Abstract
Arginine-rich cell-penetrating peptides (AR-CPPs) are very promising tools for the delivery of therapeutic macromolecules such as peptides, proteins, and nucleic acids. These peptides allow efficient internalization of the linked cargos intracellularly through the endocytic pathway. However, when linked to bulky cargos, entrapment in the endocytic vesicles is a major limitation to the application of these peptides in cytosolic delivery. Attachment of a compatible endosomal escape device is, therefore, necessary to allow cytosolic delivery of the peptide-attached cargo. This review presents different endosomal escape devices currently in application in combination with AR-CPPs. Applications of fusogenic lipids, membrane-disruptive peptides, membrane-disruptive polymers, lysosomotropic agents, and photochemical internalization to enhance the cytosolic delivery of AR-CPPs-attached cargos are presented. The properties of each system and its mechanism of action for the enhancement of endosomal escape are discussed, together with its applications for the delivery of different macromolecules in vitro and, if applicable, in vivo.
Key words: arginine-rich cell-penetrating peptides, endosomal escape, membrane-disruptive peptides, oligoarginine, protein transduction domains
INTRODUCTION
Intracellular delivery of biological macromolecules is a major topic in the field of drug delivery. Many biological macromolecules, including proteins, peptides, and nucleic acids, have proven useful for the treatment of various health problems; however, efficient application of such macromolecules is hindered by their inefficient delivery in sufficient concentration to the target sites. The delivery of macromolecules in vivo faces many extracellular and intracellular barriers. Extracellular barriers include instability in the hostile biological environment, delivery to the target tissue or population of cells, and avoidance of nontarget cell binding (1). A major intracellular barrier is the cell membrane barrier that impedes the penetration of hydrophilic macromolecules. Therefore, therapeutic macromolecules are generally delivered through endocytosis, as the most common internalization pathway. After uptake, the macromolecules are challenged by the acidic pH of endosomes/lysosomes, the digestive enzymes of lysosomes, and the endosomal membrane (2). If the molecule manages to escape endosome/lysosome degradation, then in order to elicit its effect, it should be stable in the cytosol. In addition, it should be able to bind the target intracellular organelle and avoid nonspecific binding to other organelles. In the case of plasmid DNA (pDNA) delivery, the nuclear membrane is an additional barrier to overcome.
One way to overcome the cell membrane barrier is to link the cargo macromolecule to one of the cell-penetrating peptides (CPPs), also known as protein transduction domains (PTDs). These peptides are a class of short peptide sequences that are capable of entering cells efficiently, either alone or linked to bulky cargos such as peptides, proteins, oligonucleotides, pDNA (3), or liposomes (4). These peptides carry promise for application in the delivery of macromolecules in vivo, since they have achieved great success in vitro. Among CPPs, arginine-rich cell-penetrating peptides (AR-CPPs) are the most widely studied (5–8). Therefore, this review will elaborate on the applications of AR-CPPs for the delivery of therapeutic macromolecules. Given that endosomal escape is believed by many to be the rate-limiting step in efficient intracellular delivery of cargos linked to AR-CPPs, emphasis will be placed on ways to overcome this barrier. The current knowledge of how to overcome this barrier will be presented, along with future directions, in the hope that this review will stimulate new ideas to improve the cytosolic availability of AR-CPP-linked cargos.
AR-CPPS AND ENDOSOMAL ENTRAPMENT
Since the observation published by Frankel and Pabo that the human immunodeficiency virus transactivator of transcription (HIV-TAT) protein can penetrate cells and activate HIV-1-specific genes (9), followed by the discovery of the structure of Drosophila antennapedia peptide (10) called penetratin as the first member, the AR-CPPs have gained a great deal of attention as delivery tools for macromolecules. Besides penetratin, these peptides include a short basic segment of the transactivator of transcription (TAT) protein (amino acid sequence 48–60), a flock house virus (FHV) coat peptide (sequence 35–49), and oligoarginines (11,12). Despite the controversy surrounding the uptake mechanism that accompanied their discovery, it is now widely accepted that AR-CPPs are internalized via endocytosis (13–16). The authors and others have suggested that macropinocytosis is one of the predominant AR-CPP uptake pathways (4,17–19). Some reports, however, have suggested clathrin-mediated endocytosis and caveolin-mediated endocytosis as alternative endocytic pathways (for review, check references (20,21)). In all cases, after endocytic uptake, the internalized AR-CPPs (either alone or linked to cargos) should escape from the endocytic vesicles to the cytosol to avoid degradation. Escape from the endocytic vesicles is believed to be a bottleneck in the efficient intracellular delivery of functional macromolecules.
Whether or not free AR-CPPs can escape from endocytic vesicles after uptake is a debatable issue. Some groups have reported that AR-CPPs are unable to escape from endocytic vesicles after endocytic uptake (13,22). Other groups claim that fluorescently labeled free peptides can escape from endosomes (23,24). There have even been some models proposed for the mode of endosomal escape of free AR-CPPs. One model suggested that AR-CPPs may form a nonpolar ion pair with negatively charged components of cell membranes, followed by partitioning of this ion pair to cell membranes, as a mode for AR-CPPs to cross biological membranes (25–27). Hitz and colleagues suggested that oligoarginine binds to membranes, causing their rigidification and resulting in leakiness and rupture of the membrane (28). Another model also indicated the importance of the pH gradient across the endosomal membrane (29) and the presence of a minimum threshold concentration of penetratin for translocation across membranes without rupturing them (30). Fuchs and Raines also stressed the need for a high local concentration of free nonaarginine to rupture the membranes and allow endosomal escape (14). These models, although they may help to explain the escape of free peptides, are not applicable to peptides linked to bulky cargos such as biological macromolecules. Several studies have shown that AR-CPPs linked to quantum dots (31), oligonucleotides (32), pDNA (33), peptides (34,35), or proteins (36) remain trapped in endocytic vesicles after uptake, unless an endosomal escape device is included in the system. In the next section, details of the different endosomal escape devices applied for the release of entrapped cargos attached to AR-CPPs from endocytic vesicles to the cytosol will be discussed.
TOOLS TO ENHANCE ENDOSOMAL ESCAPE OF AR-CPPS-BOUND MACROMOLECULES
Fusogenic Lipids
Liposomes are one of the most common vectors currently used for the delivery of macromolecules. Cationic liposomes, first introduced in 1987 (37), are able to form a complex with nucleic acids, known as lipoplex, that can result in efficient gene transfection. Inclusion of a neutral helper lipid such as dioleoylphosphatidylethanolamine (DOPE) in the lipoplex has been shown to enhance the expression of the complexed gene in a concentration-dependent manner (38). Zhou et al. (39) showed that the role of DOPE as a helper lipid is to mediate fusion between the liposomes and the endosomal membrane after endocytosis, which results in the destabilization of this membrane and release of the complexed DNA to the cytosol. DOPE is known to exist in two forms: the lamellar phase and the hexagonal phase. Transformation from the lamellar to the inverted hexagonal phase can be stimulated upon a decrease in pH, similar to what happens inside the endosome. The inverted lipid phase is known to promote fusion of lipid bilayers, resulting in the escape of the enclosed cargo from the endosome (for review, check reference (40)). When TAT, a model AR-CPP, was simply mixed with DOTAP liposomes or the commercial transfection agent lipofectin (composed of the cationic lipid DOTMA together with DOPE) before mixing with pDNA, the resulting complexes showed enhanced GFP transfection in A549 cells. However, when these complexes were used to transfect nondividing primary tracheal epithelial cells, they failed (41). It is of interest that, after complexation of pDNA with lipofectin and pDNA, the resulting complexes had a negative charge, even at high concentrations of the peptide TAT. This shows the importance of topology control that guarantees the presentation of the CPP on the surface of the complex.
In 2004, our group introduced a multifunctional envelope-type nanodevice (MEND) as a gene delivery system that utilized octaarginine (R8) as an AR-CPP, together with DOPE as fusogenic lipid, to enhance the release from endosomes after endocytosis (for detailed review, check references (42,43)). A new strategy was followed in the design of MEND in which anionic lipids (such as cholesteryl hemisuccinate [CHEMS] or phosphatidic acid) were used, rather than cationic lipids. The AR-CPP octaarginine (R8) was then added at a later step to impart a positive charge to the system. This allowed better control of the topology of R8 and insured that it was presented on the surface of the system. MEND, described in the first study, was composed of a condensed plasmid DNA core encapsulated in a lipid bilayer (DOPE/CHEMS, in 9:2 molar ratio) (44). The surface of MEND was decorated with R8 linked to a stearyl moiety that served as an anchor into the lipid bilayer. This MEND was as efficient as adenovirus in transfecting cells in cultures, and it could transfect skin cells after topical administration in vivo, while the commercial reagent LipofectAmine failed (45). Besides its use for delivery of pDNA (4,44–47), MEND was used for the delivery of oligodeoxynucleotides (48), siRNA (49), and proteins (50). In another application of the combination of the fusogenic lipid DOPE and R8, our group developed a mitochondria-selective protein delivery system called MITO-Porter (51). This system could deliver encapsulated protein to mitochondria through a novel mechanism of fusion to the mitochondrial membrane.
Fusogenic liposomes modified with R8 were also useful in the field of immunization. Modification of the surface of liposomes composed of DOPE/CHEMS/egg phosphatidylcholine with R8 proved to deliver an encapsulated antigen, ovalbumin, to the cytosol of dendritic cells, resulting in antigen presentation through the major histocompatibility complex class I pathway (52). Since the major histocompatibility complex class I presentation requires the release of the antigens to the cytosol, these results indicated the efficiency of the combination of AR-CPP octaarginine and DOPE in mediating the cytosolic release of ovalbumin. This efficient presentation was consistent with the significant in vivo antitumor effect of R8-modified liposomes when they were used to immunize mice before challenge with tumor cells. It was even shown that other arginine-rich peptides could be used to modify MEND and result in efficient delivery of the encapsulated cargo. A novel arginine-rich peptide, identified by in vivo phage display and called IRQ peptide (having the sequence IRQRRRR), was checked for delivery of siRNA. The peptide used to modify MEND with a lipid coat composed of DOPE/CHEMS (9:2) and encapsulating antiluciferase siRNA resulted in significant silencing of luciferase expression when checked in HeLa cells stably expressing luciferase (53).
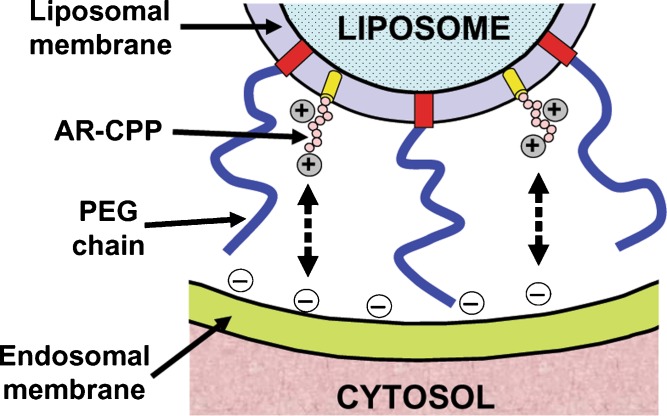
Polyethylene glycol (PEG) is widely used during the preparation of lipoplexes for in vivo applications. PEG, when used to modify the lipoplexes, prolongs blood circulation time, decreases nonspecific interaction with the reticuloendothelial system, and improves the size uniformity of particles. On the other hand, PEG modification results in a decrease in gene expression in culture cells due to a decrease in the cellular uptake of particles. One possible solution to this is to include AR-CPPs in the system to overcome the problem of low cellular uptake. When TAT was used to modify liposomes composed of DOTAP/DOPE/DSPE-PEG2000, either before or after complexation with pDNA, TAT modification improved the gene expression twofold at 30% DOTAP content, while TAT decreased the gene expression when DOTAP content was 50% (33). It is worth noting that the internalized particles colocalized almost completely with the endosome marker, LysoTracker, until 1 h post-transfection. This indicated that the presence of DOPE did not sufficiently enhance the endosomal escape of the lipoplexes. Holland et al. (54) showed that decoration of DOPE-containing liposomes with PEG stabilized the lamellar structure of DOPE and hindered the hexagonal phase formation that is necessary for fusion. In addition, and as the model in Fig. 1 shows, despite the relative electrostatic attraction between the cationic AR-CPPs, modifying the liposome surface, and the anionic components in the endosomal membrane, the presence of PEG chains on the liposome may prevent the fusion between the two membranes due to steric hindrance by the bulky hydrophilic PEG chains. Therefore, there is a need for the removal of PEG from the lipid bilayer prior to cellular uptake, or just after uptake, in order for DOPE to function and mediate fusion.
Fig. 1.
PEGylation of liposomes linked to AR-CPPs hinders fusion between the liposomes and endosomal membrane. Despite the electrostatic attraction between the AR-CPPs on the liposome surface and the negatively charged components in endosomal membrane, fusion does not take place due to steric hindrance by the bulky hydrophilic PEG chains
Torchilin et al. are currently working on the development of a “smart” carrier whose surface will be modified with pH-sensitive PEG linked to DOPE and TAT linked to short PEG (55–57). According to their proposed strategy, in the acidic microenvironment of tumor tissue, the acid-sensitive hydrazone linkage between PEG and DOPE will be broken, allowing the exposure of TAT to bind to the cell and enhance the internalization of the liposomal carriers. Detachment of PEG from DOPE is also expected to allow DOPE to transform to the hexagonal phase in endosomes, which is expected to facilitate fusion to endosomes. Thus far, they have shown proof of the concept that the removal of PEG in tumor tissue enhances the internalization of carriers injected intratumorally (56,57). Their next step will be to prove that carriers injected intravenously will specifically accumulate in tumors and result in efficient cellular uptake and gene expression.
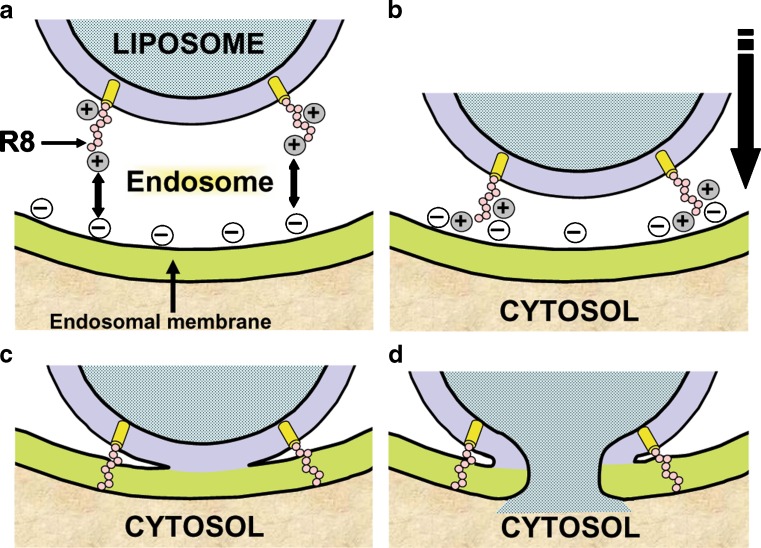
Table I summarizes the applications of the fusogenic lipid DOPE along with AR-CPPs for intracellular delivery of different biological macromolecules. The high number of applications for the delivery of different cargos both in vitro and in vivo indicates that such a combination of the fusogenic lipid DOPE and AR-CPPs is a promising formulation. In order to explain why this combination was so efficient for the delivery of different macromolecules intracellularly, the exact role played by octaarginine, as a model for AR-CPPs, in endosomal escape in the presence of DOPE was studied in comparison with octalysine. The study showed that the presence of R8 on the surface of liposomes composed of DOPE/phosphatidic acid broadened the fusogenic spectrum of these liposomes to cover both neutral and acidic pH (58). It is interesting to note that when the surface of liposomes was modified with octalysine peptide, as a model for a cationic peptide not rich in arginine, the liposomes could fuse to the endosomal membrane only at neutral pH, resulting in low endosomal escape efficiency of the encapsulated cargo inside the liposomes. When the same liposomes were modified with R8, as a model for AR-CPPs, they could fuse to membranes at both acidic and neutral pH, resulting in enhanced endosomal escape of encapsulated cargo. Comparison of our gene delivery system, MEND, modified with K8 to that modified with R8 proved that R8-modified MEND resulted in improvement in gene expression by more than 300 times over unmodified and 17 times over K8-modified MEND. In a similar finding, when antiluciferase siRNA was encapsulated into MEND, the silencing effect obtained from R8-modified MEND was higher than that of K8-modified MEND (58). These results confirmed that AR-CPPs might play an additional role in mediating endosomal escape, along with their well-recognized role in enhancing cellular uptake. The results also pointed to the pivotal role that the guanidinium group, not cationic groups, plays in the function of AR-CPPs. A proposed model for the role of octaarginine in fusion between liposomes and endosomal membrane is summarized in Fig. 2. It was reported that, for fusion to take place between different membranes, three steps are required: (1) specific recognition of the site of fusion, (2) close apposition of membranes, and (3) fusion or coalescence of membranes (59). Apparently, AR-CPPs, exemplified by R8 in the reported study, have the ability to boost all the three steps of fusion resulting in efficient cytosolic delivery of the cargo enclosed inside the liposomes (Fig. 2).
Table I.
Applications of the Fusogenic Lipid DOPE for the Delivery of Various Therapeutic Macromolecules
| AR-CPP | Lipid composition | Linkage between the AR-CPP and liposomes | Cargo delivered | Application in vitro | Application in vivo | Reference |
|---|---|---|---|---|---|---|
| TAT | DOTMA/DOPE | Simple mixing | Plasmid DNA | Yes | No | (41) |
| R8 | DOPE/CHMES | Surface modification of liposomes | Plasmid DNA | Yes | Yes | (4,44,45,47) |
| R8 | DOPE/CHMES | Surface modification of liposomes | Plasmid encoding siRNA | Yes | No | (46) |
| R8 | DOPE/CHMES | Surface modification of liposomes | Oligodeoxynucleotide | Yes | No | (48) |
| R8 | DOPE/CHMES | Surface modification of liposomes | siRNA | Yes | No | (49) |
| R8 | DOPE/CHMES | Surface modification of liposomes | GFP | Yes | No | (50) |
| R8 | DOPE/SM | Surface modification of liposomes | GFP and gold colloid | Yes | No | (51) |
| R8 | DOPE/CHEMS/EPC | Surface modification of liposomes | Ovalbumin | Yes | Yes | (52) |
| IRQ | DOPE/CHEMS | Surface modification of liposomes | siRNA | Yes | No | (53) |
| R8 | DOPE/PA | Surface modification of liposomes | Plasmid DNA | Yes | No | (58) |
| TAT | DOTAP/DOPE/DSPE-PEG | Surface modification of liposomes | Plasmid DNA | Yes | No | (33) |
| TAT | EPC/Chol/DOTAP/PE-HZ-PEG | Surface modification of liposomes | Plasmid DNA | Yes | Yes | (57) |
R8 octaarginine, DOTMA N-[1-(2,3-dioleyloxy)propyl]-N,N,N-triethylammonium, CHEMS cholesteryl hemisuccinate, GFP green fluorescence protein, SM sphingomyelin, EPC egg phosphatidylcholine, DSPE-PEG distearoylphosphatidylethanolamine–PEG conjugate, IRQ IRQRRRR peptide, PA phosphatidic acid, PE-HZ-PEG phosphatidylethanolamine linked to PEG through an acid-sensitive hydrazone linkage
Fig. 2.
Model for the role of AR-CPPs in the fusion between liposome and endosomal membrane. Steps of the fusion between liposome modified with octaarginine, a model AR-CPP, and endosome are summarized. a Recognition of the site of fusion between the liposome and endosomal membrane through electrostatic attraction between the cationic AR-CPP (R8) and the anionic components in the endosomal membrane. b Close apposition of the membranes, aided by the electrostatic attraction and neutralization of the positive charges on R8 by the endosomal membrane anionic components. c Fusion or coalescence of the endosomal membrane and the liposome. Fusion is guided by insertion of the neutralized R8 into the endosomal membrane and movement inwards under the influence of difference in endosomal transmembrane potential. d Fusion pore is formed following lipid mixing between the two membranes and resulting in the release of the liposome cargo into the cytosol
Membrane-Disruptive Peptides
One of the common ways to overcome endosomal entrapment is to mimic the mechanisms followed by viruses to escape from endosomes. Human influenza virus is one of the enveloped viruses that utilize fusion to the endosomal membrane for cytosolic delivery of their genetic material after endocytosis. The influenza virus envelope is composed largely of two major glycoproteins: hemagglutinin (HA) and neuraminidase. HA is composed of two subunits, HA1, responsible for viral cell binding, and HA2, responsible for endosomal escape. The HA2 subunit possesses a fusion domain at its N terminus, which forms an α-helix structure capable of insertion into membranes as the first step of fusion. Under the acidic pH of endosomes, a conformational change in hemagglutinin protein takes place, exposing the α-helix structure in the HA2 subunit. This is followed by insertion of the α-helix into the endosomal membrane, resulting in fusion between the virus envelope and the endosomal membrane and the release of the nucleocapsid of the virus into the cytoplasm of the infected cell (60,61).
Utilization of this fusogenic segment of HA2 protein to enhance the endosomal escape of AR-CPP-linked cargo was first reported by Wadia et al. (18). In that report, TAT linked to a fusogenic 20-amino acid N-terminal of HA2 peptide was coincubated with TAT-Cre protein. When TAT was linked to Cre protein, the result was stimulation of the uptake of the fusion proteins by macropinocytosis. In the absence of TAT-HA2 peptide, the majority of the TAT-Cre fusion protein was found trapped in macropinosomes up to 24 h after transduction. When TAT-HA2 was coincubated with TAT-Cre protein, the result was enhancement of its escape from the endocytic vesicle and Cre recombination, as judged by EGFP expression in NIH 3T3 cells. However, according to the authors, >99% of the TAT fusion protein remained trapped in macropinosomes even after the fusogenic peptide TAT-HA2 treatment (62).
Langel et al. adopted the same strategy, but they used the cell-penetrating peptide penetratin instead of TAT for the delivery of peptide nucleic acids (PNA) (63) or siRNA (64). Coincubation of the CPP-linked PNA with penetratin-HA2 resulted in minor (less than twofold) improvement in splice correction. Splice correction also remained far below that obtained in the presence of the endosome disrupting agent chloroquine, indicating that penetratin-HA2 peptide does not significantly improve endosomal escape of the PNA cargo (63). When the same group tested penetratin-HA2 for enhancement of the silencing effect of penetration/siRNA complex, it resulted in less than 40% gene silencing effect (64). One possible explanation for the low efficiency of this strategy is that the fusogenic peptide used in these studies should colocalize in the same endocytic vesicle with the cargo in order to release it from the endocytic vesicle. Since this could not be guaranteed for each cargo molecule, the enhancement of endosomal escape remained marginal. In addition, there is a possibility that some of the CPP-HA2 molecules leak out of endosomes without serious disruption of the endosomal membrane, leaving the CPP-attached cargos trapped in the endosomes.
In search of more efficient derivatives of influenza HA2 peptide, Plank et al. (65) introduced the INF7 peptide as a pH-sensitive, more potent membrane-destabilizing peptide. In this peptide, two glutamic acid moieties were introduced into the HA2 structure to extend the α-helix structure and increase the pH sensitivity. Recent studies confirmed that this peptide had more pH sensitivity than the parent HA2 peptide (66). Some researchers have used this modified HA2 peptide to improve the endosomal escape of cargos linked to AR-CPP. Sugita et al. (67) provided microscopic evidence that coincubation of fluorescently labeled TAT, antennapedia, Rev, or VP22 AR-CPPs with TAT-HA2 results in diffuse labeling of the cells with the CPPs, rather than punctuate staining of the AR-CPPs alone. This strategy was applied for cytosolic delivery of the anticancer peptide shepherdin fused to TAT in vitro, resulting in a greater cytotoxic effect of shepherdin at low doses (68). Similarly, through the inclusion of a nuclear localization signal (NLS) in this system, PM10 peptide linked to TAT could be delivered intranuclearly to cells in vitro. Cells coincubated with the NLS-PM10-TAT fusion protein, together with TAT-HA2, showed enhanced cytostatic effect of the peptide PM10 (69). Using a different strategy, Michiue et al. (70) linked the AR-CPP undecaarginine (R11) to the C-terminal of P53 protein and the modified HA2 peptide to the N-terminal of the protein. The R11 moiety was expected to enhance uptake, while the HA2 moiety would enhance the endosomal escape. The presence of HA2 peptide increased the percentage of apoptotic cancer cells from less than 10% using P53-R11 alone to more than 50% when HA2-P53-R11 fusion protein was used. The advantage of this strategy is that each cargo molecule is accompanied by the HA2 peptide in the endocytic vesicle, in stoichiometric ratio.
A few synthetic peptides have also been checked to determine whether they enhanced the endosomal escape of AR-CPP-linked cargos. The 43E peptide composed of three LAEL amino acid sequence units, which is capable of α-helix formation at acidic pH, was proven to enhance the transfection activity of plasmid DNA complexed with the cationic peptide 46 (71). Recently, Lo et al. (72) showed that the incorporation of a histidine tail onto the TAT peptide resulted in up to a 7,000-fold enhancement in gene expression over an unmodified TAT/pDNA complex. This complex, when injected into rat brain and spinal cord in vivo, resulted in gene expression levels five times lower than those obtained with polyethyleneimine (PEI) 25 kDa complexes in the brain, whereas the expression levels from the two vectors in the spinal cord were close. However, cell viability experiments showed that the modified TAT complexes did not affect cell viability, unlike PEI, which resulted in cell toxicity, especially at high N/P ratios (72). Histidine moieties are thought to mediate endosomal escape through protonation of their imidazole groups under acidic conditions of the endosomes, which may result in osmotic swelling of the endosomes followed by rupture and release of their contents (73). Table II summarizes the different applications of membrane-disruptive peptides reported to date. It is interesting that the systems reported were tested only in cellular experiments; only the histidine-modified TAT system was attempted in vivo, but it had limited success.
Table II.
Applications of Membrane-Disruptive Peptides for the Delivery of Various Therapeutic Macromolecules
| AR-CPP | Membrane-disruptive peptide | Linkage between the AR-CPP and the membrane-disruptive peptide | Cargo delivered | Application in vitro | Application in vivo | Reference |
|---|---|---|---|---|---|---|
| TAT | HA2-TAT | Coincubation | Cre recombinase protein | Yes | No | (18) |
| TAT, Pen, Tp | Penetratin-HA2 | Coincubation | PNA | Yes | No | (62) |
| Pen | Penetratin-HA2 | Coincubation | siRNA | Yes | No | (63) |
| TAT | Modified HA2-TAT | Coincubation | Shepherdin peptide | Yes | No | (67) |
| TAT | Modified HA2-TAT | Coincubation | PM10 peptide | Yes | No | (68) |
| R11 | Modified HA2-TAT | Fusion protein | P53 protein | Yes | No | (69) |
| 46 | 43E | Ternary complex | Plasmid DNA | Yes | No | (70) |
| TAT | Histidine 10 | Fusion protein | Plasmid DNA | Yes | Yes | (71) |
Pen penetratin, Tp transportan, PNA peptide nucleic acid, R11 undecaarginine
Membrane-Disruptive Polymers
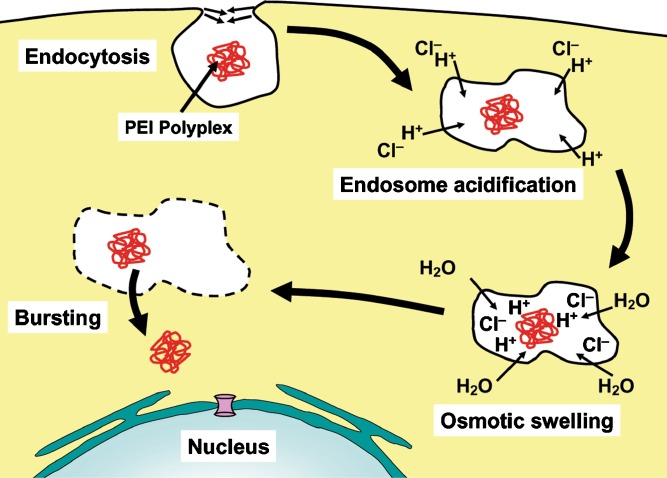
One of the methods employed to enhance the endosomal escape of AR-CPP-linked cargos is the attachment of a polymer to the system that can rupture the endosome upon acidification. The most well-established polymer in the field of gene delivery that employs this mechanism is PEI. This polymer is rich in secondary and tertiary amines that can be protonated at the acidic pH of endosomes. Upon protonation, PEI results in the rupture of endosomes by the proton sponge effect that was first suggested by Boussif et al. (74) and is summarized in reference (75). Figure 3 shows a simplified model for the mode of endosomal escape of PEI polyplexes by the proton sponge effect.
Fig. 3.
Endosomal escape of PEI polyplexes by proton sponge effect. After uptake, PEI polyplexes in the endosome act as proton sponge through protonation of the secondary and tertiary amine groups in the PEI polymer chain. Accumulation of protons together with their counter ions in the endosome stimulates entrance of water from the cytosol to balance the high osmotic pressure inside the endosome. Swelling of the endosome in the presence of PEI eventually leads to endosome bursting and release of PEI polyplexes to the cytosol
In the first trial of the combination of AR-CPPs with PEI, a ternary complex was formed by first complexing pDNA with either TAT oligomer and then adding PEI 25 kDa, or vice versa. In vitro transfection results showed that the former method of preparation resulted in higher gene expression than complexes prepared by the latter method or conventional PEI complexes. However, when the system was checked in vivo by intratracheal instillation into mice, the gene expression levels did not differ significantly from those obtained from conventional PEI complexes (76). A similar recent study showed that modification of PEI/pDNA polyplexes with antennapedia AR-CPP after preparation enhanced the gene expression of complexes over unmodified particles in brain capillary endothelial primary cells (77). On the other hand, when PEI 25 kDa was linked to TAT through a PEG spacer and used to condense pDNA, the in vitro gene expression of this system was lower than that of unmodified PEI polyplexes. However, when the same system was applied in vivo to mouse lung by intratracheal instillation, significant improvement was obtained compared with PEI polyplexes alone. This was explained by the role of PEG in stabilizing the particles in vivo while hindering transfection in vitro. PEG-modified particles are less aggregated, so particle sedimentation to cell surfaces in culture takes place at a lower rate, resulting in less in vitro transfection (78).
In a similar system, branched PEI 2 kDa covalently linked to PEG and TAT was shown to deliver exon-skipping oligonucleotides in vivo and significantly improved dystrophin expression in mdx mice over that obtained with PEI–PEG complexes (79). Alexis et al. (80) checked the effect of low-molecular-weight PEI covalently linked to TAT on its transfection activity. Interestingly, the resulting particles had in vitro gene expression values comparable to those of PEI 25 kDa complexes, but with less cell toxicity.
A more complex system was proposed by Suk and colleagues (81) in which the polyplexes of branched PEI 25 kDa with pDNA were chemically modified with PEG and then TAT moieties were linked to the free tip of PEG. This system enhanced cell uptake over unmodified PEI polyplexes and resulted in slightly higher YFP expression. Surprisingly, more than 50% of modified particles were shown to colocalize with endosome/lysosome compartments, compared to more than 60% of unmodified PEI polyplexes, raising doubts about the efficiency of PEI in mediating endosomal escape. On the other hand, a recent study showed that covalent linkage of PEI to R11 resulted in less efficient gene expression than PEI polyplexes. The authors suggested that the presence of R11 changes the uptake pathway of PEI polyplexes to a less productive one that results in less migration of the polyplexes to the perinuclear region across the microtubules (82). Different applications of combinations of AR-CPPs and PEI are listed in Table III. The table shows that low-molecular-weight PEI derivatives, though of limited application so far, seem to be a promising less toxic alternative to the conventional PEI when combined with AR-CPPs.
Table III.
Applications of PEI for the Delivery of Various Therapeutic Macromolecules
| AR-CPP | PEI structure | Linkage between the AR-CPP and liposomes | Cargo delivered | Application in vitro | Application in vivo | Reference |
|---|---|---|---|---|---|---|
| TAT oligomers | Branched PEI 25 kDa | Ternary complex | Plasmid DNA | Yes | Yes | (75) |
| Antp | Branched PEI 25 kDa | Ternary complex | Plasmid DNA | Yes | No | (76) |
| TAT | Branched PEI 25 kDa | Covalent linkage through PEG | Plasmid DNA | Yes | Yes | (77) |
| TAT | Branched PEI 2 kDa | Covalent linkage to PEI | Oligonucleotides | Yes | Yes | (78) |
| TAT | Branched PEI 600 kDa | Covalent linkage to PEI | Plasmid DNA | Yes | No | (79) |
| TAT | Branched PEI 25 kDa | Covalent linkage through PEG | Plasmid DNA | Yes | No | (80) |
| R11 | Branched PEI 25 kDa | Covalent linkage to PEI | Plasmid DNA | Yes | No | (81) |
Antp antennapedia peptide, R11 undecaarginine
Lysosomotropic Agents
Lysosomotropic agents are substances of varying chemical structures that are taken up selectively into lysosomes. The most famous among this group is chloroquine, which is a weak base that can enter the cell and accumulate in endosomes and lysosomes after being protonated. Depending on the concentration of chloroquine, it can have different functions; at low concentrations, it may act as an inhibitor to endosome acidification, preventing the accumulation of free protons in the endosome and preventing its maturation. At high concentrations, it can result in the accumulation of counter ions to protons in endosomes, resulting in endosomal swelling and rupture (83,84). At high concentrations, chloroquine can be used to enhance cytosolic delivery of many functional cargos such as nucleic acids (85–87) or proteins (18,88) that may suffer entrapment in endosomes after uptake. In the same way, chloroquine is currently used by many researchers to enhance the cytosolic delivery of cargos attached to AR-CPPs that are known to be internalized through the endocytic pathway.
In 2004, Wadia et al. (18) reported enhanced nuclear delivery of TAT fusion protein to Cre recombinase in the presence of chloroquine. However, the high concentrations of chloroquine used in this study were toxic to cells. Similarly, Caron and colleagues (88) showed that increasing concentrations of chloroquine or sucrose resulted in an increase in recombination of the TAT-Cre fusion protein. For delivery of pDNA, TAT fused to polylysine could condense plasmid DNA and result in efficient intracellular delivery of the complex formed. However, efficient expression of the EGFP plasmid was shown only in the presence of chloroquine. Recently, chloroquine has been used widely in combination with PNA for efficient splice correction. PNA linked to TAT (63,89,90), oligoarginine (89), and penetratin (63,91) all resulted in more efficient splice correction when chloroquine was included in the transfection medium. Nevertheless, despite the high efficiency of chloroquine in in vitro experiments, it still has no application for the in vivo delivery of biological macromolecules due to its pharmacological effects.
Photochemical Internalization
Photochemical internalization (PCI) is a technique in which the cells are incubated with a photosensitizer that preferentially localizes in the cell membrane, thereafter being taken in by endocytosis to localize in the endocytic vesicle membranes. Upon irradiation of the cell with light at a special wavelength, the photosensitizer will be excited, resulting in the formation of reactive oxygen species. This reactive oxygen will rupture all membranes in close proximity, particularly endocytic vesicles, releasing their contents to the cytosol (92). This strategy was applied to release the fusion protein between the apoptotic proteins P53 and R11 from the endocytic vesicles. The cells were irradiated with light at 480 nm, which excited the fluorescent label fluorescein isothiocyanate attached to the fusion protein and released the fusion protein to the cytosol (93). PCI was also applied widely for the release of PNA from the endocytic vesicles (90,94,95). A detailed protocol for the application of PCI to release PNA from endosomes was published (90). PCI, though very effective in the release of any type of drug at a localized site, has some limitations that will require more effort to overcome. Among these are the limited depth of light penetration, damage of therapeutic macromolecules that are in close proximity to the photosensitizer, and skin sensitivity to light that may appear as a side effect of the photosensitizer (92,96). There are currently no reports about in vivo application of PCI for the delivery of AR-CPPs-linked cargos.
CONCLUSIONS
AR-CPPs have many advantages that allow their application in the field of therapeutic macromolecule delivery, such as high cellular uptake, low cytotoxicity, biocompatibility, and biodegradability. However, one of their major limitations remains to be the lack of an intrinsic mechanism of escape from endocytic vesicles after uptake. Therefore, they should be attached to a system to allow their cytosolic release after uptake. This has been a review of the different systems currently in use for the enhancement of endosomal escape of cargos linked to AR-CPPs. The lack of quantitative parameters for endosomal escape efficiency and differing experimental conditions make it difficult to judge which system was the most successful. Therefore, there is a need to establish methods to quantitatively determine endosomal escape efficiency in order to develop more efficient endosomal escape devices. There is still room for the introduction of new endosomal escape devices or combinations of more than one of the current devices, since the number of systems currently in application is still limited. Another important consideration during development of delivery systems is that the endosomal escape device should not contradict the cell-penetrating function of the AR-CPPs. This contradiction can be avoided through the rational design of the system so that the AR-CPP is presented only at the cell surface during uptake, while the endosomal escape device is presented after uptake.
Future Directions
In this review, five categories of compounds to enhance the endosomal escape of cargos attached to AR-CPPs were presented. Only three of them were tested for in vivo animal experiments and none of them was tested clinically. This may indicate inefficiency in the process of extension from in vitro to in vivo application for these systems. The reason is that in vivo applications need more complex systems to overcome multiple extracellular and intracellular barriers. The system should include the following: (1) PEG, to shield the cationic charge of the AR-CPPs during circulation to avoid nonspecific interaction and uptake by the macrophages; (2) a ligand, to target a specific tissue or cell population; (3) AR-CPPs, to enhance cellular uptake; (4) an endosomal escape device; and (5) a NLS if nuclear delivery is needed. Such a complex system necessitates a special arrangement of the different functional devices in a rational design in order for every device to be presented at the specific location and time for eliciting its function. We recently adopted the concept of programmed packaging for the design of such a multifunctional device, which utilizes octaarginine as a model AR-CPP. Other groups are also working on the design of delivery systems that combine different AR-CPPs together with various devices. It is hoped that such ongoing research will result in a multifunctional system that brings clinical applications of these peptides into reality.
Acknowledgements
The authors’ research was supported, in part, by Grants-in-Aid for Scientific Research (A) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by Grants-in-Aid for Scientific Research on Priority Areas from the Japan Society for the Promotion of Science. The authors thank Dr. James L. McDonald for his helpful advice in writing the English manuscript.
References
- 1.Belting M., Sandgren S., Wittrup A. Nuclear delivery of macromolecules: barriers and carriers. Adv. Drug Deliv. Rev. 2005;57(4):505–527. doi: 10.1016/j.addr.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Kamiya H., Akita H., Harashima H. Pharmacokinetic and pharmacodynamic considerations in gene therapy. Drug Discov. Today. 2003;8(21):990–996. doi: 10.1016/S1359-6446(03)02889-7. [DOI] [PubMed] [Google Scholar]
- 3.Mae M., Langel U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr. Opin. Pharmacol. 2006;6(5):509–514. doi: 10.1016/j.coph.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Khalil I. A., Kogure K., Futaki S., Harashima H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J. Biol. Chem. 2006;281(6):3544–3551. doi: 10.1074/jbc.M503202200. [DOI] [PubMed] [Google Scholar]
- 5.Brooks H., Lebleu B., Vives E. Tat peptide-mediated cellular delivery: back to basics. Adv. Drug Deliv. Rev. 2005;57(4):559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Futaki S. Membrane-permeable arginine-rich peptides and the translocation mechanisms. Adv. Drug Deliv. Rev. 2005;57(4):547–558. doi: 10.1016/j.addr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Torchilin V. P. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008;60(4–5):548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Nakase I., Takeuchi T., Tanaka G., Futaki S. Methodological and cellular aspects that govern the internalization mechanisms of arginine-rich cell-penetrating peptides. Adv. Drug Deliv. Rev. 2008;60(4–5):598–607. doi: 10.1016/j.addr.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 10.Derossi D., Joliot A. H., Chassaing G., Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269(14):10444–10450. [PubMed] [Google Scholar]
- 11.Futaki S., Suzuki T., Ohashi W., Yagami T., Tanaka S., Ueda K., Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001;276(8):5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T., Futaki S., Niwa M., Tanaka S., Ueda K., Sugiura Y. Possible existence of common internalization mechanisms among arginine-rich peptides. J. Biol. Chem. 2002;277(4):2437–2443. doi: 10.1074/jbc.M110017200. [DOI] [PubMed] [Google Scholar]
- 13.Richard J. P., Melikov K., Vives E., Ramos C., Verbeure B., Gait M. J., Chernomordik L. V., Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. J. Biol. Chem. 2003;278(1):585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs S. M., Raines R. T. Pathway for polyarginine entry into mammalian cells. Biochemistry. 2004;43(9):2438–2444. doi: 10.1021/bi035933x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Futaki S. Oligoarginine vectors for intracellular delivery: design and cellular-uptake mechanisms. Biopolymers. 2006;84(3):241–249. doi: 10.1002/bip.20421. [DOI] [PubMed] [Google Scholar]
- 16.Duchardt F., Fotin-Mleczek M., Schwarz H., Fischer R., Brock R. A comprehensive model for the cellular uptake of cationic cell-penetrating peptides. Traffic. 2007;8(7):848–866. doi: 10.1111/j.1600-0854.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakase I., Niwa M., Takeuchi T., Sonomura K., Kawabata N., Koike Y., Takehashi M., Tanaka S., Ueda K., Simpson J. C., Jones A. T., Sugiura Y., Futaki S. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol. Ther. 2004;10(6):1011–1022. doi: 10.1016/j.ymthe.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 18.Wadia J. S., Stan R. V., Dowdy S. F. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat. Med. 2004;10(3):310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 19.Nakase I., Tadokoro A., Kawabata N., Takeuchi T., Katoh H., Hiramoto K., Negishi M., Nomizu M., Sugiura Y., Futaki S. Interaction of arginine-rich peptides with membrane-associated proteoglycans is crucial for induction of actin organization and macropinocytosis. Biochemistry. 2007;46(2):492–501. doi: 10.1021/bi0612824. [DOI] [PubMed] [Google Scholar]
- 20.Melikov K., Chernomordik L. V. Arginine-rich cell penetrating peptides: from endosomal uptake to nuclear delivery. Cell. Mol. Life Sci. 2005;62(23):2739–2749. doi: 10.1007/s00018-005-5293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wender P. A., Galliher W. C., Goun E. A., Jones L. R., Pillow T. H. The design of guanidinium-rich transporters and their internalization mechanisms. Adv. Drug Deliv. Rev. 2008;60(4–5):452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Taei S., Penning N. A., Simpson J. C., Futaki S., Takeuchi T., Nakase I., Jones A. T. Intracellular traffic and fate of protein transduction domains HIV-1 TAT peptide and octaarginine. Implications for their utilization as drug delivery vectors. Bioconjug. Chem. 2006;17(1):90–100. doi: 10.1021/bc050274h. [DOI] [PubMed] [Google Scholar]
- 23.Potocky T. B., Menon A. K., Gellman S. H. Cytoplasmic and nuclear delivery of a TAT-derived peptide and a beta-peptide after endocytic uptake into HeLa cells. J. Biol. Chem. 2003;278(50):50188–50194. doi: 10.1074/jbc.M308719200. [DOI] [PubMed] [Google Scholar]
- 24.Fischer R., Kohler K., Fotin-Mleczek M., Brock R. A stepwise dissection of the intracellular fate of cationic cell-penetrating peptides. J. Biol. Chem. 2004;279(13):12625–12635. doi: 10.1074/jbc.M311461200. [DOI] [PubMed] [Google Scholar]
- 25.Rothbard J. B., Jessop T. C., Lewis R. S., Murray B. A., Wender P. A. Role of membrane potential and hydrogen bonding in the mechanism of translocation of guanidinium-rich peptides into cells. J. Am. Chem. Soc. 2004;126(31):9506–9507. doi: 10.1021/ja0482536. [DOI] [PubMed] [Google Scholar]
- 26.Rothbard J. B., Jessop T. C., Wender P. A. Adaptive translocation: the role of hydrogen bonding and membrane potential in the uptake of guanidinium-rich transporters into cells. Adv. Drug Deliv. Rev. 2005;57(4):495–504. doi: 10.1016/j.addr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Sakai N., Takeuchi T., Futaki S., Matile S. Direct observation of anion-mediated translocation of fluorescent oligoarginine carriers into and across bulk liquid and anionic bilayer membranes. Chembiochem. 2005;6(1):114–122. doi: 10.1002/cbic.200400256. [DOI] [PubMed] [Google Scholar]
- 28.Hitz T., Iten R., Gardiner J., Namoto K., Walde P., Seebach D. Interaction of alpha-and beta-oligoarginine-acids and amides with anionic lipid vesicles: a mechanistic and thermodynamic study. Biochemistry. 2006;45(18):5817–5829. doi: 10.1021/bi060285d. [DOI] [PubMed] [Google Scholar]
- 29.Bjorklund J., Biverstahl H., Graslund A., Maler L., Brzezinski P. Real-time transmembrane translocation of penetratin driven by light-generated proton pumping. Biophys. J. 2006;91(4):L29–L31. doi: 10.1529/biophysj.106.083881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magzoub M., Pramanik A., Graslund A. Modeling the endosomal escape of cell-penetrating peptides: transmembrane pH gradient driven translocation across phospholipid bilayers. Biochemistry. 2005;44(45):14890–14897. doi: 10.1021/bi051356w. [DOI] [PubMed] [Google Scholar]
- 31.Ruan G., Agrawal A., Marcus A. I., Nie S. Imaging and tracking of Tat peptide-conjugated quantum dots in living cells: new insights into nanoparticle uptake, intracellular transport, and vesicle shedding. J. Am. Chem. Soc. 2007;129(47):14759–14766. doi: 10.1021/ja074936k. [DOI] [PubMed] [Google Scholar]
- 32.Youngblood D. S., Hatlevig S. A., Hassinger J. N., Iversen P. L., Moulton H. M. Stability of cell-penetrating peptide-morpholino oligomer conjugates in human serum and in cells. Bioconjug. Chem. 2007;18(1):50–60. doi: 10.1021/bc060138s. [DOI] [PubMed] [Google Scholar]
- 33.Vandenbroucke R. E., De Smedt S. C., Demeester J., Sanders N. N. Cellular entry pathway and gene transfer capacity of TAT-modified lipoplexes. Biochim. Biophys. Acta. 2007;1768(3):571–579. doi: 10.1016/j.bbamem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 34.Maiolo J. R., Ferrer M., Ottinger E. A. Effects of cargo molecules on the cellular uptake of arginine-rich cell-penetrating peptides. Biochim. Biophys. Acta. 2005;1712(2):161–172. doi: 10.1016/j.bbamem.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Fischer R., Bachle D., Fotin-Mleczek M., Jung G., Kalbacher H., Brock R. A targeted protease substrate for a quantitative determination of protease activities in the endolysosomal pathway. Chembiochem. 2006;7(9):1428–1434. doi: 10.1002/cbic.200600209. [DOI] [PubMed] [Google Scholar]
- 36.Rinne J., Albarran B., Jylhava J., Ihalainen T. O., Kankaanpaa P., Hytonen V. P., Stayton P. S., Kulomaa M. S., Vihinen-Ranta M. Internalization of novel non-viral vector TAT-streptavidin into human cells. BMC Biotechnol. 2007;7:1. doi: 10.1186/1472-6750-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA. 1987;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farhood H., Serbina N., Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim. Biophys. Acta. 1995;1235(2):289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhou X., Huang L. DNA transfection mediated by cationic liposomes containing lipopolylysine: characterization and mechanism of action. Biochim. Biophys. Acta. 1994;1189(2):195–203. doi: 10.1016/0005-2736(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 40.Hafez I. M., Cullis P. R. Roles of lipid polymorphism in intracellular delivery. Adv. Drug Deliv. Rev. 2001;47(2–3):139–148. doi: 10.1016/S0169-409X(01)00103-X. [DOI] [PubMed] [Google Scholar]
- 41.Hyndman L., Lemoine J. L., Huang L., Porteous D. J., Boyd A. C., Nan X. HIV-1 Tat protein transduction domain peptide facilitates gene transfer in combination with cationic liposomes. J. Control. Release. 2004;99(3):435–444. doi: 10.1016/j.jconrel.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 42.Kogure K., Akita H., Harashima H. Multifunctional envelope-type nano device for non-viral gene delivery: concept and application of Programmed Packaging. J. Control. Release. 2007;122(3):246–251. doi: 10.1016/j.jconrel.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Kogure K., Akita H., Yamada Y., Harashima H. Multifunctional envelope-type nano device (MEND) as a non-viral gene delivery system. Adv. Drug Deliv. Rev. 2008;60(4–5):559–571. doi: 10.1016/j.addr.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 44.Kogure K., Moriguchi R., Sasaki K., Ueno M., Futaki S., Harashima H. Development of a non-viral multifunctional envelope-type nano device by a novel lipid film hydration method. J. Control. Release. 2004;98(2):317–323. doi: 10.1016/j.jconrel.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 45.Khalil I. A., Kogure K., Futaki S., Hama S., Akita H., Ueno M., Kishida H., Kudoh M., Mishina Y., Kataoka K., Yamada M., Harashima H. Octaarginine-modified multifunctional envelope-type nanoparticles for gene delivery. Gene. Ther. 2007;14(8):682–689. doi: 10.1038/sj.gt.3302910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriguchi R., Kogure K., Akita H., Futaki S., Miyagishi M., Taira K., Harashima H. A multifunctional envelope-type nano device for novel gene delivery of siRNA plasmids. Int. J. Pharm. 2005;301(1–2):277–285. doi: 10.1016/j.ijpharm.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki R., Yamada Y., Harashima H. Development of small, homogeneous pDNA particles condensed with mono-cationic detergents and encapsulated in a multifunctional envelope-type nano device. Biol. Pharm. Bull. 2008;31(6):1237–1243. doi: 10.1248/bpb.31.1237. [DOI] [PubMed] [Google Scholar]
- 48.Nakamura Y., Kogure K., Yamada Y., Futaki S., Harashima H. Significant and prolonged antisense effect of a multifunctional envelope-type nano device encapsulating antisense oligodeoxynucleotide. J. Pharm. Pharmacol. 2006;58(4):431–437. doi: 10.1211/jpp.58.4.0002. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura Y., Kogure K., Futaki S., Harashima H. Octaarginine-modified multifunctional envelope-type nano device for siRNA. J. Control. Release. 2007;119(3):360–367. doi: 10.1016/j.jconrel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki R., Yamada Y., Harashima H. Efficient cytoplasmic protein delivery by means of a multifunctional envelope-type nano device. Biol. Pharm. Bull. 2007;30(4):758–762. doi: 10.1248/bpb.30.758. [DOI] [PubMed] [Google Scholar]
- 51.Yamada Y., Akita H., Kamiya H., Kogure K., Yamamoto T., Shinohara Y., Yamashita K., Kobayashi H., Kikuchi H., Harashima H. MITO-Porter: a liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim. Biophys. Acta. 2008;1778(2):423–432. doi: 10.1016/j.bbamem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Nakamura T., Moriguchi R., Kogure K., Shastri N., Harashima H. Efficient MHC class I presentation by controlled intracellular trafficking of antigens in octaarginine-modified liposomes. Mol. Ther. 2008;16(8):1507–1514. doi: 10.1038/mt.2008.122. [DOI] [PubMed] [Google Scholar]
- 53.Mudhakir D., Akita H., Tan E., Harashima H. A novel IRQ ligand-modified nano-carrier targeted to a unique pathway of caveolar endocytic pathway. J. Control. Release. 2008;125(2):164–173. doi: 10.1016/j.jconrel.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 54.Holland J. W., Hui C., Cullis P. R., Madden T. D. Poly(ethylene glycol)-lipid conjugates regulate the calcium-induced fusion of liposomes composed of phosphatidylethanolamine and phosphatidylserine. Biochemistry. 1996;35(8):2618–2624. doi: 10.1021/bi952000v. [DOI] [PubMed] [Google Scholar]
- 55.Sawant R. M., Hurley J. P., Salmaso S., Kale A., Tolcheva E., Levchenko T. S., Torchilin V. P. “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug. Chem. 2006;17(4):943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kale A. A., Torchilin V. P. “Smart” drug carriers: PEGylated TATp-modified pH-sensitive liposomes. J. Liposome. Res. 2007;17(3–4):197–203. doi: 10.1080/08982100701525035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kale A. A., Torchilin V. P. Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified pegylated liposomes. J. Drug Target. 2007;15(7–8):538–545. doi: 10.1080/10611860701498203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El-Sayed A., Khalil I. A., Kogure K., Futaki S., Harashima H. Octaarginine- and octalysine-modified nanoparticles have different modes of endosomal escape. J. Biol. Chem. 2008;283(34):23450–23461. doi: 10.1074/jbc.M709387200. [DOI] [PubMed] [Google Scholar]
- 59.Meers P., Bentz J., Alford D., Nir S., Papahadjopoulos D., Hong K. Synexin enhances the aggregation rate but not the fusion rate of liposomes. Biochemistry. 1988;27(12):4430–4439. doi: 10.1021/bi00412a033. [DOI] [PubMed] [Google Scholar]
- 60.Maeda T., Kawasaki K., Ohnishi S. Interaction of influenza virus hemagglutinin with target membrane lipids is a key step in virus-induced hemolysis and fusion at pH 5.2. Proc. Natl. Acad. Sci. USA. 1981;78(7):4133–4137. doi: 10.1073/pnas.78.7.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bullough P. A., Hughson F. M., Skehel J. J., Wiley D. C. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 62.Kaplan I. M., Wadia J. S., Dowdy S. F. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Control. Release. 2005;102(1):247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 63.El-Andaloussi S., Johansson H. J., Lundberg P., Langel U. Induction of splice correction by cell-penetrating peptide nucleic acids. J. Gene Med. 2006;8(10):1262–1273. doi: 10.1002/jgm.950. [DOI] [PubMed] [Google Scholar]
- 64.Lundberg P., El-Andaloussi S., Sutlu T., Johansson H., Langel U. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. Faseb J. 2007;21(11):2664–2671. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
- 65.Plank C., Oberhauser B., Mechtler K., Koch C., Wagner E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J. Biol. Chem. 1994;269(17):12918–12924. [PubMed] [Google Scholar]
- 66.Esbjorner E. K., Oglecka K., Lincoln P., Graslund A., Norden B. Membrane binding of pH-sensitive influenza fusion peptides. Positioning, configuration, and induced leakage in a lipid vesicle model. Biochemistry. 2007;46(47):13490–13504. doi: 10.1021/bi701075y. [DOI] [PubMed] [Google Scholar]
- 67.Sugita T., Yoshikawa T., Mukai Y., Yamanada N., Imai S., Nagano K., Yoshida Y., Shibata H., Yoshioka Y., Nakagawa S., Kamada H., Tsunoda S., Tsutsumi Y. Comparative study on transduction and toxicity of protein transduction domains. Br. J. Pharmacol. 2008;153(6):1143–1152. doi: 10.1038/sj.bjp.0707678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sugita T., Yoshikawa T., Mukai Y., Yamanada N., Imai S., Nagano K., Yoshida Y., Shibata H., Yoshioka Y., Nakagawa S., Kamada H., Tsunoda S., Tsutsumi Y. Improved cytosolic translocation and tumor-killing activity of Tat-shepherdin conjugates mediated by co-treatment with Tat-fused endosome-disruptive HA2 peptide. Biochem. Biophys. Res. Commun. 2007;363(4):1027–1032. doi: 10.1016/j.bbrc.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 69.Yoshikawa T., Sugita T., Mukai Y., Yamanada N., Nagano K., Nabeshi H., Yoshioka Y., Nakagawa S., Abe Y., Kamada H., Tsunoda S., Tsutsumi Y. Organelle-targeted delivery of biological macromolecules using the protein transduction domain: potential applications for Peptide aptamer delivery into the nucleus. J. Mol. Biol. 2008;380(5):777–782. doi: 10.1016/j.jmb.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 70.Michiue H., Tomizawa K., Wei F. Y., Matsushita M., Lu Y. F., Ichikawa T., Tamiya T., Date I., Matsui H. The NH2 terminus of influenza virus hemagglutinin-2 subunit peptides enhances the antitumor potency of polyarginine-mediated p53 protein transduction. J. Biol. Chem. 2005;280(9):8285–8289. doi: 10.1074/jbc.M412430200. [DOI] [PubMed] [Google Scholar]
- 71.Ohmori N., Niidome T., Wada A., Hirayama T., Hatakeyama T., Aoyagi H. The enhancing effect of anionic alpha-helical peptide on cationic peptide-mediating transfection systems. Biochem. Biophys. Res. Commun. 1997;235(3):726–729. doi: 10.1006/bbrc.1997.6880. [DOI] [PubMed] [Google Scholar]
- 72.Lo S. L., Wang S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials. 2008;29(15):2408–2414. doi: 10.1016/j.biomaterials.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 73.Midoux P., Monsigny M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjug. Chem. 1999;10(3):406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 74.Boussif O., Lezoualc’h F., Zanta M. A., Mergny M. D., Scherman D., Demeneix B., Behr J. P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA. 1995;92(16):7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pack D. W., Hoffman A. S., Pun S., Stayton P. S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 76.Rudolph C., Plank C., Lausier J., Schillinger U., Muller R. H., Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J. Biol. Chem. 2003;278(13):11411–11418. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- 77.Huang R. Q., Pei Y. Y., Jiang C. Enhanced gene transfer into brain capillary endothelial cells using Antp-modified DNA-loaded nanoparticles. J. Biomed. Sci. 2007;14(5):595–605. doi: 10.1007/s11373-007-9171-5. [DOI] [PubMed] [Google Scholar]
- 78.Kleemann E., Neu M., Jekel N., Fink L., Schmehl T., Gessler T., Seeger W., Kissel T. Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG–PEI. J. Control. Release. 2005;109(1–3):299–316. doi: 10.1016/j.jconrel.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 79.Sirsi S. R., Schray R. C., Guan X., Williams J. H., Erney M. L., Lutz G. J. Functionalized PEG–PEI copolymers complexed to exon-skipping oligonucleotides improve dystrophin expression in mdx mice. Hum. Gene Ther. 2008;19(8):795–806. doi: 10.1089/hum.2007.129. [DOI] [PubMed] [Google Scholar]
- 80.Alexis F., Lo S. L., Wang S. Covalent attachment of low molecular weight Poly(ethylene imine) improves Tat peptide mediated gene delivery. Adv. Mater. 2006;18(16):2174–2178. doi: 10.1002/adma.200502173. [DOI] [Google Scholar]
- 81.Suk J. S., Suh J., Choy K., Lai S. K., Fu J., Hanes J. Gene delivery to differentiated neurotypic cells with RGD and HIV Tat peptide functionalized polymeric nanoparticles. Biomaterials. 2006;27(29):5143–5150. doi: 10.1016/j.biomaterials.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Doyle S. R., Chan C. K. Differential intracellular distribution of DNA complexed with polyethylenimine (PEI) and PEI-polyarginine PTD influences exogenous gene expression within live COS-7 cells. Genet. Vaccines Ther. 2007;5:11. doi: 10.1186/1479-0556-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erbacher P., Roche A. C., Monsigny M., Midoux P. Putative role of chloroquine in gene transfer into a human hepatoma cell line by DNA/lactosylated polylysine complexes. Exp. Cell. Res. 1996;225(1):186–194. doi: 10.1006/excr.1996.0169. [DOI] [PubMed] [Google Scholar]
- 84.Ciftci K., Levy R. J. Enhanced plasmid DNA transfection with lysosomotropic agents in cultured fibroblasts. Int. J. Pharm. 2001;218(1–2):81–92. doi: 10.1016/S0378-5173(01)00623-8. [DOI] [PubMed] [Google Scholar]
- 85.Wadhwa M. S., Knoell D. L., Young A. P., Rice K. G. Targeted gene delivery with a low molecular weight glycopeptide carrier. Bioconjug. Chem. 1995;6(3):283–291. doi: 10.1021/bc00033a008. [DOI] [PubMed] [Google Scholar]
- 86.Jeon E., Kim H. D., Kim J. S. Pluronic-grafted poly-(L)-lysine as a new synthetic gene carrier. J. Biomed. Mater. Res. A. 2003;66(4):854–859. doi: 10.1002/jbm.a.10012. [DOI] [PubMed] [Google Scholar]
- 87.Katav T., Liu L., Traitel T., Goldbart R., Wolfson M., Kost J. Modified pectin-based carrier for gene delivery: cellular barriers in gene delivery course. J. Control. Release. 2008;130(20):183–191. doi: 10.1016/j.jconrel.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Caron N. J., Quenneville S. P., Tremblay J. P. Endosome disruption enhances the functional nuclear delivery of Tat-fusion proteins. Biochem. Biophys. Res. Commun. 2004;319(1):12–20. doi: 10.1016/j.bbrc.2004.04.180. [DOI] [PubMed] [Google Scholar]
- 89.Shiraishi T., Pankratova S., Nielsen P. E. Calcium ions effectively enhance the effect of antisense peptide nucleic acids conjugated to cationic tat and oligoarginine peptides. Chem. Biol. 2005;12(8):923–929. doi: 10.1016/j.chembiol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 90.Shiraishi T., Nielsen P. E. Enhanced delivery of cell-penetrating peptide–peptide nucleic acid conjugates by endosomal disruption. Nat. Protoc. 2006;1(2):633–636. doi: 10.1038/nprot.2006.92. [DOI] [PubMed] [Google Scholar]
- 91.Abes S., Turner J. J., Ivanova G. D., Owen D., Williams D., Arzumanov A., Clair P., Gait M. J., Lebleu B. Efficient splicing correction by PNA conjugation to an R6-Penetratin delivery peptide. Nucleic Acids Res. 2007;35(13):4495–4502. doi: 10.1093/nar/gkm418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hogset A., Prasmickaite L., Selbo P. K., Hellum M., Engesaeter B. O., Bonsted A., Berg K. Photochemical internalisation in drug and gene delivery. Adv. Drug Deliv. Rev. 2004;56(1):95–115. doi: 10.1016/j.addr.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 93.Matsushita M., Noguchi H., Lu Y. F., Tomizawa K., Michiue H., Li S. T., Hirose K., Bonner-Weir S., Matsui H. Photo-acceleration of protein release from endosome in the protein transduction system. FEBS Lett. 2004;572(1–3):221–226. doi: 10.1016/j.febslet.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 94.Shiraishi T., Nielsen P. E. Photochemically enhanced cellular delivery of cell penetrating peptide-PNA conjugates. FEBS Lett. 2006;580(5):1451–1456. doi: 10.1016/j.febslet.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 95.Folini M., Bandiera R., Millo E., Gandellini P., Sozzi G., Gasparini P., Longoni N., Binda M., Daidone M. G., Berg K., Zaffaroni N. Photochemically enhanced delivery of a cell-penetrating peptide nucleic acid conjugate targeting human telomerase reverse transcriptase: effects on telomere status and proliferative potential of human prostate cancer cells. Cell. Prolif. 2007;40(6):905–920. doi: 10.1111/j.1365-2184.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berg K., Hogset A., Prasmickaite L., Weyergang A., Bonsted A., Dietze A., Lou P. J., Bown S., Norum O. J., Mollergard H. M. T., Selbo P. K. Photochemical internalization (PCI): a novel technology for activation of endocytosed therapeutic agents. Med. Laser Appl. 2006;21(4):239–250. doi: 10.1016/j.mla.2006.08.004. [DOI] [Google Scholar]