Abstract
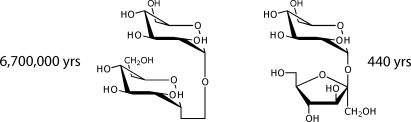
The half-lives for spontaneous hydrolysis of trehalose and sucrose at 25 °C are 6.6 × 106 years and 440 years. The half-lives for decomposition of the hydrolysis products glucose and fructose are 96 years and 70 days, respectively. Whereas sucrose and trehalose differ by a factor of 15000 in their rates of uncatalyzed hydrolysis, the reactions catalyzed by invertase (EC 3.2.1.26) and trehalase (EC 3.2.1.28) proceed at similar rates. Accordingly, the attainments of invertase as a catalyst are modest, but the rate enhancement and catalytic proficiency produced by trehalase approach the high levels achieved by polysaccharide hydrolases.
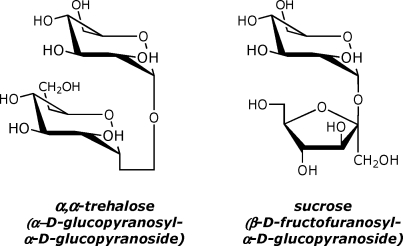
Most of the early experimental work on enzyme kinetics was based on the properties of yeast invertase (β-fructo-furanosidase, EC 3.2.1.26), whose robust activity(1) (together with the availability of a continuous polarimetric assay) enabled Michaelis and Menten to establish the existence of a quantitative relationship between the rate of an enzyme reaction and the concentration of its substrate.(2) To appreciate the proficiency of an enzyme as a catalyst, it is also desirable to have information about the relative rates of the catalyzed and uncatalyzed reactions.(3) Surprisingly, the literature discloses no report of the rate of spontaneous hydrolysis of sucrose (although there have been many studies of the acid-catalyzed reaction).(4) That information would be of special interest in view of the remarkable resistance to hydrolysis (t1/2 ∼107 y),(5) of the 1−4 “head-to-tail” glycosidic linkages that join the common glucose polymers cellulose, chitin, amylose, and glycogen. Thus, polysaccharide hydrolases (e.g., β-amylase) generate the largest rate enhancements that are known to be produced by hydrolytic enzymes that operate without the assistance of metals or other cofactors.(6) Here, we describe the uncatalyzed hydrolysis of sucrose and trehalose, in which the constituent monosaccharides are symmetrically joined, by a “head-to-head” glycosidic linkage between their carbonyl groups (Chart 1).
Chart 1. Nonreducing Disaccharides.
Samples of sucrose or trehalose (0.05 M) were incubated in potassium phosphate and carbonate buffers (0.1 M) in Teflon-lined acid digestion bombs (Parr Instrument Co. #276AC) at elevated temperatures in Barnstead-Thermolyne Corp. #47900 ovens, monitoring the temperature of the sample with an ASTM mercury thermometer. In experiments conducted at pH values below 9, samples were sealed under argon in quartz tubes (3 mm ID, 4 mm OD). At timed intervals, samples were cooled and diluted 50-fold with D2O to which pyrazine had been added as an internal integration standard. Proton NMR spectra were obtained using a Varian 500 MHz NMR spectrometer equipped with a cold probe. The decomposition of sucrose, trehalose, and glucose was monitored by following the disappearance of the anomeric proton resonances at ∼4.2 ppm, while the decomposition of fructose was followed by monitoring the disappearance of a multiplet(7) at 3.5 ppm. In potassium phosphate (0.1 M, pH 8.1) or potassium carbonate buffer (0.1 M, pH 10.3), the decomposition of each of these molecules followed simple first order kinetics to at least 95% completion under all conditions examined.
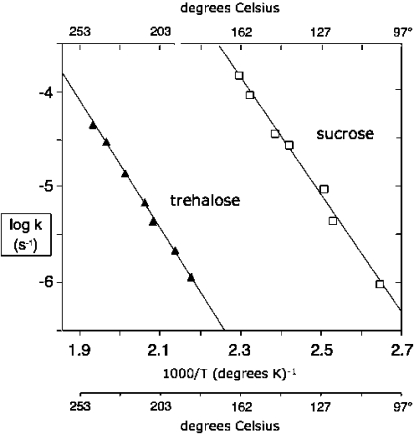
For sucrose, trehalose, glucose, and fructose, the rates of decomposition observed at various temperatures yielded linear Arrhenius plots (Figure 1) with a least-squares regression coefficient of r(2) ≥ 0.97 and n ≥ 7. But decomposition of the reducing disaccharides lactose, cellobiose, and maltose yielded Arrhenius plots that were concave upward, consistent with the availability of alternate routes for decomposition, including aldol cleavage and oxidation (Supporting Information).
Figure 1.
Arrhenius plots for hydrolysis of sucrose and trehalose (0.05 M) in 0.1 M potassium phosphate buffer, pH 8.1.
Rate constants and activation parameters observed for the hydrolysis of trehalose and sucrose, in potassium phosphate buffer (0.1 M, pH 8.1) are shown in Table 1. As in the case of 1-methylglycosides,(5) acid catalysis was observed at low pH, but rates of hydrolysis did not vary significantly in the pH range between 7 and 12. Rates of hydrolysis of sucrose and trehalose did not change with changing ionic strength in the range from 0.2 to 2 M KCl, and did not vary significantly with phosphate buffer concentrations varying between 0.065 and 1.2 M. The extrapolated rate constant for the uncatalyzed hydrolysis of trehalose at 25 °C corresponds to a half-life of 6.6 × 106 years, not very different from the rate of hydrolysis reported earlier for α-methylglucopyranoside (Table 1).(5) But the hydrolysis of sucrose was found to proceed much more rapidly, with t1/2 = 440 years at 25 °C (Table 1).
Table 1. Rate Constants and Activation Parameters for the Uncatalyzed Decomposition of Mono- And Disaccharides.
Decomposition of the product monosaccharides (fructose and glucose) was found to occur much more rapidly than disaccharide hydrolysis, accounting for their failure to appear in more than fleeting amounts during disaccharide hydrolysis. Experiments conducted on glucose (0.05 M) under an argon atmosphere over the temperature range between 70 and 130 °C showed that glucose decomposes with ΔH‡ = 28.0 kcal/mol and ΔS‡ = −0.4 cal deg−1 mol−1. The extrapolated rate constant for glucose decomposition in neutral solution at 25 °C was 2.3 × 10−10 s−1 (t1/2 = 96 years), with a corresponding t1/2 of 70 days for fructose (Supporting Information).
Trehalose cleavage occurs at the C-1 atom of one of its symmetrically situated glucopyranosyl moieties, at a rate that is the same, within a factor of 2, as the rate of cleavage of α-methylglucopyranoside (Table 1). Thus, the effect of the leaving group (methoxide vs glucopyranoside) is slight. The 15000-fold more rapid hydrolysis of sucrose suggests that sucrose cleavage occurs at another site, presumably at the fructofuranosyl moiety. The acid-catalyzed hydrolysis of β-methylfructopyranoside, which involves cleavage of the fructosyl-oxygen bond(8) is known to proceed ∼104-fold more rapidly than that of α-methylglucopyranoside.(9) These differences in reactivity are probably related to the greater stability of a tertiary oxocarbenium ion generated at C-2 of fructose than that of the secondary ion generated at C-1 of glucose; and their similar magnitudes suggest that carbocationic character may have developed to a similar extent in the transition states for the spontaneous and the acid-catalyzed reactions.
Whereas there is a 15000-fold difference between sucrose and trehalose in their rates of uncatalyzed hydrolysis, the hydrolytic reactions catalyzed by invertase (EC 3.2.1.26) and trehalase (EC 3.2.1.28) exhibit similar values of kcat and Km (Table 2). Thus, the rate enhancement produced by trehalase is much greater. Unlike invertase, trehalase produces inversion of configuration at the site of attack.(10) Accordingly the trehalase-catalyzed reaction probably resembles the uncatalyzed reaction in that both involve direct water attack on the substrate. The transition state dissociation constant of trehalase can then be estimated in the usual manner (knon/(kcat/Km)), as 8 × 10−21 M−1, not very different from the value (10−22 M) for β-amylase, another “inverting” enzyme.(5) But in the case of invertase, the catalyzed and uncatalyzed reactions proceed by different mechanisms, rendering estimation of transition state affinity in the usual sense problematic.11,12
Table 2. Kinetic Parameters for Invertase and Trehalase, pH 8.1.
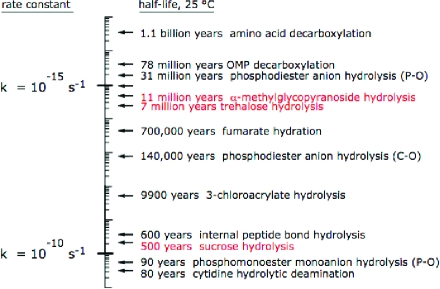
Of the unusually slow reactions that are catalyzed by enzymes, only the hydrolyses of phosphoric acid monoester dianions(13) and diester monoanions(14) appear to be more difficult than glycoside cleavage (Figure 2). Phosphate ester hydrolyses are catalyzed by enzymes containing metal ions that are capable, by themselves, of acting as strong catalysts. But glycoside hydrolases act as purely protein catalysts, and these enzymes appear to be matched only by OMP decarboxylase in their ability to catalyze very difficult reactions without the assistance of metals or other cofactors.(3)
Figure 2.
Rate constants for uncatalyzed biological reactions in water (for references, see ref (6)).
In summary, the intrinsic stabilities of sucrose, trehalose, glucose, and fructose in water have been measured for the first time. Because sucrose is so much more readily hydrolyzed than trehalose, the attainments of invertase as a catalyst are relatively modest. But the rate enhancement and catalytic proficiency produced by trehalase approach the very high levels achieved by the polysaccharide hydrolases.
Acknowledgments
This work was supported by NIH Grant No. GM-18325.
Supporting Information Available
Arrhenius plot of rate constants for decomposition of fructose and glucose; rate constants observed for the decomposition of lactose, celloboise, and maltose. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- O’Sullivan C.; Thompson F. W. J. Chem. Soc. 1890, 834–931. [Google Scholar]
- Michaelis L.; Menten M. L. Biochem. Z. 1913, 49, 333–355. [Google Scholar]
- Radzicka R; Wolfenden R. Science 1995, 267, 90–93. [DOI] [PubMed] [Google Scholar]
- Capon B. Chem. Rev. 1969, 69, 407–499. [Google Scholar]
- Wolfenden R.; Lu X.; Young G. J. Am. Chem. Soc. 1998, 120, 6814–6815. [Google Scholar]
- Wolfenden R. Chem. Rev. 2006, 106, 3379–3396. [DOI] [PubMed] [Google Scholar]
- Blanchard J. S.; Brewer C. F.; Englard S.; Avigad G. Biochemistry 1982, 21, 75–81. [DOI] [PubMed] [Google Scholar]
- Mega T. L.; Van Etten R. L. J. Am. Chem. Soc. 1988, 110, 6372–6376. [Google Scholar]
- Heidt L. J.; Purves C. B. J. Am. Chem. Soc. 1944, 66, 1385–1343. [Google Scholar]
- Sinnott M. L. In Enzyme Mechanisms; Page M. I., Williams A., Eds.; Royal Society of Chemistry: London, 1987; pp 259−297. [Google Scholar]
- Wolfenden R. Acc. Chem. Res. 1972, 5, 10–18. [Google Scholar]
- Lienhard G. E. Science 1973, 180, 149–156. [DOI] [PubMed] [Google Scholar]
- Lad D.; Williams N. H.; Wolfenden R. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 5607–5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder G. K.; Lad C.; Wyman P.; Williams N. H.; Wolfenden R. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 4052–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty J. W.; Sinnott M. L. Can. J. Chem. 2005, 83, 1516–1524. [Google Scholar]
- Neumann N. P.; Lampen J. O. Biochemistry 1967, 6, 468–475. [DOI] [PubMed] [Google Scholar]
- Lee J. H.; Tsuji M.; Nakamura M.; Nishimoto M.; Okuyama M.; Mori H.; Kimura A.; Mitsui H.; Chiba S. Biosci. Biotechnol. Biochem. 2001, 65, 2657–2665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.