Abstract
Summary
Comparison of infrared spectroscopic images of sections from biopsies of placebo-treated post-menopausal women and women treated for 3 years with 10 mg/day alendronate demonstrated significant increases in cortical bone mineral content, no alterations in other spectroscopic markers of “bone quality,” but a decrease in tissue heterogeneity.
Methods
The material properties of thick sections from iliac crest biopsies of seven alendronate-treated women were compared to those from ten comparably aged postmenopausal women without bone disease, using infrared spectroscopic imaging at ~7 µm spatial resolution. Parameters evaluated were mineral/matrix ratio, crystallinity, carbonate/amide I ratio, and collagen maturity. The line widths at half maximum of the pixel histograms for each parameter were used as measures of heterogeneity.
Results
The mineral content (mineral/matrix ratio) in the cortical bone of the treated women’s biopsies was higher than that in the untreated control women. Crystallinity, carbonate/protein, and collagen maturity indices were not significantly altered; however, the pixel distribution was significantly narrowed for all cortical and trabecular parameters with the exception of collagen maturity in the alendronate treatment group.
Conclusions
The increases in mineral density and decreased fracture risk associated with bisphosphonate treatment may be counterbalanced by a decrease in tissue heterogeneity, which could impair tissue mechanical properties. These consistent data suggest that alendronate treatment, while increasing the bone mass, decreases the tissue heterogeneity.
Keywords: Alendronate, Bone heterogeneity, Infrared spectroscopic imaging, Osteoporosis
Introduction
Fourier transform infrared (FTIR) imaging spectroscopy [1] has previously been used to characterize the distribution of mineral content, mineral crystallinity, acid phosphate content, carbonate content, and collagen maturity in human bone biopsies at ~10 µm spatial resolution [2–10]. While it is well accepted that whole bone strength is related to the bone mineral content, these other spectroscopic parameters have been correlated with whole bone and nano-indentation mechanical properties [11–12]. The mechanical parameters have also been correlated with related Raman spectroscopic parameters [13, 14]. Recently, we reported that, in both low- and high-turnover osteoporosis, the distribution of these spectroscopic properties was narrowed [9], showing a more homogeneous distribution of properties, and by analogy with composite materials [15, 16], we and others have suggested that a decrease in tissue heterogeneity was deleterious [17–21].
Fratzl has attributed the heterogeneity of bone seen on measurement of bone mineral density distribution (BMDD) to site specific variations in the individual activities of bone structural units and the extent of primary and secondary mineralization that has occurred [22]. His group has found that values and distributions of BMDD that are different from normal are associated with disease states [22]. While BMDD provides information on the mineral content of the tissue, FTIR imaging spectroscopy can also show the distribution of other material properties (mineral composition, collagen maturity) along with the distribution of mineral content.
Serial bone biopsies from patients treated with the bisphosphonate risedronate studied by FTIR imaging spectroscopy demonstrated increased mineral content, yet decreased crystallinity and matrix maturity, but tissue heterogeneity was not assessed [10]. However, earlier studies using microradiography or backscatter electron imaging found a reduction in BMDD heterogeneity with risedronate [19] and alendronate (ALN) [23, 24] treatments, which was attributed to prolonged secondary mineralization. The goal of the present study was to test the hypothesis that treatment with the more commonly used antiresorptive, alendronate, would similarly result in greater mineral content but would increase the uniformity (decrease the heterogeneity) of both this and other material properties measured by FTIR imaging. Because it is difficult to obtain serial biopsies, we took advantage of biopsies previously studied by histomorphometry [25].
Materials and methods
Specimens for analysis
Transilial bone biopsies were available from a 3-year double-blind, randomized, placebo-controlled trial of alendronate in 447 healthy postmenopausal women, aged 40–59 years with menopause 6–36 months before enrollment [25], corresponding to a time now known to be the period of maximum bone loss [26]. This was a prevention trial and BMD for all participants at baseline was within two standard deviations of the normal mean value. Women with disorders of bone and mineral metabolism (including Paget’s disease of bone, vitamin D deficiency, and hyperparathyroidism), nontraumatic fractures, previous treatment with bisphosphonates or treatment within the year before enrollment with estrogen, progestin, calcitonin, glucocorticoids, anticonvulsant drugs, phosphate-binding antacids, or more than normal daily amounts of vitamin A or D were excluded. At baseline, bone mineral density in the participants was approximately 10% below the mean values for young adult women, and the placebo recipients lost up to 3–4% of bone density at the spine, femoral neck, and trochanter during the trial, indicating these women were experiencing postmenopausal bone loss [25]. Seven-millimeter diameter biopsies with two cortices and the intervening cancellous bone intact were on hand from the placebo [initial age, 51.3±0.4 (SEM) years] and oral alendronate [initial age, 52.1±0.3 (SEM) years] at 10 mg/day groups. Seven PMMA embedded iliac crest biopsies from osteoporotic women treated (3 yrs) with alendronate, ALN (10 mg/day×3 years), and vitamin D and calcium, and ten biopsies from the placebo (vitamin D and calcium) group were provided by one of the authors (R.S.W.) under an IRB-approved protocol to reexamine the archival specimens from the trial [25].
FTIRI
The biopsies were sectioned at 2 µm on a Jung-K microtome, and three to five sections mounted on barium fluoride windows (SpectraTech, Hopewell Junction, NY, USA) and used for FTIR imaging analyses of three to five randomly selected regions, each of cortical and cancellous bone [1]. Randomly selected areas were assayed to insure inclusion of different regions of the biopsy section for each individual. In brief, spectra (see Fig. 1) were acquired with a Perkin Elmer Spectrum Spotlight 300 Imaging System (Perkin Elmer Instruments, Shelton, CT, USA), consisting of a step-scanning FTIR spectrometer with an mercury–cadmium–telluride focal plane array detector placed at an image focal plane of an IR microscope. Images were collected in transmission mode at a spectral resolution of 4 cm−1 in the frequency region comprised between 2,000 and 720 cm−1 with an IR detector pixel size (6.25×6.25 µm) with cortical and cancellous bone analyzed separately. Background (IR window only) and PMMA spectra were collected for each section analyzed, and these spectra were used for correction of the sample spectral data using ISYS software (Spectral Dimensions, Olney, MD, USA). Spectra were base-lined and the PMMA contribution subtracted using ISYS software.
Fig. 1.
Infrared spectrum of healthy adult cortical bone showing regions of interest for the current study. The spectrum has been baseline corrected and the PMMA contribution subtracted
FTIRI parameter evaluation
The parameters calculated for each image as described in detail elsewhere [1] were mineral-to-matrix ratio (linearly related to mineral content) calculated as the ratio of the integrated areas under the phosphate (920–1180 cm−1) band to that of the Amide I band (1596–1710 cm−1), and carbonate-to-phosphate ratio (level of carbonate substitution in the HA crystal) calculated as the ratio of the integrated area of the carbonate peak (852–890 cm−1) to the phosphate area. Because phosphate was found to increase while amide I was relatively constant, the carbonate to amide I ratio was also calculated. The crystallinity (XST, related to crystallite size and perfection as determined by x-ray diffraction) was calculated as the intensity ratio of phosphate subbands at 1030 and 1020 cm−1. The collagen cross-link ratio (XLR) was calculated as the intensity ratio of Amide I subbands at 1660 and 1686 cm−1; the 1686 subband differs in position based on that previously used [5] and was chosen based on curve-fitting of the amide I peak obtained after spectral subtraction of PMMA. Mean and standard deviations for the three to five images in each bone type were calculated and compared. The values were then averaged for the groups (ALN) vs. control, and differences in each parameter calculated by analysis of variance.
Heterogeneity
The spatial distribution of each parameter in each image was calculated as the full width at half maximum (FWHM) from the pixel histogram for each image, and these values averaged as above. FTIR parameters and heterogeneity of each parameter analyzed were compared by ANOVA, with Bonferroni p<0.05 accepted as significant. Data was displayed as pixel images for each of the parameters, as averaged values for each data group and average FWHM for each parameter in each data group.
Results
Retrospective data
The biopsies used for these analyses were selected from samples previously shown to have comparable starting BMD, no confounding factors to prevent them from participating in the study. The results of the 1994–1997 trial showed that alendronate at 10 mg/d increased BMD from baseline at the lumbar spine, femoral neck, trochanter, and total body by 1% to 4%, while placebo led to losses of 2% to 4% at these sites. Alendronate also decreased biochemical markers of bone turnover [25].
FTIRI
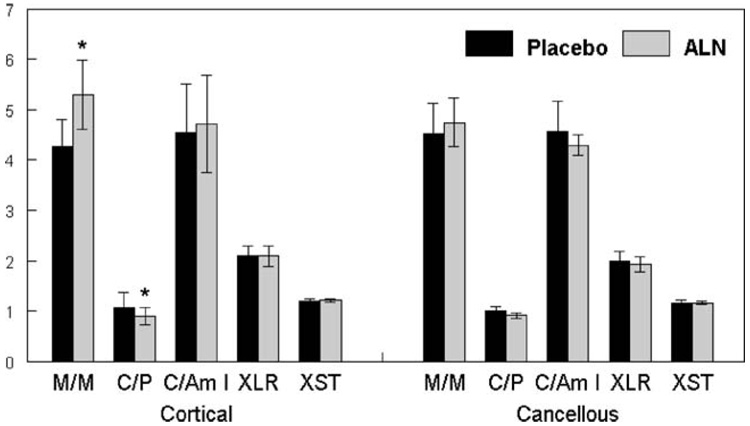
Figure 1 illustrates a typical FTIR spectrum of adult human cortical bone showing the spectral areas of interest for the analyses described here. In agreement with our primary hypothesis, as seen in Fig. 2, compared to the placebo control biopsies, alendronate treatment resulted in a significantly increased mineral-to-matrix ratio (p=0.03) in cortical bone, and the ratio tended (p=0.055) to be increased in the trabeculae. Carbonate-to-phosphate peak area ratio was significantly decreased (p<0.01) relative to control in all ALN-treated bone (cortical and trabecular); however, there was no significant change in carbonate-to-amide I ratio. Carbonate-to-amide I was not altered, indicating that the carbonate-to-phosphate ratio was decreased due to the elevated phosphate content. There was a slight but not significant increase in crystallinity in the trabecular and cortical bone of the ALN-treated group relative to the placebo group, and a slight decrease in collagen cross-link ratio in both cortical and trabecular bone relative to the placebo group.
Fig. 2.
Mean±SD for each parameter measured from three to five FTIR images of cortical and cancellous bone per biopsy in patients treated with alendronate (ALN) or a placebo. Values shown are mineral/matrix ratio (M/M); carbonate/phosphate ratio (C/P), this value has been multiplied by 100 for ease of presentation; carbonate/amide I ratio (C/Am I) also multiplied by 100; crystallinity (XST); and collagen maturity ratio (XLR). For each parameter, *p<0.05 relative to placebo control
Typical images for representative parameters in ALN and control groups are shown in Fig. 3. It is visually apparent from these figures that the mineral content is increased, as was shown by the averaged data in Fig. 2.
Fig. 3.
Typical FTIRI images and pixel histograms for cortical (left) and cancellous (right) bone from one placebo and one ALN-treated case; a mineral/matrix, b carbonate/phosphate
Heterogeneity
The FWHM was calculated from the pixel histograms for each individual image as illustrated in Fig. 4a. The width of the FWHM reflecting the heterogeneity of each of the measured parameters was decreased for all parameters in the ALN-treated cases (Fig. 4b), but significant differences in uniformity (narrowing of the bands) was seen for mineral-to-matrix ratio, carbonate-to-phosphate ratio, carbonate-to amide I ratio, and crystallinity in cortical bone, and for carbonate-to-phosphate ratio, carbonate-to-amide I ratio, crystallinity, and collagen cross-link ratio in cancellous bone, perhaps reflecting the increased turnover of cancellous bone.
Fig. 4.
Heterogeneity was calculated from FWHM of pixel histograms as illustrated in a and b. The mean±SD for FWHM for each parameter in the cortical and cancellous bones of the ALN and placebo groups are shown. Values shown for carbonate/phosphate ratio (C/P) and carbonate/amide I ratio have been multiplied by 100 for ease of presentation, crystallinity (XST), and collagen maturity ratio (XLR) values multiplied by 10. *p<0.05 relative to placebo control
Discussion
In this study of 17 biopsies of ALN-treated and placebo-treated post-menopausal women biopsied 3 years after treatment started, we found that ALN treatment resulted in greater cortical bone mineral content, a decrease in bone carbonate-to-phosphate ratio (reflecting the increased phosphate content), but no other statistically significant changes in material properties as determined by FTIR imaging. The observation that the increase in mineral content in the cortical bone was significant but that in trabecular bone was not (p=0.06) most likely reflects the much greater variation in the spatial distribution of trabecular mineral content [3]. In agreement with earlier studies based on quantitative back-scatter electron imaging of ALN-treatment of minipigs and humans, and of mice with osteogenesis imperfecta [23, 24, 27], we found that the ALN treatment decreased the bone mineral content heterogeneity. Additionally, decreases in heterogeneity were found for crystallinity, carbonate content, and collagen maturity.
Tissue heterogeneity
Bone is heterogeneous due to its constant remodeling, and the different rates at which mineralization (both primary and secondary) occur [21]. This heterogeneity is reflected in the distribution of bone mineral density, mineral crystal composition, and collagen maturity. Too high a mineral content is known to make bones more brittle [28], while too low a mineral content makes them less stiff. The heterogeneity of individual trabeculae are thought to affect the mechanical properties of the whole bone [29, 30]. There is obviously an optimal distribution of the amount of mineral, where both too much and too little uniformity leads to more brittle and more ductile, less stiff bone, respectively [28]. We suggest that it is not only the amount of mineral in the bone packets but also the properties of the mineral crystals and the matrix that affect bone strength.
The decrease in heterogeneity of the FTIR parameters seen in the alendronate-treated subjects is reminiscent of the changes noted in a back scatter electron imaging investigation of biopsies from post-menopausal women treated with the bisphosphonate risedronate [19] and their placebo-treated controls at 3 years after the start of treatment. In that study, greater mineral content and a lesser heterogeneity was also noted, although by 5 years, the heterogeneity had increased, suggesting that new bone formation was occurring. In analysis of those same biopsies, FTIR imaging analyses found that the increase in crystallinity and collagen maturity seen in the placebo was prevented by risedronate treatment at 3 and 5 years. While the heterogeneity of the FTIRI parameters was not assessed directly, the sizes of the error bars suggest that the distributions were narrowed for crystallinity but not for the collagen cross-link ratio [10]. Most interesting, in agreement with the present study, in the study of the effect of bisphosphonate on BMDD [19], the control group, which is similar to those in the present study, were treated with calcium and vitamin D, showed a broadened (more heterogeneous) BMDD peak width [29, 31]. This observation and the increase in mineral content observed in the placebo group in the BMDD study implied that vitamin D and calcium could have been suppressing remodeling resulting in older mean tissue age.
Boivin et al. [32], in a study of 53 post-menopausal women treated for a similar amount of time (3 years) with the same dose of ALN (10 mg/day) as used in the present study, reported an 11% increase in the degree of mineralization of cortical and cancellous bone assessed by quantitative microradiography, along with greater mineral homogeneity, again in agreement with our findings. The question is whether more homogeneous bone, both in terms of mineral content and the composition of the mineral crystallites, has a positive or negative effect on fracture risk and bone mechanical properties.
Bisphosphonate use
The bisphosphonates are widely recognized as effective therapies for increasing bone mass and reducing fracture risk in osteoporosis [33–36]. Even after 10 years of treatment with 10 mg/day ALN, no deleterious effects were noted in two large clinical trials [33, 35]. However, recently there have been a limited number of reports of unexplained subtrochanteric fractures in patients taking alendronate [37–40]. The increased brittleness of the bones associated with “over-suppression” of bone remodeling in this small group of patients might be accounted for by the decreased heterogeneity of the bones that we noted in our study. However, it will be necessary to expand this study to additional cases to substantiate this suggestion.
Study limitations
It must be noted that the conclusions of this study are limited by the small sample size available for analysis and the fact that we did not have pre- and post-treatment biopsies. To address this concern, we are currently doing two short-term studies of alendronate treatment in non-rodent models of osteoporosis. In the absence of matched pairs, our results and conclusions must be tempered. This is particularly true because Burr has reported that with age, there is a similar loss of new bone mineral and greater homogeneity of the tissue [18]. Both of these, increasing mineral content and decreasing heterogeneity can have a similar effect, making the tissue more brittle. However, from the results of this study, even though the age range in the two groups were similar, because we did not have baseline data, we cannot separate drug and aging effects.
The second limitation of the study is that the patients, who were osteopenic but not osteoporotic, were treated with alendronate for only 3 years. While we have seen measurable short-time changes in mineral parameters with a SERM [1], the risedronate study of material properties reported findings similar to those for alendronate at 3 years but evidence of increased heterogeneity at 5 years [10]. Since in a large clinical study, women treated with a similar dose of alendronate for as many as 10 years did not show adverse effects [33, 36], it is likely that the advantageous increase in bone mineral density in most patients far outweighs the disadvantage of decreased heterogeneity. Many more specimens would need to be evaluated to reach any stronger conclusion.
Conclusion
We suggest that alendronate, while increasing bone mineral density and thereby enhancing the mechanical properties of the bone, has the potential to have negative effects. These negative effects are not seen in the majority of treated patients; however, analyses of the tissues from a randomly selected group of post-menopausal women treated 3 years with alendronate, and Ca and vitamin D or only with Ca and vitamin D (placebo control) in the absence of adverse effects continuously demonstrated a decrease in tissue heterogeneity, which we speculate, combined with other factors, could contribute to increased brittleness of the bone in some patients.
Acknowledgment
This study was supported by NIH grant AR043125 and Core Center grant AR046121 to A.L.B. and by AR046191 to R.S.W. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program grant number C06-RR 12538-01 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Conflicts of interest None.
Contributor Information
A. L. Boskey, Musculoskeletal Integrity Program, Hospital for Special Surgery, New York, NY, USA
L. Spevak, Musculoskeletal Integrity Program, Hospital for Special Surgery, New York, NY, USA
R. S. Weinstein, Division of Endocrinology and Metabolism, Center for Osteoporosis and Metabolic Bone Diseases, Department of Internal Medicine and the Central Arkansas Veterans Healthcare System, University of Arkansas for Medical Sciences, Little Rock, AR, USA
References
- 1.Boskey AL, Mendelsohn R. Infrared spectroscopic characterization of mineralized tissues. Vib Spectrosc. 2005;38:107–114. doi: 10.1016/j.vibspec.2005.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paschalis EP, DiCarlo E, Betts F, et al. FTIR micro-spectroscopic analysis of human osteonal bone. Calcif Tissue Int. 1996;59:480–487. doi: 10.1007/BF00369214. [DOI] [PubMed] [Google Scholar]
- 3.Paschalis EP, Betts F, DiCarlo E, et al. FTIR micro-spectroscopic analysis of normal human cortical and trabecular bone. Calcif Tissue Int. 1997;61:480–486. doi: 10.1007/s002239900371. [DOI] [PubMed] [Google Scholar]
- 4.Paschalis EP, Betts F, DiCarlo E, et al. FTIR micro-spectroscopic analysis of human iliac crest biopsies from untreated osteoporotic bone. Calcif Tissue Int. 1997;61:487–492. doi: 10.1007/s002239900372. [DOI] [PubMed] [Google Scholar]
- 5.Paschalis EP, Verdelis K, Doty SB, et al. Spectroscopic characterization of collagen cross-links in bone. J Bone Miner Res. 2001;16:1821–1828. doi: 10.1359/jbmr.2001.16.10.1821. [DOI] [PubMed] [Google Scholar]
- 6.Paschalis EP, Boskey AL, Kassem M, Eriksen EF. Effect of hormone replacement therapy on bone quality in early postmenopausal women. J Bone Miner Res. 2003;18:955–959. doi: 10.1359/jbmr.2003.18.6.955. [DOI] [PubMed] [Google Scholar]
- 7.Paschalis EP, Recker R, DiCarlo E, et al. Distribution of collagen cross-links in normal human trabecular bone. J Bone Miner Res. 2003;18:1942–1946. doi: 10.1359/jbmr.2003.18.11.1942. [DOI] [PubMed] [Google Scholar]
- 8.Paschalis EP, Shane E, Lyritis G, et al. Bone fragility and collagen cross-links. J Bone Miner Res. 2004;19:2000–2004. doi: 10.1359/JBMR.040820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boskey AL, DiCarlo E, Paschalis E, et al. Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: an FT-IR microspectroscopic investigation. Osteoporos Int. 2005;16:2031–2038. doi: 10.1007/s00198-005-1992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durchschlag E, Paschalis EP, Zoehrer R, et al. Bone material properties in trabecular bone from human iliac crest biopsies after 3- and 5-year treatment with risedronate. J Bone Miner Res. 2006;21:1581–1590. doi: 10.1359/jbmr.060701. [DOI] [PubMed] [Google Scholar]
- 11.Miller LM, Little W, Schirmer A, et al. Accretion of bone quantity and quality in the developing mouse skeleton. J Bone Miner Res. 2007;22:1037–1045. doi: 10.1359/jbmr.070402. [DOI] [PubMed] [Google Scholar]
- 12.Busa B, Miller LM, Rubin CT, et al. Rapid establishment of chemical and mechanical properties during lamellar bone formation. Calcif Tissue Int. 2005;77:386–394. doi: 10.1007/s00223-005-0148-y. [DOI] [PubMed] [Google Scholar]
- 13.Silva MJ, Brodt MD, Wopenka B, et al. Decreased collagen organization and content are associated with reduced strength of demineralized and intact bone in the SAMP6 mouse. J Bone Miner Res. 2006;21:78–88. doi: 10.1359/JBMR.050909. [DOI] [PubMed] [Google Scholar]
- 14.Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res. 2006;21:466–476. doi: 10.1359/JBMR.051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumanov AT, Gunyaev GM, Lyutsau VG, Stepanychev EI. Structure, properties, and tests of carbon-reinforced plastics. Mech Compos Mater. 1975;11:167–327. [Google Scholar]
- 16.National Materials Advisory Board Committee on high-performance synthetic fibers for composites; Commission on Engineering and Technical Systems; National Research Council. High-performance synthetic fibers for composites publication NMAB-458. Washington, DC: National Academy Press; 1992. pp. 49–103. [Google Scholar]
- 17.Smith EJ, McEvoy A, Little DG, et al. Transient retention of endochondral cartilaginous matrix with bisphosphonate treatment in a long-term rabbit model of distraction osteogenesis. J Bone Miner Res. 2004;19:1698–1705. doi: 10.1359/JBMR.040709. [DOI] [PubMed] [Google Scholar]
- 18.Burr DB. Bone material properties and mineral matrix contributions to fracture risk or age in women and men. J Musculoskelet Neuronal Interact. 2002;2:201–204. [PubMed] [Google Scholar]
- 19.Zoehrer R, Roschger P, Paschalis EP, et al. Effects of 3- and 5-year treatment with risedronate on bone mineralization density distribution in triple biopsies of the iliac crest in postmenopausal women. J Bone Miner Res. 2006;21:1106–1112. doi: 10.1359/jbmr.060401. [DOI] [PubMed] [Google Scholar]
- 20.Roschger P, Dempster DW, Zhou H, et al. New observations on bone quality in mild primary hyperparathyroidism as determined by quantitative backscattered electron imaging. J Bone Miner Res. 2007;22:717–723. doi: 10.1359/jbmr.070120. [DOI] [PubMed] [Google Scholar]
- 21.Ruffoni D, Fratzl P, Roschger P, et al. The bone mineralization density distribution as a fingerprint of the mineralization process. Bone. 2007;40:1308–1319. doi: 10.1016/j.bone.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Roschger P, Pascalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–466. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Roschger P, Fratl P, Klaushofer K, Rodan G. Mineralization of cancellous bone after alendronate and sodium fluoride treatment: a quantitative backscattered electron imaging study on minipig ribs. Bone. 1997;20:393–397. doi: 10.1016/s8756-3282(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 24.Roschger P, Rinnerthaler S, Yates J, Rodan GA, et al. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29:185–191. doi: 10.1016/s8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 25.McClung M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. A double-blind, randomized, controlled trial. Alendronate Osteoporosis Prevention Study Group. Ann Intern Med. 1998;128:253–261. doi: 10.7326/0003-4819-128-4-199802150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Finkelstein JS, Brockwell SE, Mehta V, Greendale GA, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Misof BM, Roschger P, Baldini T, et al. Differential effects of alendronate treatment on bone from growing osteogenesis imperfecta and wild-type mouse. Bone. 2005;36:150–158. doi: 10.1016/j.bone.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Fratzl P, Roschger P, Fratzl-Zelman N, Paschalis EP, et al. Evidence that treatment with risedronate in women with postmenopausal osteoporosis effects bone mineralization and bone volume. Calciif Tissue Int. 2007;81:73–80. doi: 10.1007/s00223-007-9039-8. [DOI] [PubMed] [Google Scholar]
- 29.Keaveny TM, Hayes WC. A 20-year perspective on the mechanical properties of trabecular bone. Transact ASME. 1993;115:534–554. doi: 10.1115/1.2895536. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein SA. The mechanical properties of trabecular bone: dependence on anatomic location and function. J Biomech. 1987;20:1055–1061. doi: 10.1016/0021-9290(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 31.Zoehrer R, Roschger P, Duschschlag E, Fratzl P, et al. Bone mineralization density distribution in triple biopsies of the iliac crest in post-menopausal women. J Bone Miner Res. 2006;21:1106–1112. doi: 10.1359/jbmr.060401. [DOI] [PubMed] [Google Scholar]
- 32.Boivin GY, Chavassieux PM, Santora AC, et al. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone. 2000;27:687–694. doi: 10.1016/s8756-3282(00)00376-8. [DOI] [PubMed] [Google Scholar]
- 33.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 34.Bone HG, Hosking D, Devogelaer JP, et al. Ten years’ experience with alendronate for osteoporosis in post menopausal women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 35.Chavassieux PM, Arlot ME, Reda C, et al. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997;100:1475–1480. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–2938. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 37.Goh SK, Yang KY, Koh JS, et al. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br 2007. 2007;89:349–353. doi: 10.1302/0301-620X.89B3.18146. [DOI] [PubMed] [Google Scholar]
- 38.Lenart B, Lorich DG, Lane JM. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. NEJM. 2008;358:1304. doi: 10.1056/NEJMc0707493. [DOI] [PubMed] [Google Scholar]
- 39.Lee P, van der Wall H, Seibel MJ. Looking beyond low bone mineral density: multiple insufficiency fractures in a woman with post-menopausal osteoporosis on alendronate therapy. J Endocrinol Invest. 2007;30:590–597. doi: 10.1007/BF03346353. [DOI] [PubMed] [Google Scholar]
- 40.Imai K, Yamamoto S, Anamizu Y, Horiuchi T. Pelvic insufficiency fracture associated with severe suppression of bone turnover by alendronate therapy. J Bone Miner Metab. 2007;25:333–336. doi: 10.1007/s00774-007-0771-y. [DOI] [PubMed] [Google Scholar]