each of the tissues and bile tended to be lower in catfish that received BaP and MXC as compared with those that received MXC alone, although this reached statistical significance (p < 0.001) only for bile (Fig. 2). Of the tissues, the visceral fat deposits contained the highest concentration of total radioactivity, followed by liver and intestinal mucosa. The concentration of radioactivity in muscle was low and was similar to the concentration in whole blood.
FIG. 2.
Distribution of MXC and metabolites in channel catfish tissues and fluids 24 h after the final administered dose. Groups are as for Figure 2. The values shown are mean nmol MXC and metabolites per gram tissue or milliliter fluid. Bars indicate standard error and a significant difference (p < 0.001) between the groups is indicated by an asterisk
Hepatic EROD Activity
Treatment of catfish with BaP and MXC resulted in significantly higher (p < 0.001) EROD activity, 987 ± 95 pmol/min/mg protein, than treatment with MXC alone, 92.6 ± 12.8 pmol/min/mg protein.
Distribution of Radioactivity in Liver Subcellular Fractions
Each of the liver subcellular fractions was assayed for radioactivity (Table 1). Most of the MXC-derived radioactivity was present in the washed mitochondrial fraction, followed by the cytosolic fraction, the washed microsomes and the fraction containing nuclei and cell debris. The subcellular distribution of radioactivity was similar in MXC- and MXC-BaP–treated catfish (Table 1).
TABLE 1.
Distribution of Radioactivity in Hepatic Subcellular Fractions from 14C-MXC- and 14C-MXC-BaP–treated Channel Catfish as a Percentage of the Total in Each Liver
| Treatment groups |
||
| MXC | MXC-BaP | |
| Nuclei and cell debris | 9.9 ± 1.38 | 7.6 ± 1.29 |
| Mitochondria | 46.0 ± 2.22 | 45.5 ± 0.59 |
| Cytosol | 27.8 ± 1.22 | 30.8 ± 1.25 |
| Microsomes | 16.3 ± 1.18 | 16.2 ± 0.46 |
Note. Values are mean percentages ± SE; N = 4.
Extraction and Analysis of MXC Metabolites in Tissues and Bile
The extraction method developed was effective in extracting over 90% of MXC and metabolites from the liver. It was assumed that the extraction efficiency would be similar for other tissues. For parent 14C-MXC, 95.6 ± 0.1% of the spiked compound was found in the pooled organic solvent extracts. The amounts extracted for 14C-OH-MXC and a mixture of the glucuronides of OH-MXC and HPTE were 96.7± 0.1 and 92.0 ± 0.05%, respectively. In determining the concentration of each analyte in tissues following chromatographic analysis of the extracts, no correction was made for the extraction efficiency.
Following the solvent extraction of each tissue sample prior to HPLC analysis, the total radioactivity not extracted by organic solvent was monitored by digesting the final tissue pellet in sodium hydroxide, neutralizing, then quantifying by scintillation counting. For most of the tissues, an average of 90–95% of the radioactivity was extracted, consistent with the amount of MXC and metabolite standards extracted from spiked liver. In the cases of the skin (range of radioactivity extracted 38–92%) and visceral fat samples (range of radioactivity extracted 61–92%), the extraction results were very variable, some of the samples not being extracted efficiently.
Liquid chromatography was used to quantitatively analyze radiolabeled MXC and its metabolites in different tissues and bile. Analyte retention times of MXC and its metabolites for the two HPLC methods used are shown in Table 2.
TABLE 2.
HPLC Methods and Analyte Retention Times (min) of MXC and its Metabolites
| Compound | Reverse-phase (C18) method | Chiral (AGP) column method |
| HPTE-glucuronide | 6.4 | |
| OH-MXC-glucuronide | 9.2 | |
| HPTE | 13.3 | 12.6 |
| (R)-OH-MXC | 17.1 | 20.3 |
| (S)-OH-MXC | 17.1 | 30.4 |
| MXC | 20.9 | 39.2 |
| MXC-olefin | 21.8 |
Note. Separation on the C18 column was carried out at 40°C. The mobile phase, 1 ml/min, was a linear gradient over 20 min of 30–90% acetonitrile in 10mM ammonium acetate/acetic acid buffer pH 4.6, followed by a gradient to 100% acetonitrile over 5 min before a return to starting conditions. Separation on the α-glycoprotein column was carried out at room temperature. The isocratic mobile phase, 0.9 ml/min, was 14% acetonitrile in 10mM ammonium acetate/acetic acid buffer pH 7.0.
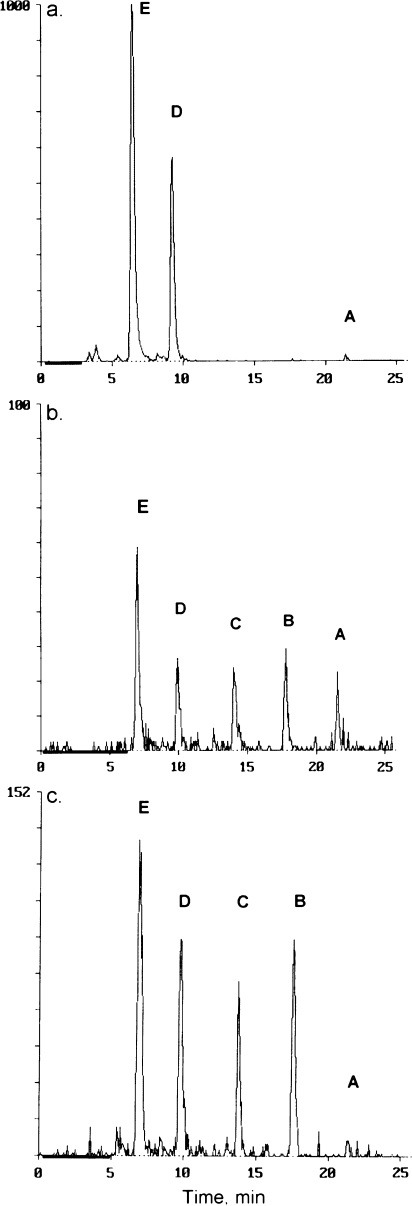
A summary of the concentrations of MXC and metabolites found in extracts of each tissue and fluid is given in Table 3. The bile contained glucuronides of HPTE and OH-MXC and very small amounts of MXC, but no sulfate conjugates or unconjugated HPTE or OH-MXC. A chromatogram of a representative bile sample is shown in Figure 3a. Analysis of the liver and the intestinal mucosa showed the presence of the glucuronides of both HPTE and OH-MXC, the phenolic metabolites OH-MXC and HPTE and in some samples the parent MXC. Liver from catfish treated with MXC alone contained more HPTE-glucuronide than catfish treated with MXC and BaP (p < 0.05). Representative chromatograms of extracts from liver (Fig. 3b) and intestinal mucosa (Fig. 3c) are given. Blood samples from the MXC-treated fish were extracted and analyzed individually, but the MXC-BaP samples were pooled because of the low concentration of 14C in blood. The metabolites seen in extracts of blood samples were mainly the phenolic metabolites and the parent compound (chromatograms not shown). Analysis of blood extracts from the MXC-treated fish showed small amounts of the glucuronides of OH-MXC or HPTE, however no glucuronides were detected in the pool of blood from MXC-BaP–treated fish. There were two minor unidentified metabolites in the chromatograms of blood extracts from some of the fish. Unknown 1, which eluted from C18 reverse-phase HPLC at 15.4 min, between HPTE and OH-MXC, was present in one fish from the MXC-treated group and in the MXC-BaP pooled blood sample. Unknown 2 eluted from C18 reverse-phase HPLC at 19.2 min, between OH-MXC and MXC, and was only evident in the extract of pooled blood from the MXC-BaP–treated samples. In muscle, visceral fat deposits, gonads and brain, only HPTE, OH-MXC, and MXC were found with no evidence for glucuronides. In the gonads, HPTE amounts were higher than MXC (p < 0.05), and the same trend was observed in muscle. In the fat deposits MXC concentrations were higher than concentrations of OH-MXC or HPTE (p < 0.05), and the same trend was observed in brain. Catfish skin samples from the two treatment groups were pooled by treatment before analysis. There was evidence of MXC and the glucuronide of HPTE in both treatment groups but the OH-MXC glucuronide was present only in the MXC-BaP group.
TABLE 3.
Metabolite Concentrations (nmol/g) from Tissues and Bile of 14C-MXC–Treated Channel Catfish
| Sample | Treatment | HPTE-Gluc | OH-MXC-Gluc | HPTE | OH-MXC | MXC |
| Bile | MXC | 3379 ± 805 | 1126 ± 82* | ND | ND | 14.1 ± 7.04 |
| MXC-BaPa | 2526 ± 1185 | 645 ± 91 | ND | ND | 6.95 ± 4.02 | |
| Liver | MXC | 24.7 ± 1.64* | 7.24 ± 1.84 | 13.7 ± 1.41 | 10.8 ± 4.40 | 1.70 ± 1.26 |
| MXC-BaP | 12.3 ± 1.53 | 3.72 ± 1.07 | 7.95 ± 2.26 | 6.33 ± 2.92 | 1.10 ± 0.96 | |
| Intestinal | MXC | 26.8 ± 2.77 | 10.9 ± 2.23 | 4.73 ± 2.02 | 5.72 ± 2.83 | 1.24 ± 0.86 |
| mucosa | MXC-BaP | 17.2 ± 4.95 | 7.78 ± 3.81 | 4.18 ± 1.04 | 5.14 ± 3.04 | 2.58 ± 1.07 |
| Blood | MXC | 0.76 ± 0.33 | 0.70 ± 0.10 | 1.73 ± 0.29 | 2.19 ± 0.45 | 1.44 ± 0.37 |
| MXC-BaP | NDb | ND | 0.76c | 1.12c | 1.85c | |
| Brain | MXC | 0.50d | ND | 8.94 ± 1.91 | 9.61 ± 3.03 | 17.7 ± 2.40 |
| MXC-BaP | 1.02d | ND | 4.38 ± 0.39 | 6.09 ± 1.71 | 15.0 ± 4.01 | |
| Visceral fat | MXCa | ND | ND | 17.7 ± 3.84 | 43.5 ± 7.89 | 241 ± 81 |
| MXC-BaP | ND | ND | 7.81 ± 0.22 | 21.0 ± 4.64 | 157 ± 24 | |
| Gonad | MXCa | ND | ND | 8.65 ± 1.47* | 1.85 ± 1.07 | 1.46 ± 0.47 |
| MXC-BaPa | ND | ND | 4.72 ± 0.58 | 2.85 ± 0.99 | 1.45 ± 1.22 | |
| Muscle | MXC | ND | ND | 3.56 ± 0.91 | 2.05 ± 0.67 | 1.63 ± 1.07 |
| MXC-BaP | ND | ND | 2.53 ± 0.55 | 1.62 ± 0.47 | 0.67 ± 0.32 | |
| Skin | MXC | 2.85c | ND | ND | ND | 9.48c |
| MXC-BaP | 2.93c | 1.14c | ND | ND | 3.09c |
Note. Values are mean ± SE, N = 4 unless otherwise noted. Within each tissue, an asterisk (*) denotes a significant difference in the concentration of a particular metabolite between MXC alone and MXC-BaP–treated catfish. The values shown were not corrected for extraction efficiency.
N = 3 for this tissue and treatment.
Not detected.
Samples from four individuals were pooled.
Detected in only one sample.
FIG. 3.
Representative HPLC radiochemical detector traces showing MXC and metabolites in (a) bile; (b) extract of liver; and (c) extract of intestinal mucosa. The chromatography was performed on a C18 column under conditions described in Table 2. Peaks are as follows: A, MXC; B, OH-MXC; C, HPTE; D, OH-MXC-glucuronide; E, HPTE-glucuronide. Similar chromatograms were obtained with other samples.
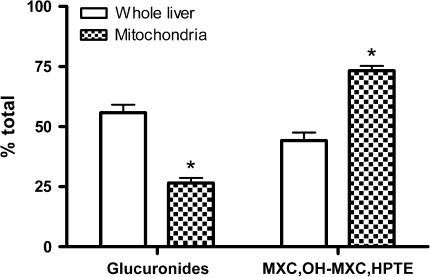
In the liver, mitochondria contained more of the MXC-derived radioactivity than other subcellular fractions. Mitochondria have been reported as a target organelle for MXC toxicity, therefore mitochondrial fractions were extracted and analyzed for metabolites. The liver mitochondria contained significantly more MXC, unconjugated OH-MXC and HPTE and less glucuronides than the whole liver sample. Figure 4 shows the results for catfish treated with MXC alone. Similar results were found with samples from catfish coexposed to BaP and MXC (data not shown).
FIG. 4.
MXC metabolites in liver and hepatic mitochondria of channel catfish dosed by gavage with MXC alone, 6 daily doses of 2 mg/kg. Bars indicate the mean percent total hepatic radioactivity found as each metabolite group in whole liver homogenate (open bars) or isolated mitochondria (cross-hatched bars). The standard error is shown. There was a greater relative amount of MXC and unconjugated OH-MXC and HPTE in mitochondria compared with whole liver (*p < 0.05), and a correspondingly lower amount of glucuronides.
Enantiomeric Composition of OH-MXC
In order to determine if channel catfish produced different amounts of (R)- or (S)-OH-MXC, standard of each enantiomer were biosynthesized by incubating 14C-MXC and NADPH with bactosomes expressing NADPH cytochrome P450 reductase and CYP1A2 or CYP2C19, respectively (Hu and Kupfer, 2002). The two enantiomers were baseline separated on the AGP chiral column under the conditions used (Table 2). Because bile contained high concentrations of radioactivity, we selected hydrolyzed bile for analysis of the enantiomeric composition of OH-MXC. Bile was incubated with β-glucuronidase both to confirm the identity of the metabolites as being glucuronides and to determine the ratio of (R)- and (S)-enantiomers of OH-MXC. Despite the presence of bile salts, which were shown to inhibit the hydrolysis of 4-methylumbelliferyl-β-D-glucuronide by up to 75% during a 30-min incubation (data not shown), a 24-h incubation achieved the hydrolysis of more than 95% of the biliary conjugates. C18 reverse-phase HPLC showed that HPTE and OH-MXC were present in the relative amounts expected from the concentrations of HPTE-glucuronide and OH-MXC-glucuronide as shown in Table 3. Chiral HPLC analysis showed that both (R) and (S)-OH-MXC were present in the bile hydrolysates. There was considerable variability between individual catfish bile samples, however the mean R/S ratios for the different treatment groups were not different. The ratio for the MXC-treated group was 1.05 ± 0.25 (N = 4) and for MXC-BaP–treated fish was 1.02 ± 0.32 (N = 3).
Glucuronidation of OH-MXC Enantiomers
The possibility of enantioselective glucuronidation of OH-MXC was examined by allowing a mixture of the two enantiomers to be glucuronidated and then comparing the average ratio of the area under the curve for (R)-OHMXC divided by that of (S)-OHMXC. A very slight preference for glucuronidation of (R)-OH-MXC was found after incubation of 500μM racemic OH-MXC with hepatic microsomes from control catfish under conditions that consumed about 10% of the OH-MXC. Analysis of the substrate before incubation, and of incubation blanks, showed the R/S ratio was 1.01 ± 0.01, N = 9 analyses, whereas analysis of extracted OH-MXC after glucuronidation gave a mean R/S ratio of 0.96 ± 0.01, N = 4 individual microsomal samples, significantly different from the substrate and incubated blanks, p < 0.01.
Lipid Quantitation
Of the tissues analyzed, the visceral fat, as expected, contained the highest percentage by weight of total lipids, at 81 ± 1%. The gonads contained 8.1 ± 0.5% and the liver 2.4 ± 0.1%.
DISCUSSION
This study showed that 14C-MXC was absorbed and distributed into tissues of the catfish, following daily oral gavage administration of 2 mg/kg. Visceral fat deposits were found to contain the highest concentrations of MXC residues. Although fat accounted for only 1% body weight in these lean fish, a high percentage of the administered dose was present in fat. This distribution pattern suggests that if fatty fish were exposed to MXC, they would retain more of the pesticide than lean fish. The muscle did not contain high concentrations of MXC-derived radioactivity, but because 60% of the weight of the catfish is muscle, the % administered dose retained in muscle was comparable to that retained in the fat. Consumers of catfish that were recently exposed to MXC would ingest MXC residues along with the filet. Bile had high concentrations of MXC-derived radioactivity in catfish treatment groups, suggesting that biliary excretion is an important route of elimination, as is true for other xenobiotics that are metabolized in the liver to glucuronide conjugates (Lewis and Lech, 1996; Statham et al., 1975). Concentrations of MXC-derived radioactivity were similar in blood, muscle and skin, suggesting there was no selective partitioning of MXC and metabolites from blood into muscle and skin. The brain and gonads, which presumably took up MXC and metabolites from the blood, contained higher concentrations of radioactivity than blood, possibly because of partitioning of MXC into these relatively lipid-rich tissues.
Effect of Coexposure to BaP on MXC-Derived Tissue Residues
Previous in vitro studies demonstrated that hepatic and intestinal MXC biotransformation was increased in catfish exposed to a single ip dose of 3-methylcholanthrene, 10 mg/kg (James et al., 2008; Stuchal et al., 2006). This was true for cytochrome P450–dependent demethylation as well as glucuronidation and sulfonation of the demethylated metabolites. In the present study it was shown that coexposure of catfish to BaP and MXC by gavage for 6 days significantly reduced the overall retention of MXC residues in tissues, compared with treatment with MXC alone. Although this study was not designed to study MXC toxicokinetics, the difference in overall retention in tissues implies that BaP treatment increased the rate of elimination of MXC in vivo, most probably through increased rates of biotransformation in intestine and liver to more readily excreted metabolites. Because fish are frequently exposed to mixtures of environmental chemicals, these results suggest that fish exposed to PAH as well as MXC will clear the MXC more rapidly than fish not exposed to PAH. There was no effect of BaP treatment on the tissue distribution pattern, as in both treatment groups the relative percentages of retained dose in the individual tissues were similar.
The BaP dietary dose used was confirmed to be sufficient to induce cytochrome P450, as hepatic microsomal EROD activity was increased 10-fold in fish coexposed to MXC and BaP, compared with MXC alone. The EROD activities in the catfish that received MXC alone were similar to activities found in control catfish liver microsomes in other studies (James et al., 1997), suggesting no effect of MXC on CYP1A expression.
MXC Metabolites
Most of the toxicity of MXC is due to its mono- and bis-demethylated metabolites, which are estrogenic and antiandrogenic (Blum et al., 2008a, b; Gaido et al., 2000), thus it is important to know the chemical form of 14C-MXC–derived radioactivity in tissues. The relative amounts of parent MXC and metabolites varied with tissue or fluid. In the expected sites of first-pass biotransformation, intestinal mucosa and liver, the demethylated metabolites, OH-MXC and HPTE and their glucuronide conjugates were detected but sulfate conjugates were not. This is consistent with in vitro results which showed that microsomes prepared from liver and intestinal mucosa readily demethylated MXC (Stuchal et al., 2006), and that the rates and efficiencies of microsomal glucuronidation of OH-MXC and HPTE were considerably higher than those of cytosolic sulfonation (James et al., 2008).
No studies in the published literature report the quantitative analysis of MXC metabolites in tissues after an in vivo treatment. Following administration of 14C-MXC to the mouse, goat, and chicken, demethylated, dechlorinated and conjugated metabolites were reported in bile and feces, but the metabolites in tissues were not examined (Davison et al., 1982, 1983, 1984; Kapoor et al., 1970). Metabolism of 5μM 14C-MXC has been reported in precision cut liver slices from rat, mouse, Japanese quail and rainbow trout incubated for 4, 7, 8, and 8 h, respectively, times at which at least 30% of the MXC was metabolized (Ohyama et al., 2004). Different quantities of the major metabolites were found in these species. In rat liver slices, the major metabolites were HPTE-glucuronide and the HPTE-glucuronide-sulfate diconjugate. In contrast OH-MXC-glucuronide was the main metabolite in liver slices from the mouse and Japanese quail, with small amounts of unconjugated OH-MXC and HPTE, and HPTE-glucuronide. A dechlorinated derivative of OH-MXC glucuronide was observed only in mouse preparations. In rainbow trout liver slices, comparable amounts of the glucuronides of both OH-MXC and HPTE were formed as the major metabolites. In liver slices from all species, including the rainbow trout, the phenolic unconjugated MXC metabolites were detected as minor products compared with the glucuronides, and with the exception of the rat double conjugate, no sulfate conjugates were found. Compared with the rainbow trout liver slice study, quantitatively but not qualitatively different amounts of MXC metabolites were found in the livers of in vivo exposed catfish. Whether treated alone or coexposed with BaP, the channel catfish liver contained similar amounts of unconjugated OH-MXC and HPTE; the amount of HPTE glucuronide was greater than of HPTE and the amount of OH-MXC-glucuronide less than the amount of OH-MXC.
Our finding that liver mitochondria contained higher concentrations of MXC, unconjugated OH-MXC and HPTE than the whole liver (Fig. 4), or than cytosol (data not shown), suggested that this organelle selectively took up MXC and demethylated MXC metabolites formed in the microsomes. This putative selective uptake may be important in the known mitochondrial toxicity of MXC (Gupta et al., 2006).
Of the channel catfish tissues examined, the fat deposits had the highest concentrations of MXC, OH-MXC, and HPTE, suggesting that the fat is a storage depot for these lipophilic compounds. The edible muscle tissue also contained MXC and unconjugated metabolites albeit at relatively low concentrations. Thus, consumers of MXC-exposed fish will be exposed not only to MXC, but also to its estrogenic and antiandrogenic metabolites. Other tissues that contained mainly MXC and unconjugated metabolites were the gonads. About 70% of the radioactivity found in the gonads was in the form of phenolic metabolites and another 10% the parent MXC. The presence of these potentially toxic compounds in the gonads is in agreement with the known reproductive toxicity of MXC to fish (Ankley et al., 2001; Blum et al., 2008b; Ortiz-Zarragoitia and Cajaraville, 2005).
Enantioselective Metabolism of MXC
Human CYP1A2 produces predominantly R-OH-MXC, which has been reported to show a lower affinity for the human estrogen receptor alpha than S-OH-MXC (Miyashita et al., 2004), thus it was of interest to determine if BaP treatment of catfish, which induced expression of CYP1A forms in liver, altered the relative formation of enantiomers of OH-MXC. The lack of difference in the R/S ratio for OH-MXC observed in bile hydrolysis products from the two radiolabeled MXC-treated catfish groups implies that in the catfish, the induction of cytochrome P450 by BaP did not affect the proportion of (R)- and (S)-enantiomers of OH-MXC. Although there was individual variability, the mean R/S ratios were the same in both groups, and were not significantly different from the R/S ratio of the racemic OH-MXC standard. In vitro incubations of 14C-MXC with hepatic microsomes from control and 3-MC–treated catfish showed that similar amounts of (R)- and (S)-OH-MXC were produced (Nyagode, 2007). Thus it appears that channel catfish do not produce either of the enantiomers of OH-MXC predominantly.
When the glucuronidation of the OH-MXC by hepatic microsomes from control catfish was monitored, a slight preference for glucuronidation of (R)-OH-MXC was suggested, because the R/S ratio of the remaining substrate was slightly, but significantly less than the ratio of the racemic substrate. Studies with different concentrations of the OH-MXC were not conducted, thus it is not yet clear if this preference for (R)-OH-MXC is due to a higher affinity of this enantiomer for one or more of the catfish UGT isoforms. In studies of the glucuronidation of OH-MXC by expressed human hepatic UDP-glucuronosyl transferases, UGT1A1, 1A3, and 1A9, but not 2B15 showed higher activity towards (S)- over (R)-OHMXC, whereas in human liver microsomes different results were observed among individual donors (Hazai et al., 2004). The results of some donors showed no differences, whereas in others there was a slight preference for glucuronidation of (R)-OHMXC. Such variability, as was seen in channel catfish too, may be attributed to the diverse composition, both of UGT isozymes as well as their quantities, in individuals.
In considering the relevance of our study to risk assessment, it should be noted that current assessments are based only on MXC, the parent compound. This study brings out the need to assess the risk due to metabolites of persistent compounds. There have been no in vivo studies of the uptake and elimination of OH-MXC or HPTE, thus although we expect these metabolites will be eliminated by glucuronidation, the rate of elimination is unknown. A number of environmental chemicals, such as hydroxylated metabolites of PCBs, triclosan and related chemicals inhibit glucuronidation, and the effect of coexposure to such inhibitors on the elimination of OH-MXC or HPTE is not known. Channel catfish is not considered a fatty fish with reported lipid content in muscle ranging from between 4 and 8% (Hedrick et al., 2005; Reigh, 1999; Seo et al., 1995), however, MXC, OH-MXC, and HPTE were found in muscle. Because we do not know the minimum amounts of OH-MXC and HPTE needed to cause adverse effects, the results of this study bring to light the importance of examining the implications of our results for highly consumed fish with higher lipid content in muscle than the channel catfish, such as salmon. Varying amounts of pesticide residues, including MXC were detected in soils and ditch sediments as well as in ditch water leading to salmon streams in several farms in Canada (Wan et al., 2005).
In summary, this study showed that MXC was readily metabolized to phenolic metabolites and their glucuronide conjugates in the catfish, that phenolic metabolites as well as MXC were present in visceral fat and edible muscle tissues, and that catfish coexposed to BaP and MXC retained less MXC and metabolites in tissues than those exposed to MXC alone.
FUNDING
National Institute of Environmental Health Sciences, National Institutes of Health grant (P42 ES007375).
Acknowledgments
We wish to thank Mrs Laura Rowland-Faux for technical assistance. This article reflects the authors' views and not any official views of National Institutes of Health.
References
- Ankley GT, Jensen KM, Kahl MD, Korte JJ, Makynen EA. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas) Environ. Toxicol. Chem. 2001;20:1276–1290. [PubMed] [Google Scholar]
- ATSDR. Toxicological Profile for Methoxychlor, US Public Health Service. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 1994. [PubMed] [Google Scholar]
- Bal HS. Effect of methoxychlor on reproductive systems of the rat. Proc. Soc. Exp. Biol. Med. 1984;176:187–196. doi: 10.3181/00379727-176-41861. [DOI] [PubMed] [Google Scholar]
- Blum JL, James MO, Stuchal LD, Denslow ND. Stimulation of transactivation of the largemouth bass estrogen receptors alpha, beta-a, and beta-b by methoxychlor and its mono- and bis-demethylated metabolites in HepG2 cells. J. Steroid. Biochem. Mol. Biol. 2008a;108:55–63. doi: 10.1016/j.jsbmb.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JL, Nyagode BA, James MO, Denslow ND. Effects of the pesticide methoxychlor on gene expression in the liver and testes of the male largemouth bass (Micropterus salmoides) Aquat. Toxicol. 2008b;86:459–469. doi: 10.1016/j.aquatox.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison KL, Feil VJ, Lamoureux CH. Methoxychlor metabolism in goats. J. Agric. Food Chem. 1982;30:130–137. doi: 10.1021/jf00109a029. [DOI] [PubMed] [Google Scholar]
- Davison KL, Lamoureux CH, Feil VJ. Methoxychlor metabolism in goats. 2. Metabolites in bile and movement through skin. J. Agric. Food Chem. 1983;31:164–166. doi: 10.1021/jf00115a038. [DOI] [PubMed] [Google Scholar]
- Davison KL, Lamoureux CH, Feil VJ. Methoxychlor metabolism in chickens. J. Agric. Food Chem. 1984;32:900–908. [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: Structure-activity studies. Mol. Pharmacol. 2000;58:852–858. [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL, Lasley BL, Natarajan K, Tarantal AF. Effects of exogenous estrogenic agents on pubertal growth and reproductive system maturation in female rhesus monkeys. Toxicol. Sci. 2003;74:103–113. doi: 10.1093/toxsci/kfg090. [DOI] [PubMed] [Google Scholar]
- Gray LE, Jr, Ferrell JM, Ostby JS. Alteration of behavioral sex differentiation by exposure to estrogenic compounds during a critical neonatal period: Effects of zearalenone, methoxychlor, and estradiol in hamsters. Toxicol. Appl. Pharmacol. 1985;80:127–136. doi: 10.1016/0041-008x(85)90107-3. [DOI] [PubMed] [Google Scholar]
- Guo JY, Zeng EY, Wu FC, Meng XZ, Mai BX, Luo XJ. Organochlorine pesticides in seafood products from southern China and health risk assessment. Environ. Toxicol. Chem. 2007;26:1109–1115. doi: 10.1897/06-446r.1. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol. Appl. Pharmacol. 2006;216:436–445. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hazai E, Gagne PV, Kupfer D. Glucuronidation of the oxidative cytochrome P450-mediated phenolic metabolites of the endocrine disruptor pesticide: Methoxychlor by human hepatic UDP-glucuronosyl transferases. Drug Metab. Dispos. 2004;32:742–751. doi: 10.1124/dmd.32.7.742. [DOI] [PubMed] [Google Scholar]
- Hedrick RL, Popma TJ, Davis DA. Pond production and fatty acid profiles of the fillets of channel catfish reared on diets with different protein sources. N. Am. J. Aquac. 2005;67:304–311. [Google Scholar]
- Hinck JE, Blazer VS, Denslow ND, Echols KR, Gross TS, May TW, Anderson PJ, Coyle JJ, Tillitt DE. Chemical contaminants, health indicators, and reproductive biomarker responses in fish from the Colorado River and its tributaries. Sci. Total Environ. 2007;378:376–402. doi: 10.1016/j.scitotenv.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Hu Y, Kupfer D. Enantioselective metabolism of the endocrine disruptor pesticide methoxychlor by human cytochromes P450 (P450s): Major differences in selective enantiomer formation by various P450 isoforms. Drug Metab. Dispos. 2002;30:1329–1336. doi: 10.1124/dmd.30.12.1329. [DOI] [PubMed] [Google Scholar]
- James MO, Altman AH, Morris K, Kleinow KM, Tong Z. Dietary modulation of phase 1 and phase 2 activities with benzo(a)pyrene and related compounds in the intestine but not the liver of the channel catfish, Ictalurus punctatus. Drug Metab. Dispos. 1997;25:346–354. [PubMed] [Google Scholar]
- James MO, Kleinow KM. Trophic transfer of chemicals in the aquatic environment. In: Malins DC, Ostrander GK, editors. Aquatic Toxicology: Molecular, Biochemical and Cellular Perspectives. Boca Raton, FL: Lewis Publishers; 1994. pp. 1–35. [Google Scholar]
- James MO, Pritchard JB. Pesticide metabolism in aquatic species. In: Frehse H, editor. Pesticide Chemistry, Advances in International Research, Development and Legislation. Germany and New York: VCH Weinheim; 1991. pp. 277–286. [Google Scholar]
- James MO, Stuchal LD, Nyagode BA. Glucuronidation and sulfonation, in vitro, of the major endocrine-active metabolites of methoxychlor in the channel catfish, Ictalurus punctatus, and induction following treatment with 3-methylcholanthrene. Aquat. Toxicol. 2008;86:227–238. doi: 10.1016/j.aquatox.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor IP, Metcalf RL, Nystrom RF, Sangha GK. Comparative metabolism of methoxychlor, methiochlor, and DDT in mouse, insects, and in a model ecosystem. J. Agric. Food Chem. 1970;18:1145–1152. doi: 10.1021/jf60172a017. [DOI] [PubMed] [Google Scholar]
- Kleinow KM. Experimental techniques for pharmacokinetic data collection in free swimming fish. In: Mayes MA, Barron MG, editors. Aquatic Toxicology and Risk Assessment. Philadelphia, PA: American Society for Testing and Materials; 1991. pp. 131–138. [Google Scholar]
- Krisfalusi M, Eroschenko VP, Cloud JG. Methoxychlor and estradiol-17beta affect alevin rainbow trout (Oncorhynchus mykiss) mortality, growth, and pigmentation. Bull. Environ. Contam. Toxicol. 1998;61:519–526. doi: 10.1007/s001289900793. [DOI] [PubMed] [Google Scholar]
- Lewis SK, Lech JJ. Uptake, disposition, and persistence of nonylphenol from water in rainbow trout (Oncorhynchus mykiss) Xenobiotica. 1996;26:813–819. doi: 10.3109/00498259609046751. [DOI] [PubMed] [Google Scholar]
- Lijinsky W. The formation and occurrence of polynuclear aromatic hydrocarbons associated with food. Mutat. Res. 1991;259:251–261. doi: 10.1016/0165-1218(91)90121-2. [DOI] [PubMed] [Google Scholar]
- Michel CM, Squibb KS, O'Connor JM. Pharmacokinetics of sulphadimethoxine in channel catfish (Ictalurus punctatus) Xenobiotica. 1990;20:1299–1309. doi: 10.3109/00498259009046628. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Shimada T, Nakagami S, Kurihara N, Miyagawa H, Akamatsu M. Enantioselective recognition of mono-demethylated methoxychlor metabolites by the estrogen receptor. Chemosphere. 2004;54:1273–1276. doi: 10.1016/j.chemosphere.2003.10.035. [DOI] [PubMed] [Google Scholar]
- Nyagode BA. Ph.D. Dissertation. Gainesville, FL: University of Florida; 2007. Biotransformation of methoxychlor and selected xenobiotics in the channel catfish (Ictalurus punctatus) and largemouth bass (Micropterus salmoides) p. 188. [Google Scholar]
- Ohyama K, Maki S, Sato K, Kato Y. In vitro metabolism of [14C]methoxychlor in rat, mouse, Japanese quail and rainbow trout in precision-cut liver slices. Xenobiotica. 2004;34:741–754. doi: 10.1080/00498250400003455. [DOI] [PubMed] [Google Scholar]
- Ortiz-Zarragoitia M, Cajaraville MP. Effects of selected xenoestrogens on liver peroxisomes, vitellogenin levels and spermatogenic cell proliferation in male zebrafish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005;141:133–144. doi: 10.1016/j.cca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Ottinger MA, Quinn MJ, Jr, Lavoie E, Abdelnabi MA, Thompson N, Hazelton JL, Wu JM, Beavers J, Jaber M. Consequences of endocrine disrupting chemicals on reproductive endocrine function in birds: Establishing reliable end points of exposure. Domest. Anim. Endocrinol. 2005;29:411–419. doi: 10.1016/j.domaniend.2005.02.038. [DOI] [PubMed] [Google Scholar]
- Ousterhout J, Struck RF, Nelson JA. Estrogenic activities on methoxychlor metabolites. Biochem. Pharmacol. 1981;30:2869–2871. doi: 10.1016/0006-2952(81)90429-9. [DOI] [PubMed] [Google Scholar]
- Reigh RC. Production characteristics of pond-raised channel catfish Ictalurus punctutus fed diets with and without animal protein for three growing seasons. J. World Aquac. Soc. 1999;30:154–160. [Google Scholar]
- Schlenk D, Stresser DM, Rimoldi J, Arcand L, McCants JC, Nimrod AC, Benson WH. Biotransformation and estrogenic activity of methoxychlor and its metabolites in channel catfish (Ictalurus punctatus) Mar. Environ. Res. 1998;46:159–162. [Google Scholar]
- Seo CW, Kowtha BJ, Williamson S. Cholesterol and total fat content in farm-raised channel catfish cultured in North Carolina. J. Am. Oil Chem. Soc. 1995;72:1583–1585. [Google Scholar]
- Statham CN, Pepple SK, Lech JJ. Biliary excretion products of 1-[1-14C]naphthyl-N-methylcarbamate (carbaryl) in rainbow trout (Salmo gairdneri) Drug Metab. Dispos. 1975;3:400–406. [PubMed] [Google Scholar]
- Stuchal LD, Kleinow KM, Stegeman JJ, James MO. Demethylation of the pesticide methoxychlor in liver and intestine from untreated, methoxychlor-treated, and 3-methylcholanthrene-treated channel catfish (Ictalurus punctatus): Evidence for roles of CYP1 and CYP3A family isozymes. Drug Metab. Dispos. 2006;34:932–938. doi: 10.1124/dmd.105.009068. [DOI] [PubMed] [Google Scholar]
- Tsai WT, Mi HH, Chang YM, Yang SY, Chang JH. Polycyclic aromatic hydrocarbons (PAHs) in bio-crudes from induction-heating pyrolysis of biomass wastes. Bioresour. Technol. 2007;98:1133–1137. doi: 10.1016/j.biortech.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Vorkamp K, Riget F, Glasius M, Pecseli M, Lebeuf M, Muir D. Chlorobenzenes, chlorinated pesticides, coplanar chlorobiphenyls and other organochlorine compounds in Greenland biota. Sci. Total Environ. 2004;331:157–175. doi: 10.1016/j.scitotenv.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Wan MT, Kuo JN, Pasternak J. Residues of endosulfan and other selected organochlorine pesticides in farm areas of the Lower Fraser Valley, British Columbia, Canada. J. Environ. Qual. 2005;34:1186–1193. doi: 10.2134/jeq2004.0361. [DOI] [PubMed] [Google Scholar]