INTRODUCTION
It is likely that the use of biomarkers will become one of the most important genetic tools available to the clinician. With the increased understanding of the molecular basis of carcinogenesis has come a realization that surrogate markers can be used to enable the physicians to estimate cancer risk, detect cancers at microscopic dimensions, determine the most appropriate therapies for gastrointestinal (GI) cancers, and monitor the effectiveness of those therapies. All of these applications are being investigated in various forms in several different GI cancers. Driving this biomarker discovery is a host of new technologies that can delve into the proteonomics, kinenomics, genomics, and other “omics” to discern exactly what a cancer truly is made from. The proliferation of microarrays and platforms that allow the scientists to scan thousands of proteins, genes, or virtually any molecule, simultaneously allows a more precise view of the neoplasms than ever hoped for previously. These technologies can also be performed in days or weeks and are increasing in speed as fast as the microchips that power our personal computers. It is likely that within a decade, each patient’s genome will be sequenced so that precise information can be obtained.
Like any new technology, one has to first determine if the older technology really no longer functions adequately. The current “markers” of GI neoplasms are all based on structural information regarding the tumor. The diagnosis of a tumor requires structural imaging to determine the location and dimensions of the tumor. Histology must be obtained, whose interpretation depends on the criteria developed over a hundred years ago based on the size and shape of the cells, their nuclei, their organization, and their invasion of the surrounding tissue. This is, of course, a nonobjective interpretation, with the only quantification being the “grade” of the tumor that in many GI tumors has no real relationship to the tumor behavior. The tumor size in cancers such as those of the pancreas has a prognostic significance, but in others, like those of the esophagus or colon, it does not have much clinical usefulness.
By definition, an ideal biomarker is a characteristic or a factor that could be evaluated or measured and that could help in the initial risk assessment and the subsequent management of a certain patient. Biomarkers could be the indicators of normal biologic processes, pathologic processes, or pharmacologic responses to therapeutic interventions (1) (Figure 1). The prevalence of an ideal biomarker should be low in precancerous lesions and should go up in early cancer stages like dysplasia. This is the rationale for the use of several of the known genetic pathways in the pathogenesis of cancer as candidate biomarkers. The discovery and validation of biomarkers prior to clinical use is not an easy task. The National Cancer Institute recommends five phases to facilitate this process (2): a preclinical exploratory phase, a clinical assay phase, a retrospective longitudinal phase, a prospective screening phase, and a cancer control phase. The detailed discussion of these phases is beyond the scope of this review.
Figure 1.
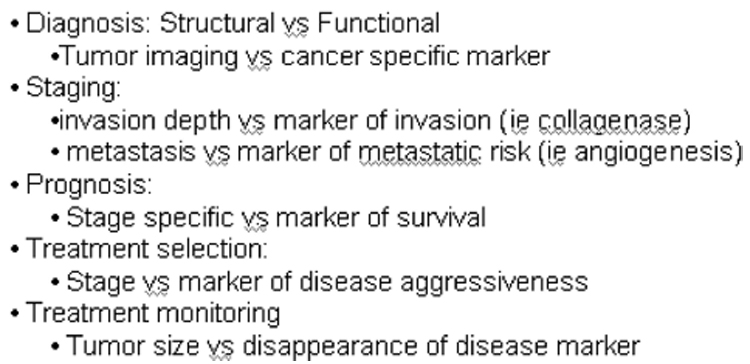
Current vs Future Technology
GENES AND GENETIC INSTABILITY
There are several classes of genes involved in carcinogenesis. Tumor suppressor genes (TSG) are present in all cells and help to regulate the cell cycle. They are often involved in regulating the cell growth and in determining when damaged cells are beyond repair and should degenerate through a process known as apoptosis. These genes promote carcinogenesis when inactivated. An example of that would be the p53 and the RB (retinoblastoma) genes. Proto-oncogenes, on the other hand, promote cellular proliferation and become oncogenes when mutated. K-ras is the best example of an oncogene in the GI tract. In general, oncogenes act dominantly, so a single mutated copy will drive the cell growth. Other classes of genes include the “housekeeping genes” or DNA repair genes. All of these genes interact with a variety of other genes and are usually the effectors of cell change when they receive the proper signals. The signaling pathways usually involve the means by which an external stress is translated by the receptors into phosphate kinases or phosphorylases. These can permit the cell signals to activate other genes or can silence the signal by inactivating the protein.
Below is a summary of the main diagnostic and predictive biomarkers in different GI cancers (Table 1).
Table 1.
Biomarkers That Have Been Proposed for Gastrointestinal Tumors
| Colon Biomarkers | Esophagus Biomarkers | Pancreas Biomarkers | Stomach Biomarkers |
|---|---|---|---|
| FAP | Ki-67, P53, P16, COX-2, EGFR, | CA19-9, CEA, K-ras, | cagA, vacA, babA2, IL1 |
| APC | EGF, TGF-α, β-catenin, | P53, P16, MIC-1, | gene, PGI, PGII, IgG |
| HNPCC | E-cadherin, c-erb-b2, cyclin | cyclin D2, TSLC1, | H. pylori |
| hMSH2, hMLH1, hPMS1, hPMS2, | D1 | SPARC | |
| hMSH3, hMSH6 | LOH: 17p/P53, 9p/P16 | Mitochondrial DNA | |
| Sporadic | Aneuploidy, tetraploidy, | mutation | |
| Tumor suppressor: P53, DCC, APC | Y-chromosome loss | ||
| Oncogenes: Myc, ras, src, erbB2, | |||
| MMR genes |
COLON ADENOCARCINOMA
The molecular basis of colorectal cancer (CRC) has undergone many refinements since its introduction by Vogelstein in 1990 (3). As genetic changes are fundamental to the neoplastic process in colon cancer, a molecular biomarker approach for screening seems very attractive. Commonly inherited CRC (APC [adenomatosis polyposis coli], hereditary nonpolyposis colorectal cancer [HNPCC]) (hereditary non-polyposis colon cancer) are thought to result from the germline mutations, whereas sporadic CRC are thought to result from the accumulation of multiple somatic mutations. The adenoma–carcinoma sequence in colon cancer has been extensively studied. It describes the common pathway taken by the neoplasms that arise as a result of the progressive accumulation of genetic changes. Mutation of the APC gene, which is common to both inherited and sporadic CRCs, happens early, whereas P53 mutations happen late in the process. Ras mutations are found in up to 50% of sporadic CRCs, and 50% of colonic adenomas larger than 1 cm (4). Familial adnenomatous polyposis (FAP) is a dominantly inherited syndrome caused by a germline mutation in the APC gene. Clinically, it is characterized by the development of hundreds of thousands of polyps by the second or third decade of life. Nearly all patients will develop cancer before the fifth decade. Patients with FAP are at an increased risk of small intestinal tumors and adenomas, particularly the duodenal periampullary adenomas. The other familial colon cancer syndrome that has been well studied is the Lynch syndrome or HNPCC, which is also transmitted as an autosomal-dominant disorder. Clinically, Lynch syndrome is characterized by early-onset right-sided colon cancer as well as extracolonic tumors. A mutation in any of the four mismatch repair (MMR) genes (MSH2, MLH1, MSH6, and PMS2) is the cause of Lynch syndrome. Microsatellite instability (MSI) is useful in patients thought to have Lynch syndrome (based on published criteria) as most of these patients will have MSI in their tumors (5). Tumors with MSI tend to occur in the proximal colon, have a greater mucinous component, and are more often poorly differentiated. MSI is a good predictor of the lack of response to 5-FU (5-fluorouracil) treatment because patients with MSI cancers have a worse response to chemotherapy than others. A marker of poor prognosis in CRC is the loss of 18q (long arm of chromosome 18) (6); however, this has not been translated into clinical application yet. The methylation of TSGs has been described in CRCs including P16, APC, and many others. But again, this could be physiologic, and it is hard to translate this into clinical practice. Although the evolution of CRC seems to stem from the loss of function of the APC gene, many other genetic and epigenetic instabilities are strongly involved. In addition, other factors such as viruses could also contribute to the adenoma–carcinoma sequence. The JC (Jakob Creutzfeldt) virus (human polyomavirus) has been implicated in the induction of chromosomal instability (CIN) and has been found in nearly 90% of CRC (7). It is worth noting that some phenotypic appearances in CRC and colon polyps are closely linked to genetic instability and certain biomarkers. For example, raised or pedunculated polyps are more likely to have mutations in the K-ras gene (8). Subtle alterations in the regular pattern of the intestinal crypts are one of the first histologically detectable changes. These are called “aberrant crypt foci” (ACF) and may be associated with the development of CRC. Magnifying endoscopy is showing some promises in the early detection of ACF (9). Several trials looked at the usefulness of fecal biomarkers in CRC (10). Ideally, this would be a noninvasive, sensitive, and cost-effective approach to CRC screening. To date, the performance of such tests has been limited. A panel of DNA markers failed to identify the majority of neoplastic lesions identified by colonoscopy and had a false-positive rate of 5–6% (10). When compared to hemoccult testing, however, multitargeted fecal DNA testing detected a higher proportion of important lesions (10).
ESOPHAGEAL ADENOCARCINOMA
Although Barrett’s esophagus is a strong risk factor for esophageal adenocarcinoma, only a minority of patients with adenocarcinoma of the esophagus have an antecedent diagnosis of Barrett’s metaplasia (11). Biomarkers are extremely important in esophageal carcinoma, in general, and in Barrett’s esophagus, in particular, given the controversies about the diagnosis of “dysplasia.” The molecular features of esophageal adenocarcinoma are less characterized than those of colon cancer. The adenoma–carcinoma sequence is different in both cancers, although many of the involved genes are the same. It is still not clear whether invasive esophageal carcinoma arise from a single clone of cells or from multiple oligoclonal lesions (12).
Different biomarkers have been looked at in esophageal adenocarcinoma. Dysplasia, on histology, in Barrett’s esophagus seems to be one of the most commonly used biomarkers to predict adenocarcinoma. There are new advances in molecular diagnostic techniques like “fluorescent in situ hybridization” (FISH), polymorphism analysis, and cytogenetics; however, these techniques have not been established in the clinical setting.
There is evidence that cell proliferation is one of the earliest steps in the development of esophageal cancer in Barrett’s esophagus (13). This is evidenced by an increase in metabolic labeling as well as immunohistochemistry showing an increase in Ki-67, a nuclear antigen expressed only during the proliferating phase of the cell cycle (14). Interestingly, the proliferation diminishes when dysplasia regresses. The P53 gene and the RB gene are essential TSGs that govern this control. As everyone normally has a copy of a gene from each parent, we are heterozygotes with two copies of each gene. The loss of heterozygosity (LOH) means having a loss of one or both copies of a gene from its original chromosome that is associated with gene inactivation. Alternatively, TSGs could be suppressed by other factors. For example, the P16 gene inhibits and inactivates the RB protein, and the murine double minute clone 2 protein (Mdm2) downregulates the level of P53. P53 gene mutation is also found in 85–89% in esophageal adenocarcinoma (15) and increases with higher degrees of dysplasia in Barrett’s esophagus (16). Although P16 gene mutations are less frequent, there is a high prevalence of hypermethylation in the promoter region of P16 in patients with dysplasia or adenocarcinoma of the esophagus, which also leads to loss of gene transcription and function (17). Other biomarkers that have been studied include protooncogenes like cyclin D1 as well as structural chromosomal abnormalities and aneuploidy. Aneuploidy and tetraploidy are found more frequently with increasing degrees of dysplasia (18). The loss of the Y chromosome is seen in up to 90% of the patients with esophageal adenocarcinoma (19). An aberrant or reduced expression of adhesion molecules like β-catenin, E-cadherin, and α-catenin has also been described. Advances in chemoprevention research, similar to those done in selective COX-2 inhibitors, will also help unravel new biomarkers. These would hopefully be detected early in the carcinoma sequence and would be easier to be detected by endoscopic biopsies than dysplasia, which could be patchy.
PANCREATIC CANCER
Because pancreatic cancer rarely presents at an early stage, investigators have tried hard to find accurate biomarkers that would help in detecting early stages of this deadly cancer. The current standard serum marker CA19–9 is used mainly to monitor the responses to therapy, and not as a diagnostic marker (20). Given that CA19–9 is a sialylated Lewis blood group antigen, its use is limited by the false-negative results in 5–10% of the populations that do not express the Lewis antigens. Furthermore, CA19–9 cannot always differentiate between benign and malignant pancreatic diseases. Pancreatic juice CEA and CA19–9 are also commonly used in the evaluation of pancreatic cystic neoplasms, but there is no consensus on the diagnostic accuracy of these and many clinicians do not rely solely on them. Many other pancreatic serum biomarkers have been investigated like macrophage inhibitory cytokine 1 (MIC-1); however, none proved to be of great clinical or diagnostic utility (21). Some pancreatic juice genetic and epigenetic markers that have been looked at include K-ras, P53, and P16 mutations, mitochondrial DNA mutations, and DNA hypermethylation.
Mitochondrial mutations are common in pancreatic cancer, and preliminary results of the use of these as biomarkers of pancreatic cancer in pancreatic juice and urine needs further confirmation (22). Another promising diagnostic strategy that will require further investigation is the detection of aberrant gene methylation like P16, cyclin D2, TSLC1, SPARC, and others in the pancreatic juice (23).
K-ras is probably the only biomarker that has been most extensively studied in pancreatic cancer. K-ras is readily detected using molecular assays, and mutations in this gene are present in the majority of ductal adenocarcinoma. In fact, it is thought that pancreatic cancers evolve through the sequential appearance of intraepithelial neoplastic lesions (PanINs), which appear to be mediated by the sequential appearance of K-ras mutations, inactivation of P16, followed by inactivation of P53 genes and genes on chromosome 18q called the DPC genes (deleted in pancreatic cancer) (24). Unfortunately, K-ras mutations are not specific for invasive pancreatic cancer and could be found in patients with chronic pancreatitis, in smokers, and in patients with PanINs (25).
GASTRIC CANCER
Gastric cancer is the second most frequent cause of cancer mortality in the world. As most cases of gastric cancer arise in the setting of inflammation and Helicobacter pylori (H. pylori), most biomarker research for this cancer focused on the evaluation of gastritis for cancer prevention and screening. Many factors are involved in the inflammation and cancer process (cagA, vacA, babA2, cytokine genes like IL-1) (26). More recently, serum levels of pepsinogen I and II (PGI/II), IgG H. pylori antibodies, and antiparietal cell antibodies (APCA) have been looked at as potential useful biomarkers (27). To date, however, there is still no clinically useful biomarker for the early detection of gastric cancer. Further studies are warranted to validate the existing markers and discover new ones for early gastric cancer diagnosis.
SUMMARY
We have summarized the characteristics of the clinically useful or potentially useful biomarkers in a number of GI cancers. This field is growing, and the predictive and diagnostic value of the biomarkers as a measure of carcinogenesis is still in evolution. It is essential for the clinician to have an understanding of the basic molecular biology behind GI cancers because, in the future, many clinicians will be routinely ordering biomarker testing as part of patient screening. The biomarkers in GI cancers are useful not only for screening, diagnosis, and prognosis but also for prediction of the response to mechanism-based interventions, such as chemoprevention. Ongoing assessment of these diagnostic and predictive factors will probably lead to a change in the current staging of many GI cancers.
Acknowledgments
Financial support: The study was supported by NIH (National Institutes of Health) grants R01 CA111603, R01 CA097048, and R21 CA122426.
Footnotes
Guarantor of the article: Kenneth K. Wang, M.D.
Specific author contributions: Rami Badreddine: literature review and manuscript drafting; and Kenneth K. Wang: manuscript drafting and revision.
Potential competing interests: The senior author has a financial interest in a patent on fluorescent in situ hybridization probes applied in Barrett’s esophagus.
REFERENCES
- 1.Ilyin SE, Belkowski SM, Plata-Salaman CR. Biomarker discovery and validation: Technologies and integrative approaches. Trends Biotechnol. 2004;22:411–416. doi: 10.1016/j.tibtech.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 3.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 4.Takayama T, Ohi M, Hayashi T, et al. Analysis of K-ras, APC, and beta-catenin in aberrant crypt foci in sporadic adenoma, cancer, and familial adenomatous polyposis. Gastroenterology. 2001;121:599–611. doi: 10.1053/gast.2001.27203. [DOI] [PubMed] [Google Scholar]
- 5.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 6.Jen J, Kim H, Piantadosi S, et al. Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med. 1994;331:213–221. doi: 10.1056/NEJM199407283310401. [DOI] [PubMed] [Google Scholar]
- 7.Enam S, Del Valle L, Lara C, et al. Association of human polyomavirus JCV with colon cancer: Evidence for interaction of viral T-antigen and beta-catenin. Cancer Res. 2002;62:7093–7101. [PubMed] [Google Scholar]
- 8.Yashiro M, Carethers JM, Laghi L, et al. Genetic pathways in the evolution of morphologically distinct colorectal neoplasms. Cancer Res. 2001;61:2676–2683. [PubMed] [Google Scholar]
- 9.Hawk ET, Umar A, Viner JL. Colorectal cancer chemoprevention—an overview of the science. Gastroenterology. 2004;126:1423–1447. doi: 10.1053/j.gastro.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Fecal DNA versus fecal occult blood for colorectal cancer screening in an average-risk population. N Engl J Med. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 11.Conio M, Cameron AJ, Romero Y, et al. Secular trends in the epidemiology and outcome of Barrett’s oesophagus in Olmsted county, Minnesota. Gut. 2001;48:304–309. doi: 10.1136/gut.48.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankowski JA, Wright NA, Meltzer SJ, et al. Molecular evolution of the metaplasia-dysplasia-adenocarcinoma sequence in the esophagus. Am J Pathol. 1999;154:965–973. doi: 10.1016/S0002-9440(10)65346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pellish LJ, Hermos JA, Eastwood GL. Cell proliferation in three types of Barrett’s epithelium. Gut. 1980;21:26–31. doi: 10.1136/gut.21.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Hamelin R, Flejou JF, Muzeau F, et al. TP53 gene mutations and p53 protein immunoreactivity in malignant and premalignant Barrett’s esophagus. Gastroenterology. 1994;107:1012–1018. doi: 10.1016/0016-5085(94)90225-9. [DOI] [PubMed] [Google Scholar]
- 16.Younes M, Lebovitz RM, Lechago LV, et al. p53 protein accumulation in Barrett’s metaplasia, dysplasia, and carcinoma: A follow-up study. Gastroenterology. 1993;105:1637–1642. doi: 10.1016/0016-5085(93)91058-p. [DOI] [PubMed] [Google Scholar]
- 17.Klump B, Hsieh CJ, Holzmann K, et al. Hypermethylation of the CDKN2/p16 promoter during neoplastic progression in Barrett’s esophagus. Gastroenterology. 1998;115:1381–1386. doi: 10.1016/s0016-5085(98)70016-2. [DOI] [PubMed] [Google Scholar]
- 18.Krishnadath KK, Tilanus HW, van Blankenstein M, et al. Accumulation of genetic abnormalities during neoplastic progression in Barrett’s esophagus. Cancer Res. 1995;55:1971–1976. [PubMed] [Google Scholar]
- 19.Moskaluk CA, Hu J, Perlman EJ. Comparative genomic hybridization of esophageal and gastroesophageal adenocarcinomas shows consensus areas of DNA gain and loss. Genes Chromosomes Cancer. 1998;22:305–311. [PubMed] [Google Scholar]
- 20.American Gastroenterological Association medical position statement: Epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. Gastroenterology. 1999;117:1463–1484. doi: 10.1016/s0016-5085(99)70297-0. [DOI] [PubMed] [Google Scholar]
- 21.Koopmann J, Buckhaults P, Brown DA, et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin Cancer Res. 2004;10:2386–2392. doi: 10.1158/1078-0432.ccr-03-0165. [DOI] [PubMed] [Google Scholar]
- 22.Maitra A, Cohen Y, Gillespie SE, et al. The human MitoChip: A high-throughput sequencing microarray for mitochondrial mutation detection. Genome Res. 2004;14:812–819. doi: 10.1101/gr.2228504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato N, Fukushima N, Maitra A, et al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735–3742. [PubMed] [Google Scholar]
- 24.Hruban RH, Goggins M, Parsons J, et al. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 25.Berger DH, Chang H, Wood M, et al. Mutational activation of K-ras in nonneoplastic exocrine pancreatic lesions in relation to cigarette smoking status. Cancer. 1999;85:326–332. doi: 10.1002/(sici)1097-0142(19990115)85:2<326::aid-cncr9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 26.Zambon CF, Navaglia F, Basso D, et al. Helicobacter pylori babA2, cagA, and s1 vacA genes work synergistically in causing intestinal metaplasia. J Clin Pathol. 2003;56:287–291. doi: 10.1136/jcp.56.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watabe H, Mitsushima T, Yamaji Y, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: A prospective endoscopic cohort study. Gut. 2005;54:764–768. doi: 10.1136/gut.2004.055400. [DOI] [PMC free article] [PubMed] [Google Scholar]


