Abstract
Very low-density lipoproteins (VLDL) are triglyceride-rich particles. VLDL is synthesized in hepatocytes and secreted from the liver in a pathway that is tightly regulated by insulin. Hepatic VLDL production is stimulated in response to reduced insulin action, resulting in increased release of VLDL into the blood under fasting conditions. Circulating VLDL serves as a vehicle for transporting lipids to peripheral tissues for energy homeostasis. Conversely, hepatic VLDL production is suppressed in response to increased insulin release after meals. This effect is critical for preventing prolonged excursion of postprandial plasma lipid profiles in normal individuals. In subjects with obesity and type 2 diabetes, the ability of insulin to regulate VLDL production becomes impaired due to insulin resistance in the liver, resulting in excessive VLDL secretion and accumulation of triglyceride-rich particles in the blood. Such abnormality in lipid metabolism characterizes the pathogenesis of hypertriglyceridemia and accounts for increased risk of coronary artery disease in obesity and type 2 diabetes. Nevertheless, the molecular basis that links insulin resistance to VLDL overproduction remains poorly understood. Our recent studies illustrate that the forkhead transcription factor FoxO1 acts in the liver to integrate hepatic insulin action to VLDL production. Augmented FoxO1 activity in insulin resistant livers promotes hepatic VLDL overproduction and predisposes to the development of hypertriglyceridemia. These new findings raise an important question: Is FoxO1 a therapeutic target for ameliorating hypertriglyceridemia? Here we discuss this question in the context of recent advances toward our understanding of the pathophysiology of hypertriglyceridemia.
Keywords: hypertriglyceridemia, FoxO1, MTP, ApoB, VLDL
Etiology of Hypertriglyceridemia
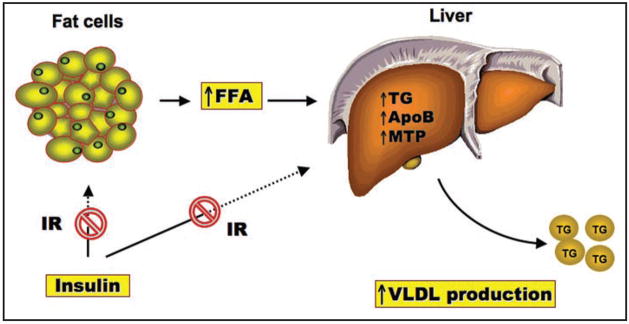
Hypertriglyceridemia is a hallmark of metabolic syndrome and is characterized by a triad plasma lipid profile, i.e., increased triglyceride (TG) and low-density lipoprotein (LDL) levels, and decreased high-density lipoprotein (HDL) levels.1,2 Due to its pro-atherogenic potential, hypertriglyceridemia is considered an independent risk factor for coronary artery disease.3–12 Hypertriglyceridemia increases the incidence of cardiovascular disease by 32% in men and 76% in women, independent of plasma HDL-C levels.13–15 To date, the pathophysiology of hypertriglyceridemia is incompletely understood. Its close association with adiposity and type 2 diabetes implicates insulin resistance as a causative factor in the development of hypertriglyceridemia.10,16–18 As a result of insulin resistance, adipose tissue undergoes unrestrained fat mobilization, resulting in elevated plasma free fatty acid (FFA) levels. An increased FFA flux into the liver stimulates hepatic lipogenesis and promotes VLDL-TG overproduction, contributing to the pathogenesis of hypertriglyceridemia (Fig. 1).16,19,20
Figure 1.
Hepatic VLDL overproduction. Unrestrained fat mobilization, resulting from peripheral insulin resistance (IR), leads to increased FFA flux into the liver. This effect stimulates hepatic lipogenesis and promotes VLDL overproduction, contributing to hypertriglyceridemia in insulin resistant subjects with obesity and type 2 diabetes.
Treatment of Hypertriglyceridemia
Hypertriglyceridemia is often associated with visceral obesity, insulin resistance, hyperinsulinemia, hypertension or diabetes, a cluster of conditions that are collectively termed “metabolic syndrome”. As a result, patients with hypertriglyceridemia are primarily recommended with exercise or dietary supplements of omega-3 fatty acid-enriched food or congestion of fish oil. If these non-pharmacological interventions fail to correct atherogenic lipid profiles, pharmacological therapies are instituted with fibrates or niacin. These two commonly prescribed triglyceride-lowering agents act to reduce plasma triglyceride levels via distinct mechanisms, but each with its own limitations. Fibrates are fibric acid derivatives that act as agonists of peroxisome proliferator-activated receptor alpha (PPAR-α) to enhance fatty acid oxidation in peripheral tissues and promote triglyceride VLDL-TG clearance from plasma.21–23 Niacin appears to target G protein-coupled receptors GPR109A and GPR109B (also known as HM74A and HM74, respectively) in adipose tissues to limit fat mobilization and reduce plasma levels of FFA, the substrate for hepatic VLDL production.24–27 More detailed discussions on pharmacological intervention of hypertriglyceridemia have been reported elsewhere.28–31 Here we focus our review on the molecular basis that governs insulin-dependent regulation of VLDL production, while bearing in mind the question of how hepatic VLDL production becomes unrestrained in insulin resistant subjects with metabolic syndrome.
Insulin Regulation of VLDL Production
VLDL is assembled and produced in liver, which depends on substrate availability and is tightly regulated by insulin.32,33 Under fasting conditions, hepatic VLDL production is induced, resulting in increased VLDL secretion into the blood. In response to postprandial insulin release, hepatic VLDL production is suppressed to limit plasma triglyceride excursion.34–37 Such an acute inhibitory mechanism of insulin action on VLDL production is critical for rapid adaptation by the liver to metabolic shift between fasting and refeeding for maintaining plasma lipids within the physiological range.
VLDL assembly in hepatocytes is conducted by microsomal triglyceride transfer protein (MTP), an endoplasmic reticulum resident protein. MTP (MW, 88 kDa) is regarded as a molecular chaperone. When heterodimerized with its small subunit protein disulphide isomerase (PDI, 58 kDa) in the endoplasmic reticulum (ER), MTP catalyzes the transfer of lipid to nascent apolipoprotein B (apoB), a rate-limiting step in hepatic VLDL production.38–40 MTP is also produced in the intestine and is responsible for lipidation of apoB48 for the production of chylomicrons.40–45 In humans, a lack of MTP activity, resulting from genetic lesions in its gene, causes abetalipoproteinemia or Bassen-Kornzweig syndrome, a rare autosomal recessive disorder that is characterized by defects in the assembly and secretion of triglyceride-rich lipoproteins. Patients with abetalipoproteinemia manifest severe lipid disorders and multiple vitamin deficiencies, due to the impairment in dietary fat absorption secondary to defects in intestinal chylomicron secretion.46–50 This clinical condition is recapitulated in mice with genetic MTP depletion, as MTP−/− homozygous mice are associated with the inability to manufacture VLDL and die at the E10.5 stage during embryonic development.50–52 MTP haploinsufficiency is associated with increased hepatic fat deposition due to markedly reduced VLDL secretion in MTP+/− heterozygous mice.49 In contrast, hepatic MTP overproduction results in excessive VLDL-TG secretion and significantly elevated plasma TG levels.38 Pharmacological inhibition of MTP activity is shown to reduce VLDL production and decrease plasma cholesterol levels in subjects with familial hypercholesterolemia.53 DNA polymorphism at -493G/T in the human MTP promoter is linked to altered triglyceride metabolism and increased risk of coronary heart disease.54–58 Furthermore, there is emerging evidence that diminished MTP activity is a compounding factor for advanced alcoholic liver disease.59,60 Together these data highlight the critical role of MTP in VLDL assembly and secretion in health and disease. This has spurred intensive investigations to understand hepatic regulation of MTP gene expression in order to gain insights into the pathophysiology of hypertriglyceridemia associated with obesity and diabetes.
Hepatic Regulation of MTP Production
Consistent with its importance in triglyceride metabolism, hepatic MTP production is regulated in response to physiological cues. Hagan et al.,61 first report that MTP gene expression is negatively regulated by insulin in cultured HepG2 cells. This observation is corroborated by Lin et al.,62 who show in HepG2 cells that insulin inhibits MTP expression in a dose- and time-dependent manner. In parallel with these findings, hepatic MTP mRNA levels are significantly upregulated, correlating with augmented VLDL-TG secretion in a number of animal models with insulin resistance and aberrant triglyceride metabolism, including non-diabetic obese Zucker rats,63,64 high fat-induced obese mice,65,66 and high fructose-induced hypertriglyceridemic hamsters.67–70 Pharmacological intervention, which improves insulin resistance and ameliorates metabolic dyslipidemia, is associated with reduced MTP expression and diminished VLDL-TG output in hypertriglyceridemic models.67,69,71,72 These data are consistent with the idea that insulin exerts an inhibitory effect on MTP gene expression in the liver. Loss of insulin inhibition is thought to be a contributor for unrestrained MTP expression and VLDL overproduction in insulin resistant subjects.
To account for the underlying mechanism, Au et al.,73 show in HepG2 cells that insulin regulates MTP gene expression via the activation of mitogen-activated protein kinase (MAPK). However, a caveat of this study is the use of a relatively shorter version of the human MTP promoter (−250/+86 nt) in their luciferase reporter system. Furthermore, targeted blockage of MAPK activity only results in partial inhibition of insulin-mediated reduction in VLDL-apoB production,74 suggesting that other mechanisms are involved in insulin-dependent inhibition of MTP and VLDL-TG production in the liver.
Wolfrum and Stoffel75 show that the forkhead box A2 (Foxa2) contributes to hepatic regulation of MTP expression. Foxa2 in complex with its co-activator PGC-1β stimulates hepatic MTP mRNA expression, contributing to increased VLDL secretion from the liver.76 In response to insulin action, Foxa2 is phosphorylated and dissociated from PGC-1β, contributing to the reduction in hepatic MTP and VLDL production.76 However, Foxa2 is predominantly localized in the cytoplasm in response to hyperinsulinemia,76 arguing against its direct action in promoting MTP gene expression in insulin resistant states. This has led to the postulation that the absence of Foxa2 in the nucleus in the liver is compensated by other factors such as hepatocyte nuclear factor 4 (HNF4) and peroxisome proliferator-activated receptor alpha (PPAR-α) under hyperinsulinemic conditions.77 Indeed, a recent study by Sheena et al.,72 demonstrates that hepatocyte nuclear factor 4-alpha (HNF-4α) targets the human MTP promoter for trans-activation in cultured HepG2 cells. This effect is complemented by hepatocyte nuclear factor 1-alpha (HNF-1α) and counteracted by β,β-tetramethyl-hexadecanedioic acid acting as an HNF-4α antagonist. Nevertheless, the significance of this finding in the pathogenesis of hypertriglyceridemia remains to be determined.
Peroxisome proliferator-activated receptor alpha (PPAR-α) is shown to stimulate hepatic MTP mRNA expression in primary cultures of mouse and rat hepatocytes.78 However, this finding seems paradoxical, as targeted activation of PPAR-α with anti-hypertriglyceridemia therapy such as fibrates helps attenuate hepatic MTP activity and curb VLDL-TG overproduction in animal models with diet-induced dyslipidemia.67,69,71,79 PPAR-α is known to bind specifically to a highly conserved DNA sequence that comprises 2-hexamer repeats with one nucleotide in between (AGGTCAXAGGTCA) in target promoters. Such a consensus PPAR-α binding site is lacking within a 5-kb DNA region (−5000/+1 nt) of human and mouse MTP promoters. Further studies are needed to reconcile the stimulatory action of PPAR-α on hepatic MTP production with the ameliorating effect of PPAR-α agonists on hypertriglyceridemia.
Although MTP plays an obligatory role in the lipidation of apoB for VLDL assembly and secretion, there is evidence that hepatic VLDL production is upregulated without significantly altering hepatic MTP mRNA levels in genetically modified mice that over-express human apoB but lack brown adipose tissue (Batless).80 In keeping with this observation, two independent groups show that the late addition of core lipids to apoB molecules, a critical step for VLDL maturation in the lumen of ER, is independent of MTP, as selective inhibition of MTP activity does not seem to affect apoB100 secretion during the later stages of lipoprotein assembly.81,82 While MTP is required for transferring lipids to nascent apoB polypeptides in coupling with translation during the initial phase of VLDL assembly, these data argue against the requirement of MTP in the late stage of VLDL assembly, in which bulk core lipids are incorporated into poorly lapidated apoB for the production of mature VLDL particles.81,83 Thus, it remains an unsettled question of whether MTP is absolutely necessary for the late stage of VLDL assembly. Studies are needed to further delineate the VLDL secretion pathway for better understanding of the molecular basis of hypertriglyceridemia.
Hepatic Regulation of apoB Expression
ApoB is a structural component of triglyceride-rich lipoproteins. There are two forms of apoB, namely apoB100 and apoB48 that are differentially expressed in humans.84 ApoB100 is expressed in the liver and is responsible for VLDL-TG production in the post-absorptive phase, whereas apoB48 is produced in the intestine and required for postprandial chylomicron secretion. In mice and rats, apoB48 is also produced in the liver.85 It is noteworthy that apoB48 is translated from its distinct mRNA that is derived from apoB100 mRNA editing, a posttranscriptional process by which a C is converted to a U at nucleotide 6666. This single nucleotide change results in the conversion of CAA (Gln-2153) to a stop codon UAA. As a result, apoB48 comprises the N-terminal 48% of apoB100.86,87
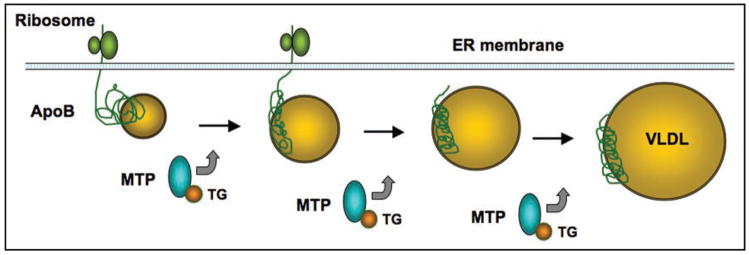
Unlike MTP that is an ER-resident chaperone, apoB is a secretory glycoprotein whose lipidation by MTP is essential for VLDL assembly and secretion. As depicted in Figure 2, this dynamic process of VLDL assembly depends on substrate availability, which is counteracted by insulin.33,88–91 Consistent with this model is the evidence that the synthesis of apoB is closely coordinated with MTP protein activity.92,93 In the presence of lipids, nascent apoB undergoes rapid lipidation that is facilitated by MTP. This process is kinetically coupled with translation and translocation of apoB polypeptides into the ER lumen for VLDL assembly and secretion. In the absence of lipids, nascent apoB molecules are unable to engage in lipidation and are destined for proteasome-mediated degradation.83,94 This fine-tuning mechanism serves as a quality control process for allowing physiological secretion of VLDL particles in the access of lipids.
Figure 2.
Hepatic VLDL assembly. The assembly of VLDL initiates with the lipidation of nascent apoB polypeptides in a process that is mechanistically coupled with apoB translation and translocation into the ER lumen. MTP acts as a molecular chaperone for transporting lipids to nascent apoB molecules, resulting in the production of triglyceride (TG)-rich VLDL particles. It remains a debatable issue of whether MTP is required for the late stage of VLDL assembly, in which bulk core lipids are incorporated into poorly lapidated apoB for the maturation of VLDL particles.
Given its importance in lipoprotein metabolism, the molecular mechanism underlying insulin- and/or substrate-dependent regulation of apoB secretion has received intensive investigations. One potential mechanism suggests that insulin inhibits apoB secretion by stimulating apoB degradation. Consistent with this notion is the observation that insulin suppresses apoB expression and promotes proteasome-mediated apoB degradation in cultured HepG2 cells, primary rat hepatocytes, and perfused rat livers.91,95–100 Alternatively, insulin inhibits hepatic apoB secretion by limiting its substrate FFA availability.91,101,102 Indeed, insulin has been shown to restrain FFA mobilization from adipose tissue by inhibiting the hormone-sensitive lipase.103 It is thought that such an inhibitory action of insulin along with substrate availability is critical for the liver to adjust the rate of hepatic VLDL-TG secretion in response to changes in metabolic states. In insulin resistant states, an increased FFA flux into the liver, resulting from unrestrained fat mobilization in adipose tissue, augments apoB secretion, contributing to hepatic VLDL-TG overproduction and the development of dyslipidemia. Indeed, hepatic apoB production is markedly elevated, accompanied by increased VLDL-TG secretion in animal models with whole-body insulin resistance and altered triglyceride metabolism.67,70,94,104–110 There is clinical evidence that elevated plasma apoB levels, which reflect the number of small, dense LDL particles in plasma, are a significant predictor of cardiovascular risk in subjects with metabolic syndrome.111–116
As discussed above, hepatic apoB production is tightly regulated at the posttranslational level. This process has been viewed as a safeguarding mechanism for protecting against the development of steatosis by enhancing VLDL secretion in the response to lipid overload into the liver. This view raises an important question: why the liver cannot rid of excessive lipids and avoid steatosis by accelerating VLDL secretion in the face of lipid excess such as in obesity? A significant clue to this question derives from the study by Ota et al.,107 who show that hepatic apoB production is subject to regulation by ER stress, an adaptive response that is elicited by the accumulation of unfolded or misfolded proteins in the ER lumen. They show that an increased lipid infiltration into the liver induces ER stress and compromises the secretory pathway. This effect inhibits hepatic apoB secretion and instigates lipid accumulation, contributing to the development of steatosis.107 This lipid-induced hepatic ER stress along with concomitant steatosis is detectable in both genetic and dietary models of obese mice.107,117,118 Likewise, we and others show that high fructose-induced hypertriglyceridemic hamsters also exhibit hepatic ER stress, accompanied by excessive fat deposition in the liver.67,70,108,119 Together these data elucidate that lipid-induced ER stress links aberrant apoB secretion to the development of hepatic steatosis associated with obesity.
FoxO1 Integrates Insulin Signaling to Hepatic VLDL Production
FoxO1 is a nuclear transcription factor that belongs to a protein family characterized by a highly conserved DNA binding motif, termed “forkhead” domain, including FoxO1 (FKHR), FoxO3a (FKHRL1), FoxO4 (AFX) and FoxO6 in mammals.120–122 These fork-head proteins are substrates of the Akt/PKB and SGK kinases and play important roles in insulin action.120,121,123–128 Insulin exerts its inhibitory effect on gene expression via a highly conserved insulin response element (IRE) with its core nucleotide sequence (TG/ATTTT/G) in the promoter. In the absence of insulin, FoxO1 resides in the nucleus (Fig. 3A) and binds as a trans-activator to IRE, enhancing promoter activity. In response to insulin, FoxO1 is phosphorylated through the PI3K-dependent pathway, resulting in its nuclear exclusion (Fig. 3B) and inhibition of target gene expression.123,129–133 Failure in phosphorylation of FoxO1 results in its constitutive nuclear localization and trans-activation of gene expression.130,134
Figure 3.
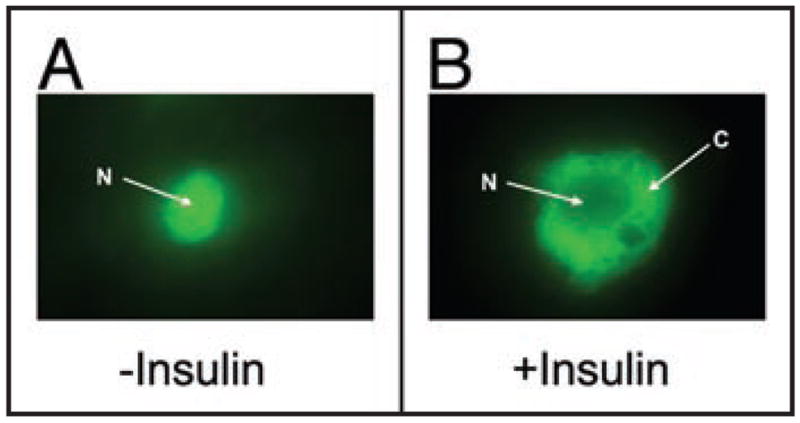
Insulin-mediated FoxO1 protein trafficking in HepG2 cells. (A) FoxO1 is localized to the nucleus (N) in the absence of insulin. (B) In response to insulin, FoxO1 is translocated to the cytoplasm (C). HepG2 cells pre-transfected with a plasmid encoding FoxO1-GFP fusion protein are exposed to insulin (100 nM) for 15 min, followed by immunofluorescent microscopy.
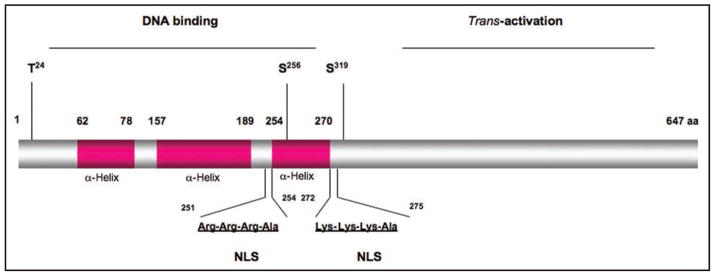
As shown in Figure 4, FoxO1 comprises two structural domains, the amino forkhead domain that is necessary for DNA binding and the carboxyl trans-activation domain that is responsible for stimulating promoter activity.131 These two domains are functionally separable, as the carboxyl domain, when fused with a heterologous Gal4-DNA binding domain, is capable of stimulating Gal4 promoter activity.135 In contrast, a truncated version of FoxO1 containing the forkhead domain is able to bind IRE DNA, but its binding does not result in trans-activation of promoter activity.136 This unique property accounts for its dominant-negative phenotype in suppressing target gene expression.129,136
Figure 4.
Schematic depiction of FoxO1 protein. FoxO1 comprises the amino DNA binding domain and carboxyl trans-activation domain. The DNA binding domain is formed by three α-helix structural motifs. Within the DNA binding domain are two consensus nuclear localization signals (NLS) and three highly conserved phosphorylation sties (T24, S256 and S319). Phosphorylation of FoxO1 in response to insulin promotes FoxO1 translocation from the nucleus to cytoplasm. This effect results in inactivation of FoxO1 transcriptional activity and inhibition of target gene expression, as FoxO1 is removed from its active nuclear location.
Recently, we demonstrate that FoxO1 mediates insulin-dependent regulation of MTP expression in modulating hepatic VLDL secretion.66 We show in cultured HepG2 cells that hepatic MTP production is stimulated by FoxO1 and inhibited by insulin. This effect correlates with the ability of FoxO1 to bind at its target site in the MTP promoter, resulting in trans-activation of MTP promoter activity. Deletion or mutation of the FoxO1 binding site disables FoxO1 binding to the MTP promoter and abrogates insulin-dependent regulation of hepatic MTP production. FoxO1 gain-of-function, resulting from either FoxO1 transgenic expression or adenovirus-mediated FoxO1 production in the liver, augments hepatic MTP expression and promotes apoB secretion, contributing to a significant induction in both the number and size of VLDL-TG particles. Both VLDL-TG production and apoB secretion are increased in response to elevated FoxO1 production in HepG2 cells and mice. Conversely, FoxO1 loss-of-function, caused by RNAi-mediated FoxO1 knockdown in the liver, suppresses hepatic MTP expression and reduces hepatic VLDL-TG output in mice.
These data shed light on the mechanism that the liver has evolved to adjust hepatic VLDL-TG assembly and secretion to maintain lipid homeostasis and energy balance in different physiological states. It follows that in response to postprandial insulin release, FoxO1 is phosphorylated and excluded from the nucleus, resulting in the inhibition of hepatic MTP expression. This effect acts to abate VLDL-TG production and limit postprandial lipid excursion in the blood. As a result of insulin resistance, FoxO1 is preferentially localized in the nucleus due to the inability of FoxO1 to undergo insulin-dependent phosphorylation and unclear exclusion. This effect augments FoxO1 transcriptional activity in promoting hepatic MTP and VLDL-TG overproduction. These data suggest that unleashed FoxO1 activity in insulin resistant livers plays an important role in linking impaired insulin action to excessive VLDL-TG secretion, contributing to the development of hypertriglyceridemia in obesity and type 2 diabetes.66,137 Consistent with this model, we show that hepatic MTP abundance and hepatic VLDL-TG production are markedly upregulated, correlating with augmented hepatic FoxO1 activity in multiple models of mice with altered triglyceride metabolism, including high fat-induced obese mice, diabetic db/db mice, FoxO1-transgenic mice, and high fructose-induced hypertriglyceridemic hamsters.66,67,108,129,138,139
These data are consistent with the idea that FoxO1 dysregulation is associated with aberrant hepatic metabolism.139–141 Further support of this notion is provided by Valenti et al.,142 who report that an enhanced FoxO1 activity is associated with nonalcoholic steatohepatitis in humans. This raises an important hypothesis that selective inhibition of FoxO1 activity in insulin resistant livers would curb hepatic VLDL-TG overproduction and ameliorate hypertriglyceridemia. As a proof of the concept, Samuel et al.,143 show that targeted inhibition of FoxO1 by an anti-sense oligonucleotide approach results in significant improvement in peripheral insulin sensitivity, glucose and lipid metabolism in high fat-induced obese mice. Likewise, we demonstrate that functional inhibition of FoxO1 by adenovirus-mediated production of FoxO1 dominant-negative mutant in the liver improves whole-body insulin sensitivity and reduces hyperinsulinemia, contributing to improved carbohydrate metabolism in diabetic db/db mice.129 Furthermore, FoxO1 haplo-insufficiency protects from high fat-induced insulin resistance and lipid disorders in insulin receptor-deficient diabetic mice.144 In keeping with observation, Dong et al., show that liver-specific depletion of FoxO1 is sufficient to restore the metabolic abnormality in diabetic mice with genetic deletion of both insulin receptor substrate 1 (IRS1) and 2 (IRS2) genes.145
Our recent data of using RNAi-mediated FoxO1 knockdown approach to curb hepatic VLDL-TG overproduction have validated the concept that FoxO1 deregulation contributes to aberrant hepatic metabolism and selective inhibition of FoxO1 in insulin resistant livers contributes to improved glucose and lipid metabolism.139,140 It also prompts an urgent call for the development of an antagonist compound for targeted inhibition of FoxO1 activity in vivo for testing its therapeutic value in preclinical models of diabetic dyslipidemia.
Conclusion
FoxO1 has emerged as an important player in integrating insulin signaling to downstream target gene expression in carbohydrate metabolism. FoxO1 mediates the inhibitory effect of insulin on the expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase), two key enzymes in hepatic gluco-neogenesis.120,121,139 Under fasting conditions, FoxO1 expression along with its nuclear distribution is increased, accounting for its augmented transcriptional activity to promote hepatic gluconeogenesis. Under fed conditions, FoxO1 is phosphorylated and translocated to the cytoplasm, resulting in inhibition of gluconeogenesis in the liver. These two reciprocal mechanisms play a critical role in maintaining blood glucose levels within a narrow physiological range in different metabolic states.120,121
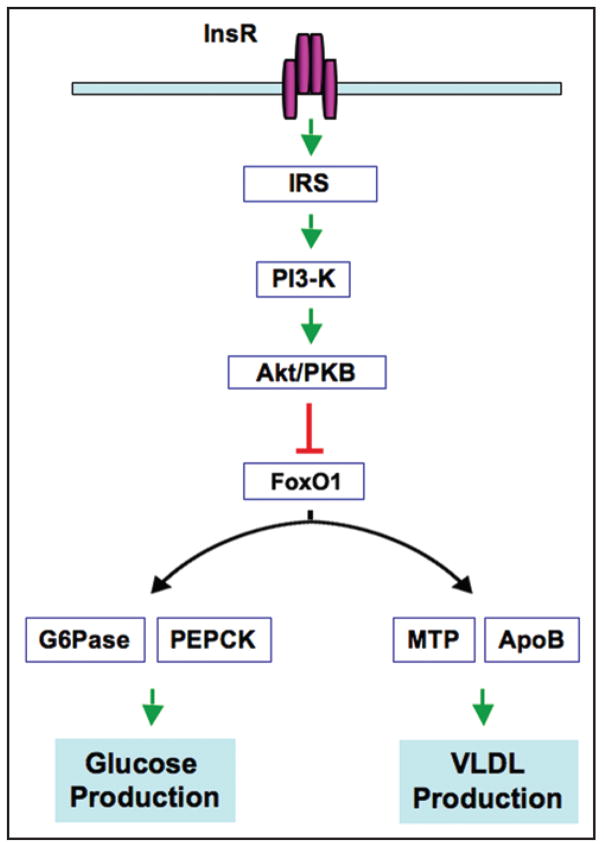
Our recent data suggest that a similar mechanism is exploited by the liver to regulate hepatic VLDL-TG secretion for maintaining normal triglyceride metabolism.66 This is achieved via FoxO1-mediated regulation of MTP and apoB production in response to insulin action in the liver. As illustrated in Figure 5, hepatic insulin signaling bifurcates at FoxO1 to target different sets of genes in glucose and lipid metabolism. Such a FoxO1-accentuated regulatory mechanism is evolved for synchronizing insulin-dependent regulation of hepatic glucose production and VLDL-TG secretion for priming the liver to respond to metabolic shift between fasting and refeeding states. However, an impaired ability of insulin to check FoxO1 activity consequently triggers concomitant perturbations in glucose and lipid metabolism, accounting for concurrent manifestations of both hyperglycemia and hypertriglyceridemia in insulin resistant subjects with obesity and/or type 2 diabetes.
Figure 5.
FoxO1 integrates insulin signaling to hepatic glucose and VLDL production. Insulin binds to its receptors (Insr) at cell surface, resulting in the activation of a cascade of events including insulin receptor substrate (IRS), PI3-kinase, and Akt (known as protein kinase B, PKB). Insulin inhibits FoxO1 activity via Akt/PKB-dependent phosphorylation and nuclear exclusion. This effect is instrumental for liver to curb hepatic glucose and VLDL-TG production and limit postprandial glucose and lipid excursion. Loss of insulin inhibition of FoxO1 activity in insulin resistant livers results in excessive production of both glucose and VLDL-TG, contributing to the dual pathogenesis of hyperglycemia and hypertriglyceridemia in diabetes.
It is of note that FoxO1 is a nuclear transcriptional factor that is ubiquitously expressed. However, a comprehensive survey of FoxO1 function in other insulin sensitive tissues, including the brain,146,147 skeletal muscle,148,149 pancreas150,151 and adipose tissue,152,153 is beyond the scope of this article. Due to space limitation in this article, we have centered our review on recent advances made toward our understanding of the underlying mechanism of VLDL overproduction in the liver, a prominent pathological feature of hypertriglyceridemia. In addition, there is emerging evidence that intestinal overproduction of apoB48-containing lipoproteins is a compounding factor for the pathogenesis of postprandial lipaemia and diabetic dyslipidemia in insulin resistant subjects.64,154–156 In accordance with these findings, intestinal MTP and apoB48 expression levels are markedly elevated in enterocytes isolated from hyperlipidemic animals.157–159 Nevertheless, the molecular pathway from insulin resistance to aberrant production of apoB48-containing lipoprotein particles remains largely undefined. It is noteworthy that FoxO1 is also expressed in the intestine,138 but its role in regulating intestinal MTP and apoB48 expression in response to insulin action remains obscure. Further studies are needed to decipher the molecular basis underlying intestinal overproduction of apoB48-containing lipoproteins for better understanding the pathophysiology of postprandial lipaemia that are closely associated with obesity and type 2 diabetes.
Acknowledgments
This study was supported in part by American Diabetes Association and National Health Institute grant DK066301. We thank Drs. Steve Ringquist, German Perdomo, Dongming Su, Dae Hyun Kim and Sandra Slusher for critical proofreading of this manuscript.
Abbreviations
- FoxO1
forkhead box O1
- MTP
microsomal triglyceride transfer protein
- ApoB
apolipoprotein B
- IRS
insulin receptor substrate
- PEPCK
phosphoenolpyruvate carboxykinase
- G6Pase
glucose-6-phosphatase
- PPARα
peroxisome proliferator activated receptor alpha
- PGC1β
PPARgamma coactivator-1beta
- Foxa2
forkhead box a2
- HNF4α
hepatocyte nuclear factor 4alpha
- HNF1α
hepatocyte nuclear factor 1alpha
- VLDL
very low-density lipoprotein
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- TG
triglyceride
- FFA
free fatty acid
Footnotes
Previously published online as a Cell Cycle E-publication:
References
- 1.Rapp RJ. Hypertriglyceridemia: a review beyond low-density lipoprotein. Cardiol Rev. 2002;10:163–72. doi: 10.1097/00045415-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Moller DE, Kaufman KD. Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Heiss G, Cohn R, Cowan LD, Suchindran CM, Bangdiwala S, Kritchevsky S, Jacobs DR, Jr, O’Grady HK, Davis CE. Plasma triglyceride level and mortality from coronary heart disease. N Engl J Med. 1993;328:1220–5. doi: 10.1056/NEJM199304293281702. [DOI] [PubMed] [Google Scholar]
- 4.Hopkins PN, Heiss G, Ellison RC, Province MA, Pankow JS, Eckfeldt JH, Hunt SC. Coronary artery disease risk in familial combined hyperlipidemia and familial hypertriglyceridemia: a case-control comparison from the National Heart, Lung and Blood Institute Family Heart Study. Circulation. 2003;108:519–23. doi: 10.1161/01.CIR.0000081777.17879.85. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins PN, Wu LL, Hunt SC, Brinton EA. Plasma triglycerides and type III hyperlipidemia are independently associated with premature familial coronary artery disease. J Am Coll Cardiol. 2005;45:1003–12. doi: 10.1016/j.jacc.2004.11.062. [DOI] [PubMed] [Google Scholar]
- 6.Stewart MM, Laker MF, Alberti KG. The contribution of lipids to coronary heart disease in diabetes mellitus. J Intern Med Suppl. 1994;736:41–6. [PubMed] [Google Scholar]
- 7.Defronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med. 1997;50:191–7. doi: 10.1016/s0300-2977(97)00012-0. [DOI] [PubMed] [Google Scholar]
- 8.Krauss RM. Atherogenicity of triglyceride-rich lipoproteins. Am J Cardiol. 1998;81:13–7. doi: 10.1016/s0002-9149(98)00032-0. [DOI] [PubMed] [Google Scholar]
- 9.Brewer HBJ. Hypertriglyceridemia: changes in the plasma lipoproteins associated with an increased risk of cardiovascular disease. Am J Cardiol. 1999;83:3–12. doi: 10.1016/s0002-9149(99)00308-2. [DOI] [PubMed] [Google Scholar]
- 10.Bard JM, Charles MA, Juhan-Vague I, Vague P, Andre P, Safar M, Fruchart JC, Eschwege E. Accumulation of triglyceride-rich lipoprotein in subjects with abdominal obesity. Arterioscher Thromb Vasc Biol. 2001;21:407–14. doi: 10.1161/01.atv.21.3.407. [DOI] [PubMed] [Google Scholar]
- 11.Oliveieri O, Stranieri C, Bassi A, Zaia B, Girelli D, Pizzolo F, Trabetti E, Cheng S, Grow MA, Pignatti PF, Corrocher R. ApoC-III gene polymorphisms and risk of coronary artery disease. J Lipid Res. 2002;43:1450–7. doi: 10.1194/jlr.m200145-jlr200. [DOI] [PubMed] [Google Scholar]
- 12.Assmann G, Cullen P, Schulte H. The Munster Heart Study (PROCAM). Results of follow-up at 8 years. European heart journal. 1998;19:2–11. [PubMed] [Google Scholar]
- 13.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. Journal of cardiovascular risk. 1996;3:213–9. [PubMed] [Google Scholar]
- 14.Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. The American journal of cardiology. 1998;81:7–12. doi: 10.1016/s0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 15.Hokanson JE. Hypertriglyceridemia and risk of coronary heart disease. Curr Cardiol Rep. 2002;4:488–93. doi: 10.1007/s11886-002-0112-7. [DOI] [PubMed] [Google Scholar]
- 16.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocrine Rev. 2002;23:201–29. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 17.Chan DC, Watts GF, Barrett PH, Mamo JCL, Redgrave TG. Markers of triglyceride-rich lipoprotein remnant metabolism in visceral obesity. Clin Chem. 2002;48:278–83. [PubMed] [Google Scholar]
- 18.Nieves DJ, Cnop M, Retzlaff B, Walden CE, Brunzell JD, Knopp RH, Kahn SE. The atherogenic lipoprotein profile associated with obesity and insulin resistance is largely attributable to intra-abdominal fat. Diab. 2003;52:172–9. doi: 10.2337/diabetes.52.1.172. [DOI] [PubMed] [Google Scholar]
- 19.Kissebah AH, Adams PW, Wynn V. Interrelationships between insulin secretion and plasma free fatty acid and triglyceride kinetics in maturity onset diabetes and the effect of phenethybriguanide (phenformin) Diabetologia. 1974;10:119–30. doi: 10.1007/BF01219667. [DOI] [PubMed] [Google Scholar]
- 20.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. J Biol Chem. 2000;275:8416–25. doi: 10.1074/jbc.275.12.8416. [DOI] [PubMed] [Google Scholar]
- 21.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–35. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 22.Minnich A, Tian N, Byan L, Bilder G. A potent PPARalpha agonist stimulates mitochondrial fatty acid beta-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:270–9. doi: 10.1152/ajpendo.2001.280.2.E270. [DOI] [PubMed] [Google Scholar]
- 23.Nagai Y, Nishio Y, Nakamura T, Maegawa H, Kikkawa R, Kashiwagi A. Amelioration of high fructose-induced metabolic derangements by activation of PPARalpha. Am J Physiol Endocrinol Metab. 2002;282:1180–90. doi: 10.1152/ajpendo.00471.2001. [DOI] [PubMed] [Google Scholar]
- 24.Kamanna VS, Kashyap ML. Nicotinic acid (niacin) receptor agonists: will they be useful therapeutic agents? The American journal of cardiology. 2007;100:53–61. doi: 10.1016/j.amjcard.2007.09.080. [DOI] [PubMed] [Google Scholar]
- 25.Pike NB, Wise A. Identification of a nicotinic acid receptor: is this the molecular target for the oldest lipid-lowering drug? Curr Opin Investig Drugs. 2004;5:271–5. [PubMed] [Google Scholar]
- 26.Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, Brown AJ, Dowell SJ, Szekeres PG, Hassall DG, Marshall FH, Wilson S, Pike NB. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–74. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 27.Kamanna VS, Kashyap ML. Mechanism of action of niacin. The American journal of cardiology. 2008;101:20–6. doi: 10.1016/j.amjcard.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Backes JM, Gibson CA, Ruisinger JF, Moriarty PM. Fibrates: what have we learned in the past 40 years? Pharmacotherapy. 2007;27:412–24. doi: 10.1592/phco.27.3.412. [DOI] [PubMed] [Google Scholar]
- 29.Yuan G, Al-Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. Cmaj. 2007;176:1113–20. doi: 10.1503/cmaj.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scanu AM, Bamba R. Niacin and lipoprotein(a): facts, uncertainties, and clinical considerations. The American journal of cardiology. 2008;101:44–7. doi: 10.1016/j.amjcard.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 31.Barter PJ, Rye KA. Is there a role for fibrates in the management of dyslipidemia in the metabolic syndrome? Arterioscler Thromb Vasc Biol. 2008;28:39–46. doi: 10.1161/ATVBAHA.107.148817. [DOI] [PubMed] [Google Scholar]
- 32.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–66. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.den Boer MA, Voshol PJ, Kuipers F, Romijn JA, Havekes LM. Hepatic glucose production is more sensitive to insulin-mediated inhibition than hepatic VLDL-triglyceride production. Am J Physiol Endocrinol Metab. 2006;291:1360–4. doi: 10.1152/ajpendo.00188.2006. [DOI] [PubMed] [Google Scholar]
- 34.Malmstrom R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, Shepherd J, Taskinen MR. Effects of insulin and acipimox on VLDL1 and VLDL2 apolipoprotein B production in normal subjects. Diabetes. 1998;47:779–87. doi: 10.2337/diabetes.47.5.779. [DOI] [PubMed] [Google Scholar]
- 35.Malmstrom R, Packard CJ, Watson TD, Rannikko S, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, Shepherd J, Taskinen MR. Metabolic basis of hypotriglyceridemic effects of insulin in normal men. Arterioscler Thromb Vasc Biol. 1997;17:1454–64. doi: 10.1161/01.atv.17.7.1454. [DOI] [PubMed] [Google Scholar]
- 36.Lewis GF, Steiner G. Acute effects of insulin in the control of VLDL production in humans. Implications for the insulin-resistant state. Diabetes care. 1996;19:390–3. doi: 10.2337/diacare.19.4.390. [DOI] [PubMed] [Google Scholar]
- 37.Sparks JD, Sparks CE. Insulin regulation of triacylglycerol-rich lipoprotein synthesis and secretion. Biochim Biophys Acta. 1994;1215:9–32. doi: 10.1016/0005-2760(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 38.Tietge UJ, Bakillah A, Maugeais C, Tsukamoto K, Hussain M, Rader DJ. Hepatic over-expression of microsomal triglyceride transfer protein (MTP) results in increased in vivo secretion of VLDL triglycerides and apolipoprotein B. J Lipid Res. 1999;40:2134–9. [PubMed] [Google Scholar]
- 39.Jamil H, Chu CH, Dickson JK, Jr, Chen Y, Yan M, Biller SA, Gregg RE, Wetterau JR, Gordon DA. Evidence that microsomal triglyceride transfer protein is limiting in the production of apolipoprotein B-containing lipoproteins in hepatic cells. J Lipid Res. 1998;39:1448–54. [PubMed] [Google Scholar]
- 40.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 41.Swift LL, Zhu MY, Kakkad B, Jovanovska A, Neely MD, Valyi-Nagy K, Roberts RL, Ong DE, Jerome WG. Subcellular localization of microsomal triglyceride transfer protein. J Lipid Res. 2003;44:1841–9. doi: 10.1194/jlr.M300276-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Gordon DA, Wetterau JR, Gregg RE. Microsomal triglyceride transfer protein: a protein complex required for the assembly of lipoprotein particles. Trends Cell Biol. 1995;5:317–21. doi: 10.1016/s0962-8924(00)89054-6. [DOI] [PubMed] [Google Scholar]
- 43.Hui TY, Olivier LM, Kang S, Davis RA. Microsomal triglyceride transfer protein is essential for hepatic secretion of apoB-100 and apoB-48 but not triglyceride. J Lipid Res. 2002;43:785–93. [PubMed] [Google Scholar]
- 44.Manchekar M, Richardson PE, Forte TM, Datta G, Segrest JP, Dashti N. Apolipoprotein B-containing lipoprotein particle assembly: lipid capacity of the nascent lipoprotein particle. J Biol Chem. 2004;279:39757–66. doi: 10.1074/jbc.M406302200. [DOI] [PubMed] [Google Scholar]
- 45.Swift LL, Jovanovska A, Kakkad B, Ong DE. Microsomal triglyceride transfer protein expression in mouse intestine. Histochem Cell Biol. 2005;123:475–82. doi: 10.1007/s00418-005-0772-7. [DOI] [PubMed] [Google Scholar]
- 46.Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu Rev Nutr. 2000;20:663–97. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- 47.Liao W, Hui TY, Young SG, Davis RA. Blocking microsomal triglyceride transfer protein interferes with apoB secretion without causing retention or stress in the ER. J Lipid Res. 2003;44:978–85. doi: 10.1194/jlr.M300020-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Bjorkegren J, Beigneux A, Bergo MO, Maher JJ, Young SG. Blocking the secretion of hepatic very low density lipoproteins renders the liver more susceptible to toxin-induced injury. J Biol Chem. 2002;277:5476–83. doi: 10.1074/jbc.M108514200. [DOI] [PubMed] [Google Scholar]
- 49.Leung GK, Veniant MM, Kim SK, Zlot CH, Raabe M, Bjorkegren J, Neese RA, Hellerstein MK, Young SG. A deficiency of microsomal triglyceride transfer protein reduces apolipoprotein B secretion. J Biol Chem. 2000;275:7515–20. doi: 10.1074/jbc.275.11.7515. [DOI] [PubMed] [Google Scholar]
- 50.Raabe M, Veniant MM, Sullivan MA, Zlot CH, Bjorkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287–98. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raabe M, Flynn LM, Zlot CH, Wong JS, Veniant MM, Hamilton RL, Young SG. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc Natl Acad Sci USA. 1998;95:8686–91. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang BH, Liao W, Li L, Nakamuta M, Mack D, Chan L. Liver-specific inactivation of the abetalipoproteinemia gene completely abrogates very low density lipoprotein/low density lipoprotein production in a viable conditional knockout mouse. J Biol Chem. 1999;274:6051–5. doi: 10.1074/jbc.274.10.6051. [DOI] [PubMed] [Google Scholar]
- 53.Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–56. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 54.Bernard S, Touzet S, Personne I, Lapras V, Bondon PJ, Berthezene F, Moulin P. Association between microsomal triglyceride transfer protein gene polymorphism and the biological features of liver steatosis in patients with type II diabetes. Diabetologia. 2000;43:995–9. doi: 10.1007/s001250051481. [DOI] [PubMed] [Google Scholar]
- 55.Ledmyr H, Karpe F, Lundahl B, McKinnon M, Skoglund-Andersson C, Ehrenborg E. Variants of the microsomal triglyceride transfer protein gene are associated with plasma cholesterol levels and body mass index. J Lipid Res. 2002;43:51–8. [PubMed] [Google Scholar]
- 56.Ledmyr H, McMahon AD, Ehrenborg E, Nielsen LB, Neville M, Lithell H, MacFarlane PW, Packard CJ, Karpe F. The microsomal triglyceride transfer protein gene-493T variant lowers cholesterol but increases the risk of coronary heart disease. Circulation. 2004;109:2279–84. doi: 10.1161/01.CIR.0000130070.96758.7b. [DOI] [PubMed] [Google Scholar]
- 57.Juo SH, Han Z, Smith JD, Colangelo L, Liu K. Common polymorphism in promoter of microsomal triglyceride transfer protein gene influences cholesterol, ApoB, and triglyceride levels in young african american men: results from the coronary artery risk development in young adults (CARDIA) study. Arterioscler Thromb Vasc Biol. 2000;20:1316–22. doi: 10.1161/01.atv.20.5.1316. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Garcia AB, Gonzalez C, Real JT, Martin de Llano JJ, Gonzalez-Albert V, Civera M, Chaves FJ, Ascaso JF, Carmena R. Influence of microsomal triglyceride transfer protein promoter polymorphism -493 GT on fasting plasma triglyceride values and interaction with treatment response to atorvastatin in subjects with heterozygous familial hypercholesterolaemia. Pharmacogenet Genomics. 2005;15:211–8. doi: 10.1097/01213011-200504000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Day CP. Genes or environment to determine alcoholic liver disease and non-alcoholic fatty liver disease. Liver Int. 2006;26:1021–8. doi: 10.1111/j.1478-3231.2006.01323.x. [DOI] [PubMed] [Google Scholar]
- 60.Sugimoto T, Yamashita S, Ishigami M, Sakai N, Hirano K, Tahara M, Matsumoto K, Nakamura T, Matsuzawa Y. Decreased microsomal triglyceride transfer protein activity contributes to initiation of alcoholic liver steatosis in rats. Journal of hepatology. 2002;36:157–62. doi: 10.1016/s0168-8278(01)00263-x. [DOI] [PubMed] [Google Scholar]
- 61.Hagan DL, Kienzle B, Jamil H, Hariharan N. Transcriptional regulation of human and hamster microsomal triglyceride transfer protein genes. Cell type-specific expression and response to metabolic regulators. J Biol Chem. 1994;269:28737–44. [PubMed] [Google Scholar]
- 62.Lin MC, Gordon D, Wetterau JR. Microsomal triglyceride transfer protein (MTP) regulation in HepG2 cells: insulin negatively regulates MTP gene expression. J Lipid Res. 1995;36:1073–81. [PubMed] [Google Scholar]
- 63.Kuriyama H, Yamashita S, Shimomura I, Funahashi T, Ishigami M, Aragane K, Miyaoka K, Nakamura T, Takemura K, Man Z, Toide K, Nakayama N, Fukuda Y, Lin MC, Wetterau JR, Matsuzawa Y. Enhanced expression of hepatic acyl-coenzyme A synthetase and microsomal triglyceride transfer protein messenger RNAs in the obese and hypertriglyceridemic rat with visceral fat accumulation. Hepatology. 1998;27:557–62. doi: 10.1002/hep.510270233. [DOI] [PubMed] [Google Scholar]
- 64.Phillips C, Owens D, Collins P, Tomkin GH. Microsomal triglyceride transfer protein: does insulin resistance play a role in the regulation of chylomicron assembly? Atherosclerosis. 2002;160:355–60. doi: 10.1016/s0021-9150(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 65.Bartels ED, Lauritsen M, Nielsen LB. Hepatic expression of microsomal triglyceride transfer protein and in vivo secretion of triglyceride-rich lipoproteins are increased in obese diabetic mice. Diabetes. 2002;51:1233–9. doi: 10.2337/diabetes.51.4.1233. [DOI] [PubMed] [Google Scholar]
- 66.Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–64. doi: 10.1172/JCI32914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qu S, Su D, Altomonte J, Kamagate A, He J, Perdomo G, Tse T, Jiang Y, Dong HH. PPAR{alpha} mediates the hypolipidemic action of fibrates by antagonizing FoxO1. Am J Physiol Endocrinol Metab. 2007;292:421–34. doi: 10.1152/ajpendo.00157.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taghibiglou C, Carpentier A, Van Iderstine SC, Chen B, Rudy D, Aiton A, Lewis GF, Adeli K. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem. 2000;275:8416–25. doi: 10.1074/jbc.275.12.8416. [DOI] [PubMed] [Google Scholar]
- 69.Carpentier A, Taghibiglou C, Leung N, Szeto L, Van Iderstine SC, Uffelman KD, Buckingham R, Adeli K, Lewis GF. Ameliorated hepatic insulin resistance is associated with normalization of microsomal triglyceride transfer protein expression and reduction in very low density lipoprotein assembly and secretion in the fructose-fed hamster. J Biol Chem. 2002;277:28795–802. doi: 10.1074/jbc.M204568200. [DOI] [PubMed] [Google Scholar]
- 70.Basciano H, Federico L, Adeli K. Fructose, insulin resistance and metabolic dyslipidemia. Nutr Metab (Lond) 2005;2:5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chong T, Naples M, Federico L, Taylor D, Smith GJ, Cheung RC, Adeli K. Effect of rosu-vastatin on hepatic production of apolipoprotein B-containing lipoproteins in an animal model of insulin resistance and metabolic dyslipidemia. Atherosclerosis. 2006;185:21–31. doi: 10.1016/j.atherosclerosis.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Sheena V, Hertz R, Nousbeck J, Berman I, Magenheim J, Bar-Tana J. Transcriptional regulation of human microsomal triglyceride transfer protein by hepatocyte nuclear factor-4alpha. J Lipid Res. 2005;46:328–41. doi: 10.1194/jlr.M400371-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Au WS, Kung HF, Lin MC. Regulation of microsomal triglyceride transfer protein gene by insulin in HepG2 cells: roles of MAPKerk and MAPKp38. Diabetes. 2003;52:1073–80. doi: 10.2337/diabetes.52.5.1073. [DOI] [PubMed] [Google Scholar]
- 74.Allister EM, Borradaile NM, Edwards JY, Huff MW. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 2005;54:1676–83. doi: 10.2337/diabetes.54.6.1676. [DOI] [PubMed] [Google Scholar]
- 75.Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–32. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 76.Wolfrum C, Stoffel M. Coactivation of Foxa2 through Pgc-1beta promotes liver fatty acid oxidation and triglyceride/VLDL secretion. Cell metabolism. 2006;3:99–110. doi: 10.1016/j.cmet.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Koo SH, Montminy M. Fatty acids and insulin resistance: a perfect storm. Molecular cell. 2006;21:449–50. doi: 10.1016/j.molcel.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Ameen C, Edvardsson U, Ljungberg A, Asp L, Akerblad P, Tuneld A, Olofsson SO, Linden D, Oscarsson J. Activation of peroxisome proliferator-activated receptor alpha increases the expression and activity of microsomal triglyceride transfer protein in the liver. J Biol Chem. 2005;280:1224–9. doi: 10.1074/jbc.M412107200. [DOI] [PubMed] [Google Scholar]
- 79.Sheena V, Hertz R, Berman I, Nousbeck J, Bar-Tana J. Transcriptional suppression of human microsomal triglyceride transfer protein by hypolipidemic insulin sensitizers. Biochemical pharmacology. 2005;70:1548–59. doi: 10.1016/j.bcp.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Siri P, Candela N, Zhang YL, Ko C, Eusufzai S, Ginsberg HN, Huang LS. Post-transcriptional stimulation of the assembly and secretion of triglyceride-rich apolipoprotein B lipoproteins in a mouse with selective deficiency of brown adipose tissue, obesity and insulin resistance. J Biol Chem. 2001;276:46064–72. doi: 10.1074/jbc.M108909200. [DOI] [PubMed] [Google Scholar]
- 81.Pan M, Liang Js JS, Fisher EA, Ginsberg HN. The late addition of core lipids to nascent apolipoprotein B100, resulting in the assembly and secretion of triglyceride-rich lipoproteins, is independent of both microsomal triglyceride transfer protein activity and new triglyceride synthesis. J Biol Chem. 2002;277:4413–21. doi: 10.1074/jbc.M107460200. [DOI] [PubMed] [Google Scholar]
- 82.Gordon DA, Jamil H, Gregg RE, Olofsson SO, Boren J. Inhibition of the microsomal triglyceride transfer protein blocks the first step of apolipoprotein B lipoprotein assembly but not the addition of bulk core lipids in the second step. J Biol Chem. 1996;271:33047–53. doi: 10.1074/jbc.271.51.33047. [DOI] [PubMed] [Google Scholar]
- 83.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277:17377–80. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 84.Kane JP. Apolipoprotein B: structural and metabolic heterogeneity. Annual review of physiology. 1983;45:637–50. doi: 10.1146/annurev.ph.45.030183.003225. [DOI] [PubMed] [Google Scholar]
- 85.Elovson J, Huang YO, Baker N, Kannan R. Apolipoprotein B is structurally and metabolically heterogeneous in the rat. Proc Natl Acad Sci USA. 1981;78:157–61. doi: 10.1073/pnas.78.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen SH, Habib G, Yang CY, Gu ZW, Lee BR, Weng SA, Silberman SR, Cai SJ, Deslypere JP, Rosseneu M, et al. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science. 1987;238:363–6. doi: 10.1126/science.3659919. [DOI] [PubMed] [Google Scholar]
- 87.Powell LM, Wallis SC, Pease RJ, Edwards YH, Knott TJ, Scott J. A novel form of tissue-specific RNA processing produces apolipoprotein-B48 in intestine. Cell. 1987;50:831–40. doi: 10.1016/0092-8674(87)90510-1. [DOI] [PubMed] [Google Scholar]
- 88.Chirieac DV, Chirieac LR, Corsetti JP, Cianci J, Sparks CE, Sparks JD. Glucose-stimulated insulin secretion suppresses hepatic triglyceride-rich lipoprotein and apoB production. Am J Physiol Endocrinol Metab. 2000;279:1003–11. doi: 10.1152/ajpendo.2000.279.5.E1003. [DOI] [PubMed] [Google Scholar]
- 89.Lewis GF. Fatty acid regulation of very low density lipoprotein production. Curr Opin Lipidol. 1997;8:146–53. doi: 10.1097/00041433-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Steiner G, Lewis GF. Hyperinsulinemia and triglyceride-rich lipoproteins. Diabetes. 1996;45:24–6. doi: 10.2337/diab.45.3.s24. [DOI] [PubMed] [Google Scholar]
- 91.Sparks JD, Sparks CE. Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. J Biol Chem. 1990;265:8854–62. [PubMed] [Google Scholar]
- 92.Pan X, Hussain MM. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J Biol Chem. 2007;282:24707–19. doi: 10.1074/jbc.M701305200. [DOI] [PubMed] [Google Scholar]
- 93.Hussain MM, Rava P, Pan X, Dai K, Dougan SK, Iqbal J, Lazare F, Khatun I. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr Opin Lipidol. 2008;19:277–84. doi: 10.1097/MOL.0b013e3282feea85. [DOI] [PubMed] [Google Scholar]
- 94.Avramoglu RK, Adeli K. Hepatic regulation of apolipoprotein B. Reviews in endocrine & metabolic disorders. 2004;5:293–301. doi: 10.1023/B:REMD.0000045100.66675.92. [DOI] [PubMed] [Google Scholar]
- 95.Durrington PN, Newton RS, Weinstein DB, Steinberg D. Effects of insulin and glucose on very low density lipoprotein triglyceride secretion by cultured rat hepatocytes. J Clin Invest. 1982;70:63–73. doi: 10.1172/JCI110604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sparks CE, Sparks JD, Bolognino M, Salhanick A, Strumph PS, Amatruda JM. Insulin effects on apolipoprotein B lipoprotein synthesis and secretion by primary cultures of rat hepatocytes. Metabolism: clinical and experimental. 1986;35:1128–36. doi: 10.1016/0026-0495(86)90026-0. [DOI] [PubMed] [Google Scholar]
- 97.Patsch W, Franz S, Schonfeld G. Role of insulin in lipoprotein secretion by cultured rat hepatocytes. J Clin Invest. 1983;71:1161–74. doi: 10.1172/JCI110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pullinger CR, North JD, Teng BB, Rifici VA, Ronhild de Brito AE, Scott J. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res. 1989;30:1065–77. [PubMed] [Google Scholar]
- 99.Dashti N, Williams DL, Alaupovic P. Effects of oleate and insulin on the production rates and cellular mRNA concentrations of apolipoproteins in HepG2 cells. J Lipid Res. 1989;30:1365–73. [PubMed] [Google Scholar]
- 100.Sparks JD, Sparks CE, Miller LL. Insulin effects on apolipoprotein B production by normal, diabetic and treated-diabetic rat liver and cultured rat hepatocytes. Biochem J. 1989;261:83–8. doi: 10.1042/bj2610083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jackson TK, Salhanick AI, Elovson J, Deichman ML, Amatruda JM. Insulin regulates apolipoprotein B turnover and phosphorylation in rat hepatocytes. J Clin Invest. 1990;86:1746–51. doi: 10.1172/JCI114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, Chen S, McGarry JD. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97:2728–35. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Holm C, Osterlund T, Laurell H, Contreras JA. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Annu Rev Nutr. 2000;20:365–93. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]
- 104.Adeli K, Taghibiglou C, Van Iderstine SC, Lewis GF. Mechanisms of hepatic very low-density lipoprotein overproduction in insulin resistance. Trends Cardiovasc Med. 2001;11:170–6. doi: 10.1016/s1050-1738(01)00084-6. [DOI] [PubMed] [Google Scholar]
- 105.Avramoglu RK, Basciano H, Adeli K. Lipid and lipoprotein dysregulation in insulin resistant states. Clinica chimica acta; international journal of clinical chemistry. 2006;368:1–19. doi: 10.1016/j.cca.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 106.Taghibiglou C, Rashid-Kolvear F, Van Iderstine SC, Le-Tien H, Fantus IG, Lewis GF, Adeli K. Hepatic very low density lipoprotein-ApoB overproduction is associated with attenuated hepatic insulin signaling and overexpression of protein-tyrosine phosphatase 1B in a fructose-fed hamster model of insulin resistance. J Biol Chem. 2002;277:793–803. doi: 10.1074/jbc.M106737200. [DOI] [PubMed] [Google Scholar]
- 107.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–32. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L, Perdomo G, Kim DH, Qu S, Ringquist S, Trucco M, Dong HH. Proteomic analysis of fructose-induced fatty liver in hamsters. Metabolism: clinical and experimental. 2008;57:1115–24. doi: 10.1016/j.metabol.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, Vehkavaara S, Hakkinen A, Olofsson SO, Yki-Jarvinen H, Boren J. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–65. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 110.Adiels M, Westerbacka J, Soro-Paavonen A, Hakkinen AM, Vehkavaara S, Caslake MJ, Packard C, Olofsson SO, Yki-Jarvinen H, Taskinen MR, Boren J. Acute suppression of VLDL1 secretion rate by insulin is associated with hepatic fat content and insulin resistance. Diabetologia. 2007;50:2356–65. doi: 10.1007/s00125-007-0790-1. [DOI] [PubMed] [Google Scholar]
- 111.Onat A, Can G, Hergenc G, Yazici M, Karabulut A, Albayrak S. Serum apolipoprotein B predicts dyslipidemia, metabolic syndrome and, in women, hypertension and diabetes, independent of markers of central obesity and inflammation. International journal of obesity (2005) 2007;31:1119–25. doi: 10.1038/sj.ijo.0803552. [DOI] [PubMed] [Google Scholar]
- 112.Bamba V, Rader DJ. Obesity and atherogenic dyslipidemia. Gastroenterology. 2007;132:2181–90. doi: 10.1053/j.gastro.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 113.Batista MC, Welty FK, Diffenderfer MR, Sarnak MJ, Schaefer EJ, Lamon-Fava S, Asztalos BF, Dolnikowski GG, Brousseau ME, Marsh JB. Apolipoprotein A-I, B-100, and B-48 metabolism in subjects with chronic kidney disease, obesity, and the metabolic syndrome. Metabolism: clinical and experimental. 2004;53:1255–61. doi: 10.1016/j.metabol.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 114.Sierra-Johnson J, Romero-Corral A, Somers VK, Lopez-Jimenez F, Walldius G, Hamsten A, Hellenius ML, Fisher RM. ApoB/apoA-I ratio: an independent predictor of insulin resistance in US non-diabetic subjects. European heart journal. 2007;28:2637–43. doi: 10.1093/eurheartj/ehm360. [DOI] [PubMed] [Google Scholar]
- 115.Sierra-Johnson J, Fisher RM, Romero-Corral A, Somers VK, Lopez-Jimenez F, Ohrvik J, Walldius G, Hellenius ML, Hamsten A. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. European heart journal. 2008 doi: 10.1093/eurheartj/ehn347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cummings MH, Watts GF, Umpleby AM, Hennessy TR, Naoumova R, Slavin BM, Thompson GR, Sonksen PH. Increased hepatic secretion of very-low-density lipoprotein apolipoprotein B-100 in NIDDM. Diabetologia. 1995;38:959–67. doi: 10.1007/BF00400586. [DOI] [PubMed] [Google Scholar]
- 117.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 118.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–51. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 119.Morand JP, Macri J, Adeli K. Proteomic profiling of hepatic endoplasmic reticulum-associated proteins in an animal model of insulin resistance and metabolic dyslipidemia. J Biol Chem. 2005;280:17626–33. doi: 10.1074/jbc.M413343200. [DOI] [PubMed] [Google Scholar]
- 120.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–9. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 121.Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation and transformation. Cell. 2004;117:421–6. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- 122.Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/fork-head transcription factors. Gene Dev. 2000;14:142–6. [PubMed] [Google Scholar]
- 123.Nakae J, Park B-C, Accili D. Insulin stimulates phosphorylation of the forkhead transcription factor FKHR on serine 253 through a wortmannin-sensitive pathway. J Biol Chem. 1999;274:15982–5. doi: 10.1074/jbc.274.23.15982. [DOI] [PubMed] [Google Scholar]
- 124.Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem. 1999;274:17184–92. doi: 10.1074/jbc.274.24.17184. [DOI] [PubMed] [Google Scholar]
- 125.Nakae J, Kitamura T, Ogawa Y, Kasuga M, Accili D. Insulin regulation of gene expression through the forkhead transcription factor Foxo1 (Fkhr) requires kinases distinct from Akt. Biochemistry. 2001;40:11768–76. doi: 10.1021/bi015532m. [DOI] [PubMed] [Google Scholar]
- 126.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 127.Kops GJ, Burgering BM. Forkhead transcription factors: new insights into protein kinase B (c-akt) signaling. J Mol Med. 1999;77:656–65. doi: 10.1007/s001099900050. [DOI] [PubMed] [Google Scholar]
- 128.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (Foxo3a) Mol Cell Biol. 2001;21:953–65. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Altomonte J, Richter A, Harbaran S, Suriawinata j, Nakae J, Thung SN, Meseck M, Accili D, Dong H. Inhibition of Foxo1 Function Is Associated with Improved Fasting Glycemia in Diabetic Mice. Am J Physiol. 2003;285:718–28. doi: 10.1152/ajpendo.00156.2003. [DOI] [PubMed] [Google Scholar]
- 130.Biggs WH, III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC. Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA. 1999;96:7421–6. doi: 10.1073/pnas.96.13.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nakae J, Barr V, Accili D. Differential regulation of gene expression by insulin and IGF-1 receptors correlates with phosphorylation of a single amino acid residue in the forkhead transcription factor FKHR. EMBO J. 2000;19:989–96. doi: 10.1093/emboj/19.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Durham SK, Suwanichkul A, Scheimann AO, Yee D, Jackson JG, Barr FG, Powell DR. FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology. 1999;140:3140–6. doi: 10.1210/endo.140.7.6856. [DOI] [PubMed] [Google Scholar]
- 133.Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of Glucose-6-phosphatase Gene Expression by Protein Kinase Balpha and the Forkhead Transcription Factor FKHR. J Biol Chem. 2000:36324–33. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- 134.Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem. 1999;274:17179–83. doi: 10.1074/jbc.274.24.17179. [DOI] [PubMed] [Google Scholar]
- 135.Tomizawa M, Kumar A, Perrot V, Nakae J, Accili D, Rechler M. Insulin inhibits the activation of transcription by a C-terminal fragment of the forkhead transcription factor FKHR. J Bio Chem. 2000;275:7289–95. doi: 10.1074/jbc.275.10.7289. [DOI] [PubMed] [Google Scholar]
- 136.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–67. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sparks JD, Sparks CE. Overindulgence and metabolic syndrome: is FoxO1 a missing link? J Clin Invest. 2008;118:2012–5. doi: 10.1172/JCI35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. Foxo1 Mediates Insulin Action on ApoC-III and Triglyceride Metabolism. J Clin Invest. 2004;114:1493–503. doi: 10.1172/JCI19992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qu S, Altomonte J, Perdomo G, He J, Fan Y, Kamagate A, Meseck M, Dong HH. Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology. 2006;147:5641–52. doi: 10.1210/en.2006-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matsumoto M, Han S, Kitamura T, Accili D. Dual role of transcription factor FoxO1 in controlling hepatic insulin sensitivity and lipid metabolism. J Clin Invest. 2006;116:2464–72. doi: 10.1172/JCI27047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo1 in liver. Cell metabolism. 2007;6:208–16. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 142.Valenti L, Rametta R, Dongiovanni P, Maggioni M, Ludovica Fracanzani A, Zappa M, Lattuada E, Roviaro G, Fargion S. Increased Expression and Activity of the Transcription Factor Foxo1 in Nonalcoholic Steatohepatitis. Diabetes. 2008 doi: 10.2337/db07-0714. [DOI] [PubMed] [Google Scholar]
- 143.Samuel VT, Choi CS, Phillips TG, Romanelli AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg RA, Shulman GI, Veniant MM. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes. 2006;55:2042–50. doi: 10.2337/db05-0705. [DOI] [PubMed] [Google Scholar]
- 144.Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–29. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- 145.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell metabolism. 2008;8:65–76. doi: 10.1016/j.cmet.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–40. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 147.Yuan Z, Becker EB, Merlo P, Yamada T, DiBacco S, Konishi Y, Schaefer EM, Bonni A. Activation of FOXO1 by Cdk1 in cycling cells and postmitotic neurons. Science. 2008;319:1665–8. doi: 10.1126/science.1152337. [DOI] [PubMed] [Google Scholar]
- 148.Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, downregulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem. 2004;279:41114–23. doi: 10.1074/jbc.M400674200. [DOI] [PubMed] [Google Scholar]
- 149.Wu AL, Kim JH, Zhang C, Unterman TG, Chen J. Forkhead box protein O1 negatively regulates skeletal myocyte differentiation through degradation of mammalian target of rapamycin pathway components. Endocrinology. 2008;149:1407–14. doi: 10.1210/en.2007-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kitamura T, Ido Kitamura Y. Role of FoxO Proteins in Pancreatic beta Cells. Endocrine journal. 2007;54:507–15. doi: 10.1507/endocrj.kr-109. [DOI] [PubMed] [Google Scholar]
- 151.Buteau J, Accili D. Regulation of pancreatic beta-cell function by the forkhead protein FoxO1. Diabetes, obesity & metabolism. 2007;9:140–6. doi: 10.1111/j.1463-1326.2007.00782.x. [DOI] [PubMed] [Google Scholar]
- 152.Nakae J, Cao Y, Oki M, Orba Y, Sawa H, Kiyonari H, Iskandar K, Suga K, Lombes M, Hayashi Y. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57:563–76. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 153.Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation. Cell metabolism. 2007;6:105–14. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hsieh J, Hayashi AA, Webb J, Adeli K. Postprandial dyslipidemia in insulin resistance: Mechanisms and role of intestinal insulin sensitivity. Atheroscler Suppl. 2008 doi: 10.1016/j.atherosclerosissup.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 155.Vine DF, Takechi R, Russell JC, Proctor SD. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 2007;190:282–90. doi: 10.1016/j.atherosclerosis.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 156.Zoltowska M, Ziv E, Delvin E, Sinnett D, Kalman R, Garofalo C, Seidman E, Levy E. Cellular aspects of intestinal lipoprotein assembly in Psammomys obesus: a model of insulin resistance and type 2 diabetes. Diabetes. 2003;52:2539–45. doi: 10.2337/diabetes.52.10.2539. [DOI] [PubMed] [Google Scholar]
- 157.Mangat R, Su J, Scott PG, Russell JC, Vine DF, Proctor SD. Chylomicron and apoB48 metabolism in the JCR: LA corpulent rat, a model for the metabolic syndrome. Biochemical Society transactions. 2007;35:477–81. doi: 10.1042/BST0350477. [DOI] [PubMed] [Google Scholar]
- 158.Qin B, Qiu W, Avramoglu RK, Adeli K. Tumor necrosis factor-alpha induces intestinal insulin resistance and stimulates the overproduction of intestinal apolipoprotein B48-containing lipoproteins. Diabetes. 2007;56:450–61. doi: 10.2337/db06-0518. [DOI] [PubMed] [Google Scholar]
- 159.Haidari M, Leung N, Mahbub F, Uffelman KD, Kohen-Avramoglu R, Lewis GF, Adeli K. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J Biol Chem. 2002;277:31646–55. doi: 10.1074/jbc.M200544200. [DOI] [PubMed] [Google Scholar]