Abstract
Because poor comprehension has been associated with small cerebral volume and there is a high comorbidity between developmental dyslexia, ADHD, and specific language impairment, the goal of this study was to determine if cerebral volume is reduced in dyslexia and ADHD in general, as some suggest, or if reduction in volume corresponds with poor receptive language functioning regardless of diagnosis. Participants included 46 children with and without dyslexia and ADHD, ages 8-12 years. Results indicated that cerebral volume was comparable between those with and without dyslexia and ADHD overall. However, when groups were further divided into those with and without receptive language difficulties, children with poor receptive language had smaller volumes bilaterally as hypothesized. Nonetheless, the relationship between cerebral volume and receptive language was not linear; rather, our results suggest small volume is associated with poor receptive language only in those with the smallest volumes in both dyslexia and ADHD.
Keywords: Dyslexia, Attention-Deficit/Hyperactivity Disorder, Magnetic Resonance Imaging
Developmental dyslexia and specific language impairment are common neurodevelopmental disorders that share about a 30% comorbidity1. Hence, of particular interest to this paper is whether the reduction in cerebral hemisphere volume occasionally seen in dyslexia is related to poor receptive semantic/syntactic functioning. This issue is pertinent as research suggests individuals with developmental language disorder present with smaller cerebral volume than controls. For example, Preis and colleagues2 found a 7% forebrain reduction in developmental language disorder, and Herbert and colleagues3 found developmental language disorder is associated with a smaller cerebral cortex. As many believe specific language impairment is due to some form of generalized deficit, rather than one limited to language per se when receptive language is affected3-9, it is not surprising that bilateral cerebral hemisphere volume is reduced in this population10. Consistent with this, intelligence often is at least mildly reduced in specific language impairment10, and intellectual functioning is positively correlated with bilateral cerebral volume in general2, 11-15.
Specific language impairment tends to be diagnosed when children have unexplained oral language deficits that extend beyond their nonverbal intellect7, 16. Although children with specific language impairment typically have deficits in the comprehension and/or expression of semantics, syntax and grammatical morphemes7, 16, 17, many also have deficits in phonological processing, including phonological awareness and phonological short-term memory17-20. In addition, children with specific language impairment often are poor readers, meeting most psychometric definitions of dyslexia, particularly when language problems continue into the school years16.
Children with dyslexia commonly present with poor phonological processing21, 22, which may include deficits in phonological awareness23-26, rapid retrieval of phonological material from long-term memory27-29, and phonological short-term/working memory30-33. The breadth and chronic nature of these problems has led some to suggest that dyslexia should be considered a developmental language disorder, where the central feature is poor phonological processing which affects word identification and spelling34, 35. In addition to poor phonological processing, deficits also have been found in speech perception, articulation, semantics, syntactic processing, and verbal memory in this population16, 34, 36, 37.
Given the overlap between dyslexia and specific language impairment, and given that both disorders present with a great deal of heterogeneity, what may be most important is the type of deficits seen. More specifically, deficits in phonological processing are associated with poor word identification, decoding, and spelling, whereas deficits in listening comprehension and other non-phonological linguistic skills are associated with poor reading comprehension12, 34, 36, 38, 39. This is true regardless of whether a child has been diagnosed with specific language impairment or dyslexia16. Because of these associations, Bishop and Snowling16 proposed a two dimensional model of dyslexia and specific language impairment, with one dimension being phonological processing and the other being non-phonological linguistic skills including semantic, syntactic, and discourse-level processing. They suggested these two dimensions may be a better depiction of predictors of reading performance than the current dyslexia/specific language impairment classifications.
Recent work by Leonard and colleagues is consistent with this two dimensional model. When studying an adult dyslexia sample, Leonard and colleagues40 found smaller cerebral hemisphere volume was associated with reduced listening comprehension, reading comprehension, and verbal intellect, whereas poor phonological processing and decoding but intact oral and written comprehension [called ‘phonological dyslexia’] were associated with rightward cerebral asymmetry, leftward cerebellar asymmetry or symmetry, leftward asymmetry of the planum temporale, and duplication of Heschl’s gyrus on the left. In subsequent studies they found a dissociation between phonological dyslexia and specific language impairment, with specific language impairment being associated with smaller, symmetrical structures in the perisylvian region and smaller cerebral hemisphere volume in general, and phonological dyslexia being associated with additional Heschl’s gryi, larger language regions, and exaggerated planum asymmetries40-42. Although this research suggests dyslexia is associated with smaller hemisphere volume primarily when oral and written comprehension deficits are present, other research suggests hemisphere volume may be reduced in dyslexia in general43-45. The extent to which reduced cerebral volume in dyslexia is associated with poor non-phonological linguistic skills requires further examination.
The debate over the best way to conceptualize dyslexia and specific language impairment has relevance to the debate in the literature over the best way to define dyslexia. Several researchers suggest the discrepancy definition should be abandoned, with focus being placed solely on poor decoding ability23, 46, 47. This change in definition was suggested as poor readers with and without an IQ discrepancy are comparable in phonological awareness23, 47, the ‘core’ deficit in developmental dyslexia26, 30, 48, 49. Nonetheless, those who meet the traditional discrepancy definition may be more likely to have deficits limited to phonological processing and decoding skill whereas poor readers who do not have a discrepancy may be more heterogeneous as a group, including those who have phonological and non-phonological linguistic deficits16, 34. Hence, the latter group may be more likely to include individuals with smaller cerebral hemisphere volumes given the literature reviewed above. Furthermore, the two groups may differ slightly in genetic contributions. Whereas aspects of phonological processing have been linked to chromosome 650, non-phonological linguistic deficits have been linked to chromosome 19, particularly poor expressive language functioning51. Clearly the best definition of dyslexia to use when conducting neurobiological research requires further examination.
Along with dyslexia and specific language impairment sharing a high comorbidity, dyslexia and Attention-Deficit/Hyperactivity Disorder (ADHD) share about a 15-40% comorbidity52, 53, and ADHD and specific language impairment share about a 31-60% comorbidity1, 54. In addition, several researchers have reported reduced cerebral hemisphere volume in ADHD, with a 3-8% reduction in cerebral volume being found55-59. However, a study by Filipek and colleagues60 failed to find a reduction in cerebral hemisphere volume in ADHD. Non-phonological linguistic deficits are common in ADHD, including poor pragmatic language functioning61-63, reduced oral comprehension1, 61, 64, and poor syntax formation65. Nevertheless, limited research has been conducted to determine whether smaller cerebral volume in ADHD is related to worse non-phonological linguistic functioning.
The primary purpose of this project was to examine cerebral hemisphere volume in dyslexia and ADHD and the extent to which reduced volume in these disorders is related to poor receptive language functioning. Based upon prior literature suggesting smaller cerebral volume is associated with worse comprehension40, 41, it was hypothesized that cerebral hemisphere volume would be reduced in dyslexia and ADHD when weaknesses in receptive language were present as opposed to being reduced in dyslexia and ADHD in general. The second purpose of this study was to examine cerebral volume in relation to the two domains of linguistic functioning: phonological and non-phonological. Given the literature reviewed, it was hypothesized that cerebral volume would be positively correlated with non-phonological linguistic skills but there would be a limited relationship between cerebral volume and phonological skills.
Methods
Participants
Approval was obtained from the Human Subjects Committee of the University of Georgia Institutional Review Board before the study commenced. Participants were recruited by a laboratory focused on dyslexia and ADHD. They included 10 children with dyslexia, 13 children with comorbid dyslexia and ADHD, 13 children with ADHD and 10 typically developing controls, ages 8 – 12 years. For the dyslexia group, participants were 90% Caucasian and 70% male. For the dyslexia/ADHD group participants were 100% Caucasian and 77% male. For the ADHD group, participants were 92% Caucasian and 77% male, and for the control group participants were 100% Caucasian and 50% male. Exclusionary criteria applied to all participants and included neurological disorder, psychiatric disorder (except ADHD), medical conditions (except allergies and asthma), and measured intelligence below 80. No child was on medication for ADHD on the day of testing per parent report.
Dyslexia
Dyslexia was defined following State of Georgia criteria for a Specific Learning Disability in reading. State criteria were consistent with the Individuals with Disabilities Education Act (IDEA) at the time of data collection and required at least a 20 point standard score discrepancy between measured intelligence and academic achievement in reading, with reading being lower, which could not be accounted for by sensory or motor difficulties, inadequate educational opportunities or mental retardation66. State criteria have since changed when IDEA requirements for a learning disability were modified in 2004. For the purposes of this study, the discrepancy required was between measured intellect as assessed by the Wechsler Intelligence Scale for Children-Third Edition67 (WISC-III) and word identification as assessed by the Reading subtest of the Wide Range Achievement Test-Third Edition68 (WRAT-3) since poor word identification is the primary feature of developmental dyslexia.
The discrepancy definition was chosen over the poor reader definition for a few reasons. First, by using a discrepancy definition we have a more stringently-diagnosed group with which to test our first hypothesis. Second, many studies on the neurobiological basis of dyslexia utilize a discrepancy definition, facilitating comparison amongst studies. Third, those who meet the discrepancy definition may be more likely to have a genetic/neurobiological basis to their disorder69; poor readers without a discrepancy may be more likely to have a stronger environmental basis to their disorder70. Fourth, participants were recruited by means of a free, written psycho-educational report, and the State of Georgia criteria required use of a discrepancy definition at the time of data collection.
ADHD
ADHD was diagnosed through a multi-modal procedure using multiple informants. The process entailed a semi-structured clinical interview to verify DSM-IV criteria were met (Schedule for Affective Disorders and Schizophrenia for School-Age Children, updated with DSM-IV criteria71) as well as multiple questionnaires completed by the parents and teachers to ensure the level of attention problems, hyperactivity and/or impulsivity were of sufficient severity to warrant diagnosis. Parent and teacher questionnaires completed included the Child Behavior Checklist72 (CBCL), the Child Behavior Checklist-Teacher Report Form73 (TRF) and the Swanson, Nolan, and Pelham checklist74 (SNAP). The process of diagnosis used has been shown to be reliable in previous research75.
Based upon the semi-structured interview and the questionnaires, 3 children had ADHD-Predominately Inattentive type (ADHD-PI) and 10 had ADHD-Combined type (ADHD-C) in the dyslexia/ADHD group, and 3 had ADHD-PI and 10 had ADHD-C in the ADHD group. ADHD severity was mild for those with ADHD and dyslexia/ADHD, and the two groups did not differ in ADHD severity as assessed by the questionnaires.
Neuropsychological Assessment
All participants underwent a battery of neuropsychological measures after informed consent was obtained from the parent and informed assent was obtained from the child. Receptive and expressive language functioning were evaluated with the Clinical Evaluation of Language Fundamentals-Revised76 (CELF-R). This test measures semantic and syntactic language functioning, although the latter is better represented by the Expressive Language composite score, whereas semantic functioning is represented in both the Receptive and Expressive Language composite scores. CELF-R Sentence Assembly was used as a measure of syntactic functioning, and CELF-R Recalling Sentences was used a measure of rote verbal short-term memory. WISC-III Vocabulary was used as a measure of semantic functioning, and WISC-III Digit Span was used as a measure of phonological short-term memory. Phonological awareness was assessed with the Elision subtest from the Comprehensive Test of Phonological Processing — Experimental Version77 (CTOPP). Rapid naming was assessed with the number/letter composite from the Rapid Automatized Naming test78, 79 (RAN). Measures of academic achievement included the Wide Range Achievement Test-Third Edition (WRAT-3) and the Woodcock Reading Mastery Test — Revised80 (WRMT-R) Word Attack and Passage Comprehension subtests.
MRI Acquisition
Magnetic Resonance Imaging (MRI) scans were conducted on a .6 Tesla scanner (Health Images, Atlanta, Georgia). The protocol utilized 15 3-D, gapless, 3.1mm slices [TR=51; TE=10 (prior to 9/23/95) or TE=13 (after 9/23/95)]. All scans were assessed by a board certified neurologist and found to be within normal limits.
Cerebral Hemisphere Measurement
Images were traced in the coronal plane using a digitizing tablet and the publicly available software program, Scion Image for Windows (Scion Corporation, 2000). This software program is the Windows-based version of NIH IMAGE. Published studies were used as guidelines to determine measurement parameters40, 81. Each hemisphere was traced on every 4th slice in the coronal plane, starting at the most anterior slice in which a hemisphere was detectable and continuing until it was no longer present caudally. Each hemisphere was measured separately. Measurements included all gray/white matter encompassed by the dura but excluded the ventricles; optic nerve, tract, and chiasm; corpus callosum; fornix; and septum pallusidum. Cavalieri’s rule was used to correct for overprojection when calculating volume82.
An asymmetry ratio was calculated as prior researchers have revealed atypical asymmetry in those with dyslexia83 and those with specific language impairment41. The following formula was used for the interhemispheric coefficient of asymmetry84: Left-Right/[(Left+Right)*0.5)]. A positive value indicates leftward asymmetry, and a negative value indicates rightward asymmetry.
Results
Group Descriptive Data
To ensure diagnostic groups differed where appropriate, those with dyslexia (dyslexia and dyslexia/ADHD) and without dyslexia (ADHD and controls) were compared using ANOVA on relevant descriptive data. The entire sample was analyzed again, comparing those with ADHD (dyslexia/ADHD and ADHD) and without ADHD (dyslexia and controls). This procedure was chosen instead of directly comparing the four groups as cerebral hemisphere volume was examined using a 2 × 2 MANOVA, comparing those with and without dyslexia and ADHD. Those with and without dyslexia were comparable in age, handedness, Full-Scale IQ (FSIQ), and Performance IQ (PIQ). They differed in Verbal IQ (VIQ), F(1,44)=4.46, p < .05, as is common in this population. When using chi-square they were comparable in gender and ethnicity. In terms of Index scores, groups were comparable in WISC-III Perceptual Organization and Processing Speed, but they differed in Verbal Comprehension [F(1,44)=3.92, p = .05] and Freedom from Distractibility [F(1,44)=7.16, p = .01]. As a result, VIQ was used as a covariate in the 2 × 2 MANCOVA on hemisphere volume. In terms of academic achievement, those with and without dyslexia differed in all areas assessed: WRAT-3 Reading [F(1,44)=46.24, p < .001], Spelling [F(1,44)=26.67, p < .001] and Arithmetic [F(1,44)=15.82, p < .001], and WRMT-R Word Attack [F(1,44)=35.38, p < .001] and Passage Comprehension [F(1,44)=28.83, p < .001]. In contrast, those with and without dyslexia were comparable on parent and teacher CBCL Attention Problems. See Table 1 for descriptive data.
Table 1.
Participant Descriptive Data
| Variable | Dyslexia | Dyslexia/ADHD | ADHD | Controls | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 10.28 | 1.35 | 9.54 | 0.89 | 9.44 | 1.05 | 9.90 | 1.06 |
| Edinburgh | 95.00 | 5.27 | 80.00 | 28.36 | 86.54 | 25.93 | 86.43 | 33.75 |
| WISC-III | ||||||||
| Full-Scale IQ | 99.80 | 12.51 | 105.31 | 17.41 | 104.92 | 15.15 | 111.90 | 12.26 |
| Verbal IQa | 97.80 | 11.70 | 103.84 | 19.88 | 106.38 | 13.16 | 117.40 | 15.94 |
| Verbal Comprehension Indexa | 100.10 | 11.86 | 104.31 | 20.67 | 108.23 | 14.02 | 116.60 | 15.43 |
| Freedom from Distractibility Indexb | 88.40 | 10.81 | 96.62 | 15.42 | 97.46 | 11.52 | 113.60 | 14.50 |
| Performance IQ | 102.50 | 13.47 | 106.23 | 14.31 | 102.84 | 16.83 | 103.60 | 9.31 |
| Perceptual Organization Index | 106.60 | 13.78 | 108.15 | 16.23 | 104.00 | 18.44 | 102.50 | 7.26 |
| Processing Speed Index | 93.60 | 14.69 | 96.83 | 9.17 | 96.31 | 12.30 | 108.11 | 14.80 |
| WRAT-3 | ||||||||
| Readingc | 79.00 | 12.16 | 81.77 | 9.04 | 101.54 | 11.69 | 111.00 | 15.62 |
| Spellingc | 81.56 | 11.33 | 85.31 | 7.26 | 95.15 | 8.40 | 107.10 | 13.30 |
| Arithmeticc | 86.56 | 11.40 | 92.23 | 11.68 | 100.92 | 12.01 | 105.70 | 7.66 |
| WRMT-R | ||||||||
| Word Attackc | 77.90 | 13.33 | 82.00 | 7.69 | 99.38 | 11.86 | 106.70 | 17.47 |
| Passage Comprehensionc | 75.30 | 14.52 | 82.54 | 9.73 | 93.62 | 11.07 | 108.60 | 12.82 |
| CBCL | ||||||||
| Parent form Attention Problemsd | 55.90 | 6.35 | 68.00 | 6.63 | 69.85 | 6.80 | 53.14 | 5.84 |
| Teacher form Attention Problemse | 60.30 | 9.09 | 69.91 | 13.41 | 69.00 | 11.83 | 56.14 | 7.88 |
Note. Edinburgh is measured in percent of tasks performed with the right hand. CBCL is in T-scores, and the rest of the measures are in standard scores.
Those with and without dyslexia differed at p ≤ .05.
Those with and without dyslexia differed at p = .01.
Those with and without dyslexia differed at p < .001.
Those with and without ADHD differed at p < .001.
Those with and without ADHD differed at p < .01.
In terms of those with and without ADHD, groups were comparable in age, race, gender, handedness, FSIQ, VIQ, and PIQ when using the statistical procedures described above. They also were comparable on the academic achievement measures. See Table 1. Groups differed significantly on the ADHD scales: CBCL Attention Problems, F(1,42)=49.55, p < .001 and TRF Attention Problems, F(1,39)=9.75, p < .01.
Cerebral Hemisphere Volume in Dyslexia and ADHD
Given the primary purpose of this study, children with and without dyslexia and ADHD were compared on right and left hemisphere cerebral volume and the asymmetry ratio using a 2 × 2 MANCOVA with VIQ as the covariate. This approach was chosen as it allows for analysis of the interaction between dyslexia and ADHD, which was of interest given the high comorbidity between the two disorders. The omnibus main effects and interaction were not significant [Fs(3,39) < 1.0]. In addition, none of the univariate ANOVAs were significant.
Because the heterogeneity of dyslexia and ADHD could have lessened group differences, the relationship between cerebral volume, reading ability, and ADHD symptom severity was examined in the total sample. None of the correlations between size of the right and left hemispheres and WRAT-3 Reading, WRMT-R Passage Comprehension, and WRMT-R Word Attack were significant when using Pearson correlations, with all rs < .10. The parent Swanson, Nolan, and Pelham checklist was used to examine symptoms of ADHD as it includes separate scales for inattention, hyperactivity, and impulsivity. In contrast to reading, bilateral hemisphere volume was moderately correlated with ADHD symptom severity, with smaller size being related to worse inattention [right r = -.40, p < .05; left r = -.38, p < .05], hyperactivity [right r = -.41, p < .05; left r = -.41, p < .05], and impulsivity [right r = -.41, p < .05; left r = -.39, p < .05].
Receptive Language and Cerebral Hemisphere Volume
As a first step in determining the relationship between cerebral volume and receptive language in dyslexia and ADHD, all participants were divided into two groups: those with and without receptive language weaknesses. Children with below average CELF-R Receptive Language composite scores (i.e., below 85) were assigned to the poor receptive language group; those with average or better Receptive Language composites (i.e., 85 or greater) were assigned to the group without receptive language deficits. This resulted in 16 children with poor receptive language and 30 children with intact receptive language. Chi-square was utilized to determine if the two groups differed in the presence of dyslexia or ADHD. Results were not significant (X2=4.29, p > .10), and percentages of receptive language weaknesses by group were consistent with what one would expect given the comorbidities between dyslexia, ADHD and specific language impairment1, 51-53. See Table 2.
Table 2.
Frequencies of Diagnosis by Receptive Language Group
| Variable | Dyslexia | Dyslexia/ADHD | ADHD | Controls |
|---|---|---|---|---|
| CELF-R Receptive < 85 | 3 | 6 | 6 | 1 |
| CELF-R Receptive ≥ 85 | 7 | 7 | 7 | 9 |
Note. CELF-R Receptive is the CELF-R Receptive Language composite score. Receptive language groups did not differ significantly in diagnostic frequency.
Next, participants with and without poor receptive language were compared on the WISC-III using MANOVA to determine if the poor receptive language group had generalized impairment as suggested by previous research3-10. As seen in Table 3, groups differed on all Indices, Verbal Comprehension [F(1,42)=15.97, p < .001], Perceptual Organization [F(1,42)=49.05, p < .001], Freedom from Distractibility [F(1,42)=14.91, p < .001], and Processing Speed [F(1,42)=6.09, p < .05], along with Full-Scale IQ [F(1,44)=37.52, p < .001]. Children with poor receptive language also had global linguistic deficits, performing worse on the CELF-R Expressive Language composite [F(1,38)=13.39, p = .001], CELF-R Recalling Sentences subtest [F(1,38)=16.73, p < .001], CTOPP Elision [F(1,38)=7.47,p < .01], WISC-III Digit Span [F(1,38)=11.25, p < .01] and rapid naming time [F(1,38)=6.11, p < .05] when using MANCOVA with the Perceptual Organization Index as the covariate.
Table 3.
Cognitive Functions by Receptive Language Group
| Variable | Poor Receptive Language | Intact Receptive Language | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| WISC-III | ||||
| Full-Scale IQ*** | 91.81 | 9.24 | 112.70 | 11.83 |
| Verbal IQ*** | 94.44 | 11.33 | 112.47 | 15.60 |
| VCI*** | 95.19 | 11.14 | 112.64 | 15.27 |
| FDI*** | 87.75 | 8.23 | 103.89 | 15.46 |
| Performance IQ*** | 90.69 | 8.84 | 110.93 | 10.04 |
| POI*** | 91.50 | 7.21 | 112.43 | 10.61 |
| PSI* | 92.06 | 9.50 | 101.79 | 13.99 |
| CELF-R Expressive*** | 75.69 | 6.63 | 98.36 | 13.28 |
| CELF-R Recall*** | 6.38 | 2.03 | 10.72 | 2.78 |
| CTOPP Elision** | 14.31 | 4.57 | 18.88 | 4.91 |
| WISC-III Digit Span** | 7.25 | 1.29 | 10.56 | 3.06 |
| RAN Number/Letter Time* | 44.25 | 11.75 | 36.72 | 10.01 |
Note. CELF-R Recall and WISC-III Digit Span subtests are in scaled scores; CTOPP Elision subtest and RAN Number/Letter Time are in raw scores; the rest are in standard scores. CELF-R Expressive is the CELF-R Expressive Language composite score. VCI is the Verbal Comprehension Index; FDI is the Freedom from Distractibility Index; POI is the Perceptual Organization Index; and PSI is the Processing Speed Index.
p < .05.
p < .01.
p < .001.
Lastly, those with and without poor receptive language were compared on cerebral volume using MANCOVA with Full-Scale IQ as a covariate, controlling for unequal cell sizes. Full-Scale IQ was significant for left [F(1,43)=5.09, p < .05] and right [F(1,43)=5.21, p < .05] hemisphere volumes but not asymmetry [F(1,43) < 1.0]. Omnibus tests were significant [F(3,41)=4.44, p < .01], as were the univariate ANOVAs for left [F(1,43)=13.38, p = .001] and right hemisphere volume [F(1,43)=13.23, p = .001]. Asymmetry was not significant [F(1,43) < 1.0]. See Table 4.
Table 4.
Cerebral Hemisphere Size by Receptive Language Group
| Variable | Poor Receptive Language | Intact Receptive Language | ||
|---|---|---|---|---|
| Mean | SE | Mean | SE | |
| Left hemisphere volume* | 1444.57 | 35.14 | 1618.25 | 23.43 |
| Right hemisphere volume* | 1419.26 | 34.94 | 1590.96 | 23.29 |
| Asymmetry ratio | .018 | .006 | .017 | .004 |
Note. Means are adjusted for group differences in WISC-III Full-Scale IQ.
p = .001.
Receptive Language and Cerebral Volume in Dyslexia and ADHD
To address the primary purpose of this study, those with and without poor receptive language were compared on cerebral volume within the dyslexia and ADHD groupings. Of the children with dyslexia, 9 had poor receptive language and 13 had intact receptive language. Using ANCOVA with Full-Scale IQ as a covariate, groups differed on left [F(1,19)=6.07, p < .05] and right [F(1,19)=5.10, p < .05] hemisphere volume, with poor receptive language being associated with smaller volume. When examining children with ADHD, 12 had poor receptive language and 14 had intact receptive language. ANCOVA with Full-Scale IQ as a covariate revealed those with poor receptive language had smaller right [F(1,23)=16.72, p < .001] and left [F(1,23)=19.83, p < .001] hemisphere volumes than those with average or better receptive language.
Cerebral Hemisphere Volume and the Two Linguistic Dimensions
Given the secondary purpose of this study, the relationship between hemisphere volume and linguistic ability was examined in an exploratory fashion using Pearson correlations in the total sample (see Table 5). All correlations between hemisphere volume and linguistic functioning were small, and only one correlation between volume and linguistic functioning was significant at the .05 level: right hemisphere volume and number/letter naming time. When examining children with dyslexia specifically, asymmetry was negatively correlated with CELF-R Recalling Sentences (r=-.42, p < .05), indicating rightward asymmetry was moderately associated with better performance. When examining children with poor receptive language (regardless of dyslexia or ADHD diagnosis), leftward asymmetry was moderately correlated with better WISC-III Vocabulary performance (r=.51, p < .05), and left hemisphere volume was moderately correlated with CELF-R Sentence Assembly (r=.50, p = .05).
Table 5.
Pearson Correlations between Hemisphere Volume and Linguistic Functioning in the Total Sample
| Variable | Left Hemisphere | Right Hemisphere |
|---|---|---|
| CELF-R Receptive Composite | .24 | .24 |
| CELF-R Expressive Composite | .17 | .19 |
| CELF-R Sentence Assembly | .16 | .15 |
| CELF-R Recalling Sentences | .11 | .18 |
| WISC-III Vocabulary | -.04 | -.06 |
| WISC-III Digit Span | .05 | .07 |
| CTOPP Elision | .06 | .02 |
| RAN Number/Letter time | -.25 | -.30* |
p < .05.
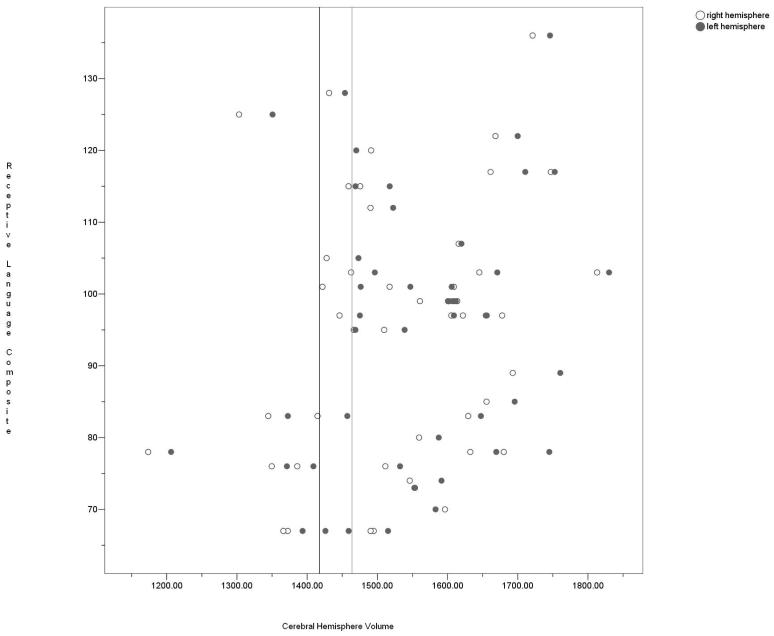
The lack of a significant relationship between the Receptive Language composite and hemisphere volume in the total sample was surprising given those with poor receptive language had smaller volumes as a group. Thus, a scatter plot of the relationship between receptive language and hemisphere volume was formed using the total sample (see Figure 1). Those with the smallest hemisphere volumes tended to have below average Receptive Language composites. However, once volume surpassed 1460cm3 on the left and 1420cm3 on the right, the relationship between receptive language and hemisphere volume became erratic. When participants were ordered according to left hemisphere volume, 8/10 of those with the smallest volumes (less than 1460cm3) had poor receptive language. Of these 8, 3 had ADHD, 4 had dyslexia/ADHD, and 1 had dyslexia. Nonetheless, the remaining 2 children had Receptive Language composites of 125 (control) and 128 (ADHD). When participants were ordered according to right hemisphere volume, 7/8 of those with the smallest volumes (less than 1420cm3) had poor receptive language, with the remaining child having the Receptive Language composite of 125 (control). The 7 with small right hemisphere volume included the same participants as the 8 with small left hemisphere volume with the exception of one child with dyslexia/ADHD.
Figure 1.
Scatterplot of the relationship between receptive language and cerebral hemisphere volume. All hemisphere volumes left of the black line are less than 1420cm3. All hemisphere volumes left of the gray line are less than 1460cm3.
Discussion
The primary purpose of this project was to examine whether reduced cerebral volume in dyslexia and ADHD is related to poor receptive language functioning. Based upon prior literature suggesting smaller cerebral volume is associated with worse language comprehension40, 41, it was hypothesized that cerebral hemisphere volume would be reduced in dyslexia and ADHD when weaknesses in receptive language were present as opposed to being reduced in dyslexia and ADHD in general. The second purpose of this study was to examine cerebral volume in relation to the two domains of linguistic functioning: phonological and non-phonological16. Based upon prior literature in the area12, 34, 36, 39, 40, it was hypothesized that cerebral volume would be positively correlated with non-phonological linguistic skills, but there would be a limited relationship between cerebral volume and phonological skills.
Cerebral Hemisphere Volume in Dyslexia and ADHD
As hypothesized, cerebral volume was quite comparable between those with and without dyslexia when using the total sample. However, cerebral volume was reduced in children with poor receptive language and dyslexia compared to those with dyslexia but intact receptive language. Similar results were found when analyzing ADHD. In addition, children with and without receptive language deficits in general differed in cerebral volume. Given these findings, at first glance it appears that cerebral hemisphere volume is only reduced in dyslexia and ADHD when poor receptive language is present, consistent with hypotheses and the work of Leonard and colleagues40-42.
Nonetheless, small cerebral volume was associated with poor receptive language functioning only in those with the smallest volumes. For the rest of the sample the relationship between receptive language and cerebral volume was rather spurious. Even for children with the smallest volumes the relationship was not absolute, as one to two children with small volumes had excellent receptive language functioning, depending on the hemisphere. In addition, children with poor receptive language had multiple cognitive weaknesses, including mildly reduced verbal and nonverbal intellect, slower processing speed, and global linguistic deficits compared to those with intact receptive language. Hence, although our findings are consistent with prior literature suggesting there are generalized deficits in individuals with poor receptive language3-5, 7-9, it is difficult to ascertain if small volume is associated with poor receptive language per se, or if it is associated with one or more of the deficits which often accompany poor receptive language. Further research is indicated to make these differentials.
Although children with and without ADHD did not differ in cerebral volume, a moderate relationship was found between cerebral volume and symptoms of ADHD in the total sample; this was true for inattention, hyperactivity, and impulsivity. These relationships likely were not mediated by linguistic functioning given the small correlations between receptive language and inattention, hyperactivity, and impulsivity (rs < .20) and the small relationship between receptive language and cerebral volume in the total sample. Hence, our results are partially consistent with prior research finding ADHD symptomotology is associated with reduced cerebral volume57, 58. It is likely that our participants with and without ADHD did not differ in volume due to our sample being largely comprised of children with mild ADHD. Nonetheless, what is informative from our study is the moderate relationship between cerebral volume and ADHD symptoms, suggesting the relationship between the two may be more continuous in nature.
Relationships between Cerebral Volume and Linguistic Ability
When examining linguistic skills comprising the phonological dimension in the total sample, the relationships between cerebral volume and phonological awareness, phonological short-term memory, word recognition, and decoding skill were quite limited. Hence, these findings are partially consistent with the work of Leonard and colleagues40, 41 who suggested that the phonological dimension may be better associated with aspects of brain morphology other than cerebral volume. Nonetheless, we did not find a linear relationship between cerebral volume and non-phonological linguistic functions in the total sample either, including semantic and syntactic oral language functioning and reading comprehension. Although this could be related to low power, the correlations were small.
When analyzing subgroups, there was a moderate relationship between rightward asymmetry and better verbatim sentence repetition in dyslexia. While the rightward nature of this relationship is surprising given traditional views on language, it is consistent with recent literature suggesting rightward asymmetry of the supramarginal gyrus is associated with better phonological short-term memory in those with dyslexia and/or ADHD85. Furthermore, findings are consistent with prior literature which suggests there is a biological contribution to phonological short-term memory performance in particular in developmental dyslexia16. Further research with a large sample is indicated to assess the relationship between cerebral volume and phonological short-term memory and whether it differs between those with and without poor phonological processing.
For those with receptive language weaknesses, there was a moderate relationship between leftward asymmetry and better vocabulary knowledge. There also was a moderate relationship between left cerebral volume and syntax formation. Hence, further research on the relationship between cerebral volume and semantic and syntactic functioning in those with poor receptive language is warranted. While replication in those with specific language impairment is required, it also would be of interest to determine if this relationship is found in other populations with non-phonological linguistic deficits such as autism. In addition, it would be of interest to assess the role of environmental contributions to this relationship. For example, do children with larger volumes but poor receptive language functioning have worse or more numerous environmental risk factors? Do children with small volumes but intact receptive language have more environmental protective factors in place?
Taken together, our findings on the relationship between cerebral volume and linguistic functioning are consistent with the review by Bishop and Snowling16 which suggests that neurobiological bases to linguistic functioning are more likely to be found when well-defined groups are used. When heterogeneous groupings are used, the sample is more likely to include participants with various environmental and neurobiological contributors to their functioning. Perhaps heterogeneity served to reduce the relationships found between cerebral volume and linguistic functioning in the total sample.
Limitations and Future Directions
First, as dyslexia was defined according to a discrepancy definition future research is warranted using the poor reader definition to determine if relationships weaken further, as a biological basis for dyslexia may be more readily found when a discrepancy definition is used16, or if poor readers have smaller cerebral volumes as a group given the increased prevalence of non-phonological linguistic deficits in this group16. Second, both the dyslexia and ADHD groups were of mild severity; thus, it would be of interest to assess whether results differ from a sample with more severe deficits. Nevertheless, often greater severity of disorders is accompanied by a greater number and severity of comorbidities, something this study tried to avoid through its inclusion and exclusion criteria. Third, receptive language functioning was assessed with a cutoff score in our study, similar to the work of Leonard and colleagues40. Hence, it would be beneficial to replicate this study using formal diagnostic procedures to determine presence or absence of specific language impairment rather than using a cut-off score. Fourth, as this study was conducted on a weak scanner, it would be beneficial to replicate this study using a stronger scanner allowing for use of more sophisticated technology (e.g., gray/white matter segmentation). Finally, as with most studies using MRI, our sample size was small. Hence, replication is required with a larger sample to test for differences in correlation values between groups (e.g., dyslexia, receptive language weaknesses, controls).
Conclusions
Of particular interest to the authors were the unusual relationships found between cerebral volume and language comprehension in our study given the work by Leonard and colleagues40-42. While not finding a continuous relationship between cerebral volume and receptive language in the total sample could be related to our sample composition and low power, it also could be that only those with the smallest volumes have this neurobiological contributor to their language comprehension and/or accompanying deficits, as opposed to there being a continuous relationship between volume and comprehension in general. Further research is indicated to investigate the relationship between cerebral volume and language comprehension in more detail, including examination of how various environmental factors may affect this relationship (e.g., maternal education, perinatal factors, quality of education, type of instruction).
Acknowledgements
Data collection occurred at the University of Georgia and was funded by a grant (R01 HD26890) awarded to the last author (GWH) from the National Institutes of Health (NIH), National Institute of Child Health and Human Development (NICHD). A grant awarded to the first author (MYK: R03 HD048752) partially supported data analysis and write-up. The data was presented in part at the International Neuropsychological Society, February 2004 conference.
References
- 1.Riccio CA, Hynd GW. Developmental language disorders in children: Relationship with learning disability and Attention Deficit Hyperacitivity Disorder. School Psychology Review. 1993;22:696–709. [Google Scholar]
- 2.Preis S, Steinmetz H, Knorr U, Jancke L. Corpus callosum size in children with developmental language disorder. Cognitive Brain Research. 2000;10:37–44. doi: 10.1016/s0926-6410(00)00020-3. [DOI] [PubMed] [Google Scholar]
- 3.Herbert MR, Ziegler DA, Makris N, et al. Larger brain and white matter volumes in children with developmental language disorder. Developmental Science. 2003;6:F11–F22. [Google Scholar]
- 4.Ahmed ST, Lombardino LJ, Leonard CM. Specific language impairment: Definitions, causal mechanisms and neurobiological factors. Journal of Medical Speech-Language Pathology. 2001;9:1–15. [Google Scholar]
- 5.Aram DM, Eisele JA. Limits to a left hemisphere explanation for specific language impairment. Journal of Speech And Hearing Research. 1994;37:824–830. doi: 10.1044/jshr.3704.824. [DOI] [PubMed] [Google Scholar]
- 6.Boucher J, Lewis V, Collis GM. Voice processing abilities in children with autism, children with specific language impairments, and young typically developing children. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2000;41:847–857. [PubMed] [Google Scholar]
- 7.Lane AB, Foundas AL, Leonard CM. The evolution of neuroimaging research and developmental language disorders. Topics in Language Disorders. 2001;21:20–41. [Google Scholar]
- 8.Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: A case for the preeminence of temporal processing. Irish Journal of Psychology. 1995;16:194–219. doi: 10.1111/j.1749-6632.1993.tb22957.x. [DOI] [PubMed] [Google Scholar]
- 9.Weismer SE, Evans JL. The role of processing limitations in early identification of specific language impairment. Topics in Language Disorder. 2002;22:15–29. [Google Scholar]
- 10.Jernigan TL, Hesselink JR, Sowell E, Tallal PA. Cerebral structure on magnetic resonance imaging in language- and learning-impaired children. Archives of Neurology. 1991;48:539–545. doi: 10.1001/archneur.1991.00530170103028. [DOI] [PubMed] [Google Scholar]
- 11.Riccio CA, Hynd GW. Measurable biological substrates to verbal-performance differences in Wechsler scores. School Psychology Quarterly. 2000;15:386–399. [Google Scholar]
- 12.Eckert MA, Leonard CM. Structural imaging in dyslexia: The planum temporale. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:198–206. doi: 10.1002/1098-2779(2000)6:3<198::AID-MRDD7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Pennington BF, Filipek PA, Lefly D, et al. A twin MRI study of size variations in the human brain. Journal of Cognitive Neuroscience. 2000;12:223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- 14.Reiss AL, Abrams MT, Singer HS, et al. Brain development, gender and IQ in children: A volumetric imaging study. Brain. 1996;119:1763–1774. doi: 10.1093/brain/119.5.1763. [DOI] [PubMed] [Google Scholar]
- 15.Willerman L, Schultz R, Rutledge J, Bigler ED. In vivo brain size and intelligence. Intelligence. 1991;15:223–228. [Google Scholar]
- 16.Bishop DVM, Snowling MJ. Developmental Dyslexia and Specific Language Impairment: Same or different? Psychological Bulletin. 2004;130:858–886. doi: 10.1037/0033-2909.130.6.858. [DOI] [PubMed] [Google Scholar]
- 17.Joanisse MF, Seidenberg MS. Specific language impairment: a deficit in grammar or processing? Trends in Cognitive Sciences. 1998;2:240–247. doi: 10.1016/S1364-6613(98)01186-3. [DOI] [PubMed] [Google Scholar]
- 18.Lane AB, Foundas AL, Leonard CM. The evolution of neuroimaging research and developmental language disorders. Topics in Language Disorders. 2001;21:20–41. [Google Scholar]
- 19.Tallal P, Piercy M. Developmental aphasia: Rate of auditory processing and selective impairment of consonant perception. Neuropsychologia. 1974;12:83–93. doi: 10.1016/0028-3932(74)90030-x. [DOI] [PubMed] [Google Scholar]
- 20.Tallal P, Stark RE, Kallman C, Mellitis D. Perceptual constancy for phonemic categories: A developmental study with normal and language impaired children. Applied Psycholinguistics. 1980;1:49–64. [Google Scholar]
- 21.Stanovich KE, Siegel LS. Phenotypic performance profile of children with reading disabilities: A regression-based test of the phonological-core variable-difference model. Journal of Educational Psychology. 1994;86:24–53. [Google Scholar]
- 22.Snowling M, Hulme C. The development of phonological skills. Philos Trans R Soc Lond B Biol Sci. 1994;346:21–27. doi: 10.1098/rstb.1994.0124. [DOI] [PubMed] [Google Scholar]
- 23.Fletcher JM, Shaywitz SE, Shankweiler DP, et al. Cognitive Profiles of Reading-Disability - Comparisons of Discrepancy and Low Achievement Definitions. Journal of Educational Psychology. 1994;86:6–23. [Google Scholar]
- 24.Mann VA, Liberman IY. Phonological awareness and verbal short-term memory. Journal of Learning Disabilities. 1984;17:592–599. doi: 10.1177/002221948401701005. [DOI] [PubMed] [Google Scholar]
- 25.Rack JP, Snowling MJ, Olson RK. The Nonword Reading Deficit in Developmental Dyslexia - a Review. Reading Research Quarterly. 1992;27:28–53. [Google Scholar]
- 26.Swank LK. Phonological coding abilities: Identification of impairments related to phonologically based reading problems. Topics in Language Disorders. 1994;14:56–71. [Google Scholar]
- 27.Badian NA, Duffy FH, Ali H, McAnulty GB. Linguistic profiles of dyslexic and good readers. Annals of Dyslexia. 1991;41:221–245. doi: 10.1007/BF02648088. [DOI] [PubMed] [Google Scholar]
- 28.Badian NA. Preschool Prediction - Orthographic and Phonological Skills, and Reading. Annals of Dyslexia. 1994;44:3–25. doi: 10.1007/BF02648153. [DOI] [PubMed] [Google Scholar]
- 29.Wolf M, Bowers PG. The double-deficit hypothesis for the developmental dyslexias. Journal of Educational Psychology. 1999;91:415–438. [Google Scholar]
- 30.Liberman IY, Shankweiler D. Phonology and beginning reading: A tutorial. In: Rieben L, Perfetti CA, editors. Learning to read: Basic Research and Its Implications. Lawrence Erlbaum Associates; Hillsdale, NJ: 1991. pp. 3–17. [Google Scholar]
- 31.Kibby MY, Marks W, Morgan S, Long CJ. Specific impairments in developmental reading disabilities: A working memory approach. Journal of Learning Disabilities. 2004;37:349–363. doi: 10.1177/00222194040370040601. [DOI] [PubMed] [Google Scholar]
- 32.Kibby MY, Cohen MJ. Memory functioning in children with reading disabilities and/or Attention-Deficit/Hyperactivity Disorder: A clinical investigation of their working memory and long-term memory functioning. Child Neuropsychology. doi: 10.1080/09297040701821752. Manuscript in press.
- 33.Wagner RK, Torgesen JK, Rashotte CA. Development of reading-related phonological processing abilities: New evidence of bidirectional causality from a latent variable longitudinal study. Developmental Psychology. 1994;30:73–87. [Google Scholar]
- 34.Catts HW. Defining dyslexia as a developmental language disorder: An expanded view. Topics in Language Disorders. 1996;16:14–29. [Google Scholar]
- 35.Kamhi AG, Catts HW, editors. Reading disabilities: A developmental language perspective. Allyn & Bacon; Boston: 1989. [Google Scholar]
- 36.Lombardino LJ, Riccio C, Hynd GW, Pinheiro S. Linguistic deficits in children with reading disabilities. American Journal of Speech Language Pathology. 1997;6:71–78. [Google Scholar]
- 37.Scarborough HS, Dobrich W. Development of children with early language delay. Journal of Speech & Hearing Research. 1990;33:70–83. doi: 10.1044/jshr.3301.70. [DOI] [PubMed] [Google Scholar]
- 38.Leonard CM. Imaging brain structure in children: Differentiating language disability and reading disability. Learning Disability Quarterly. 2001;24:158–176. [Google Scholar]
- 39.Manis F, Seidenberg M, Doi L, et al. On the bases of two subtypes of developmental dyslexia. Cognition. 1996;58:157–195. doi: 10.1016/0010-0277(95)00679-6. [DOI] [PubMed] [Google Scholar]
- 40.Leonard CM, Eckert MA, Lombardino LJ, et al. Anatomical risk factors for phonological dyslexia. Cerebral Cortex. 2001;11:148–157. doi: 10.1093/cercor/11.2.148. [DOI] [PubMed] [Google Scholar]
- 41.Leonard CM, Lombardino LJ, Walsh K, et al. Anatomical risk factors that distinguish dyslexia from Specific Language Impairment predict reading skill in normal children. Journal of Communication Disorders. 2002;35:501–531. doi: 10.1016/s0021-9924(02)00120-x. [DOI] [PubMed] [Google Scholar]
- 42.Leonard CM, Eckert MA, Given B, et al. Individual differences in anatomy predict reading and oral language impairments in children. Brain. 2006;129:3329–3342. doi: 10.1093/brain/awl262. [DOI] [PubMed] [Google Scholar]
- 43.Eckert MA, Leonard CM, Richards TL, et al. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- 44.Eliez S, Rumsey JM, Giedd JN, et al. Morphological alteration of temporal lobe gray matter in dyslexia: An MRI study. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2000;41:637–644. doi: 10.1111/1469-7610.00650. [DOI] [PubMed] [Google Scholar]
- 45.Casanova MF, Araque J, Giedd JN, Rumsey JM. Reduced Brain Size and Gyrification in the Brains of Dyslexic Patients. Journal of Child Neurology. 2004;19:275–281. doi: 10.1177/088307380401900407. [DOI] [PubMed] [Google Scholar]
- 46.Siegel LS, Ryan EB. Subtypes of developmental dyslexia: The influence of definitional variables. Reading & Writing. 1989;1:257–287. [Google Scholar]
- 47.Siegel LS. An evaluation of the discrepancy definition of dyslexia. Journal of Learning Disabilities. 1992;25:618–629. doi: 10.1177/002221949202501001. [DOI] [PubMed] [Google Scholar]
- 48.Snowling MJ. Dyslexia. 2nd Blackwell Publishers; Malden, MA: 2000. [Google Scholar]
- 49.Wagner RK, Torgesen JK. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological Bulletin. 1987;101:192–212. [Google Scholar]
- 50.Fisher SE, DeFries JC. Developmental dyslexia: Genetic dissection of a complex cognitive trait. Nature Reviews, Neuroscience. 2002;3:767–780. doi: 10.1038/nrn936. [DOI] [PubMed] [Google Scholar]
- 51.Consortium S. A genomewide scan identifies two novel loci involved in specific language impairment. American Journal of Human Genetics. 2002;70:384–398. doi: 10.1086/338649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holborow PL, Berry PS. Hyperactivity and learning difficulties. Journal of Learning Disabilities. 1986;19:426–431. doi: 10.1177/002221948601900713. [DOI] [PubMed] [Google Scholar]
- 53.Shaywitz SE, Fletcher JM, Shaywitz BA. Issues in the definition and classification of attention deficit disorder. Topics in Language Disorders. 1994;14:1–25. [Google Scholar]
- 54.D’Incau BJ. Comorbidity of language disorder with attention-deficit/hyperactivity disorder in a sample of early elementary children: A preliminary investigation. Section B: Dissertation Abstracts International. 2000.
- 55.Durston S, Hulshoff Pol HE, Schnack HG, et al. Magnetic resonance imaging of boys with attention-deficit/hyperactivity disorder and their unaffected siblings. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:332–340. doi: 10.1097/00004583-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 56.Mostofsky SH, Cooper KL, Kates WR, et al. Smaller prefrontal and premotor volumes in boys with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2002;52:785–794. doi: 10.1016/s0006-3223(02)01412-9. [DOI] [PubMed] [Google Scholar]
- 57.Rapoport JL, Castellanos FX, Gogate N, et al. Imaging normal and abnormal brain development: New perspectives for child psychiatry. Australian and New Zealand Journal of Psychiatry. 2001;35:272–281. doi: 10.1046/j.1440-1614.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 58.Krain AL, Castellanos FX. Brain development and ADHD. Clinical Psychology Review. 2006;26:433–444. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 60.Filipek PA, Semrud-Clikeman M, Steingard RJ, et al. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48:589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- 61.Bruce B, Thernlund G, Nettelbladt U. ADHD and language impairment: A study of the parent questionnaire FTF (Five to Fifteen) European Child & Adolescent Psychiatry. 2006;15:52–60. doi: 10.1007/s00787-006-0508-9. [DOI] [PubMed] [Google Scholar]
- 62.Camarata SM, Gibson T. Pragmatic language deficits in attention-deficit hyperactivity disorder (ADHD) Mental Retardation and Developmental Disabilities Research Reviews. 1999;5:207–214. [Google Scholar]
- 63.Westby CE, Cutler SK. Language and ADHD: Understanding the bases and treatment of self-regulatory deficits. Topics in Language Disorders. 1994;14:58–76. [Google Scholar]
- 64.McInnes A, Humphries T, Hogg-Johnson S, Tannock R. Listening comprehension and working memory are impaired in attention-deficit hyperactivity disorder irrespective of language impairment. Journal of Abnormal Child Psychology. 2003;31:427–443. doi: 10.1023/a:1023895602957. [DOI] [PubMed] [Google Scholar]
- 65.Javorsky J. An examination of youth with attention-deficit/hyperactivity disorder and language learning disabilities: A clinical study. Journal of Learning Disabilities. 1996;29:247–258. doi: 10.1177/002221949602900303. [DOI] [PubMed] [Google Scholar]
- 66.State of Georgia eligibility requirements for a specific learning disability as retrieved from the World Wide Web: http://www.doe.k12.ga.us/ci_exceptional.aspx
- 67.Wechsler D. Wechsler Intelligence Scale for Children -- Third Edition. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- 68.Wilkinson GS. The Wide Range Achievement Test. 3rd Jastak; Wilmington, DE: 1993. [Google Scholar]
- 69.Olson RK, Datta H, Gayan J, DeFries JC. A behavioral-genetic analysis of reading disabilities and component processes. In: Klein RM, McMullen PA, editors. Converging methods for understanding reading and dyslexia. MIT Press; Cambridge, MA: 1999. pp. 133–151. [Google Scholar]
- 70.Bishop DVM. Genetic influences on language impairment and literacy problems in children: Same or different? Journal of Child Psychology and Psychiatry. 2001;42:189–198. [PubMed] [Google Scholar]
- 71.Puig-Antich J, Chambers W. The Schedule for Affective Disorders and Schizophrenia for School-Age Children. New York State Psychiatric Institute; New York: 1978. [Google Scholar]
- 72.Achenbach TM, Edelbrock C. Child Behavior Checklist. Thomas Achenbach; Burlington, VT: 1983. [Google Scholar]
- 73.Achenbach TM, Edelbrock C. Teacher’s Report Form. Thomas Achenbach; Burlington, VT: 1986. [Google Scholar]
- 74.Atkins MS, Pelham WE, Licht MH. A comparison of objective classroom measures and teacher ratings of attention deficit disorder. Journal of Abnormal Child Psychology. 1985;13:155–167. doi: 10.1007/BF00918379. [DOI] [PubMed] [Google Scholar]
- 75.Morgan AE, Hynd GW, Riccio CA, Hall J. Validity of DSM-IV ADHD Predominantly Inattentive and Combined Types: Relationship to previous DSM diagnoses/subtype differences. American Academy of Child and Adolescent Psychiatry. 1996;35:325–333. doi: 10.1097/00004583-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 76.Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals - Revised. The Psychological Corporation; New York: 1987. [Google Scholar]
- 77.Torgesen JK, Wagner RK.Comprehensive Tests of Phonological Processing-Experimental Version Unpublished Test: Florida State University [Google Scholar]
- 78.Denckla MB, Rudel R. Rapid automatized naming (R.A.N.): Dyslexia differentiated from other learning disabilities. Neuropsychologia. 1976a;14:471–479. doi: 10.1016/0028-3932(76)90075-0. [DOI] [PubMed] [Google Scholar]
- 79.Denckla MB, Rudel R. Naming of object drawings by dyslexic children and other learning disabled children. Brain and Language. 1976b;3:1–16. doi: 10.1016/0093-934x(76)90001-8. [DOI] [PubMed] [Google Scholar]
- 80.Woodcock RW. Woodcock Reading Mastery Test - Revised. American Guidance Service; Circle Pines, MN: 1987. [Google Scholar]
- 81.Raz N, Gunning-Dixon F, Head D, et al. Age and sex differences in the cerebellum and the ventral pons: A prospective MR study of healthy adults. American Journal of Neuroradiology. 2001;22:1161–1167. [PMC free article] [PubMed] [Google Scholar]
- 82.Rosen GD, Harry JD. Brain volume estimation from serial section measurements: a comparison of methodologies. Journal of Neuroscience Methods. 1990;35:115–124. doi: 10.1016/0165-0270(90)90101-k. [DOI] [PubMed] [Google Scholar]
- 83.Kibby MY, Hynd GW. Neurobiological basis of learning disabilities. In: Keogh B, Hallahan D, editors. Research and Global Perspectives in Learning Disabilities. Earlbaum; Mahwah, NJ: 2001. pp. 25–42. [Google Scholar]
- 84.Gauger LM, Lombardino LJ, Leonard CM. Brain morphology in children with specific language impairment. Journal of Speech & Hearing Research. 1997;40:1272–1284. doi: 10.1044/jslhr.4006.1272. [DOI] [PubMed] [Google Scholar]
- 85.Kibby MY, Kroese JM, Morgan AE, et al. The relationship between perisylvian morphology and verbal short-term memory functioning in children with neurodevelopmental disorders. Brain and Language. 2004;89:122–135. doi: 10.1016/S0093-934X(03)00310-9. [DOI] [PubMed] [Google Scholar]