Abstract
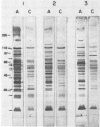
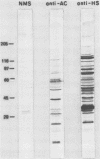
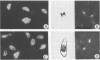
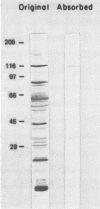
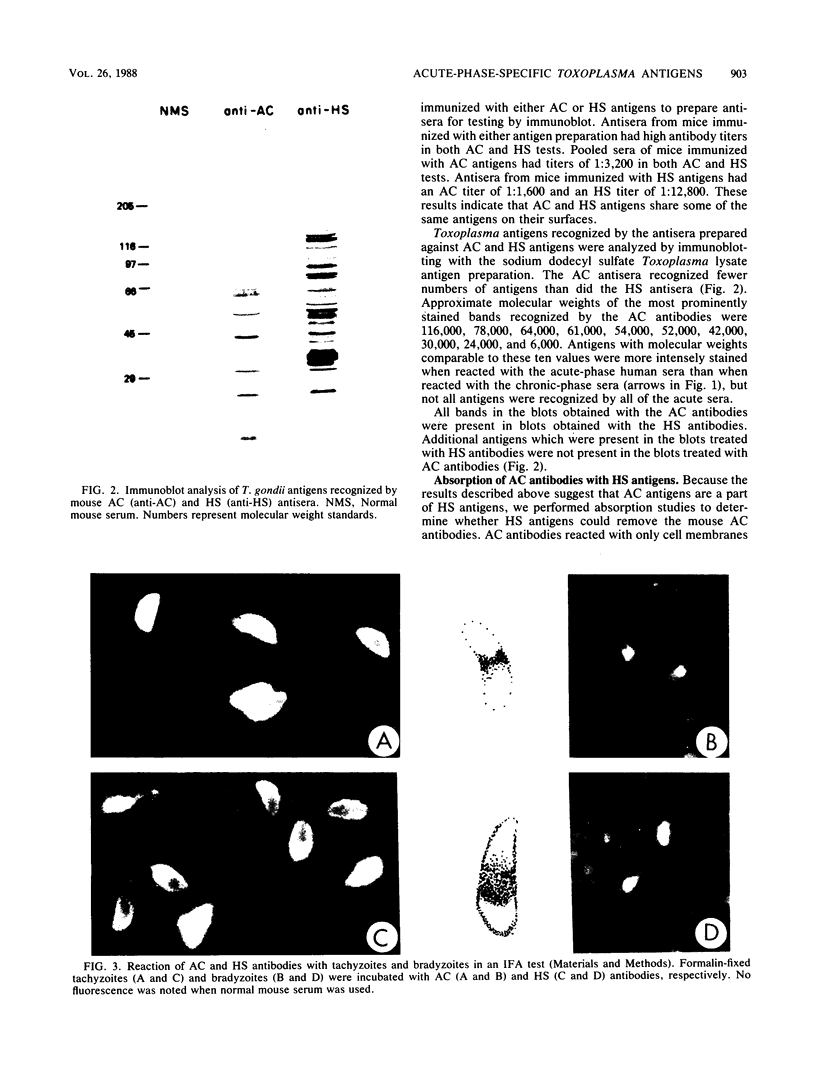
An agglutination test for immunoglobulin G (IgG) Toxoplasma antibodies with acetone-fixed tachyzoites (AC antigens; AC agglutination test) was positive only with sera from patients during the acute stage of their infection. In contrast, when the test was performed with Formalin-fixed tachyzoites (HS antigens; HS agglutination test), positive results were obtained during both the acute and chronic (latent) stages of the infection. Studies were performed to define the antigen(s) of T. gondii which are detected by IgG antibodies present only during the acute stage of the infection. Sera of mice immunized with AC antigens recognized predominantly 10 antigens of tachyzoites by immunoblot analysis. Sera from individuals with the acute but not chronic infection reacted strongly with these same 10 antigens in immunoblots. Sera of mice immunized with HS antigens recognized more Toxoplasma antigens on immunoblots than did mouse AC antibodies. Absorption of the latter with HS antigens removed detectable reactivity of AC antibodies in immunoblots and in the AC and HS agglutination tests, suggesting that AC antigens are a selected portion of HS antigens. AC antigens were specific for tachyzoites in that AC antibodies reacted with the cell membranes of both forms of the organism in an indirect fluorescent-antibody test. Tachyzoite-specific antigen(s) appear to be useful in differentiating between the acute and chronic stages of Toxoplasma infection through their detection by IgG antibodies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornelissen A. W., Overdulve J. P., Hoenderboom J. M. Separation of Isospora (Toxoplasma) gondii cysts and cystozoites from mouse brain tissue by continuous density-gradient centrifugation. Parasitology. 1981 Aug;83(Pt 1):103–108. doi: 10.1017/s0031182000050071. [DOI] [PubMed] [Google Scholar]
- Desmonts G., Remington J. S. Direct agglutination test for diagnosis of Toxoplasma infection: method for increasing sensitivity and specificity. J Clin Microbiol. 1980 Jun;11(6):562–568. doi: 10.1128/jcm.11.6.562-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich H. A., Rodgers G., Vaillancourt P., Araujo F. G., Remington J. S. Identification of an antigen-specific immunoglobulin M antibody associated with acute Toxoplasma infection. Infect Immun. 1983 Aug;41(2):683–690. doi: 10.1128/iai.41.2.683-690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS L., REMINGTON J. S., MELTON M. L. The resistance of the encysted form of Toxoplasma gondii. J Parasitol. 1960 Feb;46:11–21. [PubMed] [Google Scholar]
- Kasper L. H., Currie K. M., Bradley M. S. An unexpected response to vaccination with a purified major membrane tachyzoite antigen (P30) of Toxoplasma gondii. J Immunol. 1985 May;134(5):3426–3431. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lunde M. N., Jacobs L. Antigenic differences between endozoites and cystozoites of Toxoplasma gondii. J Parasitol. 1983 Oct;69(5):806–808. [PubMed] [Google Scholar]
- Naot Y., Remington J. S. An enzyme-linked immunosorbent assay for detection of IgM antibodies to Toxoplasma gondii: use for diagnosis of acute acquired toxoplasmosis. J Infect Dis. 1980 Nov;142(5):757–766. doi: 10.1093/infdis/142.5.757. [DOI] [PubMed] [Google Scholar]
- Potasman I., Araujo F. G., Desmonts G., Remington J. S. Analysis of Toxoplasma gondii antigens recognized by human sera obtained before and after acute infection. J Infect Dis. 1986 Oct;154(4):650–657. doi: 10.1093/infdis/154.4.650. [DOI] [PubMed] [Google Scholar]
- Sabin A. B., Feldman H. A. Dyes as Microchemical Indicators of a New Immunity Phenomenon Affecting a Protozoon Parasite (Toxoplasma). Science. 1948 Dec 10;108(2815):660–663. doi: 10.1126/science.108.2815.660. [DOI] [PubMed] [Google Scholar]
- Sharma S. D., Mullenax J., Araujo F. G., Erlich H. A., Remington J. S. Western Blot analysis of the antigens of Toxoplasma gondii recognized by human IgM and IgG antibodies. J Immunol. 1983 Aug;131(2):977–983. [PubMed] [Google Scholar]
- Siegel J. P., Remington J. S. Comparison of methods for quantitating antigen-specific immunoglobulin M antibody with a reverse enzyme-linked immunosorbent assay. J Clin Microbiol. 1983 Jul;18(1):63–70. doi: 10.1128/jcm.18.1.63-70.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulliez P., Remington J. S., Santoro F., Ovlaque G., Sharma S., Desmonts G. Une nouvelle réaction d'agglutination pour le diagnostic du stade évolutif de la toxoplasmose acquise. Pathol Biol (Paris) 1986 Mar;34(3):173–177. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton B. C., Benchoff B. M., Brooks W. H. Comparison of the indirect fluorescent antibody test and methylene blue dye test for detection of antibodies to Toxoplasma gondii. Am J Trop Med Hyg. 1966 Mar;15(2):149–152. doi: 10.4269/ajtmh.1966.15.149. [DOI] [PubMed] [Google Scholar]
- Welch P. C., Masur H., Jones T. C., Remington J. S. Serologic diagnosis of acute lymphadenopathic toxoplasmosis. J Infect Dis. 1980 Aug;142(2):256–264. doi: 10.1093/infdis/142.2.256. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]