Abstract
Objective To determine the effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking.
Design Systematic review of randomised controlled trials.
Data sources Cochrane Library, Medline, Embase, CINAHL, PsychINFO, Science Citation Index, registries of ongoing trials, reference lists, the drug company that sponsored most of the trials, and clinical experts.
Review methods Eligible studies were published or unpublished randomised controlled trials that enrolled smokers who declared no intention to quit smoking in the short term, and compared nicotine replacement therapy (with or without motivational support) with placebo, no treatment, other pharmacological therapy, or motivational support, and reported quit rates. Two reviewers independently applied eligibility criteria. One reviewer assessed study quality and extracted data and these processes were checked by a second reviewer. The primary outcome, six months sustained abstinence from smoking beginning during treatment, was assessed by individual patient data analysis. Other outcomes were cessation and reduction at end of follow-up, and adverse events.
Data synthesis Seven placebo controlled randomised controlled trials were included (four used nicotine replacement therapy gum, two nicotine replacement therapy inhaler, and one free choice of therapy). They were reduction studies that reported smoking cessation as a secondary outcome. The trials enrolled a total of 2767 smokers, gave nicotine replacement therapy for 6-18 months, and lasted 12-26 months. 6.75% of smokers receiving nicotine replacement therapy attained sustained abstinence for six months, twice the rate of those receiving placebo (relative risk (fixed effects) 2.06, 95% confidence interval 1.34 to 3.15; (random effects) 1.99, 1.01 to 3.91; five trials). The number needed to treat was 29. All other cessation and reduction outcomes were significantly more likely in smokers given nicotine replacement therapy than those given placebo. There were no statistically significant differences in adverse events (death, odds ratio 1.00, 95% confidence interval 0.25 to 4.02; serious adverse events, 1.16, 0.79 to 1.50; and discontinuation because of adverse events, 1.25, 0.64 to 2.51) except nausea, which was more common with nicotine replacement therapy (8.7% v 5.3%; odds ratio 1.69, 95% confidence interval 1.21 to 2.36).
Conclusions Available trials indicate that nicotine replacement therapy is an effective intervention in achieving sustained smoking abstinence for smokers who have no intention or are unable to attempt an abrupt quit. Most of the evidence, however, comes from trials with regular behavioural support and monitoring and it is unclear whether using nicotine replacement therapy without regular contact would be as effective.
Introduction
Smoking is one of the greatest causes of illness and premature death in developed and developing countries, but giving up smoking can prevent most of the harm. Although nearly half of all smokers in the United Kingdom try to stop every year, only 2-3% succeed.1 One reason for the low success is that many quit attempts are unplanned2 so that the most effective cessation aids may not be used.1 The most widely used cessation aid is nicotine replacement therapy.1 Standard instructions for using such therapy and guidance from the National Institute for Health and Clinical Excellence require smokers to set a day when they will abruptly stop smoking and use nicotine replacement therapy or other pharmacotherapy as a substitute for smoking. Despite 70% of smokers wanting and intending to stop at some time,3 only 12% are ready to stop smoking in the next month4 and thus only this small proportion are suitable for abrupt quit interventions.
In the UK the licence for some nicotine replacement therapies (gum, inhaler, and, most recently, lozenge) has been extended to allow longer term use in those who are not willing or able to quit abruptly, thereby aiding them to cut down smoking and to facilitate quitting. This is termed nicotine assisted reduction to stop; also called cut down then stop,5 cut down to stop, and cut down to quit. We carried out a systematic review of randomised controlled trials to determine the effectiveness of nicotine assisted reduction to stop and whether there are associated harms. Unlike previous reviews,6 7 which reported only point prevalence of cessation at end of follow-up, we focused on sustained cessation from smoking, widely considered the superior outcome measure for effectiveness.8 9 This was possible because of access to unpublished trial reports. This review is an updated extension and summary of our Health Technology Assessment on this topic.10
An ancillary paper will report on an economic analysis to determine whether nicotine assisted reduction to stop provides good value for money from the perspective of the UK National Health Service.
Methods
We electronically searched the Cochrane library, Medline, Embase, CINAHL, PsychINFO, and Science Citation Index from at least 1992 to November 2007 for relevant trials, using a combination of free text and MeSH terms (see web extra appendix 1). We contacted authors, experts, and the pharmaceutical company that sponsored most trials, and checked reference lists of retrieved documents for further trials. All titles and abstracts were screened for relevance and we obtained the full paper if appropriate.
Studies were included in the review if they were randomised controlled trials meeting the following criteria:
The population comprised smokers who were unable or unwilling to stop abruptly
The intervention was gum or inhaler nicotine replacement therapy alone or as part of combination therapy, such as motivational support. Some studies considered nicotine replacement therapy as a generic intervention and allowed a choice, and such studies were considered to meet the inclusion criteria irrespective of whether data could be disaggregated for different forms of therapy (the licensing of lozenges for gradual smoking cessation coincided with the latter stages of this review and is not dealt with specifically here)
The comparator was placebo, no treatment, non-nicotine replacement therapy drugs for smoking cessation, or psychological interventions, such as motivational support. If the intervention arm included an adjunct therapy the comparator had to include one too
The outcome was abstinence from smoking.
The criteria were applied independently by two reviewers and discrepancies resolved by discussion and with the involvement of a third reviewer if required.
Data extraction and quality assessment
The quality of included studies was assessed according to standard guidelines11 and data extracted using a data extraction form. Both tasks were undertaken by one reviewer and checked for accuracy by a second. Disagreements were resolved by discussion, and with a third reviewer if necessary. When information was missing it was sought from the authors or sponsors of trials.
Data synthesis
Studies were grouped according to outcome and comparison groups. The primary outcome for the review was six months’ sustained abstinence starting any time before the end of treatment. We regard this as definitive evidence of the effectiveness of treatment.8 9 Secondary outcomes were point prevalence abstinence at end of follow-up; sustained abstinence from early in treatment to end of follow-up; sustained reduction from week 6 to end of follow-up; point prevalence reduction at end of follow-up; and adverse events throughout follow-up—death, serious adverse events (death, admission to hospital, or permanent disability), discontinuation owing to side effects, and nausea (as an index symptom of possible nicotine overdose).
Meta-analysis was carried out using Stata (version 10). For smoking outcomes we summarised data with relative risks; the preferred statistic of the Cochrane Tobacco Addiction Review Group. For adverse events we summarised data using Peto odds ratio, which is the preferred statistic for rare occurrences.12
Developing a measure of sustained abstinence
In most studies on smoking cessation all individuals set a quit day near the beginning of the study and once they relapse they are counted forever as a sustained abstinence failure, even if they subsequently make a quit attempt and succeed. In studies of nicotine assisted reduction to stop, participants have the opportunity to use nicotine replacement therapy for a prolonged period (up to 18 months) during which time they make several quit attempts. Unlike normal studies on cessation, where the index quit attempt is the first, in studies on nicotine assisted reduction to stop, treatment continues whether or not someone attempted to stop and failed. Thus, previous failures do not nullify later success. We counted the number who had started to abstain during treatment and had maintained abstinence for at least six months. Some smokers started quit attempts late in the treatment and because follow-up did not continue for six months beyond the end of the treatment, follow-up ceased with these people having been abstinent continuously for several months, but fewer than six months. To count them as failed quitters or as successes would be inappropriate, so we developed a method to determine what proportion of these would sustain abstinence of six months if follow-up had been long enough. We applied the probability that a smoker who abstained for x months would go on to abstain for six months to those smokers who were abstinent for x months at the end of study. This calculation was based on probabilities derived from analyses using individual person data of all quit attempts made in each of the studies for which individual person data were available (see web extra appendix 2).
Results
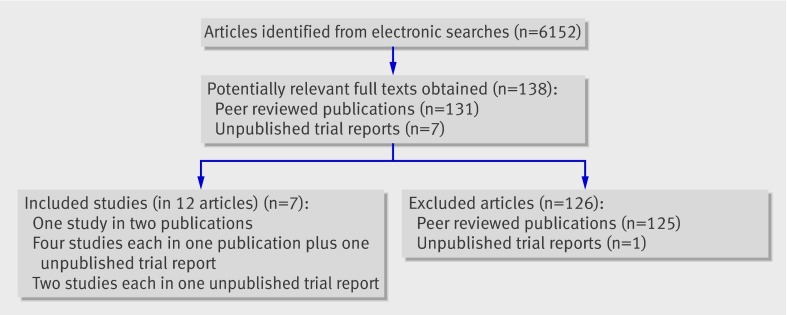
Figure 1 shows the flow of papers through the systematic review. Seven randomised controlled trialsw1-w7 (12 articles) met the inclusion criteria (see web extra appendix 3 for excluded articles).
Fig 1 Flow of papers through study
Table 1 shows the characteristics of the included studies. Six of the randomised controlled trials were sponsored by industry,w1-w6 two of which were unpublished.w3 w6 Full, unpublished trial reports were obtained for all six trials, of which five reportsw1 w3-w6 contained individual patient data allowing calculation of at least six months’ sustained abstinence. The seventh trialw7 was independent, and unpublished data were obtained from the authors.
Table 1.
Main characteristics of included studies
| Reference, country, trial dates | Treatment duration; follow-up (months) | Indication | No in group, mean age in years (% female) | Baseline cigarettes smoked/day, exhaled carbon monoxide level (ppm), Fagerström score* | NRT intervention† (nicotine content) | Comparator | Other treatment components | Main outcomes measured | Funding (trial code) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NRT | Control | NRT | Control | |||||||||
| Batra,w1 Germany and Switzerland (NR) | 12; 13 | Not intending to quit in next month; willing to change behaviour | 184, 42.6 (45.9) | 180, 43.5 (35.2) | 27.9, 29.1, 5.7 | 29.6, 28.2, 5.9 | Gum (4 mg) for 12 months | Placebo gum for 12 months | Clinic visits (n=9), telephone support, additional clinic visits as necessary | Smoking reduction; abstinence (exhaled carbon monoxide level <10 ppm)‡; NRT use (self report and records); serum cotinine and thiocyanate levels (ppm); adverse events; haematological risk factors§ | Industry (980-CHC 1013-028) | |
| Bolliger,w2 Sweden and Switzerland (Feb 1997 to May 1999) | 18; 24 | Unwilling or unable to quit; wanted to reduce cigarette consumption | 200, 46.4 (57) | 200, 45.8 (48) | 28.2, 27.1, 5.5 | 30.3, 27.1, 5.6 | Inhaler (10 mg)¶ for 18 months | Placebo inhaler as required | Clinic visits (n=9) | Smoking reduction; abstinence (exhaled carbon monoxide level <10 ppm)‡; NRT use (self report), acceptability; plasma cotinine and thiocyanate levels, (ppm); quality of life** and adverse events; haematological risk factors§ | Industry (96-NNIN 016) | |
| Haustein,w3 unpublished††, Germany (Mar 2000 to Nov 2001) | 9; 12 | Not intending to quit in next month want to reduce cigarette consumption | 97, 42.3 (50) | 96, 41.7 (50) | 24.3, 27.5, 5.4 | 24.4, 28.9, 5.5 | Gum (4 mg) as required for 9 months | Placebo gum as required for 9 months | Clinic visits (n=8) | Smoking reduction; abstinence (exhaled carbon monoxide level <10 ppm); product use; change in Fagerström score; adverse events | Industry (980 CHC-9021-0013) | |
| Rennard,w4 USA (Feb 2000 to Apr 2001) | 12; 15 | Not intending to quit within next month, wanted to reduce cigarette consumption | 215, 45.9 (59) | 214, 44.8 (54) | 29.3, 29.7, 6.5 | 30.4, 29.5, 6.6 | Inhaler (10 mg) for 12 months | Placebo inhaler for 12 months | Clinic visits (n=9) | Smoking reduction; abstinence (exhaled carbon monoxide level <10 ppm)‡; NRT use (self report), acceptability; plasma cotinine and thiocyanate levels (ppm); quality of life‡‡ and adverse events; haematological risk factors§ | Industry (98-NNIN-027) | |
| Wennike,w5 Denmark (Feb 1999 to May 2000) | 12; 24 | Not intending to quit within next month, wanted to reduce cigarette consumption | 205, 45 (65) | 206, 44 (59) | 24, 29, 6.4 | 24, 27, 6.4 | Gum (2 or 4 mg; depending on Fagerström score) for 12 months | Placebo gum for 12 months | Clinic visits (n=9) | Smoking reduction; abstinence (exhaled carbon monoxide level <10 ppm)‡; NRT use (self report) and compliance; plasma cotinine and thiocyanate levels; quality of life** and adverse events; haematological risk factors§ | Industry (98 NNCG-014) | |
| Wood-Baker,w6 unpublished, Australia (Jun 1999 to Mar 2001) | 12; 15 | Not intending to quit within next month, wanted to reduce cigarette consumption | 218, 42.9 (54) | 218, 45.3 (55) | 29.0, 25.8, 6.6 | 27.4, 25.9, 6.4 | Gum (2 or 4 mg; depending on Fagerström score) for 12 months | Placebo gum for 12 months | Clinic visits (n=9) | Smoking reduction; abstinence (exhaled carbon monoxide level <10 ppm)‡; NRT use and compliance; plasma cotinine and thiocyanate levels; quality of life** and adverse events; haematological risk factors§ | Industry (98 NNCG-017) | |
| Etter,w7 Switzerland (1999 to 2002)§§ | 6¶¶; 26 | Not intending to quit within next 6 months, wanted to reduce cigarette consumption | 265, 269, 389; 43.2, 41.7, 42.9; (46, 51, 56) | 29.8, 29.4, 30.2; NR, NR, NR; 6.0, 5.9, 6.2 | Free choice***: inhaler (10 mg), gum (4 mg), or patch (25 mg) for 6 months | Placebo NRT for 6 months and no intervention | Literature only | Smoking reduction; abstinence†††; product use; change in Fagerström score; adverse events | Government and industry (no trial code) | |||
NR=not reported; NRT=nicotine replacement therapy.
*Test for nicotine dependence.
†Gum and inhaler were Nicorette products (Pharmacia).
‡Seven day point prevalence.
§Examples include C reactive protein, fibrinogen, white blood cell count.
¶Total available nicotine 4-5 mg.
**Short form 36.
††This study had two further arms that compared short term quit intervention using gum with placebo.
‡‡Revised RAND 36 item health survey 1.0.
§§This study had a third arm in which participants received no treatment.
¶¶Quitters continued to receive NRT after six months.
***Switching between products was allowed.
†††Point prevalence for past seven days and one month.
All the studies recruited smokers who were unwilling or unable to quit abruptly, and none emphasised reduction then stop on recruitment. Consequently the primary outcome was reduction and not cessation.
Trial design
All the studies were randomised parallel group trials with nicotine replacement therapy and placebo arms. One trialw3 randomised people to four arms; two of the arms were not included in this review because participants were randomised to reduction over only one month (with active nicotine replacement therapy or placebo). Another trialw7 had three arms, comprising no pharmacotherapy, placebo, and nicotine replacement therapy. For consistency we analysed differences between nicotine replacement therapy and placebo.
Population
The populations had similar personal and smoking characteristics typical of heavy smokers attending smoking cessation clinics. Potential participants with heart disease, those receiving psychiatric drugs, pregnant or lactating women, or people with other drug problems were excluded. Recruitment was by advertisement.
Intervention
Four trials used gum,w1 w3 w5 w6 two used inhalers,w2 w4 and one used free choice of gum, inhaler, or patch.w7 Prior to randomisation in two trials,w5 w6 smokers were stratified by nicotine dependence (Fagerström score); the less nicotine dependent were given 2 mg gum whereas the more dependent received 4 mg gum. The other gum trials used 4 mg gum. The trial with three armsw7 used a 15 mg/16 hour patch, 4 mg gum, or inhaler.
Nicotine replacement therapy was available for six months in one trialw7 (although people who remained abstinent could have extended use). The other trials provided nicotine replacement therapy for nine months,w3 12 months,w1 w4-w6 and 18 months.w2
Behavioural support
The trial with three armsw7 had no clinic visits and no behavioural support, but participants received a 20 page booklet covering reasons for reducing cigarette consumption and the methods for achieving reduction.
In the other publications behavioural support was described as moderate (visits lasting 15-30 minutesw5), or participants were “instructed to reduce their smoking . . . and provided with ways to do so,”w4 or behavioural support was not really described.w1 w2 The unpublished trial reports,w1-w6 however, indicated that the behavioural support programme was similar in all these studies. Participants were given a sheet of paper with written advice on how to use gum or inhaler to reduce or stop smoking. Clinic staff followed a written behavioural support protocol giving information on how much nicotine replacement therapy to use and how to use it to substitute for cigarettes. In addition, at each visit the therapist elicited problems from the participants, helped them find solutions, and related their progress back to their goals negotiated at the start of the programme. Smokers were encouraged to quit during the study. At six and nine months, participants were instructed to stop smoking completely, regardless of reduction achieved to that point. At all visits smoking status was monitored, exhaled carbon monoxide recorded, and feedback given on progress towards agreed goals. Typically, behavioural support and clinic visits were repeated on five or more occasions up to at least a year and in some trials beyond, to 18 or 24 months.
Outcomes
The primary outcome in the trials was sustained reduction. In the industry sponsored trialsw1-w6 sustained reduction was defined as reported cigarette consumption of less than 50% of baseline from week 6 to week 16, although in some trials this was also to later visits. Sustained reduction was measured by self reported cigarettes smoked a day and validated by the carbon monoxide level that was at least 1 ppm less than at baseline on each occasion it was checked. The secondary outcomes were prolonged abstinence from the week 6 visit to end of follow-up and 7 day point prevalence abstinence and point prevalence of reduction at various follow-up times.
In the trial with three arms,w7 point prevalence abstinence and point prevalence reduction for the past seven days and four weeks were the main outcomes at six and 26 months.
Quality of included studies
Table 2 summarises the quality of the included studies. All were of high quality.
Table 2.
Summary of quality assessment of included randomised controlled trials
| Study | Was assignment of treatment really random? | Was allocation concealed and concealment method described? | Were groups similar at baseline? | Were eligibility criteria specified? | Who was blinded to treatment allocation? | Was intention to treat analysis used and were drop outs accounted for? |
|---|---|---|---|---|---|---|
| Batraw1 | Yes; computer generated list | Yes; sealed envelopes | Yes* | Yes | Participants, therapists, and outcome assessors | Yes, yes |
| Bolligerw2 | Yes; computer generated list | Yes; sealed envelopes | Yes* | Yes | Participants, therapists, and outcome assessors | Yes, yes |
| Hausteinw3 | Yes; computer generated list | Yes; sealed envelopes | Yes | Yes | Participants, therapists, and outcome assessors | Yes, yes |
| Rennardw4 | Likely, but method not described | Likely, but method not reported | Yes | Yes | Participants, therapists, and outcome assessors | Yes, yes |
| Wennikew5 | Yes (stratified by Fagerström score); computer generated list | Yes; sealed code list | Yes* | Yes | Participants, therapists, and outcome assessors | Yes, yes |
| Wood-Bakerw6 | Yes (stratified by Fagerström score); computer generated list | Yes; sealed envelopes | Yes | Yes | Participants, therapists, and outcome assessors | Yes, yes |
| Etterw7 | Yes; computer generated list | Unclear | Yes | Yes | Participants and outcome assessors | Most outcomes†, yes |
When extensive unpublished study reports were available, they were used for quality analysis.
*Except for small imbalance in sex distribution.
†Not intention to treat for product usage and for completeness of blinding of participants (determined at six months).
Although trials blinded participants to allocation, it is difficult to blind people to psychoactive drugs. At six months, participants in the three arm trialw7 guessed more accurately than would be expected by chance whether they had received active drug or placebo.
Sustained six months’ abstinence
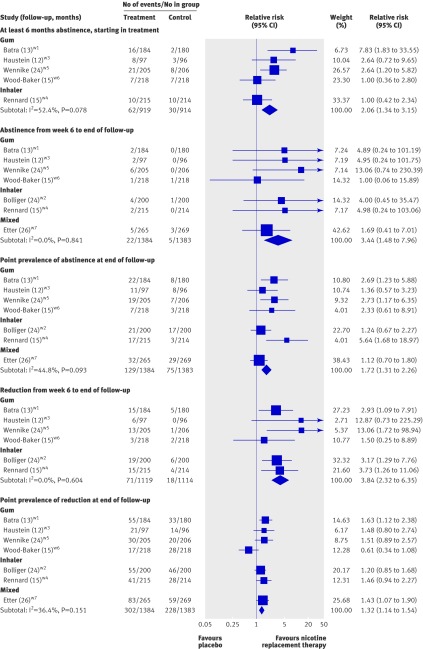
Individual person data were available from one trial using inhalerw4 and four using gumw1 w3 w5 w6 and allowed the calculation and meta-analysis of sustained abstinence of at least six months.w1 w3-w6 The proportion of smokers achieving sustained abstinence at six months with nicotine replacement therapy was double that with placebo (relative risk 2.06, 95% confidence interval 1.34 to 3.15; fig 2), but the rates were low (6.75% v 3.28%, respectively). Moderate heterogeneity was suggested (χ2=8.4, df=4, P=0.08, I2=53%). There was no evidence to indicate that this was due to the type of nicotine replacement therapy used, and the inclusion criteria and protocols of the trials were similar. By a random effects model the relative risk was 1.99 (1.01 to 3.91).
Fig 2 Meta-analysis of smoking outcomes. Pooled estimates are Mantel Haenszel relative risks (fixed effects). Heterogeneity statistic Q for at least six months’ abstinence was 8.4 (P=0.078), for abstinence from week 6 to end of follow-up was 2.74 (P=0.840), for point prevalence of abstinence at end of follow-up was 10.86 (P=0.093), for reduction from week 6 to end of follow-up was 3.63 (P=0.604), and for point prevalence of reduction at end of follow-up was 9.43 (P=0.151)
Other smoking outcomes
Sustained abstinence was measured from six weeks (two weeks in one studyw1) to the end of follow-up. Point prevalence abstinence was also measured at last follow-up, which was one month,w1 three months,w3 w4 w7 six months,w2 12 months,w5 and 20 monthsw7 after the end of treatment. Sustained reduction and point prevalence reduction was measured at these time points during treatment and at follow-up. Figure 2 summarises these results.
As might be expected of smokers unwilling or unable to quit in the short term, sustained abstinence rates starting from six weeks were low; across all studies 1.6% in the nicotine replacement therapy group and 0.4% in the placebo group. Point prevalence rates of abstinence at the end of follow-up were 9.3% and 5.4%, respectively.
Successful reduction was more common. In those receiving active nicotine replacement therapy, 21.8% had reduced consumption by more than 50% at final follow-up compared with 16.5% receiving placebo. Sustained reduction from early in treatment to final follow-up occurred in 6.3% of those receiving active treatment and 1.6% receiving placebo.
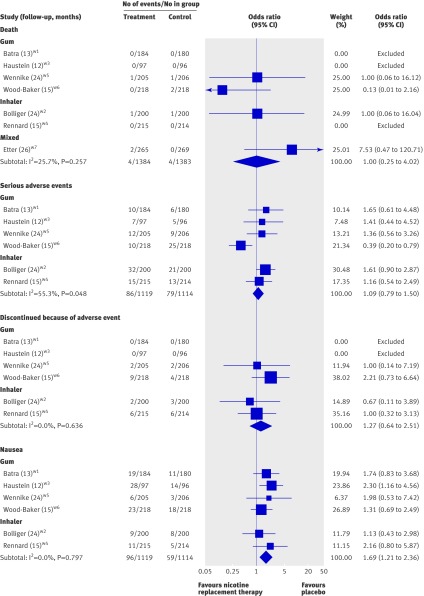
Adverse events
Overall, 1384 predominantly middle aged smokers were treated with nicotine replacement therapy for six to 18 months and 1383 were treated with placebo. Four deaths occurred in those randomised to nicotine replacement therapy and four in those randomised to placebo: odds ratio 1.00 (95% confidence interval 0.25 to 4.02; fig 3). Serious adverse events occurred in fewer than 8% of participants in both arms: 1.09 (0.79 to 1.50; fig 3). In no cases were these judged likely to have been due to treatment. Discontinuation of treatment because of adverse events was rare, with 1.7% in the nicotine replacement therapy group and 1.3% in the placebo group: odds ratio 1.27 (0.64 to 2.51; fig 3). Nausea was selected as an index symptom to indicate possible nicotine overdose. It was slightly and significantly more common in the nicotine replacement therapy group, with 8.6% experiencing nausea compared with 5.3% in the placebo group: 1.69 (1.21 to 2.36; fig 3).
Fig 3 Meta-analysis of safety outcomes; pooled estimates are Peto’s odds ratio (fixed effects). Heterogeneity statistic Q for death was 4.04 (P=0.257), for serious adverse events was 11.19 (P=0.048), for discontinuation of treatment because of adverse events was 1.70 (P 0.636), and for nausea was 2.36 (P=0.797). I2 was 0 (negative value [100×[(Q–DF)/Q)] except for serious adverse events, where I2 was 55%
Discussion
This review found evidence that nicotine assisted reduction to stop programmes can be effective in achieving sustained abstinence from smoking of six months. There was no evidence of an increase in life threatening problems, and nicotine replacement therapy was well tolerated, with almost no difference in discontinuation because of side effects in those receiving nicotine replacement therapy compared with those receiving placebo. Nausea was significantly more common with nicotine replacement therapy than with placebo, but only one in 30 users became nauseous because of treatment. The results imply that compared with placebo twice the number of smokers sustained six months’ abstinence as a result of nicotine replacement therapy. This equates to about an additional 3% of all smokers quitting who would otherwise not have done so. This is a similar effect size to treating smokers who are motivated to quit, where 4-5% might be expected to abstain for six months owing to use of nicotine replacement therapy.13 Previous data suggest that half of those who sustain six months of abstinence will maintain it for the rest of their lives.14 15
Three reviews, comprising a Health Technology Assessment,10 a Cochrane review,7 and a qualitative review6 have examined smoking cessation achieved by smokers recruited to randomised controlled trials of smoking reduction interventions. The present review is an extension and update of the Health Technology Assessment report10 and differs from the Cochrane review7 and the qualitative review.6 We report sustained abstinence rates derived from analysis of individual patient data, whereas the Cochrane and qualitative reviews were restricted to point prevalence of smoking cessation at the end of follow-up, a measure that cannot inform about the duration of cessation, which is the outcome most relevant to health. Sustained abstinence is the preferred outcome of the Society for Research in Nicotine and Tobacco8 and advised by other experts.9 We included an additional trialw6 not included in the qualitative review. Our review focused on nicotine replacement therapy whereas both the Cochrane and the qualitative reviews encompassed multiple interventions and did not meta-analyse data on safety outcomes. The qualitative review concluded that smoking reduction increased the probability of future cessation, whereas the Cochrane review concluded that people unwilling to quit were helped by nicotine replacement therapy to cut down on number of cigarettes smoked a day. Our use of sustained abstinence and measurement of safety has allowed us to draw stronger conclusions on the public health benefit of smoking reduction with nicotine replacement therapy for unwilling quitters.
The licence for nicotine replacement therapy is for reduction then stopping, whereas the trials in our review recruited smokers motivated only to reduce their consumption. We excluded one study in which participants wanted to quit by reduction,16 which was included in both the Cochrane and the qualitative reviews. The odds ratio for point prevalence of abstinence at the end of follow-up from this study was similar to our pooled effect estimate (2.34, 95% confidence interval 1.16 to 4.74); this suggests that whether smokers are motivated to reduce then quit or simply motivated to reduce may make little difference to the efficacy of nicotine replacement therapy in supporting cessation. There is further evidence that this difference between trial populations (reducers) and the smokers for whom the products are marketed (reducing to quit) is probably not important. Nearly half of surveyed American smokers planning to quit would choose reduction over abrupt cessation, and two thirds of these were interested in the assistance of drugs.17 In these smokers there was little interest in reduction as an end in itself, only as a means to stop. Even among those not planning to stop soon, cessation was the goal for half. Intentions to stop smoking are volatile18 so a stated intention to stop at a specified future time may have little long term meaning for many smokers. Instruction to stop was delivered in the trials, although the importance of this instruction has not been tested. We therefore believe that encouraging smokers prepared to reduce consumption to use nicotine replacement therapy regardless of their subsequent intention to quit is appropriate because this is the population that was included in the trials.
For health services an important issue is whether nicotine replacement therapy should be reimbursed in nicotine assisted reduction to stop programmes and whether and how such programmes should be implemented. All the industry sponsored trials took place in specialist smoking cessation clinics with extensive monitoring and moderate behavioural support. The remaining trialw7 was rather analogous to use of nicotine replacement therapy purchased directly from retail outlets, but even here a 20 page booklet was given to participants to motivate and instruct on reduction. This trial showed lower relative efficacy than the overall effect estimate and a lower absolute benefit, but whether this was due to the setting or chance is unclear.
Currently, nicotine assisted reduction to stop is licensed in the UK but recent guidance from the National Institute for Health and Clinical Excellence and a recent US Clinical Practice Guideline recommend its use only in the context of further research.19 20 Survey data show that large numbers are using nicotine replacement therapy to reduce consumption,1 but whether they are truly reading the packet inserts and seeking to follow a nicotine assisted reduction to stop programme is uncertain. Furthermore, most people who are reducing with nicotine replacement therapy are using a patch,1 which is not licensed for this use and does not come with such instructions. It is therefore unclear whether the outcomes observed in the trials are being achieved through such use.21
In summary, these trials have shown proof of concept. People who would answer “no” to “do you want to stop smoking now?” may be helped to stop over a longer period by applying drugs formerly reserved only for abrupt cessation. The contribution of the behavioural support programme is unknown, and the optimum advice to give people in reduction programmes is also unknown as these have not been manipulated in comparative trials. The importance of these trials is that they show that treating a population of smokers not ready to stop means more of them stop. Therefore it is important to examine how nicotine assisted reduction to stop can be incorporated into tobacco control programmes.
What is already known on this topic
Most smokers are not ready to quit and might not respond to interventions of abrupt cessation
Nicotine replacement therapy (NRT) is licensed for smoking reduction in smokers not ready to stop but there is no evidence that it leads to sustained abstinence
No review has assessed the safety of concurrent smoking and use of long term NRT
What this study adds
This systematic review of randomised clinical trials in smokers not ready to stop found that with NRT support twice as many quitters achieve six months of sustained abstinence
This equates to an additional 3% of sustained quitters compared with placebo
Using NRT while smoking did not lead to serious health problems
Contributors: AFS designed and implemented the searches. DW, MC, and PA extracted the data. DW and MC selected studies and did the meta-analyses. DM supervised the project and is the guarantor. All authors wrote the manuscript.
Funding: This work was funded by the UK Health Technology Assessment Programme (National Institute for Health Research).
Competing interests: PA has accepted hospitality and money from McNeil (Helsinborg, Sweden), which sponsored the trials in the report; he has not received hospitality or money in relation to any nicotine assisted reduction research.
Ethical approval: Not required.
Cite this as: BMJ 2009;338:b1024
References
- 1.West R. Smoking and smoking cessation in England, 2006. Reference paper 4. 2008. http://aspsilverbackwebsites.co.uk/smokinginengland/.
- 2.West R, Sohal T. “Catastrophic” pathways to smoking cessation: findings from national survey. BMJ 2006;332:458-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office for National Statistics. Smoking-related behaviour and attitudes. 2008. www.statistics.gov.uk/downloads/theme_health/Smoking2005.pdf.
- 4.Taylor T, Lader D, Bryant A, Keyse L, Joloza MT. Smoking-related behaviour and attitudes, 2005. London: Office for National Statistics, 2006.
- 5.Medicines and Healthcare Products Regulatory Agency, Committee on Safety of Medicines: Report of the committee on safety of medicines working group on nicotine replacement therapy. 2008. www.mhra.gov.uk/home/idcplg?IdcService=GET_FILE&dDocName=CON2023239&RevisionSelectionMethod=LatestReleased.
- 6.Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine Tob Res 2006;8:739-49. [DOI] [PubMed] [Google Scholar]
- 7.Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database Syst Rev 2007;(3):CD005231. [DOI] [PubMed] [Google Scholar]
- 8.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 2003;5:13-25. [PubMed] [Google Scholar]
- 9.West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. “Cut down to quit” with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess 2008;12:1-135. [DOI] [PubMed] [Google Scholar]
- 11.NHS Centre for Reviews and Dissemination. Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews. 2nd ed. University of York, Centre for Reviews and Dissemination, 2001.
- 12.Deeks JJ, Altman DG. Effect measures for meta-analysis of trials with binary outcomes. In: Egger M, Davey Smith G, Altman DG, ed. Systematic reviews in health care; meta-analysis in context. 2nd ed. London: BMJ Books, 2001:313-35.
- 13.Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. [update in Cochrane Database Syst Rev 2004;(3):CD000146; PMID: 15266423]. Cochrane Database Syst Rev 2002;(4). [DOI] [PubMed]
- 14.Stapleton J. Cigarette smoking prevalence, cessation and relapse. Stat Methods Med Res 1998;7:187-203. [DOI] [PubMed] [Google Scholar]
- 15.Etter JF, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control 2006;15:280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kralikova E, Kozak J, Rasmussen T, Cort N. The clinical benefits of NRT-supported smoking reduction. Nicotine Tob Res 2002;4:243. [Google Scholar]
- 17.Shiffman S, Hughes JR, Ferguson SG, Pillitteri JL, Gitchell JG, Burton SL. Smokers’ interest in using nicotine replacement to aid smoking reduction. Nicotine Tob Res 2007;9:1177-82. [DOI] [PubMed] [Google Scholar]
- 18.Hughes JR, Keely JP, Fagerstrom KO, Callas PW. Intentions to quit smoking change over short periods of time. Addict Behav 2005;30:653-62. [DOI] [PubMed] [Google Scholar]
- 19.National Institute for Health and Clinical Excellence. NICE public health guidance 10. Smoking cessation services in primary care, pharmacies, local authorities and workplaces, particularly for manual working groups, pregnant women and hard to reach communities. 2008. www.nice.org.uk/nicemedia/pdf/PH010guidance.pdf.
- 20.Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services Public Health Service, 2008.
- 21.Levy DE, Thorndike AN, Biener L, Rigotti NA. Use of nicotine replacement therapy to reduce or delay smoking but not to quit: prevalence and association with subsequent cessation efforts. Tob Control 2007;16:384-9. [DOI] [PMC free article] [PubMed] [Google Scholar]