Abstract
The rostral ventromedial medulla (RVM) has long been recognized to play a pivotal role in nociceptive modulation. Pro-nociception within the RVM is associated with a distinct functional class of neurons, ON-cells that begin to discharge immediately before nocifensive reflexes. Anti-nociceptive function within the RVM, including the analgesic response to opiates, is associated with another distinct class, OFF-cells, which pause immediately prior to nocifensive reflexes. A third class of RVM neurons, NEUTRAL-cells, does not alter firing in association with nocifensive reflexes. ON-, OFF- and NEUTRAL-cells show differential responsiveness to various behaviorally relevant neuromodulators, including purinergic ligands. Iontophoresis of semi-selective P2X ligands, which are associated with nociceptive transmission in the spinal cord and dorsal root ganglia, preferentially activate ON-cells. By contrast, P2Y ligands activate OFF-cells and P1 ligands suppress the firing of NEUTRAL cells.
The current study investigates the distribution of P2X, P2Y and P1 receptor immunoreactivity in RVM neurons. Co-localization with tryptophan hydroxylase (TPH), a well established marker for serotonergic neurons was also studied. Immunoreactivity for the four purinergic receptor subtypes examined was abundant in all anatomical subdivisions of the RVM. By contrast, TPH-immunoreactivity was restricted to a relatively small subset of RVM neurons concentrated in the nucleus raphe magnus and pallidus, as expected. There was a significant degree of co-localization of each purinergic receptor subtype with TPH-immunoreactivity. This co-localization was most pronounced for P2Y1 receptor immunoreactivity, although this was the least abundant amongst the different purinergic receptor subtypes examined. Immunoreactivity for multiple purinergic receptor subtypes was often co-localized in single neurons. These results confirm the physiological finding that purinergic receptors are widely expressed in the RVM. Purinergic neurotransmission in this region may play an important role in nociception and/or nociceptive modulation, as at other levels of the neuraxis.
Keywords: RVM, purinergic receptors, nociception, immunohistochemistry
Introduction
The rostral ventromedial medulla (RVM) has long been recognized to play a pivotal role in nociceptive modulation. Its influence may be pro-nociceptive under conditions of inflammation or nerve injury, but anti-nociceptive in response to intense environmental stress or fear, or following exposure to opiates (Fields et al., 2006, Heinricher and Ingram, 2008). The bidirectional influence of this region on nociception appears to be mediated by the actions of distinct classes of RVM neurons: “ON-cells” and “OFF-cells”. ON-cells, which are pro-nociceptive, produce a burst of activity associated with nocifensive reflexes. Activation of ON-cells gives rise to hyperalgesia. OFF-cells, which are anti-nociceptive, pause in anticipation of a nocifensive reflex. OFF-cell activation produces analgesia. The activity of the remaining RVM neurons, “NEUTRAL-cells”, does not change in relation to noxious stimuli, and their role in nociception is not definitely known. However, NEUTRAL-cells apparently include the serotonergic neurons of this region (Potrebic 1994, Winkler 2006), and serotonergic neurons may have a role in central nociceptive modulation that parallels the opioid-sensitive activity of ON- and OFF-cells (Suzuki et al., 2004, Rahman et al., 2006).
Consistent with this functional heterogeneity, ON-, OFF-, and NEUTRAL-cells also display distinct neuropharmacological profiles. Each cell class responds differentially to microinjection and/or iontophoresis of various behaviorally relevant neuromodulators (Heinricher and Ingram, 2008). Iontophoresis of various purinergic ligands has distinct effects on the ongoing activity of ON-, OFF- and NEUTRAL cells. Adenosine, which stimulates P1 purinergic receptors, selectively inhibits NEUTRAL-cells but has little effect on ON- or OFF-cells. By contrast, ATP, which stimulates P2 receptors, selectively activates ON- and OFF-cells without much effect on NEUTRAL-cells (Selden et al., 2007). Semi-selective P2X receptor ligands and semi-selective P2Y receptor ligands preferentially activate ON- and OFF-cells, respectively (Selden et al., 2007). This response pattern suggests that different classes of RVM neurons may differentially express cell surface receptors for purinergic ligands.
There are multiple subtypes of each purinergic receptor class: four subtypes of P1 receptors, seven subtypes of P2X receptors, and eight subtypes of P2Y receptors (Ralevic and Burnstock, 1998, von Kugelgen and Wetter, 2000, Fredholm et al., 2001, Abbracchio et al., 2006). Our own pharmacological evidence points to the possibility that functional P1, P2X1, P2X3, and P2Y1 receptor subtypes are present in the RVM (Selden et al., 2007). Purinergic receptor immunoreactivity has been examined in various parts of the central and peripheral nervous system, but no information exists about the distribution of these receptors in the RVM. We have therefore studied the light microscopic anatomy and distribution of purinergic receptor immunoreactivity in the RVM, as well as the co-localization of purinergic receptor- and tryptophan hydroxylase-immunoreactivity.
Experimental Procedures
All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and the Committee for Research and Ethical Issues of the International Association for the Study of Pain. All experimental protocols were in accordance with the Institutional Animal Care and Use Committee of Oregon Health & Science University and all efforts were made to minimize the number of animals used and their suffering. All experiments were performed on male Sprague-Dawley rats (250-350 grams) obtained from Taconic (Cambridge City, IN).
Immunohistochemistry
Animals were deeply anesthetized with pentobarbital (150 mg/kg i.p.) or Euthasol (390 mg/kg, i.p.) and transcardially perfused with one liter of 4% paraformaldehyde in 0.2 M phosphate buffer (PB, pH 7.4). Brains were removed and post-fixed overnight at 4°C. Coronal sections measuring 40μm were cut serially through the brainstem on a vibratome and collected in 0.1 M PB. Every third section was processed for immunohistochemistry.
Free-floating serial sections were placed into 0.1 M Tris saline buffer (TSB, pH 7.6) on a platform rotator for gentle agitation and processed for fluorescent and brightfield immunohistochemical detection of tryptophan hydroxylase (TPH), adenosine receptor P1, and the purinergic receptors P2X1, P2X3, and P2Y1. For fluorescent double labeling, tissue was washed in 0.1 M TSB, followed by incubation in a blocking solution containing 3% normal donkey serum (NDS) in TSB with 1% Triton X-100 for 30 minutes. Following blocking, the tissue was incubated overnight at room temperature in a primary antibody cocktail diluted in 3% NDS in TSB with 0.5% Triton X-100 (TSBT). The primary antibodies rabbit anti-P1 receptor (1:500, Affinity BioReagents, Golden, CO), rabbit anti-P2X1 receptor (1:1000, Neuromics, Edina, MN), rabbit anti-P2X3 receptor (1:1000, Neuromics, Edina, MN), and sheep anti-P2Y1 receptor (1:1000, a generous gift from A. Lawrence) were each combined with sheep anti-TPH (1:5000, Chemicon, Temecula CA).
After primary antibody incubation, sections were washed in TSB and then incubated in fluorescently conjugated secondary antibodies diluted in 3% NDS in TSBT. Alexa Fluor 488 donkey anti-rabbit IgG (1:500, Molecular Probes, Eugene, OR) and Alexa Fluor 555 donkey anti-sheep IgG (1:500, Molecular Probes, Eugene, OR) were used for P1, P2X1, and P2X3 receptor double labeling with TPH. A fluorescein-conjugated donkey anti-sheep F(ab')2 fragment (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was used with Alexa Fluor 555 donkey anti-sheep for labeling P2Y1 receptor with TPH. Following final washes, sections were serially mounted on gelatinized slides and coverslipped with ProLong Gold Antifade (Molecular Probes, Eugene, OR). Control sections were processed in parallel, omitting the primary antibodies.
For brightfield immunohistochemistry, tissue was washed in 0.1 M TSB, followed by incubation in 2% hydrogen peroxide for 30 minutes. After washing the tissue in TSB, tissue was incubated in a blocking solution containing 3% normal sera (NS) in TSB with 1% Triton X-100 for 30 minutes. Following blocking, the tissue was incubated overnight at room temperature in one of the primary antibodies diluted in 3% NS in TSBT. After primary antibody incubation, the sections were rinsed in TSB, and then incubated in biotinylated secondary IgG antibodies in 3% NS in TSBT for two hours. A 1:400 dilution was used for goat anti-rabbit and rabbit anti-sheep (Vector Laboratories, Burlingame, CA). Following washes in TSB, sections were reacted with standard avidin-biotin-horseradish peroxidase histochemistry (Vector Elite ABC kit, Vector Laboratories, Burlingame, CA) in TSB for 90 minutes. After further washes in TSB, the tissue was reacted with nickel enhanced DAB solution (Adams, 1981). Following subsequent washes in TSB, the sections were serially mounted onto gelatinized slides and allowed to dry overnight. When dry, the sections were dehydrated in graded alcohols, cleared, and coverslipped.
NeuN and a Nissl stain were used for estimating the total number of cells located within the RVM. Tissue sections were processed for mouse anti-NeuN (Chemicon, Temecula CA) as described above, with a primary antibody concentration of 1:1000, and Alexa Fluor 594 donkey anti-mouse secondary (1:500, Molecular Probes, Eugene, OR). Tissue sections not processed for immunohistochemistry were counterstained with methylene blue (Cetas et al., 2002).
Anatomical Analysis and Quantification
Tissue sections were initially examined using an Olympus BX51 photomicroscope equipped with fluorescent filters. Photographs were taken with a Microfire digital camera (Optronics, Goleta, CA), and processed using PictureFrame (Optronics) and Adobe Photoshop CS2 software. The RVM was defined as the nucleus raphe magnus and the adjacent reticular formation at the level of the facial nucleus, including the nucleus reticularis gigantocellularis pars alpha (Heinricher and Ingram, 2008). This ranges from the approximate interaural distance -0.68 mm to -2.80 mm in the atlas of Paxinos and Watson (Paxinos and Watson, 1986). For the purposes of this study, cell counts were taken from six sections ranging from 1.32 to 2.60 mm caudal to the interaural line. In order to calculate the approximate area of the RVM, the width and height of the RVM were measured from the lateral edges of the pyramids to the top of the facial nucleus (Fig. 1).
Fig. 1.
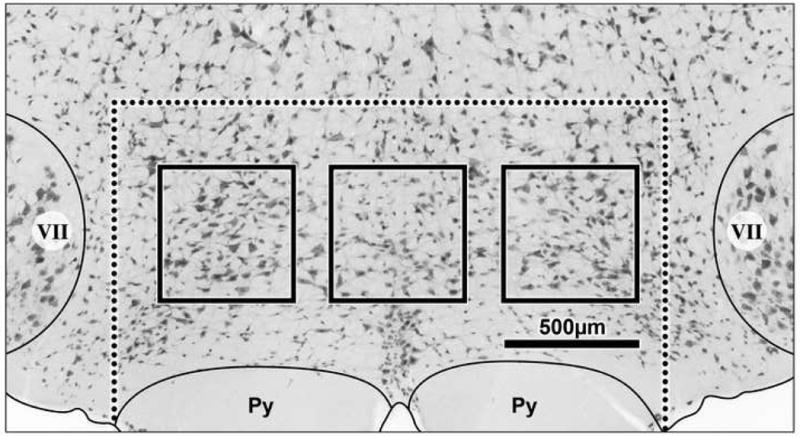
Low power photomicrograph of the RVM fluorescently labeled for NeuN and converted to grayscale. Dashed line indicates the defined border of the RVM, bold boxes indicate areas sampled for neuronal counts (see text). Scale bar equals 500μm. VII, facial nucleus; Py, pyramidal tract.
A quantitative analysis of each antibody was performed by counting the number of positive cells within sample areas in the RVM. Only clearly immunopositive neurons with a full cell body and clear cellular boundary were included in the counts. The number of immunopositive neuronal cell bodies in standardized sample areas was counted in six sections between -1.32 mm to -2.60 mm. The sample areas were 50 micron square blocks taken systematically from midline, left, and right of the RVM, as illustrated (Fig. 1). No overlap occurred between blocks. Counts were taken from tissue processed for fluorescent and brightfield immunohistochemistry. From the population of cells counted in these sample blocks, the number of labeled cells in the average RVM section was calculated. The average number of cells counted in the NeuN and Nissl sections were used as a representation of the entire population of cells present in the RVM.
Analysis of the tissue double labeled for two purinergic antibodies was done using the block method described above. Double-labeled images were created by photomerging images of the tissue taken in the same field of view. Once blocks were created from the double-labeled images, the images were separated by their respective filters to give single-labeled views. The number of single- and double-labeled cells was counted for each antibody.
Due to the specific and limited distribution of TPH immunoreactive cells in the RVM, immunopositive cells were counted individually in each of the six representative RVM sections per brain, within the entire area of the RVM as defined above. Each TPH positive cell was then assessed for co-labeling with one of the purinergic antibodies.
Results
Purinergic receptor immunoreactivity
Purinergic receptor immunoreactivity was observed throughout the brainstem. Labeled cell bodies and processes were present throughout the rostral-caudal extent of the RVM. Each antibody displayed a unique distribution of immunoreactive neurons that was visible in clear and consistent patterns. Control sections showed only a light, nonspecific background in all cases.
Staining for the adenosine receptor P1 resulted in a moderate number of labeled neurons in the RVM (Table 1). Labeling was primarily in the cytoplasm of the cell bodies and some proximal dendrites of both neurons and glial cells (Fig. 2). Positive neurons were located primarily in the nucleus raphe magnus and the raphe pallidus, and to a lesser extent in the dorsal lateral portion of the nucleus reticularis gigantocellularis pars alpha. The number of RVM neurons displaying P1 receptor immunoreactivity was 39% ± 1.7% (n = 9) of the total population of RVM neurons.
Table 1.
Purinergic, serotonergic, and double label receptor immunohistochemistry in the RVM.
| A. Receptor immunoreactivity in the RVM as a percentage of total Nissl stained neurons | |
|---|---|
| Receptor | Mean Percentage ± SEM |
| A1 | 39 ± 1.7 |
| P2X1 | 39 ± 1.1 |
| P2X3 | 42 ± 1.3 |
| P2Y1 | 25 ± 0.4 |
| TPH | 9.8 ± 1.0 |
| B. Percentage of purinergic receptor immunoreactive neurons that are TPH-positive | |
| Receptor | Mean Percentage ± SEM |
| A1 | 13 ± 0.5 |
| P2X1 | 16 ± 1.4 |
| P2X3 | 12 ± 1.4 |
| P2Y1 | 25 ± 1.2 |
| C. Percentage of TPH-positive neurons that are immunoreactive for a purinergic receptor | |
| Receptor | Mean Percentage ± SEM |
| A1 | 55 ± 1.6 |
| P2X1 | 63 ± 0.3 |
| P2X3 | 64 ± 1.1 |
| P2Y1 | 70 ± 2.6 |
Fig. 2.
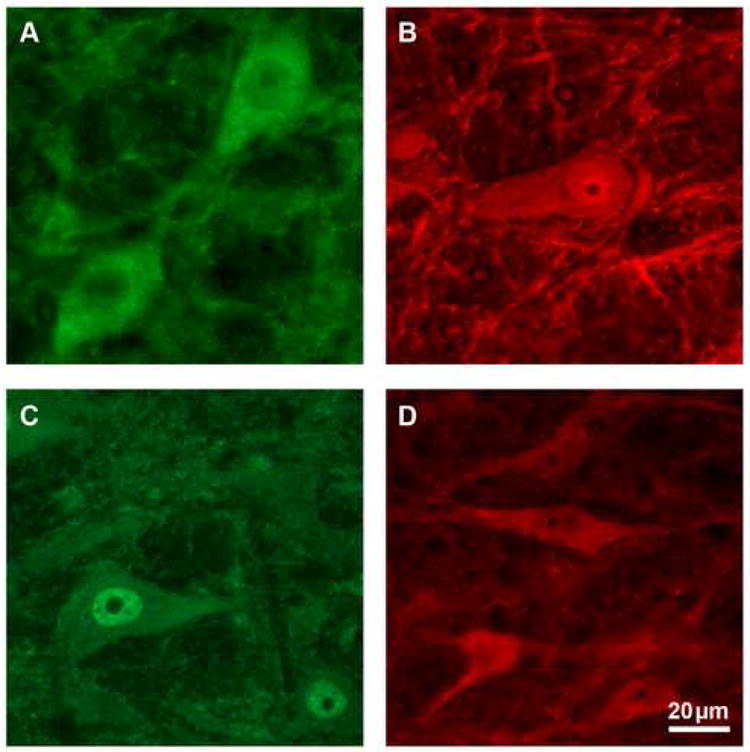
High power photomicrographs of RVM neurons immunoreactive for purinergic receptors P1 (A), P2X1 (B), P2X3 (C), and P2Y1 (D). Scale bar equals 20μm.
P2X1 receptor labeling occurred in a moderate number of neurons throughout the RVM (Table 1). Label was present in the cell body and proximal dendrites, and to a lesser extent in more distal fibers of neurons and glia (Fig. 2). Immunoreactivity was most dense in the raphe, where small and medium sized cells were uniformly distributed throughout the nucleus raphe magnus and the raphe pallidus. A diffuse lattice-like network of cells and fibers was visible immediately superior to the pyramids. P2X1 receptor immunopositive neurons accounted for 39% ± 1.1% (n = 9) of the total population of RVM neurons.
Staining for the P2X3 receptor resulted in a moderate number of labeled neurons throughout the brainstem (Table 1). The label was mainly localized in the nuclear area and the surrounding cytoplasm of the cell body in both neurons and glia, with some fiber staining present (Fig. 2). The raphe showed a slightly denser label, while staining was uniform through the dorsal aspects of the RVM. A lattice-like network of cells and fibers crisscrossed the ventral-medial area, just superior to the pyramids. Slightly more RVM neurons were positively labeled with antibodies against P2X3 receptors than for other purinergic ligands examined, with 42% ± 1.3% (n = 6) of the population labeled.
By contrast, P2Y1 receptor immunoreactivity showed lower numbers of labeled neurons in the RVM (Table 1). Staining occurred in the cell body and proximal processes, and to a lesser extent in more distal processes (Fig. 2). Staining was most dense in the raphe magnus and the dorsal lateral portion of the nucleus reticularis gigantocellularis pars alpha. Large labeled neurons were located dorsally and laterally, although smaller neurons made up the majority of labeled cells. Like P2X1 and P2X3 receptor staining, a lattice-like network of fibers crisscrossed the area immediately superior to the pyramids. P2Y1 receptor immunopositive neurons were present in 25% ± 0.4% (n = 11) of the total population of RVM neurons.
Co-labeling showed that 13% ± 0.4% (n = 3) of the total population of RVM neurons expressed both P1 and P2Y1 receptors, and 16% ± 0.7% (n = 3) of RVM neurons expressed both P2X1 and P2Y1 receptors. Of neurons expressing P2Y1 receptors, 56% ± 1.6% (n = 3) also expressed the P1 receptor, while 60% ± 1.4% (n = 3) also expressed the P2X1 receptor (Table 2).
Table 2.
Purinergic receptor colabelling in the RVM
| A. Percentage of P2Y1 neurons that are immunoreactive for a second purinergic receptor | |
|---|---|
| Receptor | Mean Percentage ± SEM |
| A1 | 56 ± 1.6 |
| X1 | 60 ± 1.4 |
| B. Double labeled neurons immunoreactive for purinergic receptors as a percentage of Nissl stained RVM neurons | |
| Receptor | Mean Percentage ± SEM |
| A1/P2Y1 | 13 ± 0.4 |
| P2X1/P2Y1 | 16 ± 0.7 |
Serotonergic immunoreactivity and its co-localization with purinergic receptors
TPH immunohistochemistry showed a clear staining pattern that varied across the rostral-caudal extent of the RVM. Individual neurons were labeled in a Golgi-like fashion (Fig. 3). Cell bodies and processes formed an extensive network throughout the nucleus. Bouton-like structures were visible on local axonal collaterals. Labeled cells were limited to the ventral RVM and the midline area, including dense labeling in the nucleus raphe magnus, the raphe pallidus, and the medial lemniscus. Staining in the ventral nucleus reticularis gigantocellularis pars alpha was denser at the lateral edges of the pyramids. Immunohistochemistry for TPH also stained smooth muscle cells of the brainstem vasculature. Overall only 9.8% ± 1.0% (n = 19) of total RVM neurons expressed TPH. Immunopositivity varied from a maximum of 14% at the caudal end to a minimum of 6.4% in the middle of the rostral-caudal extent of the RVM (Fig. 4).
Fig. 3.
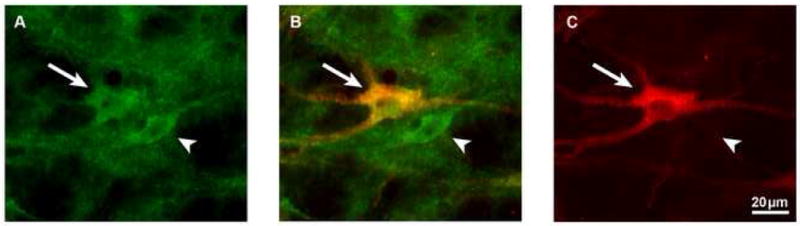
High power photomicrographs of a section double labeled with P2X1 receptor (A) and TPH (C) antibodies, and then photomerged (B). Scale bar equals 20μm. The larger neuron (arrow) is P2X1 receptor and TPH-immunoreactive, while the smaller neuron (arrowhead) is P2X1 receptor but not TPH-immunoreactive.
Fig. 4.
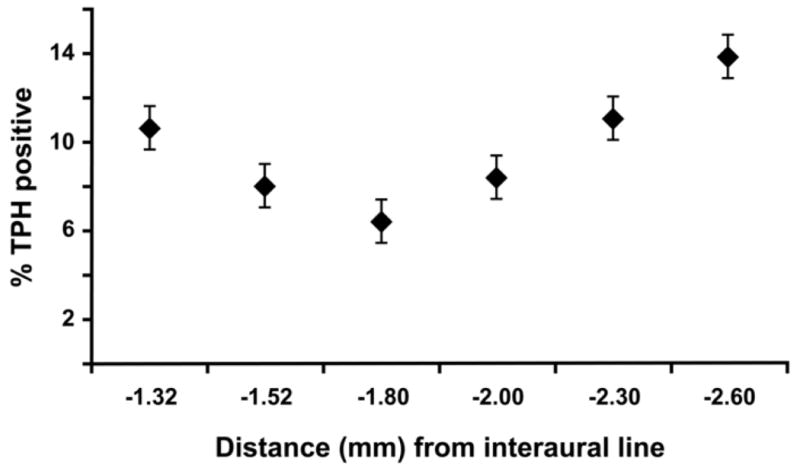
Percentage of Nissl stained neurons that are TPH-immunoreactive at each rostro-caudal level (Paxinos and Watson, 1986).
The majority of TPH positive neurons were co-labeled by purinergic receptor antibodies: 55% ± 1.6% (n = 3) of serotonergic cells were also P1 receptor positive, 63% ± 0.3% (n = 3) were P2X1 receptor positive, and 64% ± 1.1% (n = 3) were P2X3 receptor positive. A slightly higher proportion, 70% ± 2.6% (n= 3), of serotonergic neurons were P2Y1 receptor positive. Conversely, 13% ± 0.5% (n= 3) of P1 receptor positive neurons, 16% ± 1.4% (n= 3) of P2X1 receptor positive neurons, and 12% ± 1.4% (n = 3) of P2X3 receptor positive neurons co-labeled with TPH. P2Y1 receptor immonoreactive neurons showed the highest proportion of co-localization, with 25% ± 1.2% (n = 3) also labeled with TPH immunoreactivity (Table 1).
Discussion
Neurons and neuronal processes within the RVM are immunoreactive for a number of purinergic receptors, including P1, P2X1, P2X3, and P2Y1, and these neurons comprise a high proportion of neurons in the RVM. The highest percentages of immunopositivity were found for P1 and P2X receptors (both P2X1 and 3 subtypes), in approximately 40% of RVM neurons. Only 25% of identified neurons were P2Y1 positive. Purinergic receptor immunoreactive neurons were slightly more densely clustered within the midline raphe nuclei but were found scattered throughout the RVM.
P2X3 staining was also observed in RVM glia. P2 receptors on non-neural cells within both the central and peripheral nervous system have been shown to play direct or indirect roles in neurotransmission and may also be important in the RVM (Dixon et al., 2004, Zhang et al., 2005).
By contrast, a much smaller proportion, 10%, of RVM neurons showed immunoreactivity for serotonergic neuronal marker, TPH, consistent with previous reports (Moore, 1981, Fields, 2004). TPH positive neurons, as expected, were concentrated nearly universally within the bounds of the serotonergic raphe. This is the first comprehensive analysis of the prevalence of putative serotonergic cells in the functionally defined RVM using the specific neuronal marker NeuN. The mid-level of the functionally defined RVM was found to contain fewer TPH positive neurons than either the rostral or caudal ends, consistent with a previous report using cresyl violet counter stain to quantify total neuronal population (Kerman et al., 2006). Although serotonin neurons are anatomically related to ON- and OFF- cells in the RVM, the present results suggest that they are relatively sparse within the physiological core of the RVM and relatively common at its rostral and caudal extremes. The exact role of serotonin neurons in nociceptive modulation within the RVM remains unknown (Fields, 2004, Braz and Basbaum, 2008).
Purinergic receptors were commonly labeled in neurons also immunoreactive for TPH. TPH was co-localized in approximately 15% of neurons labeled with the most prevalent purinergic receptor markers in the RVM (P1, P2X1, and P2X3). By contrast, TPH was co-localized in 25% of neurons labeled with the least prevalent purinergic receptor marker, P2Y1. In all cases, the proportion of purinergic receptor-positive neurons with co-localized TPH was substantially larger than that which would be predicted by simple mathematical proportions alone (approximately 4% co-localization predicted for P1, P2X1 and P2X3 receptors; and 2.5% co-localization predicted for P2Y1 receptors). These results suggest that purinergic receptor immunoreactive neurons are more likely than other RVM neurons to be serotonergic, particularly in the case of P2Y1 neurons, although this co-localization appears to be far from complete. Conversely, a majority of serotonergic neurons appear to be purinergic-receptor positive, again most prominently so for P2Y1. Nonetheless, purinergic receptors were also identified on non-serotonergic neurons, and purines may therefore modulate both serotonergic and nonserotonergic activity in RVM.
Multiple purinergic receptor subtypes are also frequently present on the same neurons. For example, P2Y receptor positive neurons were also immunoreactive for P1 or P2X receptors more than half the time. At face value, these results contradict the relative specificity of purinergic receptor subtypes identified on individual ON-, OFF- and NEUTRAL-cells using pharmacological techniques (Selden et al, 2007). There are various possible explanations for this pattern of findings. First, RVM neurons may express both functional and non-functional purinergic receptors, with relative heterogeneity of the non-functional types. Second, because purinergic receptors are often constructed as heteromers of more than one genetic subtype, a single pharmacological receptor subtype may contain epitopes of two diverse genetic subtypes. Finally, the antibodies used here may be of limited specificity for the relevant epitopes in brainstem tissue processed according to our protocol.
The current results are limited because we did not employ stereological methodology or investigate all potential receptor subtype co-labeling combinations. Nevertheless, a number of relatively firm conclusions may be drawn. First, RVM neurons do appear to express purinergic receptors of all three major types: P1, P2X and P2Y. This observation lends support to our finding of purinergic-specific pharmacological activity for ON-, OFF- and NEUTRAL-cells in the RVM. Furthermore, these results imply that purinergic neurotransmission is important to nociception and nociceptive modulation at various levels of the neuraxis, including the RVM. Second, purinergic receptor expression appears to be relatively prevalent within RVM neurons, in all anatomical components of the RVM, including the nucleus raphe magnus and pallidus, and the nucleus reticularis gigantocellularis pars alpha. This pattern is starkly contrasted with that of TPH, which labels neurons in a Golgi-like pattern within the raphe alone. Third, purinergic receptor and TPH co-labeling is most prevalent in P2Y1 receptor immunoreactive neurons. In electrophysiological experiments, responsiveness to P2Y1 receptor ligands is a marker of OFF-cells in the RVM (Selden et al, 2007). Previous anatomical work, however, has suggested that serotonergic neurons in the RVM may be predominantly NEUTRAL-cells (Potrebic et al., 1994, Mason, 1997, Winkler et al., 2006). While it is possible that serotonergic RVM neurons may express functional P1 receptors but non-functional P2 receptors, it is also possible that serotonergic cells are not restricted to a single physiological RVM cell class.
While the current findings cannot resolve the specific pharmacological and immunological identity of different RVM functional cell classes, they do raise an important methodological consideration for continuing work in this area. Immunological identification of neurotransmitters and transmitter receptors may not reliably identify functional pharmacological responses in the same neuronal population. Given the complex sequence of molecular biological events associated with receptor protein expression and membrane targeting, this finding is not necessarily surprising, and has a number of correlates in the molecular biological literature (Mah et al., 2005, Matyas et al., 2006). This issue may be of considerable methodological importance, given that anatomical experiments using immunological methods alone are sometimes taken as proof of neurotransmitter function.
Purinergic receptor immunoreactive neurons are prevalent in all anatomical subdivisions of the RVM. Various purinergic receptor subtypes are frequently co-localized on RVM neurons. Within the raphe nuclei, purinergic receptor subtypes are also frequently co-localized with the serotonergic marker, TPH. The distribution of immunoreactivity for purinergic receptor subtypes within the RVM appears to be less specific than the purinergic pharmacological profiles of individual RVM ON-, OFF-, and NEUTRAL-cells. Purinergic neurotransmission may play an important role in nociception and nociceptive modulation at various levels of the neuraxis, including the RVM.
Acknowledgments
We thank Andrew J. Lawrence for the kind donation of purinergic antibodies, Shirley McCartney, Ph.D. for professional manuscript preparation, Andy Rekito, M.S. for figure preparation, and Kim J. Burchiel, M.D. for support of the Department of Neurological Surgery Laboratories at OHSU and personal encouragement. Supported by grants from the NIH (NS44255, N.R.S. and DA05608, M.M.H.) and the Cameron Foundation.
List of Abbreviations
- ATP
adenosine 5′-triphosphate
- DAB
3,3′-Diaminobenzidine tetrahydrochloride
- i.p
intraperitoneal injection
- NDS
normal donkey serum
- NS
normal sera
- PB
Phosphate buffer
- RVM
rostral ventromedial medulla
- TPH
tryptophan hydroxylase
- Tris
Tris(hydroxymethyl)aminomethane
- TSB
Tris saline buffer
- TSBT
Tris saline buffer with 0.5% Triton X-100
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981;29:775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Braz JM, Basbaum AI. Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits. J Comp Neurol. 2008;507:1990–2003. doi: 10.1002/cne.21665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cetas JS, Price RO, Velenovsky DS, Crowe JJ, Sinex DG, McMullen NT. Cell types and response properties of neurons in the ventral division of the medial geniculate body of the rabbit. J Comp Neurol. 2002;445:78–96. doi: 10.1002/cne.10164. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Yu R, Panupinthu N, Wilson JX. Activation of P2 nucleotide receptors stimulates acid efflux from astrocytes. GLIA. 2004;47:367–376. doi: 10.1002/glia.20048. [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fields H, Basbaum A, Heinricher M. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. 5th. London: Elsevier; 2006. pp. 125–142. [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: Bushnell MC, Basbaum AI, editors. Pain. Vol. 5. San Diego: Academic Press; 2008. pp. 593–626. [Google Scholar]
- Kerman IA, Shabrang C, Taylor L, Akil H, Watson SJ. Relationship of presympathetic-premotor neurons to the serotonergic transmitter system in the rat brainstem. Journal of Comparative Neurology. 2006;499:882–896. doi: 10.1002/cne.21129. [DOI] [PubMed] [Google Scholar]
- Mah SJ, Cornell E, Mitchell NA, Fleck MW. Glutamate receptor trafficking: endoplasmic reticulum quality control involves ligand binding and receptor function. Journal of Neuroscience. 2005;25:2215–2225. doi: 10.1523/JNEUROSCI.4573-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- Matyas F, Yanovsky Y, Mackie K, Kelsch W, Misgeld U, Freund TF. Subcellular localization of type 1 cannabinoid receptors in the rat basal ganglia. Neuroscience. 2006;137:337–361. doi: 10.1016/j.neuroscience.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Moore RY. The Anatomy of Central Serotonin Neuron Systems in the Rat Brain. In: Jacobs BL, Gelperin A, editors. Serotonin Neurotransmission and Behavior. Cambridge, Mass.: MIT Press; 1981. pp. 35–71. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain and Stereotaxic Coordinates. 2nd. New York: Academic Press; 1986. [Google Scholar]
- Potrebic SB, Fields HL, Mason P. Serotonin immunoreactivity is contained in one physiological cell class in the rat rostral ventromedial medulla. J Neurosci. 1994;14:1655–1665. doi: 10.1523/JNEUROSCI.14-03-01655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman W, Suzuki R, Webber M, Hunt SP, Dickenson AH. Depletion of endogenous spinal 5-HT attenuates the behavioural hypersensitivity to mechanical and cooling stimuli induced by spinal nerve ligation. Pain. 2006;123:264–274. doi: 10.1016/j.pain.2006.02.033. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Selden NR, Carlson JD, Cetas J, Close LN, Heinricher MM. Purinergic actions on neurons that modulate nociception in the rostral ventromedial medulla. Neuroscience. 2007;146:1808–1816. doi: 10.1016/j.neuroscience.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- von Kugelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- Winkler CW, Hermes SM, Chavkin CI, Drake CT, Morrison SF, Aicher SA. Kappa opioid receptor (KOR) and GAD67 immunoreactivity are found in OFF and NEUTRAL cells in the rostral ventromedial medulla. J Neurophysiol. 2006;96:3465–3473. doi: 10.1152/jn.00676.2006. [DOI] [PubMed] [Google Scholar]
- Zhang XF, Han P, Faltynek CR, Jarvis MF, Shieh CC. Functional expression of P2X7 receptors in non-neuronal cells of rat dorsal root ganglia. Brain Research. 2005;1052:63–70. doi: 10.1016/j.brainres.2005.06.022. [DOI] [PubMed] [Google Scholar]


