Abstract
Context
Neurofibromatosis type 1 (NF1) is among the most common genetic disorders that cause learning disabilities. Recently, it was shown that statin-mediated inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase restores the cognitive deficits in an NF1 mouse model.
Objective
To determine the effect of simvastatin on neuropsychological, neurophysiological, and neuroradiological outcome measures in children with NF1.
Design, Setting, and Participants
Sixty-two of 114 eligible children (54%) with NF1 participated in a randomized, double-blind, placebo-controlled trial conducted between January 20, 2006, and February 8, 2007, at an NF1 referral center at a Dutch university hospital.
Intervention
Simvastatin or placebo treatment once daily for 12 weeks.
Main Outcome Measures
Primary outcomes were scores on a Rey complex figure test (delayed recall), cancellation test (speed), prism adaptation, and the mean brain apparent diffusion coefficient based on magnetic resonance imaging. Secondary outcome measures were scores on the cancellation test (standard deviation), Stroop color word test, block design, object assembly, Rey complex figure test (copy), Beery developmental test of visual-motor integration, and judgment of line orientation. Scores were corrected for baseline performance, age, and sex.
Results
No significant differences were observed between the simvastatin and placebo groups on any primary outcome measure: Rey complex figure test (β=0.10; 95% confidence interval [CI], −0.36 to 0.56); cancellation test (β=−0.19; 95% CI, −0.67 to 0.29); prism adaptation (odds ratio=2.0; 95% CI, 0.55 to 7.37); and mean brain apparent diffusion coefficient (β=0.06; 95% CI, −0.07 to 0.20). In the secondary outcome measures, we found a significant improvement in the simvastatin group in object assembly scores (β=0.54; 95% CI, 0.08 to 1.01), which was specifically observed in children with poor baseline performance (β =0.80; 95% CI, 0.29 to 1.30). Other secondary outcome measures revealed no significant effect of simvastatin treatment.
Conclusion
In this 12-week trial, simvastatin did not improve cognitive function in children with NF1.
Neurofibromatosis type 1 (NF1) is a common autosomal-dominant genetic disorder (incidence 1:3000)1 caused by a mutation in the gene encoding neurofibromin, a protein that activates the hydrolysis of RAS-bound guanosine triphosphate.2 Neurofibromatosis type 1 is characterized by various neurocutaneous manifestations, problems in fine and gross motor functioning,3 as well as the frequent occurrence of cognitive disabilities. Children with NF1 have a lower mean IQ (86–94) with particular deficits in visual-spatial skills, nonverbal long-term memory, executive functions, and attention.4–7 These problems have a large impact on school performance of children with NF1.4 It has been suggested that the cognitive and motor deficits in children with NF1 are related to hyperintensities on T2-weighed magnetic resonance imaging of the brain3,8 that are characterized by high apparent diffusion coefficients (ADC values),9 but some studies failed to confirm this relationship.10
Studies using mouse models for NF1 (Nf1 mice) revealed that increased RAS/ERK signaling is primarily responsible for the neuronal plasticity deficits as well as the spatial learning and attention deficits of these mice.11–13 RAS transforming activity requires iso-prenylation (ie, farnesylation or geranylgeranylation) of RAS, which can be blocked by farnesyl transferase inhibitors and by 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors.14,15 HMG-CoA reductase is the rate-limiting enzyme in the mevalonate pathway in which cholesterol and isoprenyl groups are synthesized. Importantly, treatment of Nf1 mice with a farnesyl transferase inhibitor or HMG-CoA reductase inhibitor for just a few days reverses the cognitive deficits of these mice.11,13
These findings are not only important for NF1 but also are of great interest for other neuro-cardio-facial-cutaneous syndromes (eg, Noonan, Costello, and cardio-facio-cutaneous syndromes), which are also caused by aberrant RAS/ERK signaling, and for hamartoma syndromes (eg, Cowden disease and tuberous sclerosis complex). The genes associated with these syndromes belong to a pathway that is not only coregulated by RAS but also critically dependent on RHEB, another farnesylated protein of the RAS family.
The favorable safety profile of the HMG-CoA reductase inhibitor simvastatin in adults and children16 provided an opportunity to investigate whether the findings in the mouse model can be translated to humans. In a randomized, double-blind, placebo-controlled trial, we studied the effect of a 12-week simvastatin treatment on cognitive function of children with NF1 using neuropsychological, neurophysiological, and neuroradiological outcome measures.
METHODS
Design
A prospective, double-blind, placebo-controlled, randomized, single-site, 12-week clinical trial was conducted in children with NF1 between January 20, 2006, and February 8, 2007. The study was approved by the medical ethical committee of the Erasmus MC, Rotterdam, the Netherlands.
Participants
All participants were recruited from the multidisciplinary NF1 outpatient clinic of the Erasmus MC–Sophia Children’s Hospital, which is a university hospital and NF1 referral center in the Netherlands. Participants were enrolled by a pediatrician in the NF1 outpatient clinic (A.G.B.). Inclusion criteria were age 8 to 16 years, NF1 diagnosis according to the criteria of the National Institutes of Health,17 and oral and written informed consent from parents and children older than 12 years. Exclusion criteria were segmental NF1, pathology of the central nervous system (other than asymptomatic gliomas), deafness, severely impaired vision, use of antiepileptic drugs, insufficient comprehension or use of the Dutch language, and an IQ below 48, which was assessed at baseline using the Wechsler Intelligence Scale for Children–Revised, Dutch version.18
Protocol
Patients were randomized to simvastatin or placebo using a permuted-block, 1:1 randomization list generated by the trial statistician (S.M.F.P.) with blocks of 6 participants, in which medication numbers 1 through 62 corresponded to either simvastatin or placebo. Randomization was performed by the Erasmus MC trial pharmacist, who assigned patients a medication number in the order of their enrollment in the trial and who dispensed the medication. Patients and all other investigators were blind to the treatment allocation. Patients were treated once a day in the morning for 12 weeks with simvastatin (weeks 0–4, 10 mg/d; weeks 5–8, 20 mg/d; and weeks 9–12, 20 mg/d for children aged 8–12 years or 40 mg/d [taken as two 20-mg doses] for children aged 13–16 years) or equivalent placebo. The placebo capsules were filled with microcrystalline cellulose PH102 and treatment capsules with a filler and a tablet of 10-mg (weeks 0–4) or 20-mg (weeks 5–12) simvastatin (film-coated; Alpharma Inc; Bridge-water, New Jersey). The capsules containing placebo or simvastatin were nontransparent and identical in color, shape, and size. Patients were instructed not to open the capsules. Patients were judged adherent when they took at least 80% of their study medication during the intervention period of 12 weeks, which was assessed by counting returned capsules.
Outcome Measures
Outcome measures were assessed at baseline and after 12 weeks of treatment. For the primary outcome measures, we chose 2 neuropsychological tests that were analogous to statin-responsive tests in Nf1 mice (measuring visual-spatial memory and attention). In addition, we selected a neurophysiological and neuroradiological measure because we reasoned that these measurements would be insensitive to placebo or test-retest effects. This resulted in the following 4 primary outcome measures: performance on the Rey complex figure test (CFT) (delayed recall; assessing nonverbal long-term memory), performance on the cancellation test (speed; assessing attention), performance on a prism adaptation task (measurement of adaptation of the angle of hand movements in response to prism glass distortion,19 which is thought to be dependent on cerebellar function20,21), and mean apparent diffusion coefficient (ADC value) of the brain (mean ADC value of 7 predetermined anatomic locations predominantly affected by T2-weighed hyperintensities) as previously described.9
For the secondary outcome measures, we selected neuropsychological tests assessing domains that are specifically affected in patients with NF1: tests for attention and tests for visual-spatial skills with baseline scores of 1 SD or more below average.4,9 This resulted in the following secondary outcome measures: performance on the cancellation test (standard deviation; measuring attention fluctuations), the Stroop color word test, the block design test and object assembly test of the Wechsler Intelligence Scale for Children–Revised, the Rey CFT (copy), the Beery developmental test of visual-motor integration, and the judgment of line orientation task.22
Magnetic resonance imaging was performed by using a 1.5-tesla system (EchoSpeed; GE Healthcare, Milwaukee, Wisconsin) and a dedicated 8-channel head coil. Diffusion tensor imaging data were gathered by using a multirepetition, single-shot, echo-planar sequence with a section thickness of 3 mm with no gap. A 25-gradient directions technique was performed to obtain good diffusion tensor images (sensitivity, b = 1000 s/mm2; repetition time, 15 000 milliseconds; echo time, 82.1 milliseconds; 1 average; field of view, 240 × 240 mm2; matrix, 128 × 128; voxel size, 1.8 × 1.8 × 3.0 mm3) as described previously.9
All neuropsychological tests were developed for children, administered in their Dutch versions, and scored by 1 pediatric neuropsychologist (M.J.B.). Parallel versions of tests were applied when available to reduce the impact of practice effects. For technical reasons, left-handed children (n=7) were excluded from the prism adaptation task.
Treatment safety and adherence was assessed in the outpatient clinic at baseline, after 4 and 12 weeks, and with a telephone consult after 8 weeks. Patients were provided with a diary in which they were instructed to note any deviations from treatment protocol and possible adverse events. At each consult, a general pediatrician recorded any adverse events and serious adverse events (adverse events that were life-threatening, causing disability, or requiring hospitalization) with a standardized checklist of the adverse events and serious adverse events reported with simvastatin use,23 supported by open questions and a review of the patient’s diary. All reported adverse events were scored as being not drug related, possibly drug related, or definitely drug related prior to unblinding. During the visits to the outpatient clinic, the pediatrician (A.G.B.) performed a standardized internal and neurological assessment, and blood was drawn for laboratory examination. We examined the safety parameters (levels of alanine aminotransferase, aspartate aminotransferase, and creatine phosphokinase) and efficacy parameters (levels of total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and triglycerides) according to standard clinical laboratory protocol. Criteria for discontinuation of study medication were a persistent increase of more than 3-fold the upper limit of normal (ULN) alanine aminotransferase or aspartate aminotransferase levels, more than 10-fold the ULN for creatine phosphokinase levels with or without muscular symptoms, or 5- to 10-fold the ULN for creatine phosphokinase levels with muscular symptoms.16
Statistical Analyses
One of the prominent effects seen in statin-treated Nf1 mice was recovery of their deficit in visual-spatial memory.13 The Rey CFT (delayed recall) assesses the analogous domain of nonverbal long-term memory in humans and has good psychometric properties, and performance on this test is specifically affected in patients with NF1.24 Therefore, we based our power calculation on this test. On the assumption of a correlation of 0.70 between measurement before and after treatment, and a mean (SD) z score of −1.32 (1.01) on the Rey CFT (delayed recall) at baseline,24 we calculated that 30 persons were needed in both the placebo and treatment groups to ensure a power of 0.80 of detecting a significant (α= .05) improvement in the Rey CFT (delayed recall) score up to −0.28 (difference of 1.04) in the treatment group.
All data were analyzed using SPSS 12.0 (SPSS Inc, Chicago, Illinois). For the neuropsychological tests, z scores were used (with negative values indicating performance below the normative mean and positive values performance above the normative mean), except for the cancellation test (standard deviation) (raw score for nonnormal distribution of reference values; larger negative values indicated larger attention fluctuations). Prism adaptation was scored to occur if the change (adaptation) of the angle of hand movements was significant (P< .01) and larger than −1 SD of the mean change of age-matched healthy controls (n=16, unpublished observations). A decrease in ADC values indicates lower signal intensity.
Modified intention-to-treat analysis was performed for all patients with available 12-week test scores (n=61) without imputing missing values. Differences between the simvastatin and placebo groups at baseline were analyzed with the t test, Mann-Whitney test, and χ2 test. Differences between the simvastatin and placebo groups after 12 weeks of treatment were assessed using univariate and multivariate regression analysis. In the univariate analysis, we adjusted for baseline scores, and in the multivariate regression analysis we adjusted for baseline scores, age, and sex. Regression coefficients (β) reflect the estimated differences in mean score at follow-up between the treatment groups with 95% confidence intervals (CIs). For categorical measures (prism adaptation), the difference between the treatment groups was expressed as an odds ratio with 95% CI. Cut-off level for significance was set at P< .05. Effect modification of outcome parameters that were significantly different between the treatment and placebo groups after 12 weeks was examined using interaction terms between treatment and age and between treatment and baseline performance. The rationale for this analysis is that we expected increased plasticity in younger children and more room for improvement in children with low baseline performance, thus affecting the magnitude of response to simvastatin treatment. Subgroup analysis was performed only if effect modification was plausible (P<.10 to take into account the small size of the subgroups) for addition of the interaction term to the multivariate analysis. All P values reported are 2-sided. The outcome parameters and the method of statistical analysis, including the subgroup analyses, were defined before unblinding.
We did not correct for multiple comparisons for the following reasons. There are only 4 primary outcome measures, and they are specifically based on a priori assumptions. The outcome measures on the neuropsychological tests are potentially correlated, and correction would thus be inappropriate. By correcting for multiple comparisons, it would be very hard to detect a possible effect in a relatively small patient population. Thus we would run a high risk of discarding a promising drug while in fact there is an effect (type II error).
For ethical reasons, an interim analysis was conducted by the statistician (S.M.F.P.) after 36 patients completed the study with complete maintenance of the double-blind protocol for all others. The criterion to discontinue the study was a significant difference between the simvastatin and placebo groups on Rey CFT (delayed recall) score at 12 weeks (P<.01). The statistician communicated that this criterion was “not reached” and the study was continued as planned.
RESULTS
Participants
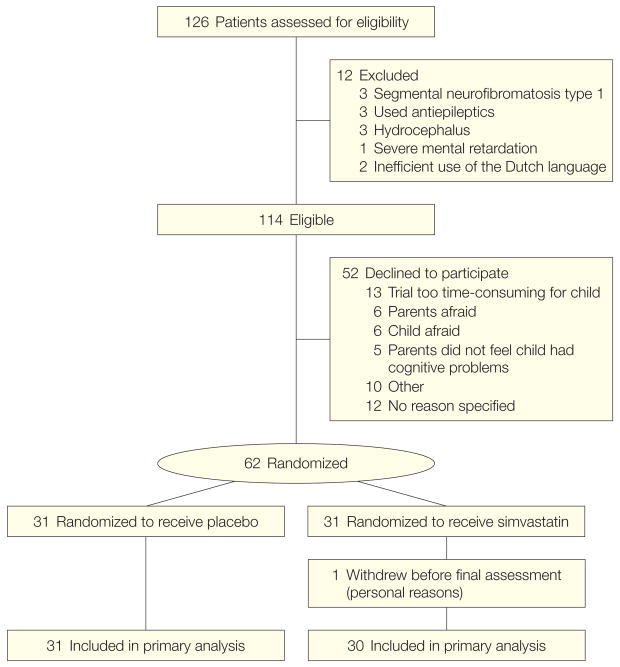
One hundred fourteen children were eligible for this study. Consent to participate was obtained for 62 children (response rate, 54%). The children who participated in the trial (n=62) did not differ significantly from the total eligible group (n=114) on age, sex, frequency of mental retardation, or disease severity (all P> .30), indicating that they were representative of the total eligible group. The 62 participants were randomly assigned to the simvastatin group (n=31) or the placebo group (n=31) (Figure 1). The baseline characteristics were similar between the simvastatin and placebo groups for all baseline parameters except for median age (Table 1 and Table 2). Mean (SD) treatment duration was 12 weeks and 3 days (6 days). There were no deviations from random allocation. One participant (2%) in the simvastatin group withdrew from the study after 10 weeks for personal reasons. Three of 62 children (5%), all in the placebo group, were not adherent according to the 80% criterion. We could not retrieve all of the medication jars for 10 of 62 children (16%; 6 in the simvastatin group and 4 in the placebo group).
Figure 1. Flowchart of Patient Inclusion.
Table 1.
Baseline Characteristics of the Study Groupsa
| No. (%) |
||
|---|---|---|
| Placebo (n = 31) | Simvastatin (n = 31) | |
| Patient characteristics | ||
| Age at randomization, median (IQR), yb | 11.5 (9.4–13.5) | 13.2 (11.3–15.2) |
| Male sex | 16 (52) | 19 (61) |
| Full-scale IQ, mean (SD) | 85 (15) | 88 (15) |
| NF1 disease severityc | ||
| Minimal | 10 (32) | 12 (39) |
| Mild | 13 (42) | 11 (35) |
| Moderate | 8 (26) | 7 (23) |
| Severe | NA | 1 (3) |
| Inheritance of NF1 | ||
| Familial | 14 (45) | 12 (39) |
| Sporadic | 16 (52) | 19 (61) |
| Unconfirmed | 1 (3) | 0 |
| Socioeconomic statusd | ||
| Low | 12 (39) | 12 (39) |
| Middle | 9 (29) | 9 (29) |
| High | 10 (32) | 10 (32) |
| Total cholesterol, mean (SD), mg/dL | 166 (31) | 163 (36) |
| LDL cholesterol, mean (SD), mg/dL | 97 (26) | 96 (32) |
| Treatment dose in weeks 9–12 | ||
| 20 mg/d | NA | 12 (39) |
| 40 mg/d | NA | 19 (61) |
| Maximal treatment dose, mg/kg, mean (SD) | NA | 0.7 (0.1) |
Abbreviations: IQR, interquartile range; LDL, low-density lipoprotein; NA, not applicable; NF1, neurofibromatosis type 1.
SI conversion factors: to convert cholesterol to mmol/L, multiply by 0.0259.
N = 62 unless otherwise indicated.
P = .03 between simvastatin and placebo group.
Disease severity of NF1 was scored according to the Riccardi scale modified to exclude cognitive aspects of NF1.4
Socioeconomic status was determined from highest parental occupation or, if not available, highest parental education, and divided into low, middle, or high.
Table 2.
Scores on Primary and Secondary Outcome Measures at Baseline and 12 Weeks
| Mean (SD) | β (95% Confidence Interval) | |||
|---|---|---|---|---|
| Baselinea | 12 Weeksb | Univariate Differenceb,c | Multivariate Differenceb,d | |
| Primary Outcome Measures | ||||
| Rey CFT (delayed recall) | ||||
| Placebo | −1.6 (0.7) | −1.5 (1.0) | ]0.07 (−0.37 to 0.51) | 0.10 (−0.36 to 0.56) |
| Simvastatin | −1.7 (0.8) | −1.4 (0.8) | ||
| Cancellation test (speed)e | ||||
| Placebo | −0.8 (1.6) | 0.4 (1.1) | ]−0.27 (−0.74 to 0.20) | −0.19 (−0.67 to 0.29) |
| Simvastatin | −1.2 (1.8) | −0.1 (1.4) | ||
| Prism adaptation, No. (%)f | ||||
| Placebo | 12 (44) | 10 (37) | ]1.57 (0.48 to 5.13)g | 2.01 (0.55 to 7.37)g |
| Simvastatin | 11 (50) | 12 (48) | ||
| Mean ADC value, × 10−3 mm2/sh | ||||
| Placebo | 8.03 (0.52) | 7.97 (0.50) | ]0.01 (−0.12 to 0.14) | 0.06 (−0.07 to 0.20) |
| Simvastatin | 8.02 (0.44) | 7.91 (0.46) | ||
| Secondary Outcome Measures | ||||
| Cancellation test (standard deviation) (raw score)i | ||||
| Placebo | −2.7 (1.2) | −1.9 (0.9) | ]−0.12 (−0.65 to 0.41) | −0.26 (−0.80 to 0.28) |
| Simvastatin | −2.8 (1.7) | −2.0 (1.5) | ||
| Stroop (speed)j | ||||
| Placebo | −0.2 (1.8) | 0.2 (1.5) | ]0.34 (−0.36 to 1.04) | 0.48 (−0.23 to 1.18) |
| Simvastatin | −0.5 (2.1) | 0.3 (1.9) | ||
| Block design | ||||
| Placebo | −1.1 (0.8) | −1.0 (1.0) | ]0.15 (−0.18 to 0.47) | 0.10 (−0.24 to 0.44) |
| Simvastatin | −0.8 (0.9) | −0.5 (1.0) | ||
| Object assembly | ||||
| Placebo | −1.1 (1.1) | −0.9 (1.3) | ]0.50 (0.05 to 0.95)k | 0.54 (0.08 to 1.01)l |
| Simvastatin | −0.8 (1.1) | −0.1 (1.0) | ||
| Rey CFT (copy) | ||||
| Placebo | −1.2 (1.2) | −0.7 (1.1) | ]−0.26 (−0.71 to 0.19) | −0.12 (−0.58 to 0.34) |
| Simvastatin | −1.4 (1.3) | −1.0 (1.2) | ||
| Beery VMI | ||||
| Placebo | −1.2 (0.9) | −1.1 (0.9) | ]−0.01 (−0.27 to 0.26) | −0.02 (−0.30 to 0.26) |
| Simvastatin | −1.2 (0.7) | −1.1 (0.7) | ||
| Judgment of line orientation test | ||||
| Placebo | −1.6 (1.4) | −1.1 (1.6) | ]−0.12 (−0.62 to 0.38) | −0.06 (−0.58 to 0.46) |
| Simvastatin | −1.1 (1.4) | −0.8 (1.6) | ||
Abbreviations: ADC, apparent diffusion coefficient; Beery VMI, Beery developmental test of visual-motor integration; CFT, complex figure test; NF1, neurofibromatosis type 1.
N = 62 (31 placebo, 31 simvastatin) unless otherwise indicated. Values indicate mean (SD) z score unless otherwise indicated in which negative values indicate performance below the normative mean and positive values performance above the normative mean.
n = 61 (31 placebo, 30 simvastatin) unless otherwise indicated; 1 loss to follow-up in the simvastatin group before final assessment. Values indicate mean (SD) z score unless otherwise indicated in which negative values indicate performance below the normative mean and positive values performance above the normative mean.
Values (regression coefficients and 95% confidence interval) indicate between-group differences in scores after 12 weeks, adjusted for baseline scores, obtained from univariate regression analysis.
Values (regression coefficients and 95% confidence interval) indicate between-group differences in scores after 12 weeks, adjusted for baseline scores, age, and sex, obtained from multivariate regression analysis.
Baseline and 12 weeks: n = 29 in the placebo group; only administered if children possessed sufficient rote memory to count groups of up to 5 dots.
Baseline: n = 49 (27 placebo, 22 simvastatin); 7 left-handed children excluded, 6 children excluded due to technical problems, including not understanding or adhering to task instructions (n = 4). 12 Weeks: n = 52 (27 placebo, 25 simvastatin); 6 left-handed children excluded, 3 children excluded due to technical problems including not understanding/adhering to task instructions (n = 2).
Odds ratio with 95% confidence interval. n = 46 (26 placebo, 20 simvastatin), 6 left-handed children excluded, 9 children excluded because of technical problems, including not adhering to task instructions (n = 6).
Baseline: n = 50 (25 placebo, 25 simvastatin); 2 missing due to artifacts, 10 were not scanned due to limited magnetic resonance imaging capacity (random). 12 Weeks: n = 46 (23 placebo, 23 simvastatin); 5 missing due to artifacts, 10 were not scanned due to limited magnetic resonance imaging capacity (random). A decrease in ADC values indicates lower signal intensity.
Baseline and 12 weeks: n = 29 in the placebo group; only administered if children possessed sufficient rote memory to count groups of up to 5 dots. Larger negative values indicate larger attention fluctuations.
Baseline: n = 59 (29 placebo, 30 simvastatin); 12 weeks: n = 58 (29 placebo, 29 simvastatin). Only administered if children could read the names of colors.
P = .03.
P = .02.
Effect of Simvastatin on Outcome Parameters
After 12 weeks of treatment, we did not observe a significant difference between the simvastatin and placebo groups on the primary outcome measures (Rey CFT [delayed recall], cancellation test [speed], prism adaptation, and mean brain ADC values). We also did not observe an effect on the secondary outcome measures (cancellation test [standard deviation], Stroop color word test, block design, Rey CFT [copy], Beery developmental test of visual-motor integration, and judgment of line orientation), except for a higher score on the object assembly test in the simvastatin group using univariate analysis (adjustment for baseline scores, β = 0.50 [95% CI, 0.05 to 0.95]) as well as multivariate analysis (adjustment for baseline scores, age, and sex, β = 0.54 [95% CI, 0.08 to 1.01]) (Table 2).
Paired t tests revealed that performance after 12 weeks was similar or better than baseline for all tests in both the simvastatin and the placebo groups. In the placebo group, the improvement between baseline and 12 weeks was significant on 4 of 9 neuropsychological outcome measures (cancellation test [speed and standard deviation], Rey CFT [copy], judgment of line orientation), leading to a performance within the normal range on the first 3 tests.
Effect Modification
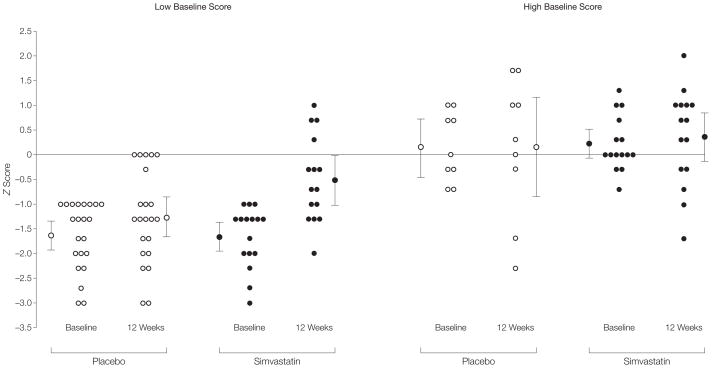
We found that baseline performance on the object assembly test was a modifier of the effect of simvastatin on this test (P= .07). Subsequent subgroup analysis showed a significant effect of simvastatin in the group with the baseline object assembly test scores −1 SD or less (β = 0.80 [95% CI, 0.29 to 1.30]; n=37), but not in the group with the baseline object assembly test score of greater than −1 SD (β = 0.47 [95% CI, − 0.64 to 1.59]; n=24) indicating that the difference in the object assembly test results between the simvastatin and placebo groups is mostly caused by an increase in score in children with a poor baseline performance in the simvastatin group (Figure 2). There was no interaction between improvement on the object assembly test and age.
Figure 2. Interaction Between Baseline Score and Effect of Simvastatin on Object Assembly Test Results.
For each subgroup, individual z scores and uncorrected group mean z scores are provided. For each subgroup, the left range shows scores at baseline and the right range, scores at 12 weeks. For the simvastatin group, n=16 for the low baseline score at baseline but n=15 for the low baseline score at 12 weeks; n=15 for the high baseline score. For the placebo group, n=22 for the low baseline score, and n=9 for the high baseline score. The difference between the simvastatin and placebo groups after 12 weeks is significant in the groups with low baseline performance (β=0.80; 95% confidence interval, 0.29 to 1.30; P=.003), but not in the groups with high baseline performance (β=0.47; 95% confidence interval, −0.64 to 1.59). Error bars represent 95% confidence intervals.
Safety and Effect on Cholesterol Levels
There were no laboratory adverse events and no serious adverse events. In total, 5 adverse events were reported by 3 of 31 children (10%) in the simvastatin group: hair loss (1 child after 4, 8, and 12 weeks), muscle weakness (1 child after 8 weeks), and constipation (1 child after 12 weeks) compared with 4 adverse events reported by 3 of 31 (10%) children in the placebo group (dizziness [1 child after 4 and 8 weeks] and constipation [1 child after 8 and 1 child after 12 weeks]). None of the reported adverse events were judged clinically significant.
After 12 weeks of simvastatin treatment, total cholesterol levels were reduced by a mean (SD) 21.1% (10.7%) of baseline values and low-density lipoprotein cholesterol by 39.4% (15.1%). There was no significant change in levels of high-density lipoprotein cholesterol or triglycerides. The change in low-density lipoprotein cholesterol level in the simvastatin group was not significantly related to the dose, sex, or age. The low-density lipoprotein cholesterol level of the children in the simvastatin group who did not return all of their medication jars was decreased by at least 34% (1 not determined because of loss to follow-up).
COMMENT
We report the results of a randomized, double-blind, placebo-controlled trial to investigate the effect of simvastatin on cognitive function in children with NF1. We used a carefully selected set of outcomes, including tests resembling measurements shown to be responsive to statins in preclinical studies, tests reflecting the specific neuropsychological deficits in NF1, and objective outcomes such as prism adaptation and brain ADC values, which are insensitive to a placebo or test-retest effect. We did not find an effect of 12-weeks of simvastatin treatment on the primary and secondary outcome parameters except for higher scores on the object assembly test.
We can conclude post hoc that the power of our study was enough to reject a possible effect on most tests. For instance, for the Rey CFT (recall) (β =0.10, SE=0.23), we can reject a change larger than 0.56, and for the cancellation test (speed) (β = −0.19, SE=0.24), we can reject a change larger than 0.28. Furthermore, we chose to interpret an improvement of 1 SD as clinically significant, and none of the outcome measures showed a difference between the simvastatin and placebo group of 1 SD or larger. Thus, given the power of the study and the overall negative findings, this study does not provide support for prescribing simvastatin to treat the cognitive deficits of children with NF1.
The object assembly test was the only outcome measure that was significantly improved. Considering that we found an improvement only on this test and that we did multiple statistical comparisons without adjusting the P value, this is probably a spurious finding.
It should be noted that the improvement in object assembly was restricted to children who performed poorly at baseline. This specific improvement in the subgroup of children with poor baseline scores is not likely to be related to a practice effect because children with high baseline scores are expected to benefit most from a practice effect.25
The object assembly test measures multiple cognitive domains, but in the context of the entire neuropsychological assessment along with the clinical behavioral observations made during the assessment, visual synthesis is probably the most damaged cognitive domain. Improved visual synthesis would affect academic performance. For instance, visual synthesis needs to be mastered for children to start reading and spelling, and visual synthesis is an important part of more advanced mathematical skills.26,27 However, whether the observed improvement in object assembly is a real effect and whether simvastatin would indeed improve academic achievement remain to be confirmed.
Our study has several limitations. First, the treatment duration used in our study might have been too short to observe a clinically significant cognitive recovery in patients with NFI. We based the length of our trial on the observation that statin treatment normalized the plasticity impairment and cognitive phenotype of Nf1 mice within days13 and the observation that treatment of some cognitive problems in children can be reached within days to weeks (for instance, in the treatment of attention deficits in attention-deficit/hyperactivity disorder, reviewed by Brown et al28). However, because precedents for translational trials of cognition are rare, we cannot exclude the possibility that the effect of simvastatin on higher cognitive functions in humans would require a longer treatment period than 12 weeks.
Second, the placebo group showed a significant improvement between baseline and 12-week scores on 4 of 9 neuropsychological outcome measures. This resulted in a performance within normal range on 3 tests. Because preclinical studies showed that statin treatment did not improve cognitive function in mice that already learned well,13 it is possible that we reached a performance ceiling that hampered detection of an effect.
Third, it is conceivable that the therapeutic effect of simvastatin on human brain function was hampered by suboptimal availability due to a first pass effect or due to inefficient crossing of the blood brain barrier. However, increasing the therapeutic dose does not seem desirable because of the lack of safety studies in children with higher doses and the increasing risk of adverse effects observed in adults.23 Furthermore, the effect of simvastatin on low-density lipoprotein cholesterol levels at 12 weeks was similar to the decrease achieved after 48 weeks of simvastatin treatment in a previous pediatric study.16 This indicates that, at least in the liver, the treatment dose was optimal with respect to inhibition of the mevalonate pathway.
Finally, there was a relatively high amount of missing data in the neuroradiological and prism adaptation results. Although this reduces the power on these outcome measures, there was no indication for a substantial bias because the distribution of observations that were missing did not significantly differ between the simvastatin and placebo groups. For the other outcome measures, the proportion of missing data was negligibly small.
The negative outcome of this trial suggests that simvastatin should not be prescribed to ameliorate the cognitive deficits associated with NF1. Further studies to evaluate a longer treatment period and whether the object assembly finding is spurious may be warranted.
Acknowledgments
Funding/Support: This trial was funded by the Hersenstichting Nederland, the Sophia Foundation for Medical Research, and the Prinses Beatrix Fonds and by a donation from the Dutch Neurofibromatosis Foundation.
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Trial Registration isrctn.org Identifier: ISRCTN14965707
Financial Disclosures: Drs Kushner and Silva reported being coapplicants on a US patent (No. 11/569,426) “Treating learning deficits with inhibitors of HMG-CoA reductase.” None of the other authors reported a potential, real, or perceived conflict of interest or financial disclosure.
Author Contributions: Ms Krab had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Krab, De Goede-Bolder, Aarsen, Pluijm, Lequin, Catsman-Berrevoets, Arts, Kushner, Silva, De Zeeuw, Moll, Elgersma.
Acquisition of data: Krab, De Goede-Bolder, Bouman, Lequin, Moll.
Analysis and interpretation of data: Krab, De Goede-Bolder, Aarsen, Pluijm, Van der Geest, Kushner, Moll, Elgersma.
Drafting of the manuscript: Krab, De Goede-Bolder, Van der Geest, Moll, Elgersma.
Critical revision of the manuscript for important intellectual content: Krab, De Goede-Bolder, Aarsen, Pluijm, Bouman, Lequin, Catsman-Berrevoets, Arts, Kushner, Silva, De Zeeuw, Elgersma.
Statistical analysis: Krab, Pluijm, Van der Geest.
Obtained funding: Krab, De Goede-Bolder, De Zeeuw, Moll, Elgersma.
Administrative, technical, or material support: Krab, De Goede-Bolder, Bouman, Van der Geest.
Study supervision: De Goede-Bolder, Aarsen, Lequin, Catsman-Berrevoets, Arts, Silva, De Zeeuw, Moll, Elgersma.
Additional Contributions: We thank the patients and their parents for their participation. Also, we thank E. Steyerberg, MD, PhD, Department of Public Health, for fruitful discussions, and J. B. C. De Klerk, MD, Department of General Pediatrics and Clinical Genetics, for laboratory safety monitoring. S. J. P. M. Van Engelen, Department of Pediatric Radiology; S. M. Goorden, MSC, M. Elgersma, BSC, P. Plak, BSC, Department of Neuroscience; and A. C. Gaemers, MA, R. Wierenga, MA, E. Barendse, MA, Department of Pediatric Neurology, helped in collecting the data. We appreciate the support of all other participants of the NF1 clinical workgroup and the NF1 Core (Cognitive Research) Team of the Erasmus MC University Medical Center, Sophia Children’s Hospital Rotterdam, the Netherlands. None of the acknowledged participants received any form of compensation for their contributions.
References
- 1.Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales: I, prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989;26(11):704–711. doi: 10.1136/jmg.26.11.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutmann DH, Wood DL, Collins FS. Identification of the neurofibromatosis type 1 gene product. Proc Natl Acad Sci U S A. 1991;88(21):9658–9662. doi: 10.1073/pnas.88.21.9658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldmann R, Denecke J, Grenzebach M, Schuierer G, Weglage J. Neurofibromatosis type 1: motor and cognitive function and T2-weighted MRI hyperintensities. Neurology. 2003;61(12):1725–1728. doi: 10.1212/01.wnl.0000098881.95854.5f. [DOI] [PubMed] [Google Scholar]
- 4.Krab LC, Aarsen FK, de Goede-Bolder A, et al. Impact of neurofibromatosis type 1 on school performance. J Child Neurol. 2008;23(9) doi: 10.1177/0883073808316366. [DOI] [PubMed] [Google Scholar]
- 5.Levine TM, Materek A, Abel J, O’Donnell M, Cutting LE. Cognitive profile of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13(1):8–20. doi: 10.1016/j.spen.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Billingsley RL, Jackson EF, Slopis JM, Swank PR, Mahankali S, Moore BD. Functional MRI of visual-spatial processing in neurofibromatosis, type I. Neuropsychologia. 2004;42(3):395–404. doi: 10.1016/j.neuropsychologia.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 7.North K, Hyman S, Barton B. Cognitive deficits in neurofibromatosis 1. J Child Neurol. 2002;17(8):605–612. doi: 10.1177/088307380201700811. [DOI] [PubMed] [Google Scholar]
- 8.North K, Joy P, Yuille D, et al. Specific learning disability in children with neurofibromatosis type 1: significance of MRI abnormalities. Neurology. 1994;44(5):878–883. doi: 10.1212/wnl.44.5.878. [DOI] [PubMed] [Google Scholar]
- 9.van Engelen SJ, Krab LC, Moll HA, et al. Quantitative differentiation between healthy and disordered brain matter in patients with neurofibromatosis type I using diffusion tensor imaging. AJNR Am J Neuroradiol. 2008;29(4):816–822. doi: 10.3174/ajnr.A0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Legius E, Descheemaeker MJ, Steyaert J, et al. Neurofibromatosis type 1 in childhood: correlation of MRI findings with intelligence. J Neurol Neurosurg Psychiatry. 1995;59(6):638–640. doi: 10.1136/jnnp.59.6.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa RM, Federov NB, Kogan JH, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415(6871):526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 12.Guilding C, McNair K, Stone TW, Morris BJ. Restored plasticity in a mouse model of neurofibromatosis type 1 via inhibition of hyperactive ERK and CREB. Eur J Neurosci. 2007;25(1):99–105. doi: 10.1111/j.1460-9568.2006.05238.x. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Cui Y, Kushner SA, et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr Biol. 2005;15(21):1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Graaf MR, Richel DJ, van Noorden CJ, Guchelaar HJ. Effects of statins and farnesyltransferase inhibitors on the development and progression of cancer. Cancer Treat Rev. 2004;30(7):609–641. doi: 10.1016/j.ctrv.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Cox AD, Hisaka MM, Graham SM, Buss JE, Der CJ. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci U S A. 1992;89(14):6403–6407. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Jongh S, Ose L, Szamosi T, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized, double-blind, placebo-controlled trial with simvastatin. Circulation. 2002;106(17):2231–2237. doi: 10.1161/01.cir.0000035247.42888.82. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health Consensus Development Conference Statement: neurofibromatosis: Bethesda, Md. USA. July 13–15, 1987. Neurofibromatosis. 1988;1(3):172–178. [PubMed] [Google Scholar]
- 18.Van Haasen PP, De Bruyn EEJ, Pijl YJ, et al. Wechsler Intelligence Scale for Children–Revised (Nederlandstalige Uitgave) Lisse, the Netherlands: Swets & Zeitlinger BV; 1986. [Google Scholar]
- 19.Van der Geest JN, Lagers-van Haselen GC, van Hagen JM, et al. Visual depth processing in Williams-Beuren syndrome. Exp Brain Res. 2005;166(2):200–209. doi: 10.1007/s00221-005-2355-1. [DOI] [PubMed] [Google Scholar]
- 20.Baizer JS, Kralj-Hans I, Glickstein M. Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol. 1999;81(4):1960–1965. doi: 10.1152/jn.1999.81.4.1960. [DOI] [PubMed] [Google Scholar]
- 21.Pisella L, Rossetti Y, Michel C, et al. Ipsidirectional impairment of prism adaptation after unilateral lesion of anterior cerebellum. Neurology. 2005;65(1):150–152. doi: 10.1212/01.wnl.0000167945.34177.5e. [DOI] [PubMed] [Google Scholar]
- 22.Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4. New York, NY: Oxford University Press; 2004. [Google Scholar]
- 23.Medicines Evaluation Board. Summary of Product Characteristics: ZCR-T-082004. The Hague, the Netherlands: Medicines Evaluation Board; 2005. [Google Scholar]
- 24.Descheemaeker MJ, Ghesquiere P, Symons H, Fryns JP, Legius E. Behavioural, academic and neuropsychological profile of normally gifted neurofibromatosis type 1 children. J Intellect Disabil Res. 2005;49(pt 1):33–46. doi: 10.1111/j.1365-2788.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 25.Rapport LJ, Brines DB, Axelrod BN, Theisen ME. Full scale IQ as mediator of practice effects: the rich get richer. Clin Neuropsychol. 1997;11(4):375–380. [Google Scholar]
- 26.Struiksma AJM, Van Der Leij A, Vieijra JPM. Diagnostics Tool of Technical Reading and Basic Spelling (Dutch version) Amsterdam, the Netherlands: VU Publisher; 2004. [Google Scholar]
- 27.Ruijssenaars AJJM, Van Lieshout ECDM, Van Luit JEH. Mathematical Problems and Dyscalculia (Dutch version) Rotterdam, the Netherlands: Lemniscaat Publisher; 2004. [Google Scholar]
- 28.Brown RT, Amler RW, Freeman WS, et al. Treatment of attention-deficit/hyperactivity disorder: overview of the evidence. Pediatrics. 2005;115(6):e749–e757. doi: 10.1542/peds.2004-2560. [DOI] [PubMed] [Google Scholar]