By introducing surface mutations, the crystal quality of a β-lactamase, Toho-1, was drastically improved. The resultant crystals showed no tendency towards merohedral twinning and diffracted to 0.97 Å resolution.
Keywords: extended-spectrum β-lactamases, crystal contacts, surface mutations, ultra-high resolution, merohedral twinning
Abstract
The β-lactamase Toho-1 exhibits a strong tendency to form merohedrally twinned crystals. Here, the crystal quality of Toho-1 was improved by using surface modification to remove a sulfate ion involved in crystal packing. The surface-modified Toho-1 variant (R274N/R276N) was crystallized under similar conditions to those used for wild-type Toho-1. R274N/R276N did not form merohedrally twinned crystals. The crystals diffracted to a significantly higher resolution (∼0.97 Å) than the wild-type crystals (1.65 Å); they belonged to the same space group and had almost identical unit-cell parameters to those of wild-type Toho-1.
1. Introduction
As a class A β-lactamase, Toho-1 is an extended-spectrum β-lactamase that efficiently hydrolyzes third-generation cephalosporins (Ishii et al., 1995 ▶). Toho-1 consists of 262 amino-acid residues and has a predicted molecular weight of 28.5 kDa. In class A β-lactamases, a strictly conserved residue Glu166 positions and activates the hydrolytic water for the deacylation at the bottom of the substrate-binding cavity (Matagne et al., 1998 ▶). Therefore, E166A mutants are often used to determine acyl-intermediate structures of class A β-lactamases (Strynadka et al., 1992 ▶; Chen & Herzberg, 2001 ▶; Shimamura et al., 2002 ▶). Structures of wild-type Toho-1 and the E166A Toho-1 variant have been determined at 1.65–2.1 Å resolution (Ibuka et al., 1999 ▶, 2003 ▶) and the two structures are nearly identical. However, despite similarities in crystallization conditions, space group and unit-cell parameters, the wild-type crystals exhibit a strong merohedrally twinning tendency that is not observed in E166A crystals. As a result, extensive screening is required to obtain untwinned crystals for structure determination (Ibuka et al., 1999 ▶). This characteristic of the wild-type protein presents a significant disadvantage in the elucidation of the detailed hydrolytic mechanisms of Toho-1 from the structures of complexes with substrates.
Surface mutagenesis is a technique that has successfully been used to improve crystal quality (D’Arcy et al., 1999 ▶; Neau et al., 2007 ▶; Liu et al., 2007 ▶; Yamada et al., 2007 ▶; Honjo et al., 2008 ▶; Mizutani et al., 2008 ▶). Surface-entropy reduction was developed to identify target residues for effective surface mutagenesis (Mateja et al., 2002 ▶; Derewenda, 2004 ▶; Cooper et al., 2007 ▶; Goldschmidt et al., 2007 ▶; Parthasarathy et al., 2008 ▶). In previous studies, most of the successful mutations have been located in crystal-packing regions. We compared the crystal contacts in wild-type Toho-1 and E166A crystals. In wild-type crystals, two surface arginine residues, Arg274 and Arg276, interact with a sulfate ion that resides close to Lys197 in the neighbouring molecule. This sulfate ion is not present in E166A crystals. Thus, we speculated that in wild-type crystals this sulfate ion causes a twinning effect and that the removal of the sulfate ion would improve the crystal quality.
In the current study, we replaced Arg274 and Arg276 of Toho-1 by asparagine. Crystals of the resultant Toho-1 variant, R274N/R276N, maintained basically the same unit-cell parameters and space group as those of the wild-type crystal. Moreover, they exhibited no tendency towards twinning and diffracted to ∼0.97 Å resolution.
2. Materials and methods
2.1. Mutagenesis
The amino-acid substitutions were introduced into Toho-1 (Ibuka et al., 1999 ▶) using the QuikChange site-directed mutagenesis kit (Stratagene). The sequences of the mutagenic primers were 5′-GA-GCAGAAGGCGGAGAATCGTAATGATATTCTGGCT-3′ and 5′-AGCCAGAATATCATTACGATTCTCCGCCTTCTGCTC-3′. The replaced bases are shown in bold.
2.2. Protein expression and purification
The Toho-1 R274N/R276N variant was expressed and purified using the methods previously described for the E166A variant (Ibuka et al., 1999 ▶). Briefly, Escherichia coli cells harbouring a Toho-1 expression plasmid were cultured in Mueller–Hinton broth at 303 K for 15 h. Expression of Toho-1 was induced by the addition of 0.1 mM IPTG. Cells were harvested by centrifugation. Cell pellets were resuspended in 20 mM MES pH 6.5, 1 mM EDTA with protease inhibitors (Roche) and then sonicated. The enzyme was purified by anion-exchange chromatography on a Toyopearl CM-650M column (Tosoh, Japan) in 20 mM MES–NaOH pH 6.5 and eluted with a linear 0–0.15 M NaCl gradient. The enzyme was more than 90% homogeneous as judged by SDS–PAGE and Coomassie Blue staining.
2.3. Crystallization
The buffer of the protein solution was exchanged to 20 mM MES–NaOH pH 6.5, 1 mM EDTA during concentration to yield a final protein concentration of approximately 10 mg ml−1. The protein concentration was determined by absorption measurements with a calculated molar absorption coefficient of 24 350 M −1 cm−1. R274N/R276N crystals were obtained using the sitting-drop vapour-diffusion technique, as previously described for wild-type Toho-1 and E166A crystals (Shimamura et al., 2002 ▶; Ibuka et al., 2003 ▶). 5 µl protein solution was mixed with an equal volume of reservoir solution [2.10 M ammonium sulfate (range of 1.95–2.10 M), 0.2 M sodium citrate buffer pH 5.5 (pH range 5.0–5.5)] and allowed to equilibrate against 500 µl reservoir solution at 298 K. Crystals appeared after a few days and stopped growing within two weeks.
2.4. X-ray data collection
Prior to data collection, crystals were soaked in a cryosolution containing 2.7 M ammonium sulfate, 30%(w/v) trehalose and 0.2 M sodium citrate pH 5.5 and then flash-cooled in liquid nitrogen. Diffraction data were collected on beamline BL26B1 of SPring-8 using a Rigaku R-AXIS V imaging-plate detector at 100 K. A total of 600 images were collected in a single step. The oscillation range, exposure time and crystal-to film-distance were 0.3°, 12 s per frame and 180 mm, respectively. All image data were processed and merged using the HKL-2000 program (Otwinowski & Minor, 1997 ▶). Refinement and model building were performed using REFMAC5 from the CCP4 program suite (Collaborative Computational Project, Number 4, 1994 ▶) and Coot (Emsley & Cowtan, 2004 ▶), respectively. All refinements were carried out with data in the 50–1.03 Å resolution range using all reflections.
3. Results and discussion
Toho-1 R274N/R276N crystals are shown in Fig. 1 ▶. The R274N/R276N crystals belonged to the same space group (P3221) and had almost identical unit-cell parameters as the wild-type Toho-1 and E166A crystals. The crystal quality of the R274N/R276N variant was drastically improved compared with that of the wild-type enzyme. We did not observe crystal twinning after examining more than 100 crystals (data not shown). Of note, the crystals diffracted to ∼0.97 Å resolution when a I/σ(I) = 2 criterion was applied. Because R merge for the last shell (0.97–0.99 Å) was very high (75%) at this resolution, we solved and refined the structure at 1.03 Å resolution, where R merge for the last shell (1.05–1.03 Å) was 35.6% (Table 1 ▶). R work/R free for the last shell is better than that for the overall data set. This is a consequence of the presence of a substantial number of overloaded low-resolution reflections because of the single-step data collection to ∼0.97 Å resolution. With one molecule in the asymmetric unit, the V M (Matthews, 1968 ▶) of the crystal was 2.62 Å3 Da−1 and the solvent content was 53.1%. Starting phases were calculated by molecular replacement with MOLREP in the CCP4 program suite using the structure of the cefotaxime–Toho-1 complex (PDB code 1iyo; Shimamura et al., 2002 ▶) as a search model. The structure refined well using all data to 1.03 Å resolution. The atomic coordinates and the structure factors were deposited in the Protein Data Bank (PDB code 2zq8). The overall structure of the R274N/R276N variant was essentially the same as the wild-type structure, with a root-mean-square deviation for Cα atoms of 0.192 Å. As expected, there was no sulfate ion corresponding to sulfate ion 1002 of the wild-type structure in the refined R274N/R276N structure (Fig. 2 ▶).
Figure 1.
Crystals of the R274N/R276N variant of Toho-1.
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the last shell.
| R274N/R276N | Wild type† | E166A‡ | |
|---|---|---|---|
| PDB code | 2zq8 | 1iys | 1bza |
| Data collection | |||
| X-ray source | SPring-8 BL26B1 | PF BL6A | PF BL6A |
| Temperature (K) | 100 | 100 | 288 |
| Space group | P3221 | P3221 | P3221 |
| Resolution (Å) | 50–0.97 (0.99–0.97) | 23.8–1.65 (1.74–1.65) | 40–1.8 |
| Unit-cell parameters (Å) | a = b = 72.511, c = 97.477 | a = b = 72.6, c = 97.9 | a = b = 73.3, c = 99.4 |
| Total observations | 1702009 | 631678 | 172393 |
| Unique observations | 174237 (8513) | 36271 (5246) | 29252 |
| Completeness (%) | 99.9 (98.2) | 100 (99.9) | Not shown |
| Rmerge§ (%) | 8.6 (75.3) | 7.2 (10.2) | 7.2 |
| Average I/σ(I) | 41.7 (2.1) | 7.3 (6.0) | Not shown |
| Multiplicity | 9.8 (7.5) | 17.4 | 5.9 |
| Refinement | |||
| Resolution (Å) | 50–1.03 (1.06–1.03) | 20–1.65 (1.73–1.65) | 6.0–1.8 |
| Rwork/Rfree¶ | 0.138/0.148 (0.125/0.137) | 0.182/0.197 (0.205/0.232) | 0.182/0.217 |
| R.m.s.d. bonds (Å) | 0.009 | 0.009 | 0.017 |
| R.m.s.d. angles (°) | 1.415 | 1.40 | 2.36 |
Values are taken from Ibuka et al. (2003 ▶).
Values are taken from Ibuka et al. (1999 ▶).
R
merge = 
 , where 〈I(hkl)〉 is the mean intensity of equivalent reflections.
, where 〈I(hkl)〉 is the mean intensity of equivalent reflections.
R
work = 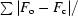
 , where F
o and F
c are the observed and calculated structure-factor amplitudes, respectively. R
free is as R
work but calculated using a test data set of 5% of the total data randomly selected from the observed reflections.
, where F
o and F
c are the observed and calculated structure-factor amplitudes, respectively. R
free is as R
work but calculated using a test data set of 5% of the total data randomly selected from the observed reflections.
Figure 2.
The crystal contact region of the R274N/R276N variant of Toho-1. The C atoms in the two adjacent molecules are shown in orange and yellow, respectively. The structure was overlaid with that of wild-type Toho-1 (magenta; PDB code 1iys) containing two sulfate ions (1002 and 1003). Sulfate ion 1002 interacts with Arg274 and Arg276 in the wild-type structure. Sulfate ion 1003 is also present in the E166A structure. The F o − F c map calculated from the refined model without Asn274, Asn276, SO4_2 and X452 (a water molecule with double conformation) is shown in green at the contour level 4.0σ.
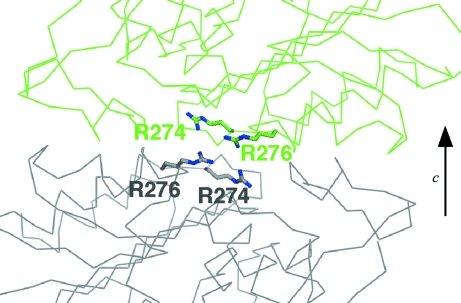
Merohedral twinning in our crystal of space group P3221 arises from irregular crystal packing along the c axis resulting from the presence of a twofold twin axis perpendicular to the crystallographic threefold axis. In the wild-type crystal, Arg274 and Arg276 are involved in crystal packing along the c axis (Fig. 3 ▶). At the interface, Arg274 and Arg276 exist in the vicinity of Lys82 and Lys197 on the neighbouring molecule and two sulfate ions bind between the two molecules (Figs. 2 ▶ and 3 ▶). There, Lys197 forms multiple conformations and one of them exists too close to sulfate ion 1002 (1.4 Å). As a result, the B factor of sulfate ion 1002 (13.19 Å2) is higher than that of sulfate ion 1003 (7.68 Å2). These fluctuations at the interface would destabilize the crystal packing along the c axis. This would explain the fact that the R274N/R276N crystal is thicker than the wild-type crystal. Additionally, we modelled the possible twinned interface by applying the twin operator (−h, −k, l) to the wild-type structure (Fig. 4 ▶). In the model, Arg274 and Arg276 of the each twin domain face each other at the interface. If sulfate ions bind to the interface to neutralize the positive charges of Arg274 and Arg276, this would stabilize the twinned interface. Apparently, our double mutant alters the relative stability of the two interfaces such that the wild-type interface becomes dominant, thereby eliminating the twinning. Considering these facts, the improvement of the crystal quality by surface mutation was attained by regulation of the crystal packing along the c axis.
Figure 3.
Crystal packing of Toho-1. The side chains of Lys82, Lys197, Arg274 and Arg276 are shown in red. Two sulfate ions, 1002 and 1003, are shown as CPK models.
Figure 4.
Possible twinned interface. A molecule in each twin domain is shown in grey and green, respectively.
Acknowledgments
We thank the staff at beamline BL26B1 of SPring-8, Japan.
References
- Chen, C. C. H. & Herzberg, O. (2001). Biochemistry, 40, 2351–2358. [DOI] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Cooper, D. R., Boczek, T., Grelewska, K., Pinkowska, M., Sikorska, M., Zawadzki, M. & Derewenda, Z. (2007). Acta Cryst. D63, 636–645. [DOI] [PubMed]
- D’Arcy, A., Stihle, M., Kostrewa, D. & Dale, G. (1999). Acta Cryst. D55, 1623–1625. [DOI] [PubMed]
- Derewenda, Z. S. (2004). Structure, 12, 529–535. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Goldschmidt, L., Cooper, D. R., Derewenda, Z. S. & Eisenberg, D. (2007). Protein Sci.16, 1569–1576. [DOI] [PMC free article] [PubMed]
- Honjo, E., Tamada, T., Adachi, M., Kuroki, R., Meher, A. & Blaber, M. (2008). J. Synchrotron Rad.15, 285–287. [DOI] [PMC free article] [PubMed]
- Ibuka, A. S., Ishii, Y., Galleni, M., Ishiguro, M., Yamaguchi, K., Frere, J. M., Matsuzawa, H. & Sakai, H. (2003). Biochemistry, 42, 10634–10643. [DOI] [PubMed]
- Ibuka, A., Taguchi, A., Ishiguro, M., Fushinobu, S., Ishii, Y., Kamitori, S., Okuyama, K., Yamaguchi, K., Konno, M. & Matsuzawa, H. (1999). J. Mol. Biol.285, 2079–2087. [DOI] [PubMed]
- Ishii, Y., Ohno, A., Taguchi, H., Imajo, S., Ishiguro, M. & Matsuzawa, H. (1995). Antimicrob. Agents Chemother.39, 2269–2275. [DOI] [PMC free article] [PubMed]
- Liu, B., Luna, V. M., Chen, Y., Stout, C. D. & Fee, J. A. (2007). Acta Cryst. F63, 1029–1034. [DOI] [PMC free article] [PubMed]
- Mateja, A., Devedjiev, Y., Krowarsch, D., Longenecker, K., Dauter, Z., Otlewski, J. & Derewenda, Z. S. (2002). Acta Cryst. D58, 1983–1991. [DOI] [PubMed]
- Matagne, A., Lamotte-Brasseur, J. & Frère, J. M. (1998). Biochem. J.330, 581–598. [DOI] [PMC free article] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Mizutani, H., Saraboji, K., Malathy Sony, S. M., Ponnuswamy, M. N., Kumarevel, T., Krishna Swamy, B. S., Simanshu, D. K., Murthy, M. R. N. & Kunishima, N. (2008). Acta Cryst. D64, 1020–1033. [DOI] [PubMed]
- Neau, D. B., Gilbert, N. C., Bartlett, S. G., Dassey, A. & Newcomer, M. E. (2007). Acta Cryst. F63, 972–975. [DOI] [PMC free article] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Parthasarathy, G., Cummings, R., Becker, J. W. & Soisson, S. M. (2008). Acta Cryst. D64, 141–148. [DOI] [PubMed]
- Shimamura, T., Ibuka, A., Fushinobu, S., Wakagi, T., Ishiguro, M., Ishii, Y. & Matsuzawa, H. (2002). J. Biol. Chem.277, 46601–46608. [DOI] [PubMed]
- Strynadka, N. C. J., Adachi, H., Jensen, S. E., Johns, K., Sielecki, A., Betzel, C., Sutoh, K. & James, M. N. G. (1992). Nature (London), 359, 700–705. [DOI] [PubMed]
- Yamada, H. et al. (2007). Protein Sci.16, 1389–1397. [DOI] [PMC free article] [PubMed]