Abstract
OBJECTIVES
To determine if capillary rarefaction persists when hypertension is treated with angiotensin converting enzyme inhibitor, thiazidic diuretic and/or beta-blocker, and to identify which microcirculatory alterations (structural and functional) persist after anti-hypertensive treatment.
METHODS
We evaluated 28 well-controlled essential hypertensive patients and 19 normotensive subjects. Nailfold videocapillaroscopy examination of the fourth finger of the left hand was used to determine the functional capillary densities at baseline, during post-occlusive hyperemia, and after venous congestion. Capillary loop diameters (afferent, apical and efferent) and red blood cell velocity were also quantified.
RESULTS
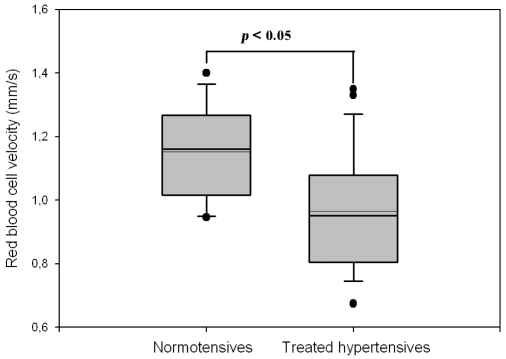
Compared with normotensive subjects, hypertensive patients showed lower mean functional capillary density at baseline (25.1±1.4 vs. 33.9±1.9 cap/mm2, p<0.01), during post-occlusive reactive hyperemia (29.3±1.9 vs. 38.2±2.2 cap/mm2, p<0.01) and during venous congestion responses (31.4±1.9 vs. 41.1±2.3 cap/mm2, p<0.01). Based on the density during venous congestion, the estimated structural capillary deficit was 25.1%. Mean capillary diameters were not different at the three local points, but red blood cell velocity at baseline was significantly lower in the hypertensive group (0.98±0.05 vs. 1.17±0.04 mm/s, p<0.05).
CONCLUSIONS
Patients treated for essential hypertension showed microvascular rarefaction, regardless of the type of therapy used. In addition, the reduced red blood cell velocity associated with capillary rarefaction might reflect the increased systemic vascular resistance, which is a hallmark of hypertension.
Keywords: Anti-hypertensive drugs, Microcirculation, Microvascular dysfunction, Videocapillaroscopy
INTRODUCTION
Increased peripheral vascular resistance is related to essential hypertension.1 The microcirculation largely determines peripheral resistance and consequently contributes to arterial hypertension.2–4 In both human arterial hypertension and animal models of hypertension, the formation and number of microvessels can be reduced by factors that are associated with hypertension, including genetic and fetal factors, among others. This microvascular rarefaction has been shown to contribute to increased peripheral resistance and raised blood pressure. Experimental studies have demonstrated rarefaction of the arterioles and capillaries in the skeletal muscles of spontaneously hypertensive rats.5,6 The first study describing microvascular rarefaction in hypertensive human subjects was published more than seventy years ago.7 Since then, several studies have reported decreased numbers of capillaries in patients with untreated hypertension.8–10
Indeed, there is controversy concerning the origin and persistence of capillary rarefaction after treatment for hypertension. In recent years, studies using intravital capillary videomicroscopy presented results that conflicted with other studies that demonstrated structural and functional capillary rarefaction.11,12 The videomicroscopy studies used techniques that recruit capillaries, such as post-occlusive reactive hyperemia and venous congestion responses.13 Venous congestion enhances the visibility of skin capillaries when using videomicroscopy. More specifically, the increase in number of red blood cells within capillaries, which is caused by venous congestion, can lead to mechanical opening of the capillaries, thus increasing their visibility by videomicroscopy.11,12
Although a wide variety of pharmacological anti-hypertensive agents can decrease blood pressure, they seem to vary in their ability to reverse structural and functional changes in microcirculation. For example, results with calcium antagonists have been variable and β-adrenoceptor blockers appear to have no effect on microcirculation changes.14 There are only a few studies that have evaluated the impact of hypertension treatment on functional capillary density (number of capillaries with moving red blood cells/mm2) and they have reported conflicting results.2,15 These conflicting data indicate that additional trials are needed to evaluate the role of anti-hypertensive drug therapy on microcirculation in hypertension.
The objectives of our study were to determine whether capillary rarefaction persisted in hypertensive patients treated with an angiotensin converting enzyme inhibitor (ACE-i), a thiazidic diuretic and/or a β-blocker, and to identify which microcirculatory alterations (structural or functional) persisted after treatment.
SUBJECTS AND METHODS
Subjects
A total of 28 patients with previous diagnoses of essential hypertension in the last ten years and 19 normotensive controls were enrolled in this study. Hypertensive patients were recruited from a pool of patients that received outpatient care at the Hypertension Clinic of a University General Hospital, and normotensive subjects were invited to participate in the research project during their routine consultations at the General Clinic in this hospital. Blacks, in whom the research technique does not work well; smokers; patients with secondary hypertension; patients with a connective tissue or cutaneous disease; and patients with diabetes mellitus were excluded. The protocol was approved by the local Ethics Committee and all subjects gave their written informed consent.
Blood Pressure and Blood Analysis
Blood pressure was measured using a mercury column sphygmomanometer whose calibration was previously tested at the Brazilian Metrology Institute (INMETRO). Each patient’s blood pressure was measured with a cuff, which was appropriate for their size, after they had been in the sitting position for 5 minutes. Three readings were obtained at 2-minute intervals; the mean value was recorded. Body weight was recorded in the morning after the patient had voided, with the patient wearing indoor clothing and no shoes. Venous blood was taken on the day of the nailfold videocapillaroscopy examination for the following biochemical tests: glucose, creatinine, total cholesterol, HDL-cholesterol and triglycerides.
Nailfold Videocapillaroscopy
Nailfold videocapillaroscopy examinations were performed as previously described.16 Briefly, the subjects acclimated in a temperature-controlled laboratory (24 ± 2°C) for 30 minutes. All individuals were seated comfortably in a fixed, high-based chair with the left upper arm raised to heart-level. The forearm and hand rested on a pedestal and the fourth finger was supported and gently fixed on an acrylic base 2 cm above palm-level with a metal device that prevented any movement. The skin temperature was monitored with a YSI Precision 4000A digital thermometer (Dayton, OH, USA). The thermistor probe was fixed 1 cm proximal to the nailfold. Capillary microcirculation was observed using a three eyepiece Leica DM/LM microscope (Wetzlar, Germany) that was equipped with an epi-illumination system (100W Xenon lamp). A JVC TK- S250 video camera was coupled to the microscope and connected to a Philips VR 999/78 VCR and a Kodo KBM 1700E monitor. All images were recorded on a super VHS tape for posterior analysis using CapImage software.17
Two magnifications were used to examine the nailfold of the fourth finger of the left hand: 250X for anatomical visualization and determination of the functional capillary density (number of capillaries with flowing red blood cells per tissue area) and 680X for measurement of the capillary loop (afferent, apical and efferent) diameters (μm) and the baseline red blood cell velocity (mm/s).
Functional Capillary Density (FCD)
We used three microscopic fields at the nailfold for measurements: the right lateral, the left lateral and the central fields. Baseline capillary density was measured along 3 mm of the distal row of capillaries. Each field was recorded for 30 seconds and the videotape recording was further analyzed by two investigators in a blinded fashion.
Importantly, the investigators used the same field (central) to compare the capillary density at baseline, at venous congestion and during reactive hyperemia responses. All patients were maintained comfortably at rest during all procedures; they did not perform any hand movements.
Venous Congestion Response
We used a miniature blood pressure cuff applied to the base of the left fourth finger inflated at 60 mmHg for 3 minutes. We visualized the capillaries for 30 seconds during the occlusion at the central field, which was previously analyzed for baseline functional capillary density. Estimated structural capillary deficit was calculated as the percentage of discrepancy of the functional capillary density between the hypertensive and normotensive subjects after the venous congestion response (Figure 1).
Figure 1.
Visualization of functional capillary density before and after venous congestion, showing the increase in the number of capillaries. The results were obtained for a normotensive subject
Reactive Hyperemia Response
The miniature digital cuff was inflated for 3 minutes to 40 mmHg above the patient’s systolic pressure in order to stop arterial blood flow to the finger. Then, the cuff was deflated abruptly and capillaroscopic images were obtained continuously for 30 seconds, under 250X magnification, for posterior evaluation of functional capillary density.
Red Blood Cell Velocity
Capillaries that were easily visualized and had intermittent blood flow were analyzed. Data on these capillaries were acquired from videotape recordings of the capillaries under 680X magnification and analyzed using CapImage software and the standard frame-to-frame analysis technique.16
Statistical Analysis
The data are presented as mean ± SEM, unless otherwise noted. Comparisons between groups were made by the Student’s t test (not paired). We tested effects of treatment types using one-way ANOVA, with Newman-Kells post-test to compare all pairs. The data were processed using Prism for Windows, version 4.02 (GraphPad Software, Inc). Differences with p≤0.05 were considered statistically significant.
RESULTS
Subjects
Mean age, body mass index (BMI) and the female to male ratio were not significantly different between the groups (Table 1). All patients in the hypertensive group reported a diagnosis of hypertension in the last 3 to 10 years and had sustained blood pressure below 140/90 mmHg for 3 consecutive months, which was considered adequate by the investigators.
Table 1.
Clinical characteristics and biochemical data for hypertensive patients and normotensive subjects
| Normotensives (n=19) | Hypertensives (n=28) | p value | |
|---|---|---|---|
| Age, years | 50.21 ± 1.5 | 52.64 ± 1.5 | NS |
| BMI, Kg/m2 | 27.6 ± 1.1 | 30.4 ± 1.4 | NS |
| Glucose, mmol/L | 5.4 ± 0.3 | 5.4 ± 0.2 | NS |
| Total cholesterol, mmol/L | 5.3 ± 0.7 | 5.4 ± 0.2 | NS |
| HDL Cholesterol, mmol/L | 1.1 ± 0.1 | 1.1 ± 0.1 | NS |
| LDL Cholesterol, mmol/L | 3.2 ± 0.2 | 3.3 ± 0.2 | NS |
| Triglycerides, mmol/L | 1.3 ± 0.1 | 1.3 ± 0.2 | NS |
| Creatinine, μmol/L | 61.68 ± 4.3 | 52.8 ± 4.4 | NS |
| Systolic BP, mmHg | 122 ± 2 | 132 ± 2 | <0.001 |
| Diastolic BP, mmHg | 75 ± 2 | 84 ± 2 | <0.001 |
| Mean BP, mmHg | 91 ± 2 | 100 ± 2 | <0.001 |
NS indicates not significant (p>0.05). BP, blood pressure; BMI, body mass index.
Blood Pressure and Blood Analysis
Although the blood pressure of each subject was considered to be under control, the systolic, diastolic and mean values in hypertensive patients were higher than those obtained in patients in the control group (Table 1). Of those patients on anti-hypertensive drugs, 11 (39.3%) were on monotherapy, 14 (50%) received two drugs and 3 (10.7%) received three drugs. The number of drugs on the patient’s treatment regimen was based on their need to achieve blood pressure levels below 140/90 mmHg. Seventeen patients used an angiotensin converting enzyme inhibitor (ACE-I; captopril 50–150 mg per day), 22 used thiazidic diuretics (hydrochlorothiazide 12.5–25 mg per day) and 6 used a β-blocker (propranolol 80–160 mg per day). These patients did not use any other types of anti-hypertensive drugs. No differences in the biochemical test results were detected among the patients (Table 1).
Nailfold Videocapillaroscopy
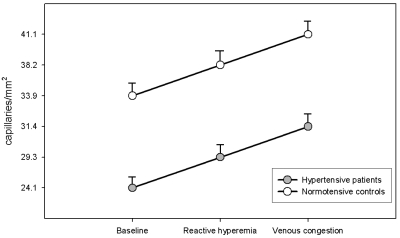
Compared with normotensive subjects, hypertensive patients showed significantly lower mean functional capillary densities at baseline, during post-reactive hyperemia and during venous congestion responses (Table 2). These differences were observed even with two recruitment maneuvers (Figure 2). Based on the functional capillary density during venous congestion for both groups, the estimated structural capillary deficit was 25.1%.
Table 2.
Measured microcirculatory parameters by nailfold videocapillaroscopy
| Normotensives (n=19) | Hypertensives (n=28) | p value | |
|---|---|---|---|
| Capillary density (capillaries/mm2) | |||
| - Baseline | 33.9 ± 1.9 | 25.1 ± 1.4 | <0.01 |
| - Reactive Hyperemia | 38.2 ± 2.2 | 29.3 ± 1.9 | <0.01 |
| - Venous Congestion | 41.1 ± 2.3 | 31.4 ± 1.9 | <0.01 |
| Red blood cell velocity (mm/s) | 1.17 ± 0.04 | 0.98 ± 0.05 | <0.05 |
| Capillary loop diameter (μm) | |||
| - Afferent | 5.3 ± 0.2 | 5.2 ± 0.3 | NS |
| - Apical | 7.2 ± 0.4 | 6.1 ± 0.2 | NS |
| - Efferent | 6.5 ± 0.4 | 6.0± 0.4 | NS |
NS indicates not significant (p > 0.05).
Figure 2.
Functional capillary densities in normotensive (n=19) and hypertensive (n=28) subjects at baseline and during post-occlusive reactive hyperemia and venous congestion responses. **p<0.01 for treated hypertensive subjects compared with normotensive controls
Mean capillary loop diameters were not different among the three points (Table 2), but the red blood cell velocity measured at baseline was significantly lower in the hypertensive group (Figure 3).
Figure 3.
Mean red blood cell velocity in capillaries at baseline, comparison between normotensive controls (n=19) and treated hypertensive patients (n=28)
To prevent bias related to the type of anti-hypertensive drug used, we performed a sub-analysis in which we compared the total hypertensive group with sub-groups of patients without ACEi, thiazidic diuretic or β-blocker treatment. The results were similar for all groups, indicating that none of the drugs by themselves were able to increase either the functional capillary density or the red blood cell velocity (Table 3).
Table 3.
Measurements performed by nailfold videocapillaroscopy on hypertensive patients per group of anti-hypertensive drugs
| Without ACEi (n=11) | Without Beta-Blocker (n=22) | Without Thiazidic (n=6) | All Hypertensives (n=28) | P value | |
|---|---|---|---|---|---|
| FCD – Baseline (cap/mm2) | 23.4±2.1 | 26.5±2.0 | 26.5±3.2 | 25.1±1.4 | NS |
| FCD - Reactive Hyperemia (cap/mm2) | 27.8±3.0 | 30.2±2.2 | 31.7±4.0 | 29.3±1.9 | NS |
| FCD - Venous Congestion (cap/mm2) | 29.4±2.7 | 32.1±2.3 | 32.0±4.2 | 31.4±1.9 | NS |
| Red blood cell velocity (mm/s) | 1.01±0.10 | 0.93±0.05 | 1.04±0.06 | 0.98±0.05 | NS |
ACEi, angiotensin converting enzyme inhibitor; FCD, functional capillary density; cap, capillaries; NS indicates not significant.
DISCUSSION
Altered microcirculation is commonly found in patients with essential hypertension, although the cause of this change has not been completely elucidated. Our study shows that the mean functional capillary densities at rest, during post-occlusive hyperemia and during venous congestion responses were all significantly lower in patients treated for essential hypertension than in normotensive controls. Structural deficits and significant functional changes contributed to the observed microvascular rarefaction. We have also shown that treated hypertensive patients showed significant reductions in red blood cell velocity measured at the resting state. This relatively newly observed relationship may result from increased arteriolar sensitivity to vasoconstrictive substances, reduction of endothelium-dependent dilation and increased oxidative stress.18
Venous congestion is generally considered a valid mechanism by which to enhance the visualization of all existing capillaries.19,20 Through use of this approach, we found that structural capillary rarefaction was approximately 25.1% in our treated hypertensive subjects. Another study, which used intravital videomicroscopy, reported an approximately 20% reduction in capillary density in patients that had never been treated for hypertension.8 A comparable structural capillary deficit was observed on the forearm skin of hypertensive patients using intravital fluorescein angiography.9 The baseline capillary densities detected in the present investigation were lower than those reported in the above-mentioned studies. The observed discrepancies between these studies could be explained by differences in the methods and settings chosen to evaluate microcirculation.
In the case of hypertension, capillary rarefaction may induce a rise in blood pressure, a relative decrease in tissue perfusion and an increased risk of cardiovascular disease.21,22 It is widely accepted that capillary rarefaction is present in patients with untreated essential hypertension11,12 and possibly in normotensive offspring of hypertensive individuals.23 Persistence of this state after treatment is a matter of debate. Furthermore, persistence may depend on the type of anti-hypertensive drug that was used during the course of treatment.14 Our findings confirmed the results reported by Antonios and co-workers showing that capillary density was significantly lower in treated patients compared with patients who were normotensive.24 Recently, Debbadi and collaborators reported different results. In their study, skin capillary density was higher in the treated hypertensive group compared with either the untreated hypertensive patients or the normotensive controls.15 Battegay and coworkers reported that specific anti-hypertensive drugs, such as angiotensin-converting enzyme inhibitors and angiotensin-1 receptor blockers, can induce angiogenesis and reduce or even reverse microvascular rarefaction; however, this was not observed in our study.2
The post-occlusive reactive hyperemic response is aimed to simulate, in a controlled setting, an ischemic situation similar to what is commonly found in the capillaries and in microcirculation in different diseases. Our objective was to produce a dilator response mediated by metabolic and/or in situ chemical factors secondary to local auto-regulation of blood flow after a period of ischemia. The significant difference between baseline capillary density in the normotensive group and the maximal functional recruitment in the hypertensive group during the reactive hyperemia response suggested both a decreased functional tissue blood flow at rest and impaired functional recruitment among the hypertensives. Thus, treated hypertensive subjects presented decreased capillary densities, both at rest and during tissue ischemia.
In the present study, we observed reduced red blood cell velocity at baseline in hypertensive subjects. This is an interesting finding because it emphasizes the importance of functional changes on microvascular rarefaction associated with essential hypertension. While, in theory, a microcirculatory dysfunction should revert following treatment, only a few trials have studied the influence of anti-hypertensive drugs on red blood cell velocity and these studies have reported conflicting results.25,26 Importantly, reduced red blood cell velocity may contribute to target organ damage in hypertension, but further studies are necessary to confirm this hypothesis.
Increased systemic vascular resistance observed in essential hypertension has been attributed to alterations in the microvasculature.2,14,18,21,27 These alterations have been demonstrated in the skin as well as in other tissues, such as skeletal muscle.8 It has been suggested that the capillary network may contribute to resistance control by regulating the narrow diameter of the capillary or by decreasing the number of perfused capillaries.11 We did not observe any significant reductions in capillary diameters at any of the three local points. On the other hand, the change in red blood cell velocity may have affected resistance control. If this is the case, the significantly lower capillary flow found in treated patients could be a consequence of pre-capillary vasoconstriction (which is well documented for essential hypertension).
This study has some limitations. Despite anti-hypertensive treatment, maintenance of sustained blood pressure control in the optimal range in hypertensive patients may not have been sufficient to decrease microvascular rarefaction. Indeed, three months of blood pressure control may have been too short of a time period to allow for structural changes in the microcirculation. Lastly, the sample size in this study was small; our findings should be confirmed in studies with larger sample sizes. The number of hypertensive patients employed in this study limited the statistical power such that we may not have observed differences between the groups, especially when comparing the effects of the various drugs.
In conclusion, our study has shown that patients treated for essential hypertension present microvascular rarefaction at the resting state. We confirmed that impaired structural and functional factors persisted and that they were independent of treatment with therapeutic agents. Reduced red blood cell velocity was found to be associated with capillary rarefaction and this may have contributed to the increased systemic vascular resistance, which is a hallmark of the disease. This feature is an important functional component of microvascular dysfunction; it likely contributes to the pathophysiology of essential hypertension. These findings emphasize the need for additional studies of microcirculation involving hypertensive subjects in order to identify functional factors that may be related to target organ damage, especially for cerebrovascular, cardiac and renal diseases. Consequently, anti-hypertensive drugs should be tested and differentiated in terms of their effectiveness to prevent or reverse the microcirculatory damage associated with hypertension.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Research Council (CNPq) and from the Research Supporting Agency of Rio de Janeiro State (FAPERJ). Preliminary results were presented during the Inter-American Society of Hypertension Meeting and the Consortium for Southeastern Hypertension Control in 2007.
REFERENCES
- 1.Conway J. Hemodynamic aspects of essential hypertension in humans. Physiol Rev. 1984;64:617–60. doi: 10.1152/physrev.1984.64.2.617. [DOI] [PubMed] [Google Scholar]
- 2.Battegay EJ, de Miguel LS, Petrimpol M, Humar R. Effects of anti-hypertensive drugs on vessel rarefaction. Curr Opin Pharmacol. 2007;7:151–7. doi: 10.1016/j.coph.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Mattar AL, Machado FG, Fujihara CK, Malheiros DM, Zatz R. Persistent hypertension and progressive renal injury induced by salt overload after short term nitric oxide inhibition. Clinics. 2007;62:749–56. doi: 10.1590/s1807-59322007000600015. [DOI] [PubMed] [Google Scholar]
- 4.Farah V M, De Angelis K, Joaquim LF, Candido GO, Bernardes N, Fazan R, Jr, Schaan BD, Irigoyen MC. Autonomic modulation of arterial pressure and heart rate variability in hypertensive diabetic rats. Clinics. 2007;62:477–82. doi: 10.1590/s1807-59322007000400015. [DOI] [PubMed] [Google Scholar]
- 5.Chen II, Prewitt RL, Dowell RF. Microvascular rarefaction in spontaneously hypertensive rat cremaster muscle. Am J Physiol. 1981;241:H306–10. doi: 10.1152/ajpheart.1981.241.3.H306. [DOI] [PubMed] [Google Scholar]
- 6.Hutchins PM, Darnell AE. Observation of a decreased number of small arterioles in spontaneously hypertensive rats. Circulation Res. 1974;34–35(suppl I):I-161. [Google Scholar]
- 7.Ruedeman AD. Conjuctiva vessels. Jama. 1933;101:1477–81. [Google Scholar]
- 8.Gasser P, Buhler FR. Nailfold microcirculation in normotensive and essential hypertensive subjects, as assessed by video-microscopy. J Hypertens. 1992;10:83–6. doi: 10.1097/00004872-199201000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Prasad A, Dunnill GS, Mortimer PS, MacGregor GA. Capillary rarefaction in the forearm skin in essential hypertension. J Hypertens. 1995;13:265–8. [PubMed] [Google Scholar]
- 10.Henrich HA, Romen W, Heimgartner W, Hartung E, Baumer F. Capillary rarefaction characteristic of the skeletal muscle of hypertensive patients. Klin Wochenschr. 1988;66:54–60. doi: 10.1007/BF01713011. [DOI] [PubMed] [Google Scholar]
- 11.Antonios TF, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Structural skin capillary rarefaction in essential hypertension. Hypertension. 1999;33:998–1001. doi: 10.1161/01.hyp.33.4.998. [DOI] [PubMed] [Google Scholar]
- 12.Serne EH, Gans RO, ter Maaten JC, Tangelder GJ, Donker AJ, Stehouwer CD. Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension. 2001;38:238–42. doi: 10.1161/01.hyp.38.2.238. [DOI] [PubMed] [Google Scholar]
- 13.Antonios TF, Rattray FE, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Maximization of skin capillaries during intravital video-microscopy in essential hypertension: comparison between venous congestion, reactive hyperaemia and core heat load tests. Clin Sci. 1999;97:523–8. [PubMed] [Google Scholar]
- 14.Agabiti-Rosei E. Structural and functional changes of the microcirculation in hypertension: influence of pharmacological therapy. Drugs. 2003;63:19–29. Spec No 1. [PubMed] [Google Scholar]
- 15.Debbabi H, Uzan L, Mourad JJ, Safar M, Levy BI, Tibirica E. Increased skin capillary density in treated essential hypertensive patients. Am J Hypertens. 2006;19:477–83. doi: 10.1016/j.amjhyper.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Dancour MA, Vaz JL, Bottino DA, Bouskela E. Nailfold videocapillaroscopy in patients with systemic lupus erythematosus. Rheumatol Int. 2006;26:633–7. doi: 10.1007/s00296-005-0033-z. [DOI] [PubMed] [Google Scholar]
- 17.Klyscz T, Junger M, Jung F, Zeintl H. [Cap image--a new kind of computer-assisted video image analysis system for dynamic capillary microscopy] Biomed Tech (Berl) 1997;42:168–75. doi: 10.1515/bmte.1997.42.6.168. [DOI] [PubMed] [Google Scholar]
- 18.Vicaut E. Hypertension and the microcirculation. Arch Mal Coeur Vaiss. 2003;96:893–903. [PubMed] [Google Scholar]
- 19.Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, et al. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest. 1997;99:1873–9. doi: 10.1172/JCI119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts CC, Stanton AW, Pullen J, Bull RH, Levick JR, Mortimer PS. Skin microvascular architecture and perfusion studied in human postmastectomy oedema by intravital video-capillaroscopy. Int J Microcirc Clin Exp. 1994;14:327–34. doi: 10.1159/000178851. [DOI] [PubMed] [Google Scholar]
- 21.Mourad JJ, Laville M. Is hypertension a tissue perfusion disorder? Implications for renal and myocardial perfusion. J Hypertens Suppl. 2006;24:S10–6. doi: 10.1097/01.hjh.0000240041.43214.8a. [DOI] [PubMed] [Google Scholar]
- 22.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, et al. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108:2230–5. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 23.Antonios TF, Rattray FM, Singer DR, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart. 2003;89:175–8. doi: 10.1136/heart.89.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonios TF, Kaski JC, Hasan KM, Brown SJ, Singer DR. Rarefaction of skin capillaries in patients with anginal chest pain and normal coronary arteriograms. Eur Heart J. 2001;22:1144–8. doi: 10.1053/euhj.2000.2442. [DOI] [PubMed] [Google Scholar]
- 25.Albrecht M, Haustein KO. The effects of 2 beta-receptor blocking agents on the microcirculation of healthy subjects and of hypertensive patients. Int J Clin Pharmacol Ther. 1997;35:580–6. [PubMed] [Google Scholar]
- 26.Martina B, Frach B, Surber C, Drewe J, Battegay E, Gasser P. Capillary blood cell velocity in finger nailfold: effect of enalapril and mibefradil in patients with mild to moderate hypertension. Microvasc Res. 1999;57:94–9. doi: 10.1006/mvre.1998.2125. [DOI] [PubMed] [Google Scholar]
- 27.Struijker Boudier HA, le Noble JL, Messing MW, Huijberts MS, le Noble FA, van Essen H. The microcirculation and hypertension. J Hypertens. 1994;12:717–28. [PubMed] [Google Scholar]