Abstract
Eukaryotic initiation factor 2B (eIF2B), a five subunit guanine nucleotide exchange factor (GEF), plays a key role in the regulation of mRNA translation. Expression of its ε-subunit is specifically upregulated in certain conditions associated with increased cell growth. Therefore, the purpose of the present study was to examine the effect of repressing eIF2Bε expression on growth rate, protein synthesis, and other characteristics of two tumorigenic cell lines that display upregulated expression of the ε-subunit. Experiments were designed to compare spontaneously transformed fibroblasts (TMEF’s) to TMEFs infected with a lentivirus containing a short hairpin (sh)RNA directed against eIF2Bε. Cells expressing the shRNA displayed a reduction in eIF2Bε abundance to 30% of the value observed in uninfected TMEF’s with no change in the expression of any of the other four subunits. The repression of eIF2Bε expression was accompanied by reductions in GEF activity and global rates of protein synthesis. Moreover, repressed eIF2Bε expression led to marked reductions in cell growth rate in culture, colony formation in soft agar, and tumor progression in nude mice. Similar results were obtained in MCF-7 human breast cancer cells in which eIF2Bε expression was repressed through transient transfection with a siRNA directed against the ε-subunit. Overall, the results support a role for eIF2Bε in the regulation of cell growth and suggest that it might represent a therapeutic target for the treatment of human cancer.
Keywords: eIF2B5, transformation, shRNA, siRNA
Introduction
Translation of mRNA occurs through a series of events that can be grouped into three distinct stages: initiation, elongation, and termination. The majority of regulatory mechanisms of protein synthesis identified thus far mediate control of initiation of mRNA translation. The reactions that mediate the initiation of mRNA translation can be briefly summarized as follows: an initiator methionyl-tRNA (met-tRNAi) binds to the 40S ribosomal subunit forming the preinitiation complex that binds to the mRNA and localizes to the AUG start codon. The initiation factors associated with the 40S ribosomal complex are then released allowing the 60S ribosomal subunit to join to form the 80S ribosomal complex that proceeds to the elongation phase of translation. Binding of met-tRNAi to the 40S ribosomal subunit is mediated through eukaryotic initiation factor 2 (eIF2), which is complexed to GTP. The GTP bound to eIF2 is hydrolyzed to GDP when eIF2 is released from the 40S subunit, leaving behind the met-tRNAi and allowing translation to progress. However, eIF2 must be bound to GTP to proceed to another round of initiation. Under physiological conditions, eIF2 has a higher affinity for GDP than GTP (1). Consequently, a catalyst is needed to promote exchange of the GDP for GTP. Regeneration of the eIF2•GTP is mediated by the guanine nucleotide exchange factor (GEF) eIF2B. Therefore, the activity of eIF2B is critical for allowing mRNA translation to proceed.
eIF2B is a five subunit complex consisting of five subunits: eIF2Bα, eIF2Bβ, eIF2Bγ, eIF2Bδ, and eIF2Bε, which are encoded by the genes EIF2B1, EIF2B2, EIF2B3, EIF2B4, and EIF2B5, respectively. The best characterized mechanism through which eIF2B is regulated involves phosphorylation of the α-subunit of eIF2 on Ser51, an event that converts eIF2 from a substrate into a competitive inhibitor of eIF2B (2). Moreover, the GEF activity of eIF2B is also altered by direct phosphorylation of the ε-subunit of the protein by at least four different protein kinases including casein kinase (CK)-I, CK-II, glycogen synthase kinase (GSK)-3 (3, 4), and the dual-specificity tyrosine-phosphorylation kinase (DYRK) (5).
The ε-subunit of eIF2B is believed to be responsible for the catalytic activity of the complex because it is the only subunit that individually shows activity in vitro (6, 7). Although eIF2Bε exhibits some GEF activity when expressed alone, this activity is approximately 10% of that observed when it is expressed together with at least the β-, γ-, and δ- subunits of eIF2B (7, 8). The α-, β-, and δ-subunits of eIF2B exhibit protein sequence homology, and are believed to sense the phosphorylation state of eIF2α through a poorly defined mechanism (6, 9). The γ- and ε-subunits of eIF2B bind directly to the substrate, eIF2 (10). Furthermore, deletion of the α- subunit of eIF2B prevents eIF2α phosphorylation-mediated inhibition of eIF2B (7, 11).
The role of eIF2B in human cancer is not well understood. However, recent evidence suggests that it may serve as an oncogene. Phosphorylation of eIF2α on Ser-51 is elevated in mammary carcinoma cell lines compared to nontransformed mammary epithelial cell lines (12). One would expect that phosphorylation of eIF2α would decrease protein synthetic rates via competitive inhibition of eIF2B; however no such repression is observed. This suggests that higher activity or expression of eIF2B compensates for the expected repression due to eIF2α Ser-51 phosphorylation, or that eIF2B has become unresponsive to this inhibitory mechanism. More recently, it has been demonstrated that spontaneously transformed mouse embryonic fibroblasts (TMEFs) display elevated eIF2B activity relative to genetically matched parental control cells (13). The increase in eIF2B activity leads to an approximate doubling in protein synthesis and cell growth. Unexpectedly, expression of the catalytic ε-subunit of eIF2B, but not the other four subunits, is upregulated in TMEFs, a finding that may account for the relative insensitivity of eIF2B activity and protein synthesis to increases in eIF2α phosphorylation. Further evidence linking upregulated eIF2Bε expression to oncogenesis is provided by studies showing that eIF2Bε mRNA is upregulated in a variety of tumors compared to normal surrounding tissue, suggesting that part of the transformation process involves loss of regulation of this gene (13). These results suggest that the catalytic subunit of eIF2B, i.e. eIF2Bε, might be a target for cancer therapy.
The present study was designed to examine the contribution of eIF2Bε overexpression to the transformed phenotype of two cell lines, TMEFs and MCF-7 cells. Expression of eIF2Bε was repressed using either lentiviral delivery of a short hairpin RNA (shRNA) or transfection of a small interfering RNA (siRNA) duplex, both specifically targeting eIF2Bε. Knockdown of eIF2Bε expression in transformed cells resulted in a reduction in eIF2B activity, global rates of protein synthesis, and cell growth rates, as well as an impairment in growth in soft agar and nude mice.
Materials and Methods
Abbreviations
eIF, eukaryotic initiation factor; Met-tRNAi, initiator methionyl-tRNA; GEF, guanine nucleotide exchange factor; eIF2Bε, epsilon subunit of eIF2B; MEF, mouse embryonic fibroblast; PAGE, polyacrylamide gel electrophoresis.
Reagents and Antibodies
The monoclonal antibodies against mouse eIF2Bε, eIF2Bγ, eIF2Bδ and eIF2Bα were developed in our laboratory as described (7, 14, 15). Antibody against human eIF2Bε was acquired from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-actin antibody and all other reagents were from Sigma (St. Louis, MO), except as listed below.
Cell Culture
Spontaneously transformed mouse embryonic fibroblasts, wild type MEFs (both generous gifts from Dr. Glen Barber, University of Miami), and MCF7 cells were maintained in high-glucose Dulbecco’s modified eagle medium (DMEM) (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Atlas Biologicals, Ft. Collins, CO) and 1% (v/v) penicillin/streptomycin (Gibco/Invitrogen). Unless otherwise noted, 4 × 105 cells were seeded onto 60 mm dishes and incubated in culture medium overnight before use.
For experiments involving siRNA transfection, 4 × 105 cells were seeded onto 60 mm dishes and grown in culture medium without penicillin/streptomycin overnight. The following day, cells were transfected using DeliverX Plus siRNA Transfection Kit (Panomics) according to the manufacturer’s protocol using 30 nM siRNA.
Plasmids and Virus Production
HEK-293T cells (1.0×106) were seeded onto 100 mm tissue culture plates and incubated overnight at 37° in 5% CO2 in the absence of antibiotics. The following day, cells were transfected with 1.5 µg pCMV-VSVG packaging plasmid, 1.5 µg pCMV-dR8.2 δvpr and 3 µg of either the eIF2Bε-shRNA (targeted against sequence 5’-AAGUGGUGCCAUCCUACGUCC 3’) in the vector pLKO.1 or the empty pLKO.1 vector as a control using Fugene 6 (Roche) as described (16). The following day, the medium was changed on transfected cells. Forty-eight hours post seeding of cells into plates, target TMEFs were plated in 100 mm dishes and transfected cells were allowed to incubate for another 24 h. The next day, the medium was harvested from transfected HEK-293T cells and was filtered through a 45 µm filter to remove cellular debris. TMEF’s were infected by addition of filtered virus-containing media (2 ml) followed by incubation for 8 h. Infection medium was then removed and replaced with growth medium and cells were permitted to recover overnight. Stably infected cells were selected with puromycin and clonal (2Bε-1, 2Bε-2) and a pooled collection of transfected cells (2Bε-3) were selected for expansion.
The mouse eIF2Bε, in the expression plasmid pCMV-Sport6 (M-2Be), was acquired from I.M.A.G.E Consortium and was purchased through Invitrogen (MGC:103029 (IMAGE:5342410)).
Plasmid Transfection
For experiments involving HEK293 cells, cells (2.5×104) were plated into individual wells of 6 well dishes. Twenty-four hours later, cells were transfected with 2 µg of either pcDNA3.1 or a plasmid (M-2Be) expressing mouse eIF2Bε per well via calcium phosphate as described previously (17). Twenty-four hours post transfection, cells were counted or protein synthesis was measured as described below. For experiments involving MEF cells, cells (2 ×106 of either WT MEF or 2Bε-1 shRNA expressing cells) were transfected with 10 µg of either pcDNA3.1 or M-2Be by electroporation using a protocol optimized for MEF cells by Amaxa. Equal numbers of transfected cells were then seeded into 3 wells of a 6-well dish and maintained in growth media for 24 hours.
Western Blotting
Cells were washed twice in ice-cold PBS and then scraped either directly into 1× SDS sample buffer or 4E lysis buffer (20 mM HEPES, 2 mM EGTA, 50 nM NaF, 100 mM KCl, 0.2 mM EDTA, 50 mM β-glycerophosphate, 2.5% Triton X-100, 0.25% deoxycholate). After scraping into 4E lysis buffer, an aliquot of the lysate was centrifuged at 1,000 × g for 3 min at 4°C and a volume of the supernatant was combined with an equal volume of 2× SDS sample buffer. The samples were boiled for 5 min at 95°C and resolved by SDSPAGE. Proteins were then electrophoretically transferred onto a PVDF membrane, incubated with the appropriate primary and secondary antibodies, and visualized using Pierce ECL Western Blotting Substrate (Pierce, Rockford, IL) or ECL Plus Western Blotting Detection System (Amersham Biosciences, Piscataway, NJ).
RNA Isolation
Total cellular RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol and resuspended in RNA storage solution (Ambion, Austin, TX). RNA samples were analyzed for quality using the Agilent 2100 bioanalyzer microfluidics platform (Agilent Biotechnologies, Palo Alto, CA) and standard spectrophotometric techniques.
Quantitative Real-Time (qRT) PCR
qRT-PCR was conducted on the RNA samples derived from intact cells. RNA from each sample was converted to cDNA using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen). The resulting cDNA was assayed to quantify the relative abundance of various mRNA species using the QuantiTect SYBR Green Real-Time PCR kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. For assessment of individual mRNA abundance from intact cells, relative expression values were normalized to relative GAPDH mRNA expression. For analysis of individual mRNA, the data were expressed as the percentage of the total relative mRNA expression compared to controls. The primers sets used were eIF2Bε sense (5’-TCCCCCATCTCCAAGGACC-3’) and anti-sense (5’-TCGATCAGCGCGACATTG-3’), and GAPDH sense (5’-GGGCTGCCTTCTCTTGTGA-3’) and anti-sense (5’-TGAACTTGCCGTGGGTAGA-3’).
Protein Synthesis
Cells were seeded into 60 mm dishes such that they would be 50% confluent the following day. Treated cells were then metabolically labeled for 30 min using 100 µCi/ml [35S] methionine/cysteine followed by preparation of cell lysates in 4E lysis buffer. Cell lysate was applied to absorbent filters and protein was precipitated in the filter with 10% TCA followed by washing 3× with 5% TCA. Filters were then dried and protein was solubilized followed by scintillation counting. DPM were normalized to overall protein levels in the lysate and are reported as a percent of the control value. Protein from the cell lysate was also subjected to Western blot analysis using anti-eIF2Bε antibodies as described above.
Soft Agar Assay
Nontransformed MEFs or TMEFs infected with either a lentivirus encoding an shRNA against eIF2Bε or a control virus not expressing an shRNA were mixed into 0.5 ml of 0.35% agar containing growth medium and layered over a base of 0.5% agar to prevent anchorage-dependent cell growth. Once this layer was solidified it was overlayed with 1 ml normal growth medium, which was replaced every 2 days for 14 days. A colony is defined as a cell aggregate larger than 100 µm. Pictures were taken and visible colonies were counted after 14 and 28 days.
eIF2B Activity Assay
GEF activity of eIF2B in lysates from TMEF’s infected with control and eIF2Bε shRNA was measured as previously described (18, 19). Briefly, eIF2 was complexed to [3H]GDP for 10 min. The assay was started with the addition of cell lysate to eIF2-[3H]GDP. Aliquots were taken at 0, 2, 4, and 6 min, and the remaining eIF2-[3H]GDP complex was captured on nitrocellulose filters, and β-radiation was quantified using liquid scintillation counting, with appropriate correction for quench due to the dissolved filters.
Tumor Formation in Nude Mice
The animal protocol used for the studies described herein was reviewed and approved by the Institutional Animal Care and Use Committee of The Pennsylvania State University College of Medicine. Nontransformed MEFs and TMEFs infected with either a virus encoding an shRNA against eIF2Bε or a control virus not expressing an shRNA were dispersed in 200 µL of serum free DMEM and injected subcutaneously into nude mice. Tumor progression and size were measured with calipers daily until animal sacrifice when tumors reached 20 mm in width. Tumors were removed from mice by dissection, protein lysates were prepared and subjected to SDS-PAGE, and Western blots for actin and eIF2Bε were performed as described above.
Statistical Calculations
One way ANOVA analysis was carried out in Prism 4 (Graphpad Software) to determine assess statistical differences among groups. When significant differences were detected by ANOVA, test conditions were compared to controls using a standard t-test. Probability values of < 0.05 were considered significant for all comparisons and data are presented as means ± SD.
Results
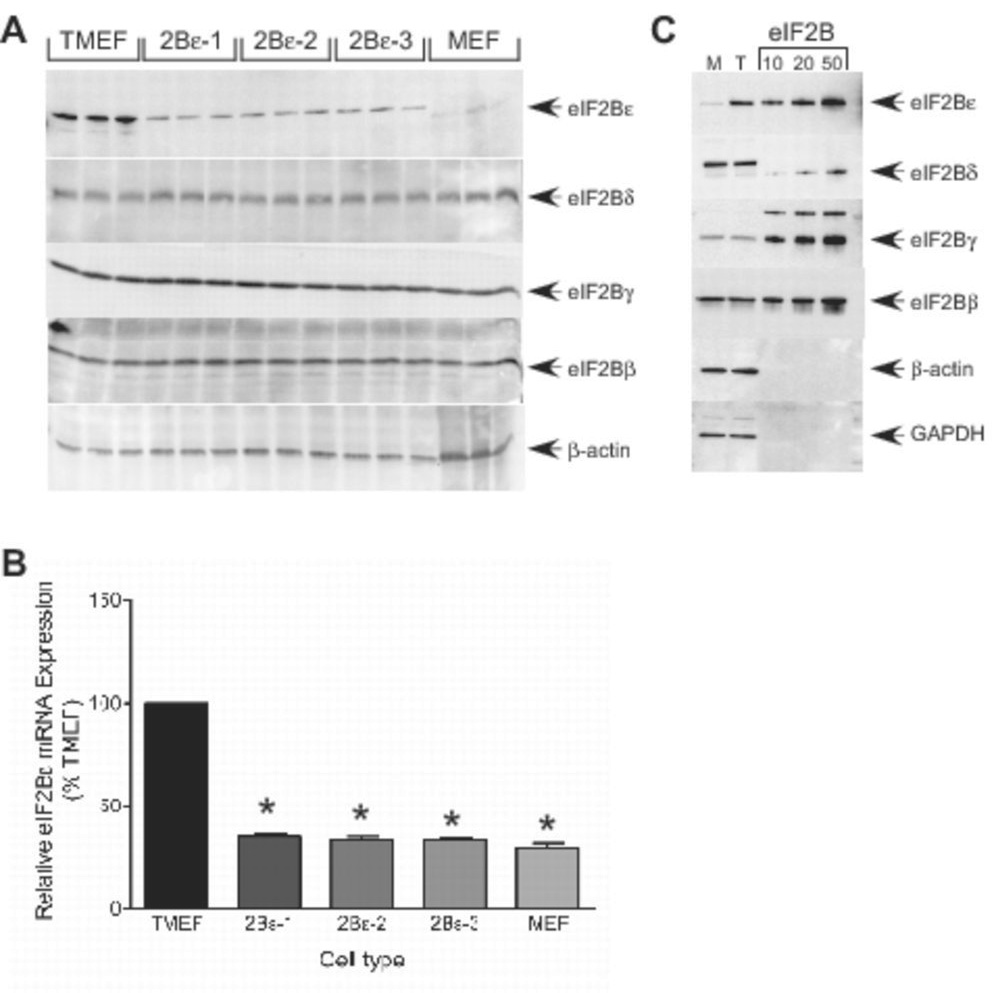
The TMEF and MCF7 cells used in the present study, as well as many other transformed cell lines and human cancers, display a relative overexpression of eIF2Bε compared to nontransformed cells (13, 20). Therefore, we sought to investigate the contribution of the overexpression of eIF2Bε to the upregulated growth phenotype of these cells. To accomplish this, an shRNA developed against eIF2Bε was delivered to the cells via a lentiviral delivery system. Clones 2Bε-1 and 2Bε-2 as well as a pooled sample of infected cells (2Bε-3) were then verified for repressed eIF2Bε protein expression via Western blot analysis (Fig. 1A) and mRNA expression via Real-Time PCR (Fig. 1B). In agreement with previous studies (13), the expression of eIF2Bε in TMEFs was more than 10-fold above the level in control MEFs, although the expression of three other subunits of the protein (β, γ, and δ) was similar in TMEFs and control MEFs. Stable expression of shRNA against eIF2Bε in TMEFs reduced expression of the protein to approximately 35% of the level in TMEFs infected with a virus not expressing an shRNA. Interestingly, although eIF2Bε mRNA expression was returned to the value observed in control MEFs in response to expression of shRNA (Fig. 1B), the protein remained consistently elevated. This finding suggests that both transcriptional and post-transcriptional regulation of eIF2Bε protein expression is involved in the elevation in eIF2Bε expression in TMEFs. The reduction in expression was specific, as there were no observed effects on eIF2B β, γ, or δ (Fig. 1A). Although eIF2Bε exhibits GEF activity when expressed in the absence of the other four subunits of the protein, its activity is significantly greater when present in the five subunit holocomplex (7). To assess whether the other eIF2B subunits are present in TMEFs in quantities sufficient to allow formation of the holocomplex, the amounts of the β, γ, δ, and ε subunits were measured by Western blot analysis. As shown in Fig. 1C, the intensity of signal observed for eIF2Bγ and eIF2Bε was similar in both MEF and TMEF cells and equivalent to approximately 16 µg of purified eIF2B analyzed on the same blot. Surprisingly, eIF2Bγ content (approximately 5 µg) was less than that of either the β- or δ-subunits, but was similar in both cell lines. In MEF and TMEF cells, the eIF2Bε content was equivalent to approximately 3 and 14 µg of purified eIF2B, respectively. The results suggest that in MEFs both the γ- and ε-subunits are present in limiting amounts and that in TMEFs the γ-subunit is limiting for formation of the eIF2B holoprotein.
Figure 1.
shRNA-induced knockdown of eIF2Bε protein and mRNA expression in TMEFs. Transformed mouse embryonic fibroblasts (TMEFs) were infected with either a lentivirus encoding an shRNA against eIF2Bε or a virus not expressing an shRNA (pLKO.1) as a control. Cell lysates were prepared from equal numbers of TMEFs, two clonal isolates of TMEFs infected with virus expressing eIF2Bε shRNA (designated 2Bε-1 and 2Bε-2), a pool of several groups of TMEFs expressing eIF2Bε shRNA (designated 2Bε-3), and non-transformed MEFs. (A) Protein from the cell lysates was separated by SDS-PAGE and then electrophoretically transferred onto a PVDF membrane and blotted using antibodies against eIF2Bε, eIF2Bδ, eIF2Bγ, eIF2Bβ or β-actin. Within each experiment, three individual dishes of cells were analyzed, and the results of a typical experiment are shown. (B) mRNA was isolated from the lysates using TRIZOL, reversed transcribed, and analyzed for eIF2Bε and GAPDH mRNA expression using SYBR Green qRT-PCR as described under “Materials and Methods”. Values for eIF2Bε mRNA were normalized to GAPDH mRNA and the results are shown as mean ± SEM for 3 experiments (* p<0.01) (C) 50 µg of protein from MEF (M) and TMEF (T) cell lysates were subjected to SDS-PAGE and Western blot analysis using antibodies against eIF2Bε, eIF2Bδ, eIF2Bγ, eIF2Bβ, β-actin, and GAPDH. A series of dilutions of eIF2B purified from rat liver (15) was analyzed in parallel lanes.
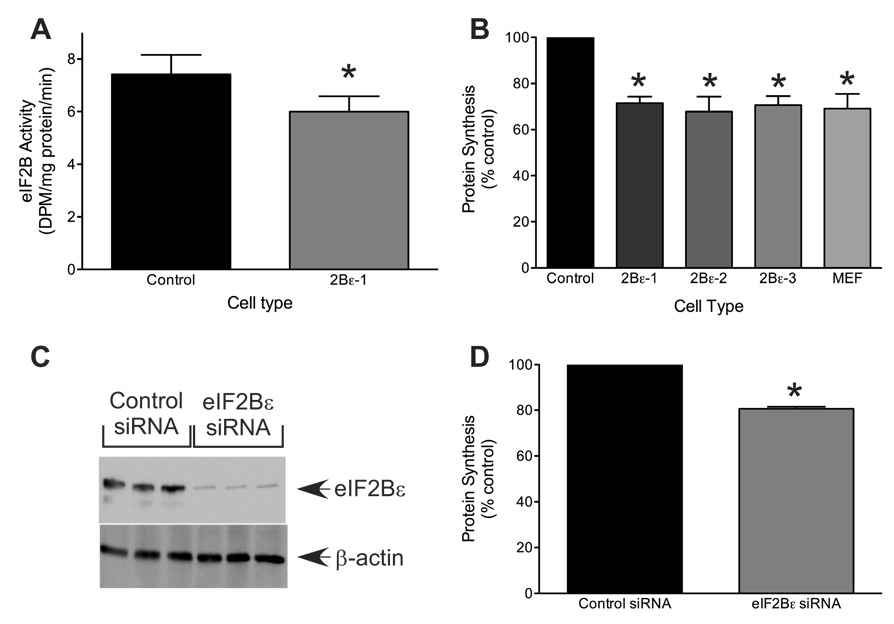
To assess the effectiveness of reducing eIF2Bε expression on eIF2B function, eIF2B GEF activity was measured in cell lysates. As shown in Fig. 2A, knockdown of eIF2Bε expression significantly repressed eIF2B GEF activity to approximately 80% of that observed in the control cells (p<0.05). Inhibition of eIF2B GEF activity, e.g. by increased phosphorylation of eIF2α, typically results in a decrease in global rates of protein synthesis (21). To determine whether the magnitude of the shRNA-induced reduction in eIF2B activity was sufficient to alter protein synthesis, the cells were subjected to metabolic labeling with [35S]methionine/cysteine, and the rate of incorporation of radiolabel into protein was measured. As shown in Fig. 2B, protein synthesis in TMEFs expressing eIF2Bε shRNA was approximately 70% of the control value (p<0.05), a result consistent with the central role of eIF2B in regulating translation initiation. To confirm that the results obtained using shRNA were specific for eIF2Bε, we also employed a siRNA knockdown approach that targeted a different portion of the eIF2Bε mRNA. TMEFs subjected to siRNA knockdown exhibited a reduction in eIF2Bε protein expression to approximately 30% of the control value (p<0.05; Fig. 2C) and a corresponding 25% decrease in protein synthesis (p<0.05; Fig. 2D), strongly suggesting that shRNA knockdown of eIF2Bε was specifically responsible for the reduction of global rates of protein synthesis. To further verify that the results observed were not due to off-target effects, we sought to restore the parental phenotype of the TMEFs expressing the eIF2Bε shRNA through exogenous expression of the targeted protein, i.e. eIF2Bε. As shown in Supplemental Fig. 1A, transfection of a plasmid expressing mouse eIF2Bε in the TMEFs already expressing eIF2Bε shRNA resulted in a 4-fold increase in expression of the protein, a value intermediate between the parental cells (TMEF) and the cells expressing the shRNA, but transfected with an empty plasmid (2Bε-1). Similarly, exogenous expression of eIF2Bε in TMEFs expressing the shRNA resulted in a significant increase in protein synthetic rate that was intermediate between that observed in TMEF and 2Bε-1 cells.
Figure 2.
shRNA- and siRNA-mediated knockdown of eIF2Bε expression in TMEFs. Cell lysates were prepared as described in the legend to Fig. 1. (A) eIF2B GEF activity was measured as described under the Materials and Methods. The data is expressed as the rate of exchange of [3H]GDP bound to eIF2 for nonradioactive GDP. The data represent the mean ± SEM of 3 experiments (*p<0.05 compared to TMEFs). (B) Cells were seeded onto 60 mm dishes and global rates of protein synthesis were measured as described under “Materials and Methods.” Values are expressed relative to the rate observed in TMEFs and the results are presented as mean ± SEM (* p<0.05 compared to TMEFs). (C and D) TMEF’s cells were transfected with either scrambled (Control) or eIF2Bε siRNA targeted against a different region of eIF2Bε than that of the shRNA used above. Twenty-four hours later, 100µCi of [35S]methionine/cysteine was added to the cell culture medium and the cells were returned to the incubator for 30 min. (C) Protein was separated by SDS-PAGE and electrophoretically transferred onto a PVDF membrane and blotted using antibodies against eIF2Bε or β-actin, and (D) global rates of protein synthesis were measured as described under “Materials and Methods.” Results from the siRNA-treated cells are reported as a percentage of the control value and are presented as mean ± SEM of 3 experiments. (* p<0.05 compared to control siRNA).
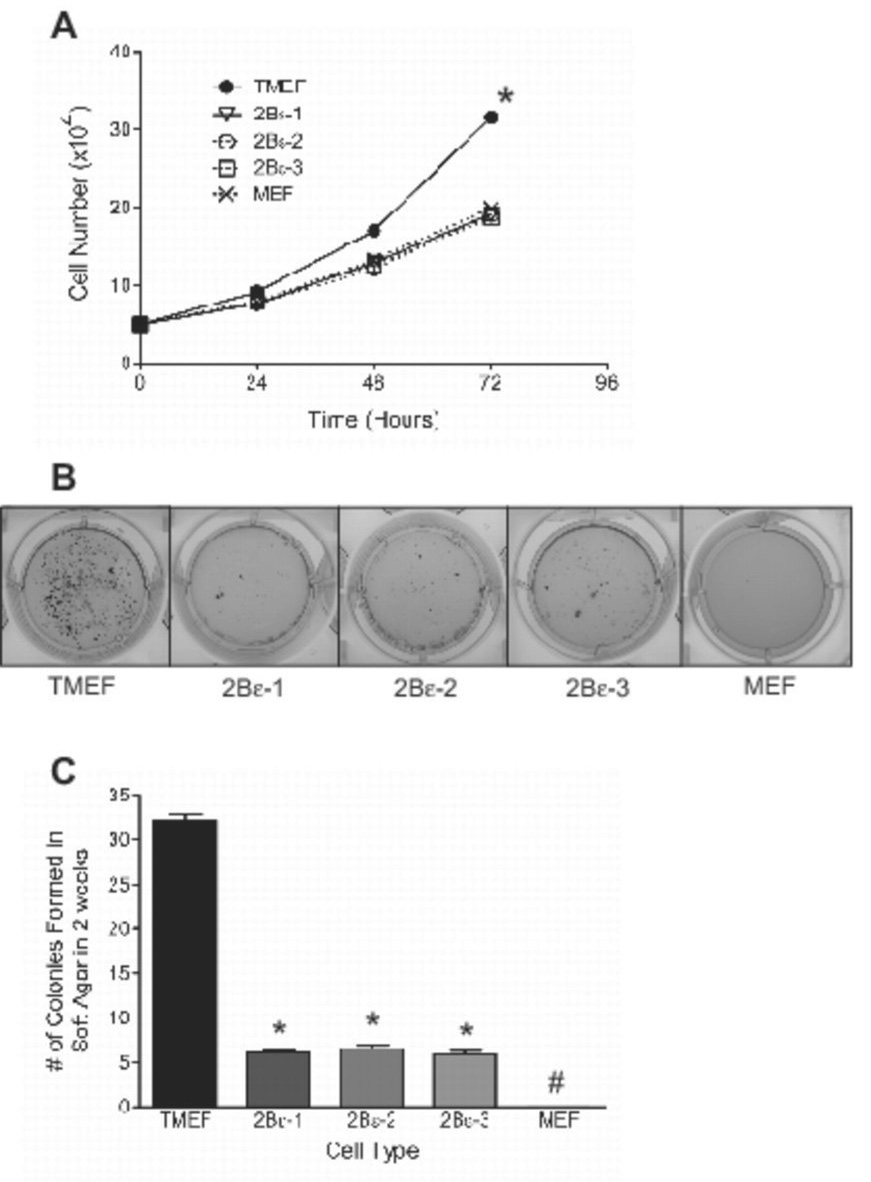
Because protein synthesis is an essential component to cell growth and replication, the reduced rate observed in cells expressing eIF2Bε shRNA would be expected to affect cell growth. To determine whether the reduced rate of protein synthesis was sufficient to repress cell growth, equal numbers of TMEFs expressing eIF2Bε shRNA or not expressing an shRNA were seeded into culture dishes and, at 24 h intervals, the number of cells on each plate was measured. Nontransformed MEFs were used as a control. A shown in Fig. 3A, the growth rate of control TMEFs was significantly greater than that of nontransformed MEFs. Moreover, as observed for protein synthesis, knockdown of eIF2Bε resulted in a decrease in growth rate to a value that was indistinguishable from non-transformed MEFs.
Figure 3.
Growth rate and colony formation in soft agar of TMEFs after shRNA-induced knockdown of eIF2Bε expression. (A) The cells described in the legend to Fig. 1 were seeded onto 100 mm dishes, harvested at the times indicated in the figure, and counted. The data are presented as mean ± SEM for 3 experiments ( growth rate *p<0.01 using ANOVA between cell types). (B) Cells were seeded in soft agar as described under “Materials and Methods”. Representative results after 2 weeks of growth are shown for TMEFs infected with a control virus (TMEF), 2Bε-1, 2Bε-2, 2Bε-3, and MEFs. (C) Colonies were counted 2 weeks after seeding, and the values are presented as mean ± SEM for 3 experiments. (* p<0.01 compared to TMEF, # no detectable colonies observed).
The ability to grow in soft agar is a characteristic shared by many transformed cells (22–24). Indeed, as previously reported (13), the TMEFs used in the present study exhibited a large number of colonies two weeks after being plated in soft agar (Fig. 3B). In contrast, wild type MEFs failed to form colonies under identical conditions. Expression of eIF2Bε shRNA in the TMEFs greatly reduced, such that the number of colonies formed in cells expressing eIF2Bε shRNA was only 20% of the value in the control TMEFs (Fig. 3C). A possible explanation for the limited colony formation in TMEFs expressing eIF2Bε shRNA was that the reduced rate of cell growth prolonged the time needed for colonies to reach a size that could be visualized. Therefore, incubation of the cultures was extended for another 2 weeks. Although the existing colonies became larger, no new colonies were observed in TMEFs expressing eIF2Bε shRNA (data not shown), demonstrating that the reduction in colony formation was not due simply to decreased cell growth but likely due to at least a partial reversal in transformation.
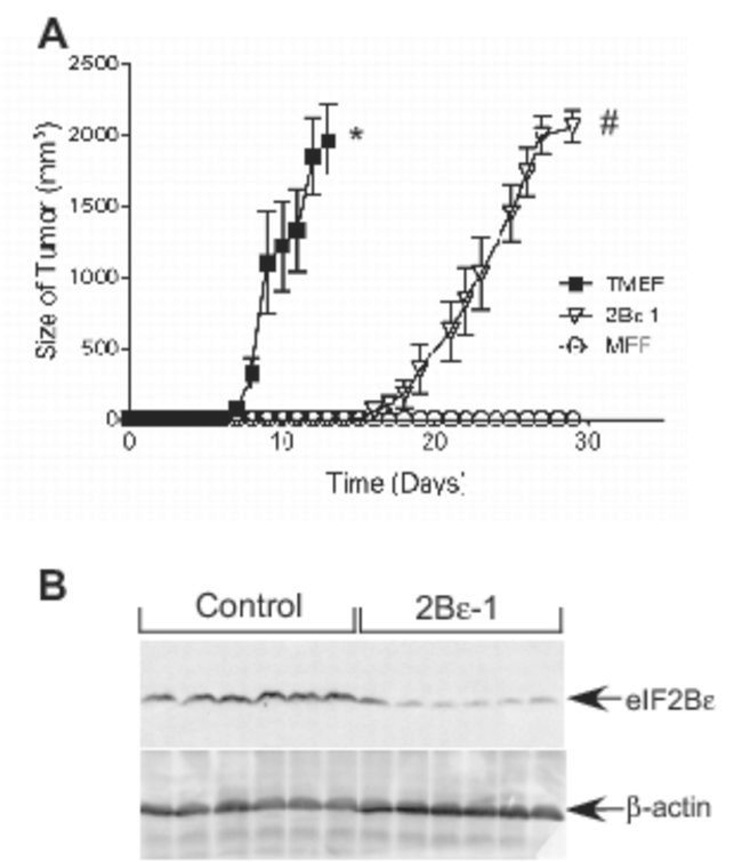
To obtain further evidence that knockdown of eIF2Bε expression attenuated cell transformation, nude mice were inoculated with either TMEFs infected with a control virus, TMEFs infected with virus expressing eIF2Bε shRNA, or wild type MEFs, and the mice were examined every 24 h thereafter for signs of tumor development. Once visible, tumor size was recorded daily. Within 8 days of inoculation, all of the control TMEF-inoculated animals exhibited tumors, and the tumors grew aggressively until animal sacrifice at day 13 (Fig. 4A). In contrast, the onset of tumor appearance in the animals inoculated with eIF2Bε-shRNA expressing TMEFs was delayed compared to animals inoculated with control TMEFs, with the first animals having visible tumor formation on day 17. The tumors also grew slower, such that animals did not have to be sacrificed until approximately day 28. Animals inoculated with wild type MEFs showed no tumor formation during the 28 day experiment. Since tumor formation was not completely ablated by expression of eIF2Bε shRNA, we hypothesized that perhaps a small subset of cells had escaped the shRNA-mediated knockdown of eIF2Bε followed by clonal expansion, thereby allowing the protein to return to the levels present in the control TMEFs. Therefore, at the time of sacrifice, tumors were excised and homogenized, and eIF2Bε protein expression was quantitated by Western blot analysis. As shown in Fig. 4B, even after 28 days of growth, the tumors excised from the animals inoculated with eIF2Bε-shRNA expressing cells still had reduced levels of eIF2Bε protein compared to levels observed in tumors from mice inoculated with control TMEFs.
Figure 4.
Time of onset and rate of tumor progression in nude mice after shRNA induced knockdown of eIF2Bε expression in TMEFs. (A) 4–6 week old nude mice were injected subcutaneously with 5×106 cells of TMEFs infected with either a control virus (TMEF), a virus expressing eIF2Bε-shRNA (2Bε-1), or non-infected, non-transformed MEFs cells. Tumor size was measured daily from the time of appearance until the animals were sacrificed. (B) Tumors were isolated from mice injected with TMEFs infected with a control virus (Control) or with TMEFs infected with a virus expressing eIF2Bε-shRNA (2Bε-1) and then homogenized. The homogenates were subjected to Western blot analysis using antibodies against eIF2Bε or β-actin as described under “Materials and Methods”. The blot shows results for 6 tumors randomly selected from mice injected with control TMEFs and TMEFs expressing eIF2Bε shRNA. The results represent the mean ± SEM of 2 experiments; within each experiment, 6 or 10 mice were analyzed per condition, respectively (* p<0.01 compared to 2Bε-1 or MEFs; # p<0.01 compared to MEFs).
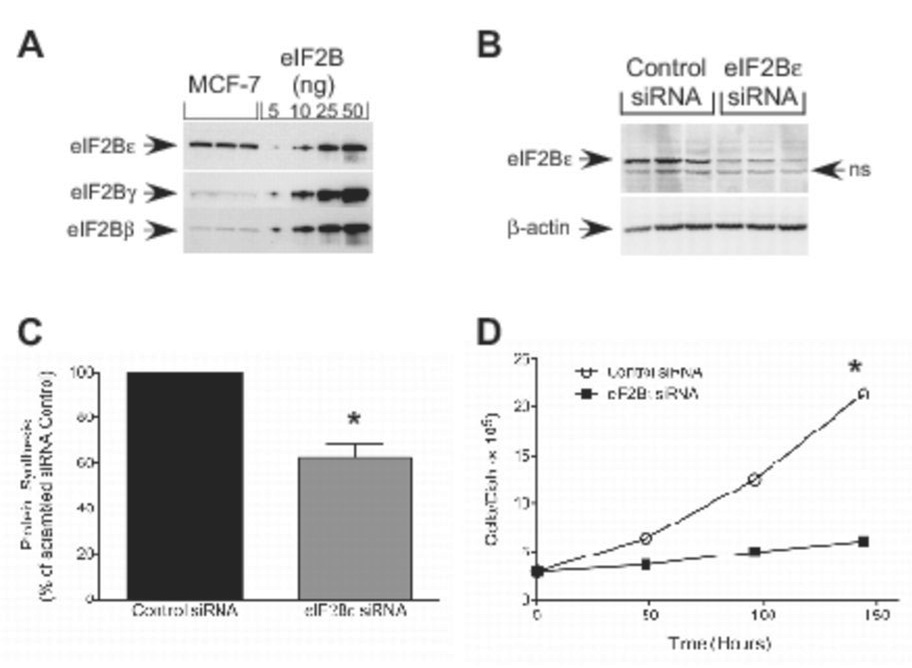
To confirm the findings shown above using TMEFs, the effect of repressing eIF2Bε expression on cell growth was examined in another cell line that specifically overexpresses eIF2Bε. As shown in Fig. 5A, MCF7 cells exhibit a specific elevation of eIF2Bε protein compared to eIF2Bβ and δ. To repress eIF2Bε expression, MCF7 cells were transiently transfected with the same siRNA targeting the protein as was used in the studies shown in Fig. 2C and 2D. Similar to the results obtained in TMEFs, eIF2Bε expression was reduced in cells transfected with eIF2Bε siRNA to less than 40% of the level observed in cells transfected with a control siRNA (Fig. 5B). Moreover, global rates of protein synthesis in cells transfected with eIF2Bε siRNA were reduced to approximately 60% of the value observed in cells transfected with the control siRNA (p<0.01; Fig. 5C). Similar to that observed for TMEFs, the decrease in eIF2Bε expression and protein synthesis engendered by eIF2Bε siRNA was sufficient to significantly reduce the rate of cell growth in MCF7 cells such that 150 hours after transfection, cell number was reduced to less than 30% of the control value (p<0.01; Fig. 5D).
Figure 5.
Protein synthesis and cell growth rate after siRNA-induced knockdown of eIF2Bε expression in MCF-7 cells. (A) MCF-7 homogenates from three dishes of cells and eIF2B purified from rat liver were subjected to Western blot analysis using antibodies against eIF2Bε, eIF2Bδ, and eIF2Bβ. A series of dilutions of eIF2B purified from rat liver (15) was electrophoresed in parallel lanes. (B–D) MCF-7 cells were transfected with either scrambled (Control) or eIF2Bε siRNA. Twenty-four hours later, cells were incubated with 100 µCi of [35S]methionine/cysteine in normal growth medium for 30 min. Cell lysates were prepared and (B) protein was separated by SDS-PAGE and subjected to Western blot analysis using antibodies against eIF2Bε or β-actin (ns, non-specific) and (C) the amount of radioactivity incorporated into protein was measured by scintillation counting. Results are presented as a percentage of the control value and are expressed as mean ± SEM for 3 experiments (* p<0.01 compared to control). (D) Cells were transfected with the siRNAs described above, and 3×105 cells were seeded onto 60 mm dishes. Cells were harvested at the times indicated in the figure and counted. The data are presented as mean ± SEM of 3 experiments (*p<0.01 compared to cells transfected with the scrambled siRNA).
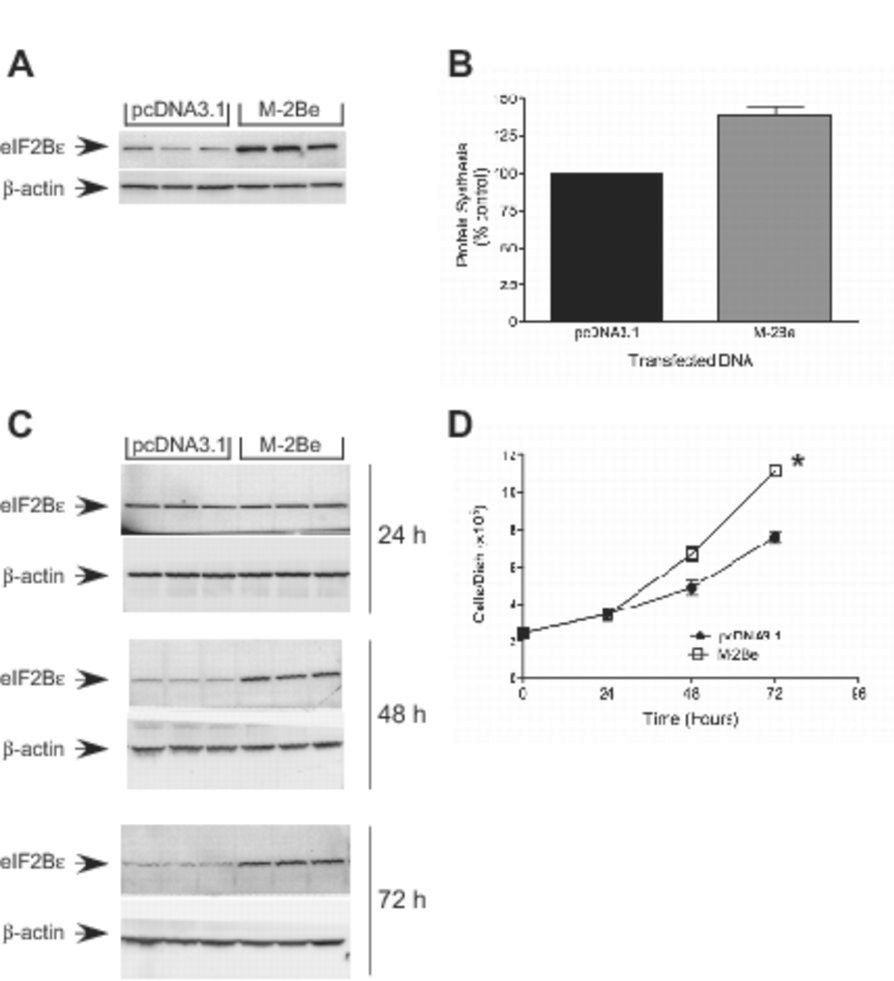
The studies described above suggest that overexpression of eIF2Bε in TMEFs is largely responsible for the increased rates of protein synthesis and cell growth. This idea was further explored by exogenous expression of the protein in HEK293 cells. As shown in Fig. 6A, HEK293 cells transfected with a plasmid expressing mouse eIF2Bε exhibited an approximate 5- fold increase in expression of the protein. Moreover, cells exogenously expressing eIF2Bε displayed both increased rates of protein synthesis (Fig. 6B) and growth (Fig. 6C). Overall, the results suggest that upregulated expression of eIF2Bε is sufficient to increase cell growth rates and transformation.
Figure 6.
Protein Synthesis and cell growth in HEK293 cells overexpression of eIF2Bε. HEK293 cells were transfected with either pcDNA3.1 (Control) or a plasmid expressing mouse eIF2Bε (M-2Be). Twenty-four hours later, cells were incubated with 100 µCi of [35S]methionine/cysteine in normal growth medium for 30 min. Cell lysates were prepared and (A) protein was separated by SDS-PAGE and subjected to Western blot analysis using antibodies against eIF2Bε or β-actin and (B) the amount of radioactivity incorporated into protein was measured by scintillation counting. Results are presented as a percentage of the control value and are expressed as mean ± SEM for 3 experiments (* p<0.01 compared to control using standard t-test). (C+D) HEK293 cells (2×105) were seeded into 6 well dishes and subsequently transfected with either control or a mouse eIF2Bε containing plasmid M-2Be plasmids. Cells were harvested at the times indicated in the figure and counted. Cell lysates were prepared and protein was separated by SDS-PAGE followed by Western blot analysis using antibodies against eIF2Bε or β-actin. The data represent the mean ± SEM of 3 experiments (growth rate *p<0.01 compared to cells transfected with the pcDNA3.1).
Discussion
There are at least two steps in translation initiation that can be rate controlling, the binding of met-tRNAi and the binding of mRNA to the 40S ribosomal subunit. Increased expression of translation initiation factors that participate in either step has been linked to tumorigenesis. For example, the mRNA cap binding protein, eIF4E, is a potent oncogene whose expression is upregulated in a variety of tumors including breast, head and neck, colon, cervix, and lung (reviewed in 25, 26). Moreover, exogenous overexpression of eIF4E in cells in culture leads to cellular transformation (27–30). The importance of the mRNA binding step in the transformation process is highlighted by the observation that expression of eIF4A and eIF4G, proteins that function in a complex with eIF4E to mediate the binding of mRNA to the 40S ribosomal subunit, is also increased in certain types of cancer (31–33), and exogenous expression of eIF4G leads to malignant transformation (34). The finding that exogenous expression of the eIF4E binding protein 1 (4E-BP1), a protein that binds to eIF4E and prevents it from assembling into the eIF4F complex with eIF4A and eIF4G, reverses the transformed phenotype of cells overexpressing eIF4E (35, 36) suggests that pharmaceutical interventions that target the mRNA binding step in initiation may be beneficial in treating tumors that exhibit upregulation of proteins that mediate this step in initiation. This idea is supported by the results of a more recent study showing that antisense oligonucleotide-mediated knockdown of eIF4E is also effective in repressing tumor growth (37).
Modulation of the met-tRNAi binding step in translation initiation has also been linked to tumorigenesis. In this regard, upregulated expression of eIF2α has been identified in mammary tumors (38) and Non-Hodgkin’s Lymphoma (39) as well as in cells transformed by myc, src, or abl (40, 41). Other studies have shown that the interferon-regulated eIF2α kinase, protein kinase R (PKR), acts as a tumor suppressor (42–44) and that exogenous expression of an eIF2α variant that cannot be phosphorylated on Ser51 results in transformation (42). In addition, exogenous expression of p58, a protein that acts to repress PKR function, leads to transformation (45). Collectively, the available evidence suggests that increases in the availability of the met-tRNAi binding protein, eIF2, enhances the transformation process, whereas inhibition of eIF2 function, e.g. by upregulating eIF2α phosphorylation on Ser51, attenuates the process.
A more recent study (13) suggests that increased expression of eIF2Bε also leads to transformation and tumorigenesis. Thus, eIF2Bε expression is specifically upregulated in a variety of transformed cell lines compared to non-transformed controls. eIF2B GEF activity is also higher in TMEFs compared to control MEFs, and, in comparison to control MEFs, the activity is relatively insensitive to inhibition by eIF2α phosphorylation. Furthermore, expression of eIF2Bε mRNA is up-regulated in a variety of human tumors, including a high percentage of those isolated from ovary, cervix, stomach, lung, and testis (13).
In the present study, the importance of eIF2Bε overexpression in the transformation process was examined using shRNA and siRNA approaches targeted against eIF2Bε to knockdown its expression in two different cell lines that overexpress the protein, TMEFs and MCF7 cells. The results show that in TMEFs, knockdown of eIF2Bε expression leads to a reduction in eIF2B activity and overall protein synthesis. Moreover, knockdown of eIF2Bε expression reduces cell growth and proliferation to levels similar to those observed for the non transformed parental MEF cell line. More importantly, eIF2Bε knockdown appears to impede the ability of transformed cells to form tumors, as shown by the studies examining growth in soft agar and tumor formation in nude mice, and the tumors that do form grow more slowly. The findings are not unique to TMEFs. Indeed, siRNA-mediated reduction of eIF2Bε expression in MCF7 cells similarly resulted in reductions in eIF2B GEF activity, protein synthesis, and cell growth rate.
The mechanism by which eIF2Bε overexpression promotes tumorigenesis is unknown, but because the only known function of eIF2B is to promote guanine nucleotide exchange on eIF2, it probably involves changes in mRNA translation. Thus, the increase in global rates of protein synthesis observed in cells that overexpress eIF2Bε is almost certainly involved in the enhanced growth rate. It is also possible that increased eIF2B activity upregulates the translation of mRNAs encoding certain proteins that mediate the transformation process and/or that overexpression of eIF2Bε allows for continued translation of mRNAs encoding specific proteins that would normally be repressed under conditions that promote eIF2α phosphorylation. For example, in non-transformed cells, phosphorylation of eIF2α leads to repressed global rates of mRNA translation, but upregulated translation of mRNAs encoding proteins such as ATF4 (46) and ATF5 (47) that function to adapt the cell to the stress that induced the phosphorylation. If the stress is not relieved, apoptosis ensues. In cells in which eIF2Bε expression is specifically upregulated, global rates of protein synthesis would not be repressed to the same extent as incontrol cells, and induction of translation of mRNAs such as that encoding ATF4 and ATF5 would also be blunted. It should also be noted that, in contrast to most GEFs that are typically single subunit proteins (48–50), eIF2B is relatively large and is comprised of five dissimilar subunits. Thus, a possible role for eIF2Bε distinct from mRNA translation cannot be discounted.
Overall, the data presented herein strongly suggest that downregulation of eIF2Bε expression reduces cell growth rate. Whether lowering expression of the protein leads to a complete reversal of the transformation process is equivocal. For example, the finding that reducing eIF2Bε expression in TMEFs dramatically decreases the number of colonies formed in soft agar suggests that the transformed phenotype is at least partially reversed in such cells. Although unproven, it is tempting to speculate that the incomplete reversal of the transformed phenotype is due to a failure to completely repress eIF2Bε expression in TMEFs to the level in control MEFs. A similar explanation could be proposed for the finding that, although the rate of appearance and growth of tumors in nude mice was slowed, tumors eventually formed in mice injected with TMEFs expressing eIF2Bε shRNA. Regardless of whether decreasing eIF2Bε by itself is sufficient to completely reverse the transformed phenotype, the results of the present study suggest that, at least in tumors that specifically overexpress eIF2Bε, the protein represents an unexplored target for therapeutic intervention to control tumor growth.
Supplementary Material
Reversal of eIF2Bε shRNA-mediated inhibition of protein synthesis. TMEFs infected with either a control virus (TMEF) or a virus expressing eIF2Bε-shRNA (2Bε-1) were transfected with either a plasmid expressing mouse eIF2Bε (M-2Be) or a control empty plasmid (pcDNA3.1). (A) Protein from lysates of transfected cells was resolved by SDS-PAGE and then electrophoretically transferred onto a PVDF membrane and analyzed using antibodies against eIF2Bε or β-actin as shown. Bands were quantitated through densitometry and the level of eIF2Bε expression was calculated and the results are presented as mean ± SEM of 3 experiments. (B) Global rates of protein synthesis was measured as described under “Materials and Methods.” Results are presented as a percentage of the 2Bε-1 cells transfected with the control plasmid and are presented as mean ± SEM of 3 experiments. (*p<0.05 compared to 2Bε-1, # p<0.01 compared to 2Bε-1 and M-2Be).
Acknowledgements
The authors would like to thank Dr. Philip Lazarus for critical reading of the manuscript prior to submission and Lydia Kutzler for technical assistance. We would also like to thank Dr. Glen Barber for generously providing the transformed MEFs that were used in many of the experiments. This study was supported by grants from the National Institutes of Health (DK-13499 and DK-15658) and a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds.
References
- 1.Erickson FL, Hannig EM. Ligand interactions with eukaryotic translation initiation factor 2: role of the gamma-subunit. EMBO J. 1996;15:6311–6320. [PMC free article] [PubMed] [Google Scholar]
- 2.Rowlands AG, Panniers R, Henshaw EC. The catalytic mechanism of guanine nucleotide exchange factor action and competitive inhibition by phosphorylated eukaryotic initiation Factor 2. J Biol Chem. 1988;263:5526–5533. [PubMed] [Google Scholar]
- 3.Wang X, Janmaat M, Beugnet A, Paulin FE, Proud CG. Evidence that the dephosphorylation of Ser(535) in the epsilon-subunit of eukaryotic initiation factor (eIF) 2B is insufficient for the activation of eIF2B by insulin. Biochem J. 2002;367:475–481. doi: 10.1042/BJ20020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welsh GI, Miller CM, Loughlin AJ, Price NT, Proud CG. Regulation of eukaryotic initiation factor eIF2B: glycogen synthase kinase-3 phosphorylates a conserved serine which undergoes dephosphorylation in response to insulin. FEBS Letters. 1998;421:125–130. doi: 10.1016/s0014-5793(97)01548-2. [DOI] [PubMed] [Google Scholar]
- 5.Woods YL, Cohen P, Becker W, et al. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem J. 2001;355:609–615. doi: 10.1042/bj3550609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavitt GD, Ramaiah KVA, Kimball SR, Hinnebusch AG. eIF2 independently binds two distinct eIF2B subcomplexes that catalyze and regulate guanine-nucleotide exchange. Genes Dev. 1998;12:514–526. doi: 10.1101/gad.12.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabian JR, Kimball SR, Jefferson LS. Reconstitution and purification of eukaryotic initiation factor 2B (eIF2B) expressed in Sf21 insect cells. Protein Expr Purif. 1998;13:16–22. doi: 10.1006/prep.1998.0860. [DOI] [PubMed] [Google Scholar]
- 8.Fabian JR, Kimball SR, Heinzinger ND, Jefferson LS. Subunit assembly and guanine nucleotide exchange activity of eukaryotic initiation factor-2B (eIF-2B) expressed in Sf9 cells. J Biol Chem. 1997;272:12359–12365. doi: 10.1074/jbc.272.19.12359. [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Hinnebusch AG. Identification of a regulatory subcomplex in the guanine nucleotide exchange factor eIF2B that mediates inhibition by phosphorylated eIF2. Molec Cell Biol. 1996;16:6603–6616. doi: 10.1128/mcb.16.11.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimball SR, Heinzinger NK, Horetsky RL, Jefferson LS. Identification of interprotein interactions between the subunits of eukaryotic initiation factors eIF2 and eIF2B. J Biol Chem. 1998;273:3039–3044. doi: 10.1074/jbc.273.5.3039. [DOI] [PubMed] [Google Scholar]
- 11.Hannig EM, Williams NP, Wek RC, Hinnebusch AG. The Translational Activator GCN3 Functions Downstream from GCN1 and GCN2 in the Regulatory Pathway that Couples GCN4 Expression to Amino Acid Availability in Saccharomyces cerevisiae. Genetics. 1990;126:549–562. doi: 10.1093/genetics/126.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raught B, Gingras AC, James A, Medina D, Sonenberg N, Rosen JM. Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein beta isoform and up-regulation of the eukaryotic translation initiation factor 2alpha are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res. 1996;56:4382–4386. [PubMed] [Google Scholar]
- 13.Balachandran S, Barber GN. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell. 2004;5:51–65. doi: 10.1016/s1535-6108(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizawa F, Kimball SR, Jefferson LS. Modulation of translation initiation in rat skeletal muscle and liver in response to food intake. Biochem Biophys Res Commun. 1997;240:825–831. doi: 10.1006/bbrc.1997.7652. [DOI] [PubMed] [Google Scholar]
- 15.Kimball SR, Everson WV, Myers LM, Jefferson LS. Purification and characterization of eukaryotic initiation factor 2 and a guanine nucleotide exchange factor from rat liver. J Biol Chem. 1987;262:2220–2227. [PubMed] [Google Scholar]
- 16.Stewart SA, Dykxhoorn DM, Palliser D, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright JL, Jordan M, Wurm FM. Transfection of partially purified plasmid DNA for high level transient protein expression in HEK293-EBNA cells. Journal of Biotechnology. 2003;102:211–221. doi: 10.1016/s0168-1656(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 18.Farrell PA, Fedele MJ, Vary TC, Kimball SR, Lang CH, Jefferson LS. Regulation of protein synthesis after acute resistance exercise in diabetic rats. Am J Physiol Endocrinol Metab. 1999;276:E721–E727. doi: 10.1152/ajpendo.1999.276.4.E721. [DOI] [PubMed] [Google Scholar]
- 19.Kimball SR, Jefferson LS. Effect of diabetes on guanine nucleotide exchange factor activity in skeletal muscle and heart. Biochem Biophys Res Commun. 1988;156:706–711. doi: 10.1016/s0006-291x(88)80900-8. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Forman AP, Mathews MB, Gunnery S. Human breast cancer cells contain elevated levels and activity of the protein kinase, PKR. Oncogene. 2000;19:3086–3094. doi: 10.1038/sj.onc.1203632. [DOI] [PubMed] [Google Scholar]
- 21.Sudhakar A, Ramachandran A, Ghosh S, Hasnain SE, Kaufman RJ, Ramaiah KV. Phosphorylation of serine 51 in initiation factor 2α (eIF2α) promotes complex formation between eIF2α (P) and eIF2B and causes inhibition in the guanine nucleotide exchange activity of eIF2B. Biochemistry. 2000;39:12929–12938. doi: 10.1021/bi0008682. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Bander JA, Santore TA, et al. Expression of Q227L-Gαs in MCF-7 human breast cancer cells inhibits tumorigenesis. Proc Natl Acad Sci U S A. 1998;95:2648–2652. doi: 10.1073/pnas.95.5.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang RF, Moore T, Arumugam T, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao WJ, Lin H, Sun T, Samanta AK, Arlinghaus R. BCR-ABL oncogenic transformation of NIH 3T3 fibroblasts requires the IL-3 receptor. Oncogene. 2007 doi: 10.1038/sj.onc.1210979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Culjkovic B, Topisirovic I, Borden KLB. Controlling gene expression through RNA regulons: The role of the eukaryotic initation factor eIF4E. Cell Cycle. 2007;6:65–69. doi: 10.4161/cc.6.1.3688. [DOI] [PubMed] [Google Scholar]
- 26.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–3199. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 27.De Benedetti A, Rhoads RE. Overexpression of eukaryotic protein synthesis initiation factor 4E in HeLa cells results in aberrant growth and morphology. Proc Natl Acad Sci. 1990;87:8212–8216. doi: 10.1073/pnas.87.21.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Benedetti A, Joshi B, Graff JR, Zimmer SG. CHO cells transformed by the translation factor eIF4E display increased c-myc expression but require overexpression of Max for tumorigenicity. Molec Cell Differ. 1994;2 [Google Scholar]
- 29.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]
- 30.Lazaris-Karatzas A, Smith MR, Frederickson RM, et al. Ras mediates translational initiation factor 4E-induced malignant transformation. Genes Develop. 1992;6:1631–1642. doi: 10.1101/gad.6.9.1631. [DOI] [PubMed] [Google Scholar]
- 31.Bauer C, Diesinger I, Brass N, Steinhart H, Iro H, Meese EU. Translation initiation factor eIF-4G is immunogenic, overexpressed, and amplified in patients with squamous cell lung carcinoma. Cancer. 2001;92:822–829. doi: 10.1002/1097-0142(20010815)92:4<822::aid-cncr1388>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 32.Brass N, Heckel D, Sahin U, Pfreundschuh M, Sybrecht GW, Meese E. Translation initiation factor eIF-4gamma is encoded by an amplified gene and induces an immune response in squamous cell lung carcinoma. Hum Mol Genet. 1997;6:33–39. doi: 10.1093/hmg/6.1.33. [DOI] [PubMed] [Google Scholar]
- 33.Eberle J, Krasagakis K, Orfanos CE. Translation initiation factor eIF-4A1 mRNA is consistently overexpressed in human melanoma cells in vitro. Intl J Cancer. 1997;71:396–401. doi: 10.1002/(sici)1097-0215(19970502)71:3<396::aid-ijc16>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Fukuchi-Shimogori T, Ishii I, Kashiwagi K, Mashiba H, Ekimoto H, Igarashi K. Malignant transformation by overproduction of translation initiation factor eIF4G. Cancer Res. 1997;57:5041–5044. [PubMed] [Google Scholar]
- 35.Lynch M, Fitzgerald C, Johnston KA, Wang S, Schmidt EV. Activated eIF4E-binding protein slows G1 progression and blocks transformation by c-myc without Inhibiting cell growth. J Biol Chem. 2004;279:3327–3339. doi: 10.1074/jbc.M310872200. [DOI] [PubMed] [Google Scholar]
- 36.Rousseau D, Gingras A-C, Pause A, Sonenberg N. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene. 1996;13:2415–2420. [PubMed] [Google Scholar]
- 37.Graff JR, Konicek BW, Vincent TM, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest. 2007;117:2638–2648. doi: 10.1172/JCI32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raught B, Gingras A-C, James A, Medina D, Sonenberg N, Rosen JM. Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein β isoform and up-regulation of the eukaryotic translation initiation factor 2 α Are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res. 1996;56:4382–4386. [PubMed] [Google Scholar]
- 39.Wang S, Rosenwald IB, Hutzler MJ, et al. Expression of the eukaryotic translation initiation factors 4E and 2α in Non-Hodgkin's Lymphomas. Am J Path. 1999;155:247–255. doi: 10.1016/s0002-9440(10)65118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenwald IB, Rhoads DB, Callanan LD, Isselbacher KJ, Schmidt EV. Increased expression of eukaryotic translation initiation factors eIF-4E and eIF-2α in response to growth induction by c-myc. Proc Natl Acad Sci. 1993;90:6175–6178. doi: 10.1073/pnas.90.13.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenwald IB. Upregulated expression of the genes encoding translation initiation factors eIF-4E and eIF-2α in transformed cells. Cancer Lett. 1996;102:113–123. doi: 10.1016/0304-3835(96)04171-7. [DOI] [PubMed] [Google Scholar]
- 42.Donzé O, Jagus R, Koromilas AE, Hershey JWB, Sonenberg N. Abrogation of translation initiation factor eIF-2 phosphorylation causes malignant transformation of NIH 3T3 cells. EMBO J. 1995;14:3828–3834. doi: 10.1002/j.1460-2075.1995.tb00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koromilas AE, Roy S, Barber GN, Katze MG, Sonenberg N. Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein Kinase. Science. 1992;257:1685–1689. doi: 10.1126/science.1382315. [DOI] [PubMed] [Google Scholar]
- 44.Meurs EF, Galabru J, Barber GN, Katze MG, Hovanessian AG. Tumor suppressor function of the interferon-induced double-stranded RNA-activated protein kinase. Proc Natl Acad Sci. 1993;90:232–236. doi: 10.1073/pnas.90.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barber GN, Thompson S, Lee TG, et al. The 58-kilodalton inhibitor of the interferon-induced double-stranded RNA activated protein kinase is a tetratricopeptide repeat protein with oncogenic properties. Proc Natl Acad Sci. 1994;91:4278–4282. doi: 10.1073/pnas.91.10.4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou D, Palam LR, Jiang L, Narasimhan J, Staschke KA, Wek RC. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem. 2008;283:7064–7073. doi: 10.1074/jbc.M708530200. [DOI] [PubMed] [Google Scholar]
- 48.Casanova JE. Regulation of Arf activation: the Sec7 family of guanine nucleotide exchange factors. Traffic. 2007;8:1476–1485. doi: 10.1111/j.1600-0854.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Mata R, Burridge K. Catching a GEF by its tail. Trends Cell Biol. 2007;17:36–43. doi: 10.1016/j.tcb.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Quilliam LA, Rebhun JF, Castro AF. A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog Nucleic Acid Res Mol Biol. 2002;71:391–444. doi: 10.1016/s0079-6603(02)71047-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Reversal of eIF2Bε shRNA-mediated inhibition of protein synthesis. TMEFs infected with either a control virus (TMEF) or a virus expressing eIF2Bε-shRNA (2Bε-1) were transfected with either a plasmid expressing mouse eIF2Bε (M-2Be) or a control empty plasmid (pcDNA3.1). (A) Protein from lysates of transfected cells was resolved by SDS-PAGE and then electrophoretically transferred onto a PVDF membrane and analyzed using antibodies against eIF2Bε or β-actin as shown. Bands were quantitated through densitometry and the level of eIF2Bε expression was calculated and the results are presented as mean ± SEM of 3 experiments. (B) Global rates of protein synthesis was measured as described under “Materials and Methods.” Results are presented as a percentage of the 2Bε-1 cells transfected with the control plasmid and are presented as mean ± SEM of 3 experiments. (*p<0.05 compared to 2Bε-1, # p<0.01 compared to 2Bε-1 and M-2Be).