Abstract
Previous studies demonstrated that the CXC chemokine, MGSA/GRO-α and its receptor, CXCR2, are expressed during wound healing by keratinocytes and endothelial cells at areas where epithelialization and neovascularization occur. The process of wound healing is dependent on leukocyte recruitment, keratinocyte proliferation and migration, and angiogenesis. These processes may be mediated in part by CXC chemokines, such as interleukin-8 and MGSA/GRO-α. To examine further the significance of CXC chemokines in wound healing, full excisional wounds were created on CXCR2 wild-type (+/+), heterozygous (+/−), or knockout (−/−) mice. Wounds were histologically analyzed for neutrophil and monocyte infiltration, neovascularization and epithelialization at days 3, 5, 7, and 10 postwounding. The CXCR2−/− mice exhibited defective neutrophil recruitment, an altered temporal pattern of monocyte recruitment, and altered secretion of interleukin-1β. Significant delays in wound healing parameters, including epithelialization and decreased neovascularization, were also observed in CXCR2−/− mice. In vitro wounding experiments with cultures of keratinocytes established from −/− and +/+ mice revealed a retardation in wound closure in CXCR2−/− keratinocytes, suggesting a role for this receptor on keratinocytes in epithelial resurfacing that is independent of neutrophil recruitment. These in vitro and in vivo studies further establish a pathophysiologic role for CXCR2 during cutaneous wound repair.
Keywords: angiogenesis, chemokine, CXCR2, keratinocyte, neutrophils, wound healing
Normal wound healing is a complex but highly regulated dynamic interplay between orderly expressed cell types, extracellular matrix and an array of soluble mediators. The process of wound repair involves keratinocyte and fibroblast proliferation, regeneration of the extracellular matrix, connective tissue regeneration, and blood vessel growth, also known as neovascularization. In the cutaneous setting, wound healing has been traditionally divided into three stages: inflammation, cellular proliferation and tissue remodeling, and maturation. Although these three stages sequentially follow each other, they are not mutually exclusive. Initially, neutrophils accumulate in the damaged tissue, where they form a first line of defense, and afterwards macrophages accumulate to initiate the specific immune response. The recruitment of these inflammatory cells is mediated by specific chemotactic factors or chemokines.
Chemokines play a fundamental part in host defense by recruiting neutrophils, monocytes, and lymphocytes to the site of inflammation. Chemokines consist of a number of distinct 8–10kDa molecules that exhibit varying sequence homologies. These chemokines are classified into four distinct classes, CC, CXC, CX3C, and C, based on a shared cysteine motif. Interleukin (IL)-8, melanoma growth stimulatory activity or growth-regulated protein (MGSA/GRO-α), and interferon-γ-inducible protein (IP-10) are CXC chemokines that primarily recruit neutrophils and lymphocytes, whereas monocyte chemoattractant protein (MCP 1–5), macrophage inflammatory protein (MIP-1α and MIP-1β), and regulated on activation normal T cells expressed and secreted (RANTES) are CC chemokines that recruit monocytes or lymphocytes (Devalaraja and Richmond, 1999; for review). The role of these chemokines in the process of wound healing is not well characterized. MIP-1α expression was recently shown to be critical for monocyte recruitment during wound healing; in addition, an antibody to MIP-1α was shown to delay the process of wound healing (DiPietro et al, 1998) in mice. The temporal and spatial expression of various CC and CXC chemokines (IL-8, MGSA/GRO-α, MCP-1, Mig, and IP-10) was carefully evaluated in experimental adult human incisional wounds (Engelhardt et al, 1998). The expression of these chemokines correlates well with the phase-specific recruitment and trafficking of neutrophils, macrophages, and lymphocytes (Engelhardt et al, 1998). Previous studies from our laboratory have demonstrated an upregulated expression of MGSA/GRO-α and its receptor, CXCR2, in human burn wounds at days 2–12. The CXCR2 expression was detected early after wounding in undifferentiated keratinocytes and in endothelial cells within the wound bed where neovascularization was ongoing (Nanney et al, 1995).
This study was designed to investigate the impact of CXCR2 on various cutaneous wound healing parameters. Previously, characterized CXCR2-deficient mice (Cacalano et al, 1994) were exposed to excisional punch biopsy and the process of wound healing was monitored histopathologically and biochemically. CXCR2−/− mice exhibited a delayed wound healing response. CXCR2-deficient mice exhibited impaired neovascularization, compromised neutrophil recruitment, altered monocyte recruitment, and decreased secretion of IL-β3 into the wound bed. Parallel in vitro wound healing experiments performed with primary cultures of keratinocytes demonstrated a defective proliferative response in these cells. The results clearly demonstrate a major role for CXCR2 in the participation of keratinocytes, endothelial cells, and leukocytes in cutaneous wound healing.
MATERIALS AND METHODS
Animals
BALB/C mice heterozygous (+/−) for CXCR2 were obtained from Charles River (Wilmington, MA), and bred to obtain CXCR2 knockout (−/−) mice in the Vanderbilt University Medical Center AALAC accredited facility, under pathogen-free conditions or by Charles River in a gnotobiotic facility. Mice were maintained under a 12 h light/dark cycle in accordance with the guidelines approved by Animal Protection Committee. Serologic studies on CXCR2 wild-type (+/+), heterozygous (+/−), and knockout (−/−) mice did not detect any blood-borne pathogens or evidence of infection in the mice used in these studies.
Polymerase chain reaction
Tail clippings were performed on 3–4 wk old mice in order to isolate genomic DNA and provide confirmation of the mCXCR2 status. Tail tissues were digested at 55°C overnight in lysis buffer containing 0.5mg Proteinase K per ml (Gibco BRL, Rockville MD), 0.05% sodium dodecyl sulfate 100 mM ethylenediamine tetraacetic acid, and 50 mM Tris pH 8.0. Genomic DNA that was obtained after three phenol–chloroform extractions was resuspended in sterile water. Using a 35-cycle polymerase chain reaction, the genomic DNA was amplified with specific primers for wild-type CXCR2 in an automatic temperature cycler (PTC-100, MJ Research, Waltham, MA). Primers used for mCXCR2 were 5′-GGCGGGGTAAGACAAGAATC-3′ and 5′-GGCAAGGTCAGGGCAAAGAA-3′. Primers used for the neomycin resistance gene were 5′-CGGTTCTTTTTGTCAAGAC-3′ and 5′-ATCCTCGCCGTCGGGCATGC-3′(Lee et al, 1995). Reactions were carried out in 50 μl volumes overlayered by mineral oil. Each reaction mixture contained 12.5pmol of each primer, 0.5U Taq polymerase (Gibco BRL), 0.2 mM of dNTP, and 1 μg of DNA. Polymerase chain reaction amplification products were electrophoresed through a 1% agarose gel containing ethidium bromide.
Excisional wounding procedures
Excisional punches were made as described previously with some slight modifications (Brauchle et al, 1995). Mice (8–10wk of age weighing approximately 25 g) were anesthetized with an intraperitoneal injection containing ketamine (20 mg per kg) and xylazine (0.32mg per kg) (Bayer Corporation, Pittsburgh, PA). The dorsal surface of the animal was cleaned, shaved, and sterilized with betadine solution. Two 3 mm full-thickness excisional punches (Acu-Punch, Fort Lauderdale, FL) were created on the upper paravertebral region of the mouse. Wounds were photographed daily and visually monitored for possible signs of infection. Histologic analyses were conducted on groups of mice (n = 5) at postwounding days 3, 5, 7, and 10.
Histologic analysis
Wound beds surrounded by a margin of nonwounded skin were collected at days 3, 5, 7, and 10 postwounding. Samples were fixed overnight in 4% paraformaldehyde (Fisher, Pittsburgh, PA) at 4°C. Tissues were processed through graded ethanol solutions and embedded in paraffin blocks using standard protocols. Tissue sections (6 μm) were stained with Gomori’s Trichrome or hematoxylin and eosin. The percentage of resurfacing was assessed by morphometric analysis (Nanney, 1990) using Image ProPlus software.
Inflammatory infiltration
This was assessed using standardized quantitative morphometric analysis. Tissue sections stained with hematoxylin and eosin from wounds at 3, 5, 7, and 10 d postwounding were viewed on an Olympus AH light microscope at 400× magnification. Based on the features of polymorphonuclear leukocytes, the density of these infiltrating cells was determined by manually counting the leukocytes within a standardized microscopic area. Five standard and representative areas were selected from each wound for these assessments. Two areas were chosen at each margin, two areas were chosen near the peripheral wound edges, and one area was selected in the central wound bed. The results were expressed as the total number of infiltrating leukocytes per 100,000μm2. We examined six individual wounds for each time point. Neutrophilic infiltration into the fibrin scab over the surface of the wound was not included in these measurements collected from within the wound bed. Neovascularization/capillary in-growth was also assessed in these same standardized wound areas within the wound bed of Trichrome stained sections. The area occupied by capillaries was expressed as μm2 capillary area per 100,000 μm2 of tissue.
Monocyte/macrophage infiltration
Monocyte/macrophage infiltration was assessed in similar fashion to neutrophilic infiltration. Manual counts were performed based on the presence or absence of features such as a comparatively large cytoplasmic size, the relatively large amount of cytoplasm in comparison with the nuclear size ratio, and the absence of an extremely elongated phenotype, such as is often characteristic of fibroblasts.
Isolation and culture of primary mouse keratinocytes
Epidermal keratinocytes were prepared from neonatal mice as described previously (Yuspa et al, 1976). The newborn mice (2d old) were killed by CO2 narcosis, washed in 0.01 M iodine in phosphate-buffered saline for 10min, rinsed with phosphate-buffered saline, washed in 70% ethanol for 10min, and then rinsed in phosphate-buffered saline. Tails from these pups were saved for genomic DNA extraction and further genotyping. The skin was removed from torso and head and then floated in 0.25% trypsin solution (Sigma, St Louis, MO) overnight at 4°C with dermis facing down. Epidermis was separated, minced, suspended and incubated at 37°C for 20 min in low calcium keratinocyte growth medium (KGM, Cascade Biologics, Portland, OR) supplemented with growth factors. Epidermal pieces are triturated to release individual cells, resuspended in KGM and spun down for cell pellet. Keratinocytes were seeded in 24 well plates at a density of ≈ 2–4 × 105 cells per cm2 and cultured in KGM containing 1% chelated fetal bovine serum. A circular wound about the size of 500 μm was made on the monolayer of keratinocytes using a silicon tip. The ability of keratinocytes to close this defect was assessed by measuring the area of the wound at 0, 4, 8, and 24 h postwounding using Bioquant (Nashville, TN) software.
In vitro keratinocyte growth assays
Keratinocyte cultures established from newborn CXCR2+/+, +/−, and −/− mice were maintained in KGM 154-CF (calcium free), 1% heat-inactivated dialyzed fetal bovine serum, 100 units penicillin/streptomycin (P/S), 0.05% gentamicin, 0.06 mM CaCl2, with 1% human keratinocyte growth supplement (Cascade Biologics, Portland, OR). For growth assays 30,000 cells of each genotype were seeded on to Corning 24 well tissue culture plates. After an incubation of 24 h, the number of attached cells was determined by counting four reference wells for each cell type. The medium was changed to KGM without addition of the fetal bovine serum. Cells in one 24-well plate of each genotype received 100 ng MIP-2 per ml, whereas cells of each genotype in the second 24-well plate received the KGM medium (without fetal bovine serum). The plates were incubated at 37°C in a water-jacketed incubator with 5% CO2 and on postseeding days 0, 2, and 5, four wells from each genotype (CXCR2+/+, +/−, −/−) and for each treatment (0 ng MIP-2 per ml, 100 ng per ml MIP-2) were trypsinized and cell number was determined by hemocytometer counting.
Myeloperoxidase (MPO) assay
MPO activity was estimated in the wounded skin according to the method previously described (Trush et al, 1994) with some minor modifications. Briefly, wounded tissues from days 1 to 10 were collected, snap frozen and later homogenized in 0.5–0.6ml of phosphate buffer containing hexadecyl trimethyl ammonium bromide (0.5% in 50mM phosphate buffer, pH6.0) (Sigma). Hexadecyl trimethyl ammonium bromide is a detergent that releases MPO from primary granules of neutrophils. The tissue after homogenization was sonicated on ice for 15s using a sonicator (Sonic 300). After one or two freeze–thaw cycles, the samples were centrifuged 25–30 min at 20,000 × g at 4°C. Protein concentrations were determined using the BioRad (Hercules, CA) protein determination assay and aliquots of lysate containing equivalent amounts of protein (50μg) were assayed for MPO. The supernatant aliquots were mixed with assay buffer containing 0.2% of O-Dianiside hydrochloride (Sigma) and 0.0005% H2O2. The change in absorbance at 490 nm was measured with a Spectrophotometer and the 2 min time point for each aliquot was compared between CXCR2+/+, +/−, and −/− mice.
Neovascularization
The angiogenic response was first assessed from Trichrome stained sections. The area occupied by capillaries in tissue sections was expressed as square microns per 100,000 μm2. A second technique was used to confirm the extent of neovascularization. Capillaries within wound beds were selectively immunostained with CD31 and subjected to computerized morphometry. Sections were incubated for 30 min in a 1/400 dilution of rat antibody to the surface marker cell adhesion molecule commonly found on endothelial cells, CD31[PECAM] (Research Diagnostics, Flanders, NJ) followed by detection using a goat ABC Elite kit purchased from Vector Labs (Burlingame, CA). Sites of immunoreactivity were visualized following staining with DAB chromagen.
Chemokine and cytokine measurements
IL-1β, tumor necrosis factor-α, MIP-2, KC, MIP-1α, MCP-1, RANTES, vascular endothelial growth factor (VEGF) and transforming growth factor (TGF)-β were measured by sandwich enzyme-linked immunosorbent assay (ELISA) using commercially available kits from R & D Systems (Minneapolis, MN). Sensitivities for the chemokines and cytokines were in the range of 10–20 pg per ml. Commercially available agents with the kit served as standards in all assays. Tissue homogenates were made in Tris/ethylenediamine tetraacetic acid buffer containing protease inhibitor. Final concentrations for each chemokine were normalized to pg per mg protein. For some assays ELISA data were also determined on lysates prepared for the MPO assay, using an equal aliquot of the MPO assay buffer as a diluent for the standard curve. The values obtained from the two lysates were highly similar. Except for TGF-β, which was determined with a human ELISA kit, all ELISA kits were specific for murine cytokines. As described previously (Crookston et al, 1995), the samples for TGF-β analysis were activated by incubating 200 μl of the sample with 20 μl of 1 M HCl for 10 min at room temperature and later neutralized with 15 μl of 1 M NaOH.
Statistical analysis
Results are expressed as mean ± SEM. Statistical differences between groups were determined by an analysis of variance (ANOVA) and/or by the two-tailed Student’s t test. Paired analyses were performed where the number of samples was identical between groups and unpaired t test were performed when the number of samples between groups was not the same. Significance was claimed for p < 0.05.
RESULTS
Delayed in vivo wound healing in CXCR2 knockout mice
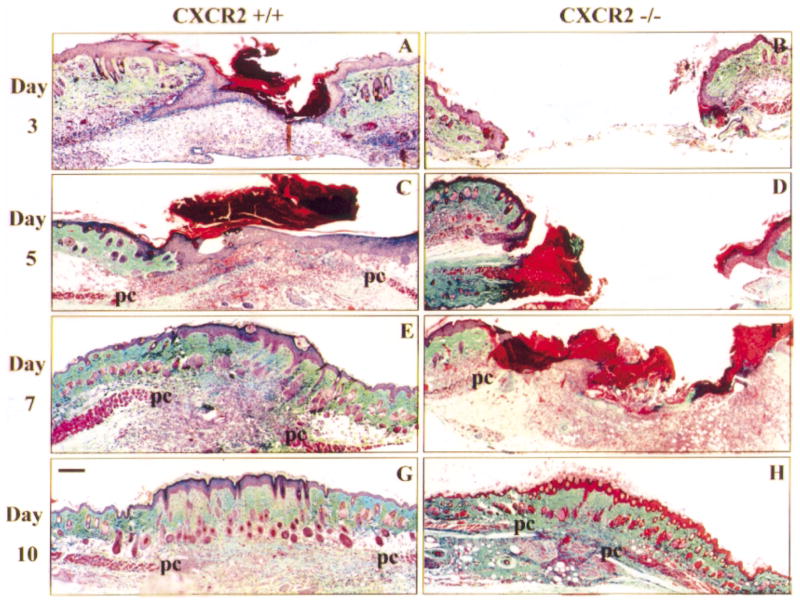
Cacalano et al (1994) have previously developed mice that lack the functional murine homolog of CXCR2. Neutrophils from these mice did not chemotax in response to MIP-2 and the thioglycollate-induced migration of neutrophils was greatly impaired. The mice exhibited lymphadenopathy and splenomegaly, as well as altered cytokine profiles, but when cultured in a germ-free facility, these abnormalities were significantly reduced (Shuster et al, 1995). Subsequently, these receptor-deficient mice were successfully employed to delineate a major role for neutrophils and the CXC chemokines in classic inflammatory conditions such as inflammatory bowel disease (Terkeltaub et al, 1998). This study investigates the significance of CXCR2 during cutaneous healing. The creation of full-thickness excisional wounds on mice expressing wild-type CXCR2 (+/+), mice heterozygous for CXCR2 (+/−), or mice deficient in CXCR2 (−/−) allowed us to examine a variety of wound healing processes affecting both the dermis and epidermis. In the first set of experiments, biopsies from these wounds taken at days 3, 5, 7, and 10 postwounding were evaluated for their histologic features and time of wound closure as determined by complete epithelialization of the wound gap with keratinocytes. The net accumulation of extracellular matrix molecules within the granulation tissue can be crudely visualized by increasing levels of green staining in histologic sections after trichrome staining. Representative micrographs of the histologic features of the wounded skin of either CXCR2 +/+ or −/− mice from days 3, 5, 7, and 10 postwounding are depicted in Fig 1. An identical series of wounds were examined from CXCR2 heterozygous mice but are not shown. In +/+ mice, both dermal and epidermal layers are reformed by postwound day 3 (Fig 1A); whereas in CXCR2−/− mice, epidermal outgrowth at the wound edges is barely detectable even by day 7 (Fig 1F) and 100% resurfacing is not generally achieved until day 10 (Fig 1H). Thus, in CXCR2−/− mice, the process of epithelialization is severely retarded. The difference between the wound healing of these two genotypes is also evident from macroscopic observations. At successive days after injury, wound areas clearly decrease in size in the CXCR2 +/+ mice and the wound beds become increasingly shallow and filled-in with granulation tissue (data not shown). Macroscopically, CXCR2 knockout mice show little or no evidence of wound contraction or healing during days 1–5. Similar experiments performed with CXCR2 +/− mice displayed an intermediary response in wound healing.
Figure 1. Delayed epithelial resurfacing and wound closure in CXCR2 −/− mice.
Representative micrographs from trichrome-stained sections in parts (A)– (H) show responses to excisional injury on days 3, 5, 7, and 10 postinjury. Parts (A), (C), (E), and (G) depict the normal sequence of repair in +/+ (wild-type) mice. Epithelialization underneath the vivid red scab is nearly complete by day 3 with a hypertrophic epidermis by day 5. Robust cellularity in the wound bed, increasing collagen deposition, and wound contracture are evident at days 5, 7, and 10. By contrast, wounds from CXCR2 knockout mice shown in (B), (D), (F), and (H) reveal impaired reparative responses. Resurfacing across the wound bed remains incomplete until days 7–8. Severely inhibited cellularity of the granulation tissue (gt) is noted at days 3–5 and delays in wound contracture are evident at days 3, 5, and 7. Immature granulation tissue is visible at day 7 followed by eventual wound closure and complete resurfacing by day 10. All wounds eventually show complete epidermal and dermal healing with no scarring. After resurfacing is achieved, sites of excisional wounding can only be detected by a discontinuity in the underlying panniculus carnosus layer (pc). Scale bars: 100 μm.
Impaired neovascularization in the wounds of CXCR2 −/− mice
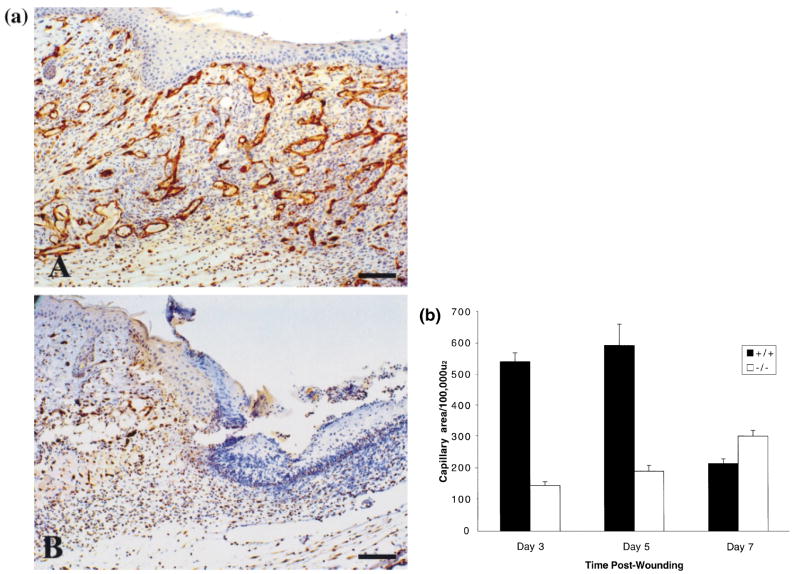
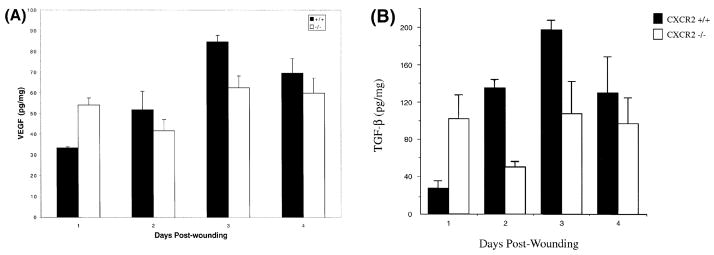
Vascularity of the wound bed serves as a quantitative parameter to gauge the extent and maturity of wound healing and provides the granulation tissue with its characteristic pink/red appearance. Several growth factors are known to promote capillary formation at the wound site, including VEGF, TGF-α, TGF-β, basic fibroblast growth factor, and CXC chemokines containing the glu-leu-arg (ELR) amino acid motif. To determine whether a neovascular deficiency was one possible mechanism contributing to the impaired wound healing observed in CXCR2 −/− mice (Fig 1), vessels were immunostained with a rat monoclonal antibody (IgG) to CD31, a marker for murine endothelial cells. The dramatic differences in the extent of vascularity in wounds at 5 d after injury is shown by CD31 immunostaining in Fig 2(a). A representative wound bed that is highly vascular is present in the +/+ mouse whereas the wound bed in the CXCR2−/− animal shows minimal immunoreactivity due to a paucity of new capillaries. The area occupied by blood vessels within the wound area was also quantitated from hematoxylin and eosin-stained tissues from wounds of CXCR2+/+ and −/− mice over days 3–7 (Roesel and Nanney, 1995). The average longitudinal and cross-sectional capillary areas within the wound bed are illustrated graphically in Fig 2(b). A statistically significant inhibition or delay in neovascularization was apparent in CXCR2−/− wounds at days 3 and 5 (p < 0.01 and p < 0.01, respectively, using the Student’s two-tailed t test; or p < 0.01 and p < 0.01, respectively, by ANOVA). By postwound day 7, the area of the capillary beds was significantly greater in the −/− mice (p<0.01, Student’s two-tailed t test, or p<0.05 by ANOVA). As expected from previous studies (Roesel and Nanney, 1995), capillary in-growth peaked at day 5 and diminished thereafter as wounds attained maturity in wild-type mice (Fig 1E, G). By contrast, capillary in-growth was delayed as well as stunted in mice deficient in CXCR2. Capillaries were present in the knockout mice only at edges of the wound as the central portion of the wound bed remained quiescent and essentially unfilled with cells or matrix for many days (Fig 1B, D). Nevertheless by day 10, 100% wound closure was eventually achieved even in knockout mice (Fig 1H). The wound attained a relative maturity by day 5 in wild-type mice whereas in CXCR2−/− mice, a similar maturity is not attained until day 7 or later (Fig 1F, H). The data shown in Fig 2(b) suggest that by day 7 neovascularization in the CXCR2 knockout mice began to approach, but did not quite reach, the level of neovascularization that characterizes the normal vascularization of the wild-type mouse. Within cutaneous wounds, both macrophages and keratinocytes are known to express and secrete cytokines that are key players in the neovascularization process (Horn and Maisel, 1992). In this study the levels of VEGF and TGF-β secreted within the wound environment were examined by ELISA over a period of time after cutaneous injury. VEGF appeared to increase during the first 3d after injury in CXCR2 wild-type mice (Fig 3A), but the VEGF secretion in the CXCR2 knockout mice did not exhibit a parallel temporal pattern. The VEGF levels were significantly lower in CXCR2+/+ mice as compared with CXCR2−/− mice on postwound day 1 (p < 0.01, ANOVA), whereas by postwound day 3, the VEGF levels were higher in wild-type mice than in CXCR2−/− mice (p<0.01, ANOVA). TGF-β levels appeared to mirror the VEGF levels. In the wild-type mice, TGF-− levels were lower than in the CXCR2−/− mice on postwound day 1, then increased steadily, peaking at day 3–4; no similar temporal pattern was observed in CXCR2-deficient mice (Fig 3B). The TGF-β levels were significantly higher in wild-type mice on postwound day 2 (p<0.01, Student’s two-tailed t test; p<0.01, ANOVA), but on postwound days 1, 3, and 4, these differences were not significant (p > 0.4, 0.09, and 0.5, respectively, Student’s two-tailed t test).
Figure 2. (a) Immunostaining for CD31 antigen outlining blood vessels within 5 d wounds.
(A) Numerous capillaries within the granulation tissue and 100% resurfacing by epithelium in CXCR2+/+ mice at postwound day 5. (B) Paucity of capillaries and incomplete resurfacing at the left and eschar at the top right. Scale bar. 100μm. (b) Delayed neovascularization in CXCR2−/− mice: Quantitative expression of capillary areas within the granulation tissue of excisional punches was assessed from hematoxylin and eosin sections as described in Materials and Methods. Values are expressed as the mean total capillary area per 100,000 (μm2 ± SEM at days 3, 5, and 7 postinjury, based upon five observations per point. CXCR2−/− mice exhibited significantly impaired neovascularization whereas CXCR2+/+ mice showed an expected peak in capillary in-growth followed by a regression in the capillary network that accompanies wound maturation (p < 0.0004, p < 0.003, p < 0.008 for postwound days 3, 5, and 7, respectively, Student’s two-tailed t test; or p = 2 E-09, p = 0.0002, and p = 0.05 for days 3, 5, and 7, respectively, by ANOVA).
Figure 3. Altered cytokine profiles in CXCR2−/− mice.
VEGF (A) and TGF-β (B) levels in the wounded skin homogenates of CXCR2+/+ show a consistent rise and fall in these cytokines in wild-type mice from day 1 to day 4 postwounding. In CXCR2−/− mice the levels of TGF-β and VEGF were higher than for wild-type mice on postwound day 1. Values represent mean ± SEM of duplicate determinations for two to four mice per time point. Statistical differences in VEGF and TGF-β levels between wild-type and CXCR2−/− mice were noted on days 1 and 3, based upon the Student’s t test and ANOVA.
Perturbed neutrophilic recruitment to the wound site correlates with CXCR2 status
Both neutrophils and monocytes are recruited from the circulating blood in response to molecular changes in the surface of endothelial cells lining the capillaries at the wound site (Riches et al, 1996). Neutrophils normally begin arriving at the wound site within minutes of injury. Traditionally, the primary role of these neutrophils was considered to be bactericidal (Detmers et al, 1991). In recent years, neutrophils have also been shown to serve as a source of pro-inflammatory cytokines. CXCR2 is widely expressed on an array of different cell types, but is an essential mediator of CXC chemokine-induced neutrophil chemotaxis into the site of inflammation (Terkeltaub et al, 1998). To assess the importance of CXCR2 on neutrophil recruitment, excisional wounds were collected and examined. Neutrophil chemoattractants in the wound bed were assessed by ELISA. Neutrophil infiltration into the wounds was assessed using two differing methods: quantitation of MPO activity from wound homogenates (Fig 4) and quantitation of neutrophils from histologic sections (Table 1). MIP-2 levels in wound tissue were rather constant in wild-type mice over postwound days 1–3, then declined by postwound day 4 (Table II). The MIP-2 level in wounds from CXCR2+/+ and CXCR2−/− mice were not significantly different (Table II). Whereas KC levels were comparable with MIP-2 levels, there were no significant differences in the KC in wounds from CXCR2+/+, +/−, and −/− mice (data not shown).
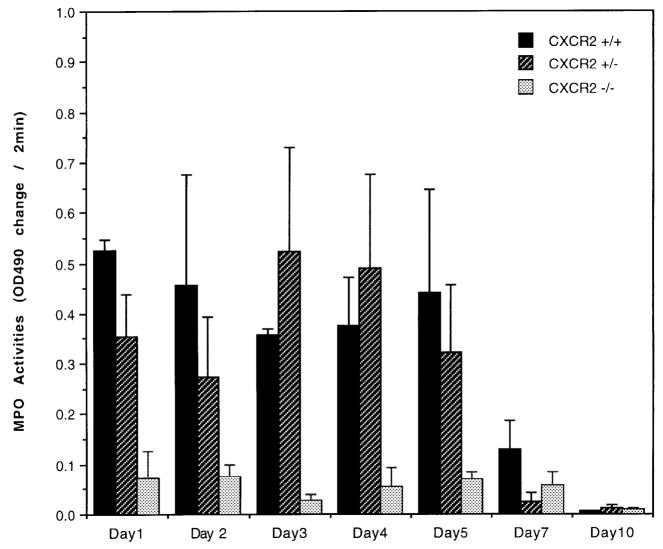
Figure 4. Reduced MPO activity in CXCR2−/− mice.
Neutrophil MPO activity was measured on 50 μg protein from wound lysate. as described in Materials and Methods on tissue homogenates from postwound day 1 to day 10. Data are graphed as change in absorbance/optical density over a period of 2 min at 490 nm wavelength each day. The bars indicate the mean ± SEM from wounds of three to four wounded animals. In the CXCR2−/− mice MPO activity is significantly lower than in wild-type mice at postwound days 1, 3, and 5, based upon the Student’s two-tailed t test and/or ANOVA. MPO activity from wild-type mice is different from CXCR2 heterozygous mice (+/−) at postwound days 1 and 2 (p<0.05, Student’s two-tailed t test).
Table I.
Delayed neutrophil recruitment in CXCR2−/−micea
| Day post-wounding | Neutrophil number |
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| 3 | 56 ± 2.80 | 32 ± 1.60 | 6.0 ± 0.47* |
| 5 | 48 ± 2.33 | 42 ± 2.05 | 2.8 ± 0.99* |
| 7 | 17 ± 1.70 | 60 ± 3.48 | 12.5 ± 1.37 |
| 10 | 11 ± 1.28 | 37 ± 2.64 | 16.5 ± 0.75* |
Neutrophil recruitment to the wound bed at days 3, 5, 7, and 10 after cutaneous wounding. Total numbers of neutrophils in CXCR2 wild-type +/+, CXCR2 heterozygous (+/−), and CXCR2 knockout mice (−/−) were manually counted within standardized areas of hematoxylin and eosin sections of the wound bed as described in Materials and Methods. Data are expressed as the mean ± SEM for the total number of neutrophils around the wounds based on six different mice at each time point after injury. The level of statistical significance of the comparison between wild-type and CXCR2−/− mice is indicated by the asterisk to denote p < 0.05 based upon the Student’s two-tailed t test and ANOVA.
Table II.
Chemokine profiles in CXCR2+/+ and −/− micea
| Day post-wounding | MIP-2 |
MIP-1α |
MCP-1 |
IL-1β |
||||
|---|---|---|---|---|---|---|---|---|
| +/+ | −/− | +/+ | −/− | +/+ | −/− | +/+ | −/− | |
| 1 | 162 ± 36 | 142 ± 36 | 172 ± 94 | 273 ± 56 | 253 ± 41 | 356 ± 158 | 305 ± 77 | 340 ± 69 |
| 2 | 138 ± 19 | 192 ± 54 | 377 ± 50 | 393 ± 167 | 207 ± 24 | 219 ± 88 | 253 ± 31 | 118 ± 41* |
| 3 | 150 ± 13 | 145 ± 23 | 273 ± 80 | 228 ± 66 | 209 ± 69 | 234 ± 75 | 283 ± 5 | 92 ± 13* |
| 4 | 119 ± 12 | 99 ± 14 | 241 ± 83 | 107 ± 28 | 122 ± 12 | 150 ± 34 | 172 ± 36 | 106 ± 32 |
Cytokines and chemokine profiles in wound fluid as measured by the sandwich ELISA technique at four different time points. Values indicate mean ± SEM from three to four independent observations. Wounded skin samples were collected and tissue homogenates were made on days 1–4 postinjury. All tissue results are expressed as pg per mg total protein recovered after homogenization of 3 mm punch biopsy. The level of statistical significance of the comparison between wild-type and CXCR2−/− mice is indicated by the asterisk to denote p-values < 0.05 based upon the Student’s two-tailed t test and ANOVA.
As expected, in the wild-type mice, the MPO activity peaked between days 1 and 5 then precipitously declined by day 7 (Fig 4), consistent with previous results where peak neutrophil infiltration comes early after wounding (Abbot et al, 1998; Yan et al, 1998). Within the histologically stained section of wound tissue from CXCR2+/+ mice, our earliest manual count of neutrophils was on day 3, when neutrophil number peaked (Table I). By contrast, CXCR2−/− mice showed a greatly diminished neutrophil recruitment into the wound sites at all time points. The MPO levels in CXCR2−/− mice were also significantly lower than in wild-type mice or heterozygous mice on postwound days 1, 3, and 4. Table I shows that neutrophilic influx was severely delayed in CXCR2−/− mice in the acute wounding period at days 3–7 (p <0.05, Student’s two-tailed t test). Moreover, MPO activity was markedly depressed, but not abolished in CXCR2 knockout mice (Fig 4). By day 10 the CXCR2−/− mice had more neutrophils than CXCR2+/+ mice (p<0.05, Student’s two-tailed t test).
The CXCR2 heterozygous mice exhibited an intermediate MPO activity on postwound days 1 and 2 and an intermediate delay in neutrophil influx for postwound days 3–4 (Table I) as compared with the other groups. Heterozygote MPO activity, however, was not significantly different than wild type on postwound days 1–10 (p>0.05 Student’s t test), even though neutrophil count in heterozygotes exceeded that in wild-type mice on postwound days 7–10. MPO activity from wounds in heterozygous mice on days 1, 3, 4, and 5 was, however, significantly greater than in CXCR2−/− mice (p < 0.05, Student’s t test). Our data do not explain why the heterozygous mice exhibit this delayed increase in neutrophil count compared with wild-type mice, or why the MPO activity does not correlate with neutrophil number on day 7 in heterozygotes, although we suspect variability in the expression of the CXCR2+ allele in heterozygous mice may contribute to these differences.
The data from this series of experiments suggest a crucial part for CXCR2 in early neutrophil influx (days 1–3), which can be partially replaced by other factors at later points in the wound healing process when CXCR2 is absent (days 7–10).
Monocyte recruitment and cytokine expression profile in wounded CXCR2−/− mice
Although neutrophils are the initial cell type attracted to the wound site, the next stage of the inflammatory response is marked by the recruitment of circulating monocytes into the wound bed. Monocyte/macrophage influx is apparently essential for effective wound healing, as wound healing is severely impaired when monocyte infiltration is prevented (Leibovitch and Ross, 1975). Activated monocytes/macrophages release a battery of growth factors and cytokines at the wound site, including TGF-β, VEGF, IL-1, and tumor necrosis factor-α, thus amplifying the earlier wound signals released by neutrophils (Brown et al, 1992; Shah et al, 1995; Frank et al, 1996). To determine whether the initial neutrophil impairment results in subsequent alterations in monocyte recruitment to the wound site, monocyte infiltration into the wound bed was manually counted on hematoxylin and eosin stained tissue sections. The total number of monocytes per wound was higher in the CXCR2−/− mice than in the CXCR2+/+ mice at postwound days 3, 5, and 7 (p < 0.05, Student’s two-tailed t test) (Table III). These data suggest that initial lack of neutrophil recruitment seen in the +/− and −/− wound beds was not accompanied by a correlative decrease in monocyte recruitment. The distribution of monocytes within the wound beds differed in CXCR2+/+ and −/− mice. Monocytes were concentrated initially on days 3–5 at the edges of the wound in CXCR2−/− mice, probably due to lack of a regenerated wound scaffold to migrate across. By day 10, the number of monocytes in the wound bed in CXCR2+/+, +/−, and −/− mice were not statistically different. MCP-1, MIP-1α, and RANTES serve as the primary monocyte chemoattractants (Yoshimura et al, 1989; Schall et al, 1990; Taub et al, 1993; Uguccioni et al, 1995). To confirm the apparent equivalence of the putative monocyte-attracting chemokine milieu within the wound sites between CXCR2+/+ and −/− mice, levels of locally secreted chemokines were estimated by commercially available ELISA kits. No significant differences in the local MCP-1 or MIP-lα concentrations at the site of the wound were detected between CXCR2+/+ and −/− mice (Table II) (p > 0.05 by Student’s two-tailed t test and by ANOVA). RANTES was not detected at the wound site in either CXCR2+/+or −/− mice (data not shown). The lack of difference in the monocyte chemoattractants secreted at the wound site, coupled with the monocyte counting data (Table III), strongly suggest that loss of CXCR2 may result in a compensatory increase in monocyte recruitment. The mechanism for this is not clear from our data, but other CC chemokines may be involved.
Table III.
Altered monocyte recruitment in CXCR2−/−rnicea
| Day post-wounding | Monocyte number |
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| 3 | 49 ± 2.30 | 50 ± 2.60 | 55 ± 3.00* |
| 5 | 31 ± 1.94 | 50 ± 3.51 | 43 ± 3.60* |
| 7 | 24 ± 2.64 | 58 ± 2.26 | 49 ± 2.44* |
| 10 | 24 ± 2.26 | 26 ± 1.94 | 29 ± 3.92 |
Monocyte infiltration to the wound bed on days 3, 5, 7, and 10 after cutaneous wounding. Total numbers of monocytes in CXCR2 wild-type (+/+), CXCR2 heterozygous (+/−), and CXCR2 knockout mice (−/−) were manually determined and counted within standardized areas of hematoxylin and eosin sections as described in Materials and Methods. Data are expressed as the mean ± SEM for monocytes in six different mice at each time point after injury. The level of statistical significance of the comparison between wild-type and CXCR2−/− mice is indicated by the asterisk to denote p < 0.05 based upon the Student’s two-tailed t test and ANOVA. RANTES was non-detectable in all the samples.
The roles of tumor necrosis factor-α and IL-1β have been well established in wound healing (Hubner et al, 1996). To determine whether differences in either of these cytokines could contribute to the impaired wound healing response observed in CXCR2−/− mice (Fig 1), ELISAs for these cytokines were also performed on wound tissue homogenates (Table II). We did not detect tumor necrosis factor-α in these wounds (data not shown). IL-1β levels were significantly lower in CXCR2−/− mice on days 2–3 (p < 0.05 by ANOVA and Student’s two-tailed t test), and this result correlates with the delayed wound healing and the impaired neutrophil recruitment of the receptor-deficient mice.
In vitro response of keratinocytes to wounding
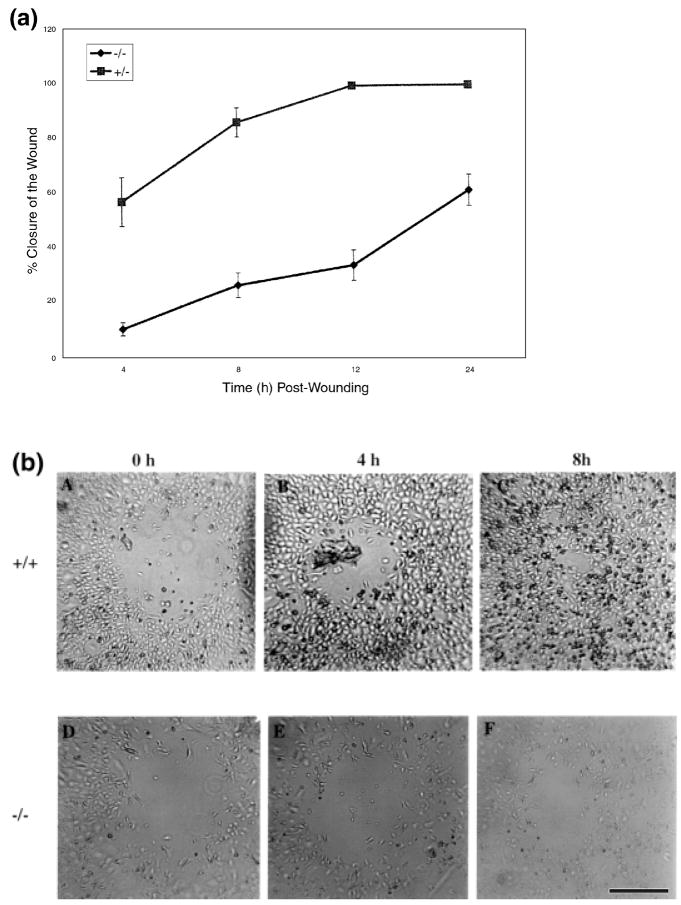
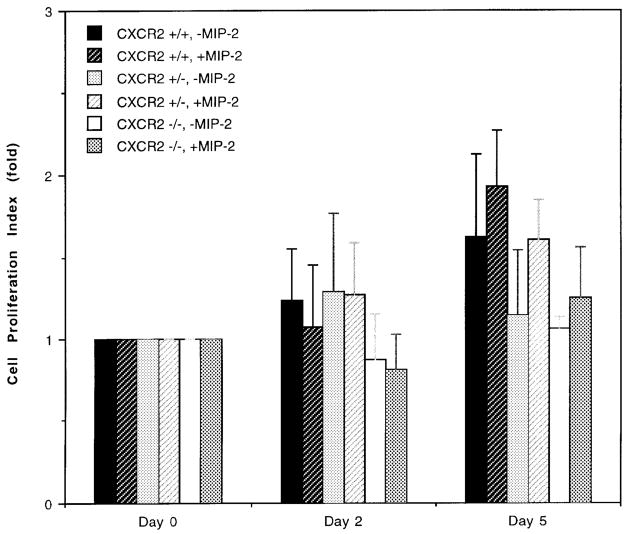
Following injury to the epidermis, keratinocytes at the immediate edge of a wound began to migrate across the wound bed, and the adjacent keratinocytes began to proliferate. This phenomenon, known as epithelialization, begins within hours of injury and eventually results in complete resurfacing. Epidermal cells undergo marked phenotypic changes during migration and proliferation (Guo et al, 1995; Haapasalmi et al, 1996; Mitcheson and Cramer, 1996; Schaffer and Nanney, 1996) and these changes are generally associated with alterations in integrins (Cavani et al, 1993; Breuss et al, 1995) and expression of matrix metalloproteinases (Stricklin et al, 1993; Stricklin and Nanney, 1994). Our series of in vitro wounding studies were designed to dissect apart the role of CXCR2 in wound epithelialization. By culturing keratinocytes from CXCR2+/+ and −/− mice in vitro, we were able to address the question of whether the in vivo delay in the epidermal resurfacing of CXCR2−/− mice was the result of inherent deficiencies in CXCR2-defective keratinocytes or was primarily controlled by the delayed recruitment of leukocytes due to loss of CXCR2 in neutrophils. We conducted in vitro wound healing experiments with isolated primary cultures of keratinocytes. The wound area was measured at 0, 4, 8, 12, and 24 h postwounding. The extent of wound closure in confluent keratinocytes represents a composite of the keratinocyte migration and the proliferative capacity that is not complicated by the dynamics of wound contraction. Keratinocytes from CXCR2+/+ mice exhibited approximately 80% closure of the defect in a confluent culture by 4 h postwounding, whereas only 60% wound closure was observed in CXCR2−/− mice even after 24 h postwounding (Fig 5a). A representative picture of the defect in wound healing in cultured keratinocytes at 4 and 8 h postwounding is shown in Fig 5(b). The pattern of wound healing exhibited by heterozygous mice was more similar to wild-type mice (data not shown). There were no statistically significant differences in the proliferation rate of keratinocyte cultures established from the CXCR2+/+, +/−, and −/− mice (p>0.05, Student’s t test) (Fig 6). The cell proliferation rate was evaluated in culture medium optimized for keratinocyte growth, containing epidermal growth factor, brain extract, and low calcium. After 5 d of culture in this optimized medium with the addition of MIP-2 (100 ng per ml), there were no differences in the means of MIP-2 treated and not treated CXCR2+/+ keratinocytes (p>0.05, Student’s t test). Keratinocytes from CXCR2−/− mice were not stimulated by addition of 100 ng per ml of MIP-2 during the 5 d assay. Though these cells appeared to grow slower than did keratinocytes from CXCR2+/+ mice, the difference was not statistically different (p>0.05). These data support a primary role for CXCR2 in the migration of wounded keratinocytes, although we cannot rule out the possibility that in vivo, cutaneous keratinocytes expressing CXCR2 also undergo a proliferative response to ligands for this receptor.
Figure 5. Delayed in vitro wound closure in keratinocytes from CXCR2−/− mice.
In vitro wounding of confluent cultures of CXCR2+/−, and −/− keratinocytes. Keratinocytes from the three genotypes (+/+, +/−, and −/−) were isolated and cultured in 24 well tissue culture plates as described in Materials and Methods. Wounds (400–500 μm) on monolayered keratinocytes were monitored over a period of 24 h and the area of the wound defect determined using Bioquant software. Keratinocytes from wild-type mice exhibited a wound closure which was not significantly different from keratinocytes of heterozygous mice. Part (a) shows the percentage of wound closure of keratinocytes from +/− mice compared with −/− mice during a 24 h period. Values represent mean ± SEM obtained from cultures derived from three different mice and five independent wounds in each well. Part (b) depicts representative in vitro photographs of wounded keratinocyte cultures from +/+ (A–C) and −/− (D–F) mice during wound closure. Scale bar. 200 μm.
Figure 6. Effect of MIP-2 on the growth of keratinocytes from CXCR2+/+, +/−, and −/− mice.
Keratinocyte cultures were established from newborn CXCR2+/+, +/−, and −/− mice according to the protocol described in Materials and Methods. Cell proliferation in the presence or absence of MIP-2 (100 ng per ml) was followed over 5 d by hemocytometer counting. Each point represents the mean of four individual determinations. The cell proliferation index on days 2 and 5 is defined as the mean number of cells per well divided by the mean number of cells attached 24 h after the initial seeding. The day the initial number of attached cells was determined is the time for the first addition of MIP-2 and this day was defined as day 0. The error bars represent the SEM.
The effects of MIP-2 on VEGF secretion were assessed on the cultured keratinocytes from wild-type mice. We did not observe significant VEGF secretion in keratinocytes from CXCR2+/+, +/−, or −/− mice in the absence or presence of MIP-2 (100 ng per ml). Culture medium from these cells contained less than 40 pg per ml of VEGF for all the cultures, except for the keratinocytes from CXCR2 heterozygotes, which had slightly higher VEGF levels. Therefore, we doubt that reduced vascularization of the CXCR2−/− mice after wounding is due to a deficiency of VEGF secretion by keratinocytes in response to MIP-2. Moreover, VEGF levels in the wounds of wild-type mice were higher on day 3 but lower on day 1 as compared with wounds in CXCR2−/− mice.
DISCUSSION
In this study mice deficient in CXCR2 exhibit delayed wound healing, in vivo and in vitro are demonstrated. The in vivo wound healing defect appears to be mediated in part through impaired neutrophilic recruitment during the acute period after wounding. As CXCR2 receptors are normally expressed on epidermal keratinocytes, endothelial cells involved in neovascularization, and neutrophils, and are upregulated in psoriasis and in response to cutaneous injury in keratinocyte and endothelial populations (Nanney et al, 1995; Kulke et al, 1998), it is not surprising that the wounding of CXCR2 knockout mice is also accompanied by disturbances in the wound healing parameters of neovascularization and epithelialization by keratinocytes.
Recruitment of inflammatory cells to the site of inflammation is essential for immune surveillance and the initial delivery of growth factors (Rappolee et al, 1988; Marikovsky et al, 1993; Schaffer and Nanney, 1996). Chemokines not only have the unique potential to direct a specific leukocyte population subset to distinct focal areas within the wound during various phases of tissue repair, they also can activate these cell types by inducing multiple cytokine signaling pathways. Using incisional adult skin wounds, Engelhardt et al (1998) demonstrated that the expression of specific type(s) of chemokines produced at the wound site is a well orchestrated and dynamic process. Neutrophils are the first cell type recruited to the wound site (Martin, 1997) and this recruitment correlates with an early expression of IL-8 and MGSA/GRO-α after wounding (Fivenson et al, 1997). MIP-2 and KC are murine homologs of IL-8 and MGSA/GRO and mouse has only one functional receptor for these chemokines, CXCR2. Several studies have reported that murine MIP-2 is much more active than KC in recruiting neutrophils (Frevert et al, 1995; Tessier et al, 1997; Yan et al, 1998). Whereas the local MIP-2 or KC levels in the wound tissue in CXCR2−/− or +/+ mice were not different, the number of neutrophils recruited to the wound site was significantly reduced in CXCR2−/− mice as indicated by both MPO assay and cell count through postwound day 10. The peak neutrophil recruitment was observed on postwound days 3 and 5 for CXCR2+/+ mice, whereas for +/− mice the peak shifted to postwound day 7. For the CXCR2−/− mice, neutrophil recruitment was reduced throughout the wound healing process and considerably delayed, showing some increase by postwound day 10. Taken together these data show a strong correlation between loss of CXCR2 expression and diminished neutrophil infiltration into the wound bed. This delay in neutrophil recruitment in CXCR2−/− mice was correlated with a decrease in IL-1 levels in the CXCR2−/− mice on postwound days 2 and 3, but not on day 1. Infiltrating neutrophils are thought to contribute significantly to the IL-1 level in the wound bed.
In contrast to human neutrophils, mouse neutrophils express CCR1, allowing for the possibility that monocyte chemoattractants that bind to CCR1 (MCP, MIP-1, RANTES, among others) may also recruit neutrophils (Johnston et al, 1999). When the levels of MCP-1 and MIP-1α in CXCR2+/+ and −/− mice were examined, these chemokines were abundant on days 1–4 (Table II). RANTES was not detected in either genotype (data not shown). As these ligands for CCR1 were abundant in the wound bed, we cannot rule out the possibility that the modest neutrophil recruitment observed in CXCR2−/− mice occurs in part in response to CC chemokines. Other neutrophil chemoattractants not requiring CXCR2 may also participate in this process.
MCP-1 and MIP-1α were previously shown to be major chemoattractants that modulate monocytic infiltration into the wound site (Ritter et al, 1996). Engelhardt et al (1998) have demonstrated in a human wound healing model that following induction of neutrophil migration into the wound bed on postwound day 1 in response to IL-8 and MGSA/GRO-α, monocyte migration peaks at day 2, correlating with the induction of MCP-1. We observed that MCP-1 is present on postwound days 1–3, and declines on day 4, whereas the rise in MIP-1α on day 2 showed a positive correlation with monocyte infiltration. Our failure to detect RANTES in the wound bed is in agreement with the findings of Engelhardt et al (1998). Although recruitment of monocytes and lymphocytes sequentially follows neutrophilic recruitment (Schaffer and Nanney, 1996), this study indicates that neutrophil recruitment is independent of monocyte recruitment; i.e., the impaired neutrophilic influx observed in CXCR2−/− mice is not correlated with a delay in monocyte recruitment into the wound bed. In fact, monocyte recruitment was elevated in CXCR2+/− and −/− mice as compared with wild-type mice. As differences were not detected in MIP-1α, MCP-1, or RANTES in CXCR2+/+ and −/− mice, the small increase in monocyte recruitment in the −/− mice does not appear to be due to differences in the levels of these chemokines in the wound bed. Rather, we propose that the higher levels of monocytes in the wound bed of CXCR2−/− mice on day 7 is a reflection of the delay in the wound healing process itself.
Within normal wounds, CXCR2 is not only present on the recruited neutrophils, but is also available on the keratinocytes and vascular endothelial cells (Nanney et al, 1995). Consistent with the hypothesis that CXCR2 is involved in angiogenesis and epithelialization, an elevated expression of both CXCR2 and its ligands during neovascularization and keratinocyte proliferation was observed in various wound models (Fivenson et al, 1997; Gibran et al, 1997; Rennekampff et al, 1997). This supports the concept that CXCR2 is not only responsible for neutrophil recruitment to the wound site, but also mediates important mitogenic and migratory signals that culminate in neovascularization. We observed a significant delay in neovascularization in CXCR2−/− mice as compared with wild-type mice during the wound healing event. We postulate this is the result of diminished angiogenic response to MIP-2 in the wound bed. We have recently demonstrated that antibody to murine CXCR2 blocks the angiogenic response to MIP-2. Additionally we have shown that CXCR2−/− mice do not exhibit angiogenic response to MIP-2 using the corneal micropocket assay (Addison et al submitted).
The local microvasculature and the tissue also depend on several other growth factors for their regeneration. The nonchemokine growth factors that are presented at the wound site and are hypothesized to be responsible for neovascularization, keratinocyte cellular proliferation, and/or re-epithelialization include epidermal growth factor, TGF-β, platelet-derived growth factor, fibroblast growth factor, and VEGF, among others (Nanney, 1994; Schaffer and Nanney, 1996). In our model, we examined the levels of VEGF and TGF-β in the wound bed by ELISA to determine whether differences in these cytokines might contribute to the delay in wound repair in the CXCR2−/− mice. VEGF is secreted by keratinocytes and macrophages, whereas TGF-β is primarily secreted by macrophages and platelets (Brown et al, 1992; Frank et al, 1996). The VEGF levels in the CXCR2+/+ mice were lower on postwound day 1 as compared with the CXCR2−/− mice, not significantly different from −/− mice on postwound day 2, slightly higher than in the CXCR2−/− mice on postwound day 3 (p<0.01, ANOVA), and not significantly different from −/− mice on postwound day 4 (Fig 3A). TGF-β levels were higher in CXCR2−/− mice on postwound day 1, but by day 2, the TGF-β values were higher in wild-type mice than in CXCR2−/− mice (Fig 3B). The primary effector functions of VEGF or TGF-β include angiogenesis and keratinocyte chemotaxis. A lack of CXCR2 on the endothelial cells and keratinocytes in CXCR2−/− mice may have resulted in reduced angiogenesis and epithelialization in response to MIP-2 in the mouse excisional wound healing model. The small increase in VEGF in CXCR2−/− mice on postwound day 1 could have been somewhat compensatory. As we did not observe any induction of VEGF by MIP-2 in cultured keratinocytes from CXCR2+/+ or −/− mice, we do not see a direct link between the loss of MIP-2 response and the decrease in VEGF in CXCR2−/− mice on day 3, but postulate that this was a secondary compensatory event.
The in vivo and in vitro wound healing studies performed here with specific CXCR2 mouse knockout mice clearly indicate that CXCR2 is essential for not only neutrophil recruitment into the wound site, but also for the overall outcome of tissue repair. Our previous work has shown that epithelialization after injury is accompanied by upregulation of CXCR2 at healing epidermal margins in human burn wounds (Nanney et al, 1995). When the present in vivo studies produced dramatic evidence that resurfacing was greatly impaired in both CXCR2+/− and −/− mice, additional studies were designed to address the possibility that delayed resurfacing may be due to an inherent deficiency in CXCR2 levels in keratinocytes that is perhaps independent of the delayed neutrophil infiltration into the wound bed. Our in vitro studies provide additional evidence that the presence of CXCR2 on keratinocytes plays an essential and pivotal part during epithelial repair. Keratinocytes totally lacking this receptor exhibit a profound delay in wound closure that is independent of neutrophil- or macrophage-derived mediators. Rennekampff et al (1997) has previously shown that the addition of MGSA to split-thickness human wounds transplanted on to nude mice enhanced the proliferation of the keratinocytes in the wound bed and hastened wound healing. Our in vitro assays using primary cultures of keratinocytes from newborn mice assayed in defined optimal medium did not reflect these in vivo observations regarding the role for MIP-2 in keratinocyte proliferation, although it is certainly possible that the inclusion of epidermal growth factor and other growth additives in the media obscured our ability to observe a proliferative response to MIP-2 in this assay.
This study distinctly demonstrated the importance of neutrophil recruitment on the overall outcome of wound repair. Our data produced dual evidence of diminished neutrophil numbers in CXCR2-deficient mice that was clearly linked to impaired cutaneous healing. At least three separate factors could contribute to the delay in wound healing observed in the CXCR2−/− mice: (i) a primary defect in the release of critical growth stimulatory signals due to delayed neutrophil recruitment could slow the neovascularization and epithelialization; (ii) loss of CXCR2 on keratinocytes may result in reduced keratinocyte migration and proliferation during the epithelialization; (iii) diminished bactericidal capabilities within subacutely infected wounds may interfere with the wound healing process. Cacalano et al (1994), however, did not observe any defect in the ability to kill Staphylococcus aureus in CXCR2−/− mice. In summary, the remarkable lack of neutrophil recruitment in CXCR2 knockout mice remains the hallmark of this initial wound healing study and warrants additional examination to determine its possible mechanisms.
Other investigators have observed wound healing disturbances when the signal transduction pathway generated through ligand binding to CXCR2 was antagonized. IP-10, a CXC chemokine that binds the receptor CXCR3, is reported to antagonize biologic effects of ligand activation of CXCR2 and to inhibit neovascularization. For example, transgenic mice overexpressing IP-10 in the epidermis, where expression of IP-10 is directed by the keratin 5 or keratin 10 promoter/enhancer, exhibit delayed wound healing (Luster et al, 1998). In a cutaneous wound healing model transgenic mice overexpressing IP-10 under the direction of the keratin 5 or keratin 10 promoter/enhancer exhibited delayed epithelialization, and a prolonged and more extensive inflammatory phase. Thus, in many ways wound healing in the CXCR2−/− mouse runs parallel to wound healing in the IP-10 transgenic mouse. Future work will be directed toward understanding how IP-10 antagonizes the biologic responses of ligands that activate CXCR2.
Acknowledgments
This work was supported by grants from the NCI (CA 34590) (AR), the Departments of Veterans Affairs and the Department of Defense (AR), a DVA career scientist award (AR), the Vanderbilt Ingram Cancer Center (CA68485), and the Vanderbilt Skin Research Center (5P30AR4194). We are indebted to investigators at Genentech for making the BALB/C CXCR2 heterozygous (+/−) mice available to us and Dr. R. Terkeltaub for making C57Bl CXCR2+/− mice available. We appreciate the assistance of Hua Lui and Thomas O. Daniel with the in vitro wound healing and Jesse Britton for help in establishing the wounding protocols. We are indebted to Amy Pruitt, Nancy Cardwell, Ben Johnston, and Steve Liou for assistance with graphing and statistical analysis of the data.
Abbreviations
- MGSA/GRO
melanoma growth stimulatory activity
- CXCR2
CXC chemokine receptor-2
- MCP
monocyte chemoattractant protein
- MIP
macrophage inflammatory protein
- RANTES
regulated on activation normal T cell expressed and secreted
- MPO
myeloperoxidase
References
- Abbot RE, Corral CJ, Maclvor DM, Lin X, Ley TJ, Mustoe TA. Augmented inflammatory response and altered wound healing in cathepsin G-deficient mice. Arch Surg. 1998;133:1002–1006. doi: 10.1001/archsurg.133.9.1002. [DOI] [PubMed] [Google Scholar]
- Addison CL, Daniel TO, Ehlert JE, et al. The CXC. chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine induced angiogenic activity. J Immunol. doi: 10.4049/jimmunol.165.9.5269. accepted. [DOI] [PubMed] [Google Scholar]
- Brauchle M, Fassler R, Werner S. Suppression of keratinocyte growth factor expression by glucocorticoids in vitro and during wound healing. J Invest Dermatol. 1995;105:579–584. doi: 10.1111/1523-1747.ep12323521. [DOI] [PubMed] [Google Scholar]
- Breuss JM, Gallo J, DeLisser HM, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- Brown LF, Yeo K, Berse B, Yeo T, Senger DR, Dvorak HF, Water LV. Expression of vascular permeability factor (Vascular Endothelial Growth Factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacalano G, Lee J, Kikly K, et al. Neutrophil and B-cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- Cavani A, Zambruno G, Marconi A, Manca V, Marchetti M, Giannetti A. Distinctive integrin expression in the newly forming epidermis during wound healing in humans. J Invest Dermatol. 1993;101:600–604. doi: 10.1111/1523-1747.ep12366057. [DOI] [PubMed] [Google Scholar]
- Crookston KP, Webb DJ, Wolf BB, Gonias SL. Classification of alpha2- macroglobulin-cytokine interactions based on affinity of noncovalent association in solution under apparent equilibrium conditions. J Biol Chem. 1994;269:1533–1540. [PubMed] [Google Scholar]
- Detmers PA, Powell DE, Walz A, Clark-Lewis I, Baggiolini M, Cohn ZA. Differential effects of neutrophil activating peptide I/IL-8 and its homologues on leukocyte adhesion and phagocytosis. J Immunol. 1991;147:4211–4217. [PubMed] [Google Scholar]
- Devalaraja MD, Richmond A. Multiple chemotactic factors: Fine control or redundancy? Trends Pharmacol Sci. 1999;236:151–156. doi: 10.1016/s0165-6147(99)01342-5. [DOI] [PubMed] [Google Scholar]
- DiPietro LA, Burdick M, Low QE, Kunkel SL, Streiter RM. MIP 1-α as critical macrophage chemoattractant in murine wound repair. J Clin Invest. 1998;101:1693–1698. doi: 10.1172/JCI1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt E, Toksoy A, Goebeler M, Debus S, Brocker E, Gillitzer R. Chemokines IL-8, GRO-α, MCP-1, IP-10 and Mig are sequentially and differentially expressed during phase-specific infiltration of leukocyte subsets in human wound healing. Am J Pathol. 1998;153:1849–1860. doi: 10.1016/s0002-9440(10)65699-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fivenson DP, Faria DT, Nickoloff BJ, Poverini PJ, Kunkel S, Burdick M, Streiter RM. Chemokine and inflammatory cytokine changes during chronic wound healing. Wound Rep Reg. 1997;5:310–322. doi: 10.1046/j.1524-475X.1997.50405.x. [DOI] [PubMed] [Google Scholar]
- Frank S, Madlener M, Werner S. Transforming Growth Factors β1, β2, and β3 and their receptors are differentially regulated during and impaired wound healing. J Biol Chem. 1996;271:10188–10193. doi: 10.1074/jbc.271.17.10188. [DOI] [PubMed] [Google Scholar]
- Frevert CW, Huang S, Donaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol. 1995;154:335–344. [PubMed] [Google Scholar]
- Gibran MS, Ferguson M, Heimbach DM, Isik FF. Monocyte chemoattractant protein-1 mRNA expression in the human burn wounds. J Surg Res. 1997;70:1–6. doi: 10.1006/jsre.1997.5017. [DOI] [PubMed] [Google Scholar]
- Guo L, Dagenstein L, Dowling J, Yu Q, Wollmann R, Perman B, Fuchs E. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Haapasalmi K, Zhang K, Tonnesen M, et al. Keratinocytes in human wounds express αvβ6 integrin. J Invest Dermatol. 1996;106:42–48. doi: 10.1111/1523-1747.ep12327199. [DOI] [PubMed] [Google Scholar]
- Horn DB, Maisel RH. Angiogenic growth factors: their effects and potential in soft tissue wound healing. Ann Otol Rhinol Laryngol. 1992;101:349–354. doi: 10.1177/000348949210100411. [DOI] [PubMed] [Google Scholar]
- Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–556. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest. 1999;103:1269–1276. doi: 10.1172/JCI5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulke R, Bornscheuer E, Schluter C, Bartels J, Rowert J, Sticherling M, Christophers E. The CXC receptor 2 is overexpressed in psoriatic epidermis. J Invest Dermatol. 1998;110:90–94. doi: 10.1046/j.1523-1747.1998.00074.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Cacalano G, Camerato T, Toy K, Moore MW, Wood WI. Chemokine binding and activities mediated by the mouse IL-8 receptor. J Immunol. 1995;155:2158–2164. [PubMed] [Google Scholar]
- Leibovitch SJ, Ross RJ. The role of macrophage in wound repair. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- Luster AD, Cardiff RD, MacLean JA, Crowe K, Granstein RD. Delayed wound healing and disorganized neovascularization in transgenic mice expressing the IP-10 chemokine. Proc Assoc Am Phys. 1998;110:183–196. [PubMed] [Google Scholar]
- Marikovsky M, Breuing K, Yu Liu P, et al. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci. 1993;90:3889–3893. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing—Aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Mitcheson TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Nanney LB. Epidermal and dermal effects of epidermal growth factor during wound repair. J Invest Dermatol. 1990;94:621–629. doi: 10.1111/1523-1747.ep12876204. [DOI] [PubMed] [Google Scholar]
- Nanney LB. Biochemical and physiological aspects of wound healing. In: Wheeland RG, editor. Cutaneous Surgery. Orlando, FL: W.B. Saunders; 1994. pp. 113–121. [Google Scholar]
- Nanney LB, Mueller SG, Bueno R, Pieper SC, Richmond A. Distributions of melanoma growth stimulatory activity or growth regulated gene and the interleukin-8 receptor B in human wound repair. Am J Pathol. 1995;147:1248–1260. [PMC free article] [PubMed] [Google Scholar]
- Rappolee DA, Mark D, Banja MJ, Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988;241:708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rennekampff HO, Hansbrough JF, Woods V, Dore C, Kiessig V, Schroder JM. Role of melanocyte growth stimulatory activity on keratinocyte function in wound healing. Arch Dermatol Res. 1997;289:204–212. doi: 10.1007/s004030050181. [DOI] [PubMed] [Google Scholar]
- Riches DWH, Hubner G, Brauchle M, Smola H, Madlener M, Fassler R, Werner S. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–556. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- Ritter U, Moll H, Brocker E-B, Velazco O, Becker I, Gillitzer R. Differential expression of chemokines in patients with localized and diffused cutaneous American leishmaniasis. J Infect Dis. 1996;173:699–709. doi: 10.1093/infdis/173.3.699. [DOI] [PubMed] [Google Scholar]
- Roesel JF, Nanney LB. Assessment of differential cytokine effects on angiogenesis using an in vivo model of cutaneous wound repair. J Sur Res. 1995;58:449–459. doi: 10.1006/jsre.1995.1071. [DOI] [PubMed] [Google Scholar]
- Schaffer CJ, Nanney LB. Cell biology of wound healing. Intern Rev Cytol. 1996;169:151–181. doi: 10.1016/s0074-7696(08)61986-5. [DOI] [PubMed] [Google Scholar]
- Schall TJ, Bacon K, Toy KJ, Goedell DV. Selective attraction of monocytes and lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MWJ. Neutralisation of TGF-β 2 or exogenous addition of TGF-β 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Shuster DE, Kehrli ME, Ackermann MR. Neutrophilia in mice that lack the murine IL-8 receptor homolog. Science. 1995;269:1590–1591. doi: 10.1126/science.7667641. [DOI] [PubMed] [Google Scholar]
- Stricklin GP, Li L, Jancic V, Wenczak BA, Nanney LB. Localization of mRNAs representing collagenase and TIMP in sections of healing human burn wounds. Am J Pathol. 1993;143:1657–1666. [PMC free article] [PubMed] [Google Scholar]
- Stricklin GP, Nanney LB. Immunolocalization of collagenase and TIMP in healing human burn wounds. J Invest Dermatol. 1994;103:488–492. doi: 10.1111/1523-1747.ep12395601. [DOI] [PubMed] [Google Scholar]
- Taub DD, Conlon KL, Loyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ Tcells in response to MIP-1α and MIP-1β. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- Terkeltaub R, Baird S, Sears P, Santiago R, Boisvert W. The murine homolog of the interleukin-8 receptor CXCR model of acute urate crystal induced gouty sinovitis. Arthritis Rheum. 1998;41:900–909. doi: 10.1002/1529-0131(199805)41:5<900::AID-ART18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Tessier P, Naccache P, Clark-Lewis I, Gladue R, Neote K, McColl S. Chemokine networks in vivo: involvement of CXC and CC chemokines in neutrophil extravasation in vivo in response to TNF-α. J Immunol. 1997;159:3595–3602. [PubMed] [Google Scholar]
- Trush MA, Egner PA, Kensler TW. Myeloperoxidase as a biomarker of skin irritation and inflammation. Food Chem Toxicol. 1994;32:143–147. doi: 10.1016/0278-6915(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Uguccioni M, D’Apuzzo M, Loetscher M, Dewald B, Baggiolini M. Actions of the chemotactic cytokines MCP-1, MCP-2, MCP-3, RANTES, MIP-1α and MIP-1β on human monocytes. Eur J Immunol. 1995;25:64–68. doi: 10.1002/eji.1830250113. [DOI] [PubMed] [Google Scholar]
- Yan XY, Tumpey TM, Kunkel SL, Oakes JE, Lausch RN. Role of MIP-2 in neutrophil migration and tissue injury in the Herpes Simplex Virus-1-infected cornea. Invest Ophthalmol Vis Sci. 1998;39:1854–1862. [PubMed] [Google Scholar]
- Yoshimura T, Yukhi N, Moore SK, Appella E, Lerman MI, Leonard EJ. Human monocyte chemoattractant protein-1 (MCP-1). Full length cDNA cloning, expression in mitogen stimulated blood mononuclear leukocytes and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- Yuspa SH, Hennings H, Dermer P, Michael D. Dimethyl sulfoxide-induced enhancement of 1,12-dimethyl benz(o)anthracene metabolism and DNA binding in differentiating mouse epidermal cell cultures. Cancer Res. 1976;36:947–951. [PubMed] [Google Scholar]