Abstract
Targeted delivery of functional nucleic acids (genes and oligonucleotides) to pulmonary endothelium may become a novel therapy for the treatment of various types of lung diseases. It may also provide a new research tool to study the functions and regulation of novel genes in pulmonary endothelium. Its success is largely dependent on the development of a vehicle that is capable of efficient pulmonary delivery with minimal toxicity. This review summarizes the recent progress that has been made in our laboratory along these research directions. Factors that affect pulmonary nucleic acids delivery are also discussed.
Key words: delivery, endothelial cells, genes, lung, oligonucleotides, pulmonary circulation, siRNA, targeting
INTRODUCTION
Pulmonary delivery via airway (inhalation) has been widely used to deliver various types of therapeutics for the treatment of many lung diseases. This approach is particularly suitable for the treatment of respiratory tract infections, asthma, and chronic obstructive pulmonary diseases and has an obvious advantage of concentrating the administered agents at the site of action and minimizing the untoward systemic effects. In recent years, the lung has also been studied as a possible route of administration for the treatment of systemic diseases (e.g., diabetes mellitus) due to the enormous surface area and relatively low enzymatic activity of the alveolar space. The application of inhalation in the delivery of conventional drugs as well as novel therapeutics (e.g., proteins, genes, siRNA, etc.) has been well addressed in several recent reviews (1–3). On the other hand, drug delivery to pulmonary endothelial cells (ECs) via airway is rather inefficient, and new strategies are being developed for systemic delivery of various types of therapeutic agents to pulmonary circulation including conventional drugs (4), radioisotopes (5), protein therapeutics (6), etc. These studies have important implications as endothelial dysfunction plays an important role in a number of pulmonary diseases such as pulmonary hypertension, adult respiratory distress syndrome, or metastatic disease to the lung. For example, targeted delivery of catalase to pulmonary ECs has been shown to offer lung protection in several models of lung injury (7–8). Nucleic acid-based drugs are currently being explored as novel therapeutics, and they also hold promise for the treatment of pulmonary vascular diseases (9). This can be achieved via either overexpression of a functional transgene (gene therapy) or silencing of a diseased gene whose overexpression is involved in the pathogenesis and/or development of a pulmonary disease (oligonucleotide or siRNA therapy). The success of either strategy, however, is largely dependent on the development of a vehicle that is capable of efficient delivery of either genes or oligonucleotides (ODN) with minimal toxicity. Delivery of nucleic acid-based therapeutics to the pulmonary circulation can be achieved via passive or active targeting. This review will discuss the pulmonary physiology that affects drug delivery to the pulmonary circulation. Special emphasis will be placed on discussion of the strategies that have been developed in our laboratory for the delivery of nucleic acid-based therapeutics to the pulmonary circulation.
PULMONARY PHYSIOLOGY THAT AFFECTS ENDOTHELIUM TARGETING
Pulmonary Circulation
Normal pulmonary circulation is a low-pressure high-capacitance bed that receives 100% of the cardiac output. The intimal lining of all blood vessels is made of a continuous single layer of ECs. In the human lung, ECs occupy a surface area of 130 m2 (10). Approximately 30% of all ECs are found in the pulmonary vasculature. In addition to its large surface area, pulmonary endothelium is also the first vascular bed the vector will interact with following systemic administration. Furthermore, ECs are readily accessible to i.v.-administered agents, and endothelium penetration is not required for EC targeting. Thus, pulmonary ECs represent an ideal target for targeted delivery of therapeutic agents via vascular route.
Surface Properties and Uptake Mechanisms of Macromolecules of Pulmonary ECs
The primary role for pulmonary endothelial lining is to serve as a nonthrombogenic semipermeable barrier for the contents in the blood and to provide a vast surface for gas exchange. ECs also function in a multitude of physiologic processes including the regulation of vasomotor tone, the maintenance of blood fluidity, the control of cellular trafficking, and the growth of new blood vessels. In addition, ECs carry out an active bulk transport of solute and macromolecules across the EC layer to the interstitial space. Transport of macromolecules is largely mediated by an active transcytosis mechanism that involves caveolae-dependent vesicles, although some of the cargo may be routed to destructive lysosome compartments (11).
ECs are coated with a glycocalyx layer enriched with sialic acid residues and sulfate groups providing net negative surface charges (12). Thus, a cationic vector may effectively interact with the negatively charged EC, which may contribute to the high efficiency in cationic polymer- or lipid-mediated delivery of nucleic acids to pulmonary endothelium via vascular route.
The ECs from different sites within the vasculature share some common features, but specific properties may differ between ECs found in small and large vessels. ECs also contain surface receptors that recognize specific macromolecules. Many of these receptors are common in different organ systems, but some of these are organ- and site-specific (13). This is very important in developing strategies for drug targeting. For example, small- and medium-sized vesicles are largely involved in the pathogenesis of pulmonary hypertension. Thus, therapeutic agents that are targeted to endothelium lining these blood vessels should be ideal for the treatment of such disease.
Several EC surface antigens have been examined as a target for cell-specific drug/gene delivery. Thrombomodulin (TM), also called CD141 or BDCA-3, was one of the first surface antigens used for targeted delivery of liposomal drugs (4), genes (14), proteins (7–8), and radioisotopes (5) to mouse lung ECs in vitro and in vivo. TM is expressed at very high levels in pulmonary ECs, and lower levels are found on ECs in other organs. Platelet/endothelial cell adhesion molecule-1 (PECAM-1) is also a commonly used target for selective delivery of gene- and protein-based therapeutics to vascular ECs including pulmonary ECs (6,15). PECAM-1 is a transmembrane adhesion molecule expressed at high levels on ECs (greater than one million copies per cell) (16). Angiotensin-converting enzyme (ACE) is another attractive surface marker for EC targeting. In the systemic circulation, only 5–15% of capillaries express ACE, compared with 100% in the lung (17). Thus, ACE may serve as an attractive target for preferential targeting to pulmonary ECs.
There are several other surface markers that also show promise as EC targets such as intercellular adhesion molecule 1 (ICAM-1), lectin-like oxidized LDL receptor (LOX-1), and P-selectin (18). P-selectin is constitutively expressed on vascular endothelium with lung ECs having the highest level of expression. However, the expression levels of this surface antigen in ECs were further upregulated when the animals were challenged with histamine or lipopolysaccharides (18). The other two molecules are normally expressed at low levels, but their expression on ECs is increased in certain disease states. For example, ICAM-1 is upregulated in vascular inflammation, oxidative stress, and thrombosis (19), while LOX-1 is upregulated in dysfunctional endothelium associated with hypertension and atherogenesis (20). Thus, these targets are particularly suitable for selective delivery of various types of therapeutics to the diseased vasculature.
Most of the published studies on endothelium targeting are conducted with antibodies as specific ligands. EC-specific peptides have also been explored as less immunogenic ligands. Phage display technology is a robust methodology for identifying peptides that bind relatively tightly to target proteins. This is especially true if the peptide is to selectively bind to a target molecule in vivo. In these applications, the first-generation peptides have a generally low Kd (10–100 μM) for their targets and typically need to be structurally altered to improve binding to the targeted molecules (21). The same rule also appears to apply to the sequences that are identified using combinatorial peptide libraries (22). We have been interested in exploring the potential of small molecule ACE inhibitors as novel EC-targeting ligands. As discussed above, ACE is an enzyme that is abundantly and selectively expressed in pulmonary ECs. In addition, ACE is further upregulated in the lungs during pulmonary hypertension (23). It is involved in the regulation of vascular resistance via converting angiotensin I to angiotensin II, the latter being a potent vasoconstrictor. A number of small molecule inhibitors targeting ACE such as lisinopril have been developed for the treatment of hypertension (24). Other than their applications in therapy, radiolabeled ACE inhibitors have been used for imaging and quantitative assessment of the ACE expression in the lungs (25–27). Based on the high specificity (Kd, 10−9∼10−10 M) and high affinity of these inhibitors toward ACE, we hypothesize that they can also serve as a targeting ligand to mediate selective delivery of various types of agents to pulmonary ECs. Our preliminary studies have shown that lisinopril-conjugated, rhodamine-labeled liposomes can be effectively targeted to lung ECs in vitro and in vivo (Wilson et al., unpublished data). The major advantage of using lisinopril as a targeting ligand lies in its excellent clinical safety profile. Its low or non-immunogenicity shall also allow repeated dosing. Furthermore, use of lisinopril as a targeting ligand overcomes the limitation of species specificity that is associated with antibody-based ligands: lisinopril can be readily used in different species including humans.
PULMONARY DELIVERY OF GENES AND OLIGONUCLEOTIDES VIA VASCULAR ROUTE
Pulmonary Delivery of Transgenes via Systemic Administration
Cationic Lipid-Mediated Pulmonary Gene Transfer
Despite the abundant uptake mechanisms in vascular ECs, delivery of genes or oligonucleotides to pulmonary endothelium without a vehicle has proven to be inefficient possibly due to lack of receptors for nucleic acids on ECs, the transient residence times of injected genes or oligonucleotides in pulmonary circulation, and the presence of nuclease degradation activities in the blood. This has prompted the development of various systems to improve the efficiency of delivery. We and others have previously shown that lungs can be preferentially and efficiently transfected following i.v. administration of a transgene expression plasmid complexed with cationic liposomes (28–32). Pulmonary gene transfer via cationic lipids is largely mediated by a passive mechanism of serum-induced aggregation of lipid vectors, although the nonspecific interaction of cationic lipid vectors with the negatively charged endothelium may also play a role (33). Following i.v. administration, blood components such as negatively charged serum proteins induce aggregation of cationic lipid vectors. The large-sized aggregates are mainly entrapped in pulmonary microvasculature since the lung has the first and the largest capillary bed the lipid vectors will interact with following systemic administration. Pulmonary delivery of a retinoblastoma (Rb) tumor suppressor transgene resulted in a significant inhibition of lung metastasis in Rb−/− mice (34). Despite the potential of this gene transfer protocol in treatment of pulmonary malignancies, its application in the management of nonmalignant pulmonary diseases may be limited by the associated proinflammatory cytokine response. Such immune response not only contributes to the complex toxicity but also causes inhibition of transgene expression (35,36). We and others have previously shown that the complex-associated immune response is largely due to the unmethylated CpG motifs in bacterial plasmid DNA (37,38). However, cationic lipids might augment the CpG immune response via enhancing the uptake of liposome/DNA complexes by immune cells such as macrophages. Cationic lipids also appear to sensitize the immune cells to the CpG-mediated immune response (Ma Z and Li S, unpublished data). While we have been actively engaged in developing strategies to counteract the CpG immune response (39–42), we also found a peculiar observation that cationic polymers appear to be rather inert with respect to enhancing the CpG immune response (15). This has led us to our recent interest in developing polymeric delivery systems for pulmonary gene transfer.
Cationic Polymer-Mediated Pulmonary Gene Transfer
Polylysine was the first cationic polymer that was used for gene transfer to hepatocytes (43) and mouse ECs (14). However, its application suffers from low transfection efficiency. Following the successful report of polyethylenimine (PEI)-mediated gene transfer in vitro and in vivo (44), there has been renewed interest in polymer-mediated gene transfer. Many new polymers have been developed and examined for applications in gene transfer to different tissues/organs (45,46). Interestingly, despite the emergence of various types of new polymers, PEI continues to be one of the most efficient vectors for in vitro transfection and pulmonary gene transfer via vascular route. Also intriguing is the observation that it is linear PEI (LPEI) but not branched PEI (BPEI) that is capable of providing a high efficiency of systemic gene delivery to lungs (47). BPEI is also associated with significantly higher levels of acute toxicity including sudden death at high doses (Li S and Gao X, unpublished data). Transgene expression is primarily limited to the alveolar region, and both endothelial and epithelial cells can be transfected (47).
The unique feature of LPEI has led us to examining its therapeutic potential in systemic delivery of mini-urokinase plasminogen activator (uPA) transgene to mouse lungs in an acute lung injury model. Acute lung injury, regardless of the underlying etiology, is often associated with activated coagulation that is accompanied by an inhibition of fibrinolytic activity (48,49). This imbalance leads to an excessive fibrin deposition in the alveolar, interstitial, and vascular spaces of the lung. Fibrin promotes the acute cellular inflammatory response, contributes to surfactant dysfunction, and provides a provisional matrix for collagen deposition in the lung (48,49). Thus, decreasing fibrin deposition within the lung is not only important for the prevention of pulmonary damage at the early stages of pulmonary injury but also beneficial for late-stage fibrosis. Upregulation of fibrinolytic activity within the lung was initially attempted by systemic administration of recombinant uPA protein (50). The efficiency of this approach is limited by the fact that only a small amount of uPA is retained in the lung via i.v. route. Hart and colleagues (51) have shown that intratracheal administration of recombinant uPA protein partially reverses the pulmonary fibrosis induced by bleomycin in a rat model. However, the rapid turnover of administered uPA protein in vivo necessitates repeated dosing which is not only costly but also potentially immunogenic. Simon and colleagues have attempted to upregulate fibrinolytic activity locally by intratracheal uPA gene delivery (52,53). A single dose of uPA adenoviral vector substantially increases fibrinolytic activity in the lung that lasts for about 2 weeks (52). Furthermore, locally overexpressed uPA significantly decreases the amount of fibrin accumulated in the lung following bleomycin-induced pulmonary injury (53). This study validates the concept of uPA gene therapy for inflammatory lung diseases and also demonstrates that gene transfer/expression of uPA can prevent the development of pulmonary fibrosis following pulmonary injury. However, the practical use of this approach may be limited by the immunogenicity of the viral vector and the poor accessibility of the administered adenovirus particles to the mucus- and fibrin-coated sites in severely inflamed lungs. We have shown that lung inflammation induced by bleomycin treatment has minimal effect on LPEI-mediated pulmonary gene transfer via vascular route (Zhang J and Li, S, unpublished data). To prolong the duration of uPA transgene expression, the uPA cDNA was subcloned into an Epstein–Barr virus (EBV)-based expression plasmid (54). To further improve the biosafety, we have deleted those sequences in uPA (growth factor-like domain and kringle linker domain) that have been shown to be associated with proinflammatory activity of wild-type uPA (55). The resultant mini-uPA is devoid of the undesired side effects but fully retains the proteolytic activity. Our data show that pulmonary delivery of mini-uPA transgene via LPEI results in a significant increase in the pulmonary fibrinolytic activity. Mini-uPA gene transfer leads to a significantly improved survival rate of the bleomycin-challenged animals which was associated with a drastically reduced pulmonary fibrosis compared to bleomycin-treated animals that were transfected with a control plasmid (Gao X, et al., unpublished data).
The mechanism of LPEI-mediated systemic gene delivery is not completely understood. Also, little is known with regard to the structural differences that contribute to the superior in vivo transfection efficiency by LPEI. Nevertheless, conjugation with an EC-specific ligand (anti-PECAM) antibody results in further improvement in LPEI-mediated pulmonary gene transfer (15). In addition, there is a further decrease in TNF-α response following conjugation with anti-PECAM antibody (15).
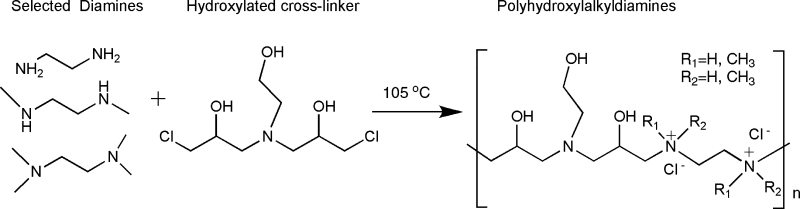
One major concern with LPEI-based gene transfer is its nonbiodegradability, which may account for its toxicity, particularly liver toxicity. To develop novel biodegradable polymers for efficient pulmonary gene transfer with minimal toxicity, we have recently generated a library of polyhydroxylalkyleneamines with hydroxyl and alkylhydroxyl groups linked to a polyamine backbone (PHA) (Gao X, et al., in revision). These polymers are generated by directly cross-linking several dichloro alkylating agents with selective diamines. Disulfide bonds can be introduced to polymer backbones by co-polymerization with cystamine. The PHA polymers have a backbone structure similar to that of LPEI, but have adjustable spacing between the amine groups. In addition, PHA polymers were designed to have less charge density, abundant hydroxyl and alkylhydroxyl “soft” groups, and potentially biodegradable disulfide bonds in the backbone. These are structural features that are potentially critical to reduce the toxicity of the polymers. Our initial in vitro screening has identified several PHA polymers that are more efficient than LPEI in transfection (Table I). More importantly, these polymers are significantly less toxic (Table I). Studies are currently underway to examine their potential in pulmonary gene transfer in intact animals.
Table I.
Properties of Polyhydroxylalkyldiamines
| |||
| Polymersa | MWb | Sizes of complexesc | Relative transfection activities (fold)d |
| bPEI25,000 | 25,000 | 125.3 ± 54.6 | 1.0 |
| C2-EDA | 7,200 | 141.6 ± 63.0 | 3.2 |
| C2-DMEDA | 4,700 | 123.4 ± 52.2 | 0.06 |
| C2-TMEDA | 5,200 | 115.3 ± 43.5 | 0.003 |
abPEI25,000 branched polyethyleneimine (MW = 25,000, Sigma-Aldrich); C2 cross-linker with a hydroxyethyl side chain group, EDA ethylendiamine, DMEDA N,N’ dimethylethylenediamine, TMEDA N,N,N’N’-tetramethylethylenediamine.
bMW was measured by gel permeation chromatography using Ultrahydrogel 500 and 1,000 columns (Waters Corp).
cSizes of DNA and polymer complexes were measured for complexes formed at nitrogen to phosphate ratios of 10, 22.5, 22.5, 22.5 for bPEI25,000, C2-EDA, C2-DMEDA, C2-TMEDA, respectively.
dTransfection activities were evaluated on COS-7 cells grown in 96-well plates, using 0.3 μg pCMVluc reporter plasmid complexed with an array of polymer concentrations ranging from 0.2–200 nmol amines. Twenty-four hours later, the cells were washed once with PBS and lysate prepared in 100 μl of lysis buffer, the luciferase activities in 50 μl of cleared supernatants were measured with 100 μl of luciferase substrate. The peak luciferase activities measured in each well was presented for each polymer. The level of transfection with bPEI25,000 was set to 1.0.
Targeted Delivery of Viral Vectors to Pulmonary Endothelium
One of the advantages of in vivo gene delivery with viral vectors is the relatively high efficiency. For certain types of viral vectors, a relatively long duration of gene expression can also be achieved. Pulmonary gene delivery with viral vectors has been largely performed via airway administration (56). Intravenous gene delivery to pulmonary endothelium using conventional viral vectors, however, is inefficient largely due to a lack of receptors on ECs for the viral vectors. A study by Reynolds and colleagues showed that an adenoviral (Ad) vector, which has a natural tropism for hepatocytes, could be redirected to pulmonary endothelium via a specific conjugate that reacts with both ECs and viral capsid (57). This conjugate was generated by linking the Fab fragment of an anti-Ad5 knob antibody (1D6.14) to the anti-ACE monoclonal antibody mAb 9B9. Premixing of the conjugate with an Ad vector resulted in a 20-fold increase in both Ad DNA localization and luciferase transgene expression in the lungs, compared to the untargeted vector (57). This was accompanied by a concomitant decrease in gene expression in liver although the absolute level of gene expression in the lung was still substantially lower than that in the liver (57). A subsequent study by the same group showed that the specificity of EC targeting can be further improved when this approach was combined with the use of an EC-specific promoter to drive the transgene expression, leading to a synergistic, 300,000-fold improvement in the selectivity of transgene expression for lung versus the liver (58). Targeted delivery of endothelial nitric oxide synthase (eNOS) transgene to ECs prevented elevation of blood pressure in stroke-prone spontaneously hypertensive rats (59). EC targeting has also been demonstrated with adeno-associated viral (AAV) vector (60). This was achieved via incorporation of an EC-specific peptide cDNA into the VP3 region of the AAV-2 capsid such that the peptide was expressed at the virion surface (60). We have shown that redirecting of an Ad vector to pulmonary endothelium can also be achieved via a simple sequential injection of cationic liposomes followed by the viral vector (61). This protocol was originally described by Liu’s group as a means to study the mechanism of cationic lipids-mediated systemic delivery of plasmid DNA (62). Interestingly a follow-up study by Tan and colleagues showed that this protocol was more effective than preformed lipid/DNA complexes in pulmonary gene transfer via vascular route (40). In addition, this protocol was associated with a reduced CpG-mediated immune response (40). Our results showed that there was about a 40-fold increase in transgene expression in the lung using the sequential protocol compared to the use of Ad vector alone (61). In addition, the expression level in the liver was significantly decreased. Premixing of adenoviral vector with cationic liposomes resulted in inactivation of the infectivity of the viral vector in a lipid dose-dependent manner (61). This contrasts with the in vitro studies in which complexing of adenoviral vector with cationic lipids enhanced the transduction efficiency. It remains unknown if this protocol can protect the adenoviral vector from inactivation by the neutralizing antibodies in vivo.
Pulmonary Delivery of Oligonucleotides via Systemic Administration
Polymer systems may not be the best choice for short nucleic acid sequences because they tend to dissociate rapidly in blood. We initially had some success with pulmonary delivery of oligonucleotides using cationic liposomes (63). Similar to large-sized plasmid DNA, oligonucleotides can readily form complexes with cationic liposomes through electrostatic interactions. However, oligonucleotides and plasmid DNA differ with respect to the optimized lipid composition of the vectors (63). Due to the fact that oligonucleotides are much smaller and it is likely that an effective sequence can be identified that is devoid of an immunostimulatory motif(s), it is relatively easy to manage the oligonucleotide-mediated immune response. Indeed, pulmonary delivery of oligonucleotides formulated in the form of LPD (a lipidic vector composed of cationic liposomes, protamine, and DNA) is associated with minimal proinflammatory cytokine response when the oligonucleotides lack a potent unmethylated CpG motif. Pretreatment of mice with LPD containing an antisense oligonucleotide targeted to ICAM-1 gene significantly decreased ICAM-1 expression in the lungs following LPS challenge (63). Despite their simplicity of use, cationic lipidic vectors are associated with an intrinsic problem of nonspecific interactions with blood components. In addition, targeted delivery with cationic liposomes is difficult due to their cell type-nonspecific nature.
Neutral liposomes are biocompatible, nontoxic, and more stable in plasma than cationic lipid vectors. Furthermore, neutral vectors can be rendered tissue-specific following conjugation with a specific ligand. Several liposomal drugs are currently used for the treatment of cancers and infectious diseases (64–66). However, as a gene or oligonucleotide delivery vehicle, conventional liposomes suffer from low encapsulation efficiency due to the limited interior size of liposomes and the poor interaction of oligonucleotides with neutral lipids. Recently, Stuart and colleagues described a method by which 80–100% of input oligonucleotides were entrapped in a charge neutral lipid vector (67). This involves the formation in and extraction of cationic lipid/ODN complex from an organic solvent. The cationic lipid/ODN complex was then mixed with other neutral lipids, and ODN-containing lipid vesicles were obtained by a reverse phase evaporation method (67). A similar method was developed by Semple and colleagues, which utilizes an ionizable aminolipid (1,2-dioleoyl-3-dimethylammonium propane, DODAP) and an ethanol-containing system (68). DODAP, a conditional cationic lipid that has a positive charge at acidic pH 4.0, was used to help the entrapment of oligonucleotides via electrostatic interactions (68). DODAP/ODN complexes were then reconstituted with other lipids [distearoylphosphatidylcholine (DSPC), cholesterol, and N-palmitoylsphingo-sine-1-succinyl-methoxypoly(ethylene-glycol)2000 (PEG-CerC16)] in an ethanol-containing co-solvent. The ODN-containing lipid vesicles were formed upon the removal of the ethanol by dialysis. Subsequent adjustment of the external pH to neutral pH values results in particles with a neutral surface charge (68). This method led to a significant increase in oligonucleotide entrapment efficiency (60–80%) with a final oligonucleotide/lipid ratio of 0.15–0.25 (w/w). This formulation has also been shown to be long circulating in the blood following systemic administration.
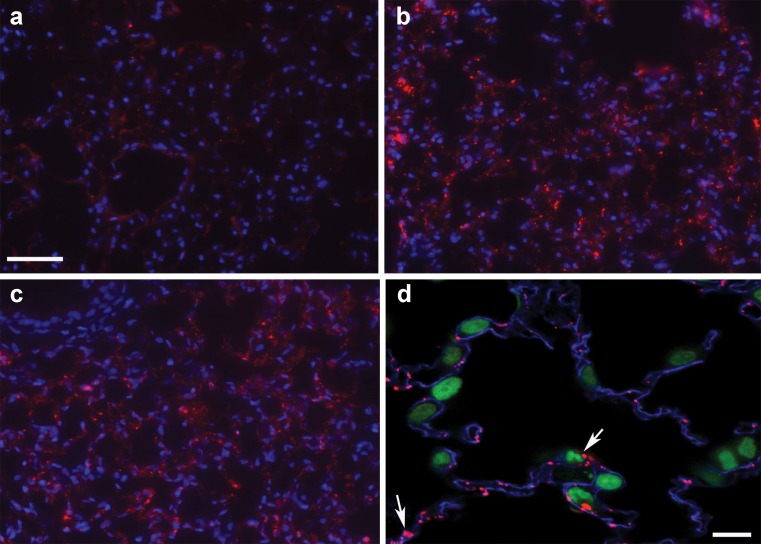
We recently examined whether a neutral lipid vector as described above can be modified with an endothelium-specific antibody to achieve targeted delivery of oligonucleotides to pulmonary ECs. The endothelium-specific antibody to TM or PECAM-1 was coupled to oligonucleotide-encapsulated lipid vesicles via a chemical reaction with para-nitrophenylcarbonyl (pNP)-PEG-phosphoethanolamine (PE) that was introduced onto the surface of the vesicles. Using this formulation, 60–80% oligonucleotide entrapment efficiency was routinely achieved. Oligonucleotides were protected from nuclease-mediated degradation. These particles are overall neutral or slightly anionic and show selective delivery of oligonucleotides into cultured ECs (69). Intravenous administration of the targeted lipidic oligonucleotides led to a substantial accumulation of oligonucleotides in the pulmonary microcirculation (Fig. 1). In contrast, little oligonucleotides were found in lungs with either free oligonucleotides or oligonucleotides formulated in a lipid vector that was conjugated with a control IgG (Fig. 1). Importantly, i.v. injection of this novel neutral lipidic vector containing oligonucleotides did not affect either: (a) pulmonary or systemic hemodynamics; or (b) blood chemistry in catheterized mice (69). More recently, we have shown that this vector can also be modified for targeted delivery of siRNA to pulmonary ECs (Wilson A., unpublished data). This system is currently being employed for delivery of endothelin-1 (ET-1) siRNA to selectively inhibit pulmonary ET-1 biosynthesis for the treatment of pulmonary hypertension. ET-1 is chosen as a target, as it is a potent vasoconstrictor, and overexpression of pulmonary ET-1 has been implicated in the development of both primary and secondary PHT (70). In addition, this system has also been used to mediate targeted delivery of therapeutic oligonucleotides to cancer cells and achieve a sequence-specific therapeutic effect (71,72).
Fig. 1.
Targeted delivery of oligodeoxynucleotides (ODN) to pulmonary ECs in vivo. Mice received a tail vein injection of 20 μg Cy3-ODN as free ODN, IgG-targeted lipidic ODN, or 34A-targeted lipidic ODN. Mice were sacrificed 15 or 45 min later and the lungs observed for Cy3-ODN uptake. a Free ODN (15 min postinjection); b 34A-targeted lipidic ODN (15 min postinjection); c 34A-targeted lipidic ODN (45 min postinjection); d 34A-targeted lipidic ODN at a higher magnification (15 min postinjection). In a, b, and c, blue represents Hoescht-stained nuclei, and red is Cy3-ODN. In d, blue is Cy5-stained PECAM, green is SYTOX green-stained nuclei, and red is Cy3-ODN. The Cy3-labeled ODN is colocalized with PECAM staining (arrows), indicating endothelium-specific targeting. Bar in (a), 50 μm for (a), (b), and (c); bar in (d), 10 μm. Reprinted by permission from Macmillan Publishers Ltd: Wilson et al. (69)
CONCLUSION
In the last few years, we have made significant progresses in developing polymeric and lipidic vectors for targeted delivery of genes and oligonucleotides to pulmonary circulation. Future work will be focused on a number of areas including: (1) continuous synthesis and screening for an improved biodegradable polymer for efficient lung gene transfer with minimal toxicity; (2) further development of more efficient small molecule EC-specific ligands for selective delivery of genes or oligonucleotides; (3) further optimization of the lipidic vector to enhance the cytoplasmic release of oligonucleotides following intracellular delivery; (4) systematic evaluation of the therapeutic efficacy and toxicity of the targeted nucleic acid therapeutics in animal models of pulmonary diseases such as lung injury and pulmonary hypertension. It should be mentioned that a number of novel nanodelivery systems have recently been developed and successfully utilized in delivery of genes or oligonucleotides to various types of tissues such as liver, tumors, etc. (73,74). These systems are likely to find applications in targeted delivery to pulmonary circulation with the use of an EC-specific ligand. Completion of these studies may help to further advance the development of novel nucleic acid-based therapeutics for the treatment of pulmonary diseases. It may also provide a new research tool for the study of functions of novel genes in pulmonary endothelium.
Acknowledgement
This work was supported by National Institutes of Health Grants HL-63080 and HL-68688 and by American Heart Association Grant 026540U.
References
- 1.Dalby R., Suman J. Inhalation therapy: technological milestones in asthma treatment. Adv. Drug Deliv. Rev. 2003;55:779–791. doi: 10.1016/S0169-409X(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 2.Scheuch G., Kohlhaeufl M. J., Brand P., Siekmeier R. Clinical perspectives on pulmonary systemic and macromolecular delivery. Adv. Drug Deliv. Rev. 2006;58:996–1008. doi: 10.1016/j.addr.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Thomas M., Lu J. J., Chen J., Klibanov A. M. Non-viral siRNA delivery to the lung. Adv. Drug Deliv. Rev. 2007;59:124–133. doi: 10.1016/j.addr.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mori A., Kennel S. J., Huang L. Immunotargeting of liposomes containing lipophilic antitumor prodrugs. Pharm. Res. 1993;10:507–514. doi: 10.1023/A:1018933632318. [DOI] [PubMed] [Google Scholar]
- 5.Kennel S. J., Lankford T. K., Foote L. J., Davis I. A., Boll R. A., Mirzadeh S. Combination vascular targeted and tumor targeted radioimmunotherapy. Cancer Biother. Radiopharm. 1999;14:371–379. doi: 10.1089/cbr.1999.14.371. [DOI] [PubMed] [Google Scholar]
- 6.Muzykantov V., Christofidou-Solomidou M., Balyasnikova I., Harshaw D., Schultz L., Fisher A., Albelda S. Streptavidin facilitates internalization and pulmonary targeting of anti-endothelial antibody (PECAM-1): a strategy for intraendothelial drug delivery. Proc. Natl. Acad. Sci. USA. 1999;96:2379–2384. doi: 10.1073/pnas.96.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kozower B. D., Christofidou-Solomidou M., Sweitzer T. D., Muro S., Buerk D. G., Solomides C. C., Albelda S. M., Patterson G. A., Muzykantov V. R. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 2003;21:392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 8.Nowak K., Weih S., Metzger R., Albrecht R. F., II, Post S., Hohenberger P., Gebhard M. M., Danilov S. M. Immunotargeting of catalase to lung endothelium via anti-angiotensin-converting enzyme antibodies attenuates ischemia–reperfusion injury of the lung in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2007;293:L162–L169. doi: 10.1152/ajplung.00001.2007. [DOI] [PubMed] [Google Scholar]
- 9.Li S., Ma Z. Non-viral gene therapy. Current Gene Therapy. 2001;1:201–226. doi: 10.2174/1566523013348814. [DOI] [PubMed] [Google Scholar]
- 10.Simionescu M. Lung endothelium: structure-function correlates. In: Crystal R. G., West J. B., editors. The Lung: Scientific Foundations. New York: Raven; 1991. [Google Scholar]
- 11.Mukherjee S., Ghosh R. N., Maxfield F. R. Endocytosis. Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 12.Simionescu M., Simionescu N., Palade G. E. Differentiated microdomains on the luminal surface of capillary endothelium: distribution of lectin receptors. J. Cell Biol. 1982;94:406–413. doi: 10.1083/jcb.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens T. Molecular and cellular determinants of lung endothelial cell heterogeneity. Chest. 2005;128(6 Suppl):558S–564S. doi: 10.1378/chest.128.6_suppl.558S. [DOI] [PubMed] [Google Scholar]
- 14.Trubeskoy V. S., Torchilin V. P., Kennel S. J., Huang L. Use of N-terminal modified poly(l-lysin)-antibody conjugate as a carrier for targeted gene delivery in mouse lung endothelial cells. Bioconjug. Chem. 1992;3:323–327. doi: 10.1021/bc00016a011. [DOI] [PubMed] [Google Scholar]
- 15.Li S., Tan Y., Viroonchatapan E., Pitt B. R., Huang L. Targeted gene delivery to the lung by anti-PECAM antibody. Am. J. Physiol. 2000;278:504–511. doi: 10.1152/ajplung.2000.278.3.L504. [DOI] [PubMed] [Google Scholar]
- 16.Newman P. The biology of PECAM-1. J. Clin. Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danilov S. M., et al. Lung uptake of antibodies to endothelial antigens: key determinants of vascular immunotargeting. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:1335–1347. doi: 10.1152/ajplung.2001.280.6.L1335. [DOI] [PubMed] [Google Scholar]
- 18.Eppihimer M. J., Wolitzky B., Anderson D. C., Labow M. A., Granger D. N. Heterogeneity of expression of E- and P-selectins in vivo. Circ. Res. 1996;79:560–569. doi: 10.1161/01.res.79.3.560. [DOI] [PubMed] [Google Scholar]
- 19.Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18) J. Cell Biol. 1990;111:3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kataoka H., Kume N., Miyamoto S., Minami M., Moriwaki H., Murase T., Sawamura T., Masaki T., Hashimoto N., Kita T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–3117. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 21.Hao J., Serohijos A. W., Newton G., Tassone G., Wang Z., Sgroi D. C., Dokholyan N. V., Basilion J. P. Identification and rational redesign of peptide ligands to CRIP1, a novel biomarker for cancers. PLoS. Comput. Biol. 2008;4:e1000138. doi: 10.1371/journal.pcbi.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh R. H., Lee T. R., Lawrence D. S. From consensus sequence peptide to high affinity ligand, a “library scan” strategy. J. Biol. Chem. 2001;276:12235–12240. doi: 10.1074/jbc.M011232200. [DOI] [PubMed] [Google Scholar]
- 23.Morrell N. W., Atochina E. N., Morris K. G., Danilov S. M., Stenmark K. R. Angiotensin converting enzyme expression is increased in small pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. J. Clin. Invest. 1995;96:1823–1833. doi: 10.1172/JCI118228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong J., Patel R. A., Kowey P. R. The clinical use of angiotensin-converting enzyme inhibitors. Prog. Cardiovasc. Dis. 2004;47:116–130. doi: 10.1016/j.pcad.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Hwang D. R., Eckelman W. C., Mathias C. J., Petrillo E. W., Jr., Lloyd J., Welch M. J. Positron-labeled angiotensin-converting enzyme (ACE) inhibitor: fluorine-18-fluorocaptopril. Probing the ACE activity in vivo by positron emission tomography. J. Nucl. Med. 1991;32:1730–1737. [PubMed] [Google Scholar]
- 26.Qing F., McCarthy T. J., Markham J., Schuster D. P. . Pulmonary angiotensin-converting enzyme (ACE) binding and inhibition in humans. A positron emission tomography study. Am. J. Respir. Crit. Care Med. 2000;161:2019–2025. doi: 10.1164/ajrccm.161.6.9907036. [DOI] [PubMed] [Google Scholar]
- 27.Femia F. J., Maresca K. P., Hillier S. M., Zimmerman C. N., Joyal J. L., Barrett J. A., Aras O., Dilsizian V. V., Eckelman W. C., Babich J. W. Synthesis and evaluation of a series of 99mTc(CO)3+ lisinopril complexes for in vivo imaging of angiotensin-converting enzyme expression. J. Nucl. Med. 2008;49:970–977. doi: 10.2967/jnumed.107.049064. [DOI] [PubMed] [Google Scholar]
- 28.Liu F., Qi H., Huang L., Liu D. Factors controlling the efficiency of cationic lipid-mediated transfection in vivo via intravenous administration. Gene Ther. 1997;4:517–523. doi: 10.1038/sj.gt.3300424. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Mounkes L. C., Liggitt H. D., Brown C. S., Solodin I., Heath T. D., Debs R. J. Factors influencing the efficiency of cationic liposome-mediated intravenous gene delivery. Nat. Biotechnol. 1997;15:167–173. doi: 10.1038/nbt0297-167. [DOI] [PubMed] [Google Scholar]
- 30.Li S., Huang L. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997;4:891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- 31.Templeton N. S., Lasic D. D., Frederik P. M., Strey H. H., Roberts D. D., Pavlakis G. N. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat. Biotechnol. 1997;15:167–173. doi: 10.1038/nbt0797-647. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Guo X., Xu Y., Barron L., Szoka F. C., Jr. Synthesis and characterization of long chain alkyl acyl carnitine esters. Potentially biodegradable cationic lipids for use in gene delivery. J. Med. Chem. 1999;41:2207–2215. doi: 10.1021/jm950802i. [DOI] [PubMed] [Google Scholar]
- 33.Li S., Tseng W. C., Stolz D. B., Wu S. P., Watkins S. C., Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6:585–594. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- 34.Nikitin A. Y., Juarez-Perez M. I., Li S., Huang L., H Lee W. RB-mediated suppression of spontaneous multiple neuroendocrine neoplasia and lung metastases in RB+/- mice. Proceedings of National Academy of Sciences of USA. 1999;96:3916–3921. doi: 10.1073/pnas.96.7.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S., P Wu S., Whitmore M., Wang L., Watkins S., Huang L. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors. Am. J. Physiol. 1999;276:796–804. doi: 10.1152/ajplung.1999.276.5.L796. [DOI] [PubMed] [Google Scholar]
- 36.Tan Y., Li S., Pitt B. R., Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum. Gene Ther. 1999;10:2153–2161. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 37.Freimark B. D., Blezinger H. P., Florack V. J., Nordstrom J. L., D Long S., Deshpande D. S., Nochumson S., Petrak K. L. Cationic lipids enhance cytokine and cell influx levels in the lung following administration of plasmid: cationic lipid complexes. J. Immunol. 1998;160:4580–4586. [PubMed] [Google Scholar]
- 38.Yew N. S., Wang K. X., Przybylska M., Bagley R. G., Stedman M., Marshall J., Scheule R. K., Cheng S. H. Contribution of plasmid DNA to inflammation in the lung after administration of cationic lipid:pDNA complexes. Hum. Gene Ther. 1999;10:223–234. doi: 10.1089/10430349950019011. [DOI] [PubMed] [Google Scholar]
- 39.Li S., Tan Y., Viroonchatapan E., Pitt B. R., Huang L. Targeted gene delivery to the lung by anti-PECAM antibody. Am. J. Physiol. 2000;278:504–511. doi: 10.1152/ajplung.2000.278.3.L504. [DOI] [PubMed] [Google Scholar]
- 40.Tan Y., Liu F., Li Z., Li S., Huang L. Sequential injection of cationic liposome and plasmid DNA effectively transfects the lung with minimal inflammatory toxicity. Molec. Ther. 2001;3:673–682. doi: 10.1006/mthe.2001.0311. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman C. R., Dileo J. P., Li Z., Li S., Huang L. Efficient in vivo gene transfer by PCR amplified fragment with reduced inflammatory activity. Gene Ther. 2001;8:71–74. doi: 10.1038/sj.gt.3301373. [DOI] [PubMed] [Google Scholar]
- 42.Ma Z., Li J., Mu Y., Yang L., Xie W., Pitt B. R., Li S. Inhibition of LPS- and CpG DNA-induced TNF-a response by oxidized phospholipids. Am. J. Physiol.: Lung Cellular Molecular Physiol. 2004;286:808–816. doi: 10.1152/ajplung.00220.2003. [DOI] [PubMed] [Google Scholar]
- 43.Wu G. Y., Wu C. H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 1987;262:4429–4432. [PubMed] [Google Scholar]
- 44.Boussif O., Lezoualc’h F., Zanta M. A., Mergny M. D., Scherman D., Demeneix B., Behr J. P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S., Ma Z. Non-viral gene therapy. Current Gene Therapy. 2001;1:201–226. doi: 10.2174/1566523013348814. [DOI] [PubMed] [Google Scholar]
- 46.Gao X., Kim K. S., Liu D. Nonviral gene delivery: what we know and what is next. AAPS J. 2007;23:92–104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goula D., Benoist C., Mantero S., Merlo G., Levi G., Demeneix B. A. Polyethylenimine-based intravenous delivery of transgenes to mouse lung. Gene Ther. 1998;5:1291–1295. doi: 10.1038/sj.gt.3300717. [DOI] [PubMed] [Google Scholar]
- 48.Idell S., James K. K., Levin E. G., Schwartz B. S., Manchanda N., Maunder R. J., Martin T. R., McLarty J., Fair D. S. Local abnormalities in coagulation and fibrinolytic pathways predispose to alveolar fibrin deposition in the adult respiratory distress syndrome. J. Clin. Invest. 1989;84:695–705. doi: 10.1172/JCI114217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit. Care Med. 2003;31:213–220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 50.Ikeda T., Hirose N., Koto H., Hirano H., Shigematsu N. Fibrin deposition and fibrinolysis in the pathogenesis of pulmonary fibrosis. Nippon Kyobu Shikkan Gakkai Zasshi. 1989;27:448–451. [PubMed] [Google Scholar]
- 51.Hart D. A., Whidden P., Green F., Henkin J., Woods D. E. Partial reversal of established bleomycin-induced pulmonary fibrosis by rh-urokinase in a rat model. Clin. Invest. Med. 1994;17:69–76. [PubMed] [Google Scholar]
- 52.Hattori N., Sisson T. H., Xu Y., Simon R. H. Upregulation of fibrinolysis by adenovirus-mediated transfer of urokinase-type plasminogen activator genes to lung cells in vitro and in vivo. Hum. Gene Ther. 1999;10:215–222. doi: 10.1089/10430349950019002. [DOI] [PubMed] [Google Scholar]
- 53.Sisson T. H., Hattori N., Xu Y., Simon R. H. Treatment of bleomycin-induced pulmonary fibrosis by transfer of urokinase-type plasminogen activator genes. Hum. Gene Ther. 1999;10:2315–23. doi: 10.1089/10430349950016960. [DOI] [PubMed] [Google Scholar]
- 54.Zhang J., Wilson A., Alber S., Ma Z., Sato E., Mazda O., Watkins S., Huang L., Pitt B., Li S. Prolonged gene expression in mouse lung endothelial cells following transfection with Epstein-Barr virus-based episomal plasmid. Gene Ther. 2003;10:822–826. doi: 10.1038/sj.gt.3301958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X. Q., Bdeir K., Yarovoi S., Cines D. B., Fang W., Abraham E. Involvement of the urokinase kringle domain in lipopolysaccharide-induced acute lung injury. J. Immunol. 2006;177:5550–5557. doi: 10.4049/jimmunol.177.8.5550. [DOI] [PubMed] [Google Scholar]
- 56.Griesenbach U., Geddes D. M., Alton E. W. Gene therapy progress and prospects: cystic fibrosis. Gene Ther. 2006;13:1061–1067. doi: 10.1038/sj.gt.3302809. [DOI] [PubMed] [Google Scholar]
- 57.Reynolds P. N., Zinn K. R., Gavrilyuk V. D., Balyasnikova I. V., Rogers B. E., Buchsbaum D. J., Wang M. H., Miletich D. J., Grizzle W. E., Douglas J. T., Danilov S. M., Curiel D. T. A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol. Ther. 2000;2:562–578. doi: 10.1006/mthe.2000.0205. [DOI] [PubMed] [Google Scholar]
- 58.Reynolds P. N., Nicklin S. A., Kaliberova L., Boatman B. G., Grizzle W. E., Balyasnikova I. V., Baker A. H., Danilov S. M., Curiel D. T. Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat. Biotechnol. 2001;19:838–842. doi: 10.1038/nbt0901-838. [DOI] [PubMed] [Google Scholar]
- 59.Miller W. H., Brosnan M. J., Graham D., Nicol C. G., Morecroft I., Channon K. M., Danilov S. M., Reynolds P. N., Baker A. H., Dominiczak A. F. Targeting endothelial cells with adenovirus expressing nitric oxide synthase prevents elevation of blood pressure in stroke-prone spontaneously hypertensive rats. Mol. Ther. 2005;12:321–327. doi: 10.1016/j.ymthe.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 60.Work L. M., Büning H., Hunt E., Nicklin S. A., Denby L., Britton N., Leike K., Odenthal M., Drebber U., Hallek M., Baker A. H. Vascular bed-targeted in vivo gene delivery using tropism-modified adeno-associated viruses. Mol. Ther. 2006;13:683–693. doi: 10.1016/j.ymthe.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 61.Ma Z., Mi Z., Wilson A., Robbins P. D., Pitt B., Li S. Redirecting adenovirus to pulmonary endothelium by cationic liposomes. Gene Ther. 2002;9:176–182. doi: 10.1038/sj.gt.3301636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song Y. K., Liu F., Liu D. Enhanced gene expression in mouse lung by prolonging the retention time of intravenously injected plasmid DNA. Gene Ther. 1998;5:1531–1537. doi: 10.1038/sj.gt.3300770. [DOI] [PubMed] [Google Scholar]
- 63.Ma Z., Zhang J., Alber S., Dileo J., Stolz D., Watkins S. C., Huang L., Pitt B. R., Li S. Lipid-mediated delivery of oligonucleotide to pulmonary endothelium. Am. J. Resir Cell Mol. Biol. 2002;27:151–159. doi: 10.1165/ajrcmb.27.2.4653. [DOI] [PubMed] [Google Scholar]
- 64.Allen T. M. Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs. 1998;56:747–756. doi: 10.2165/00003495-199856050-00001. [DOI] [PubMed] [Google Scholar]
- 65.Hussein M. Pegylated liposomal doxorubicin, vincristine, and reduced-dose dexamethasone as first-line therapy for multiple myeloma. Clin. Lymphoma. 2003;1:S18–S22. doi: 10.3816/CLM.2003.s.004. [DOI] [PubMed] [Google Scholar]
- 66.Herbrecht R., Natarajan-Ame S., Nivoix Y., Letscher-Bru V. The lipid formulations of amphotericin B. Expert Opin. Pharmacother. 2003;4:1277–1287. doi: 10.1517/14656566.4.8.1277. [DOI] [PubMed] [Google Scholar]
- 67.Stuart D. D., Semple S. C., Allen T. M. High efficiency entrapment of antisense oligonucleotides in liposomes. Methods Enzymol. 2004;387:171–188. doi: 10.1016/S0076-6879(04)87011-3. [DOI] [PubMed] [Google Scholar]
- 68.Semple S. C., Klimuk S. K., Harasym T. O., Dos Santos N., Ansell S. M., Wong K. F., Mauer N., Stark H., Cullis P. R., Hope M. J., Scherrer P. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta. 2001;1510:152–166. doi: 10.1016/S0005-2736(00)00343-6. [DOI] [PubMed] [Google Scholar]
- 69.Wilson A., Zhou W., Champion H., Alber S., Tang Z.-L., Kennel S., Watkins S., Huang L., Pitt B. R., Li S. Targeted delivery of oligodeoxynucleotides to mouse lung endothelial cells in vitro and in vivo. Molec. Ther. 2005;12:510–518. doi: 10.1016/j.ymthe.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 70.Schiffrin E. L. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 71.Zhou W., Yuan X., Wilson A., Yang L., Mokotoff M., Pitt B. R., Li S. Efficient intracellular delivery of oligonucleotides formulated in folate receptor-targeted lipid vesicles. Bioconjug. Chem. 2002;13:1220–1225. doi: 10.1021/bc025569z. [DOI] [PubMed] [Google Scholar]
- 72.Yang L., Li J., Zhou W., Yuan X., Li S. Targeted delivery of antisense oligodeoxy-nucleotides to folate receptor-overexpressing tumor cells. J. Control. Release. 2004;95:321–331. doi: 10.1016/j.jconrel.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 73.Li W., Szoka F. C., Jr. Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 74.Rytting E., Nguyen J., Wang X., Kissel T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2008;5:629–639. doi: 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]