Abstract
Colchicine, an alkaloid found in extracts of the plants Colchicum autumnale and Gloriosa superb, is effective in the treatment of acute gout and dermatological conditions like leuko-cytoclastic vasculitis, psoriasis, and Sweet’s syndrome. Oral administration of colchicine is associated with gastrointestinal side effects and its accumulation in the body leads to bone marrow suppression. In the present study, an attempt has been made for development and in vitro and in vivo evaluation of elastic liposomal formulation for topical delivery of colchicine. The in vitro skin permeation study across rat skin found transdermal flux for different elastic liposomal formulations to range between 32.8 ± 1.2 and 44.4 ± 1.9 μg h−1 cm−2, which was approximately seven to 11 times higher than obtained using drug solution (4.3 ± 0.6 μg h−1 cm−2). The results of skin deposition study showed that elastic liposomal formulation provide 12.5-fold higher skin deposition as compared to drug solution of colchicine. Confocal laser scanning microscopy also revealed better accumulation and deeper penetration (up to 200 μm) of elastic liposomes than drug solution (up to 12 μm). The biological evaluation of various vesicular formulations and drug solution was carried out using monosodium urate-induced air pouch model. The results of anti-gout activity in rats showed better and sustained biological effects in 24 h measured in terms of exudate volume (63.1 ± 5.7% and 9.6 ± 0.5% reduction with elastic liposomes and drug solution, respectively), reduction in leukocyte count (74.2 ± 6.0% and 4.1 ± 0.3% reduction with elastic liposomes and drug solution, respectively), decrease in inflammatory cells accumulation, and collagen deposition with elastic liposomal formulation than drug solution. Hence, the present study reveals that elastic liposomal formulation of colchicine possesses greater potential to enhance skin accumulation, prolong drug release, and improve the site-specificity of colchicine.
Key words: biological activity, colchicine, dermal delivery, elastic liposomes, histopathology
INTRODUCTION
Gout is an inflammatory arthritis that is triggered by crystallization of uric acid within joints and is often associated with hyperuricemia. Moreover, acute gout is one of the most painful conditions experienced by humans (1). Various therapeutic approaches such as nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, uricosuric agents, and colchicine are available for the management of gout (2). The NSAIDs are first-line therapy in the management of acute attack of gout. However, their use is restricted in patients with renal inefficiency, gastrointestinal ulceration/bleeding, or heart failure (3). Moreover, some NSAIDs such as aspirin are reported to cause uric acid retention, thereby aggravating gout (4). Uricosoric drugs like allopurinol should not be started until the acute attack has settled for 2–3 weeks, as their use can prolong the acute attack or may trigger the further episodes (5).
Colchicine, an alkaloid found in extracts of the plants Colchicum autumnale and Gloriosa superb, is effective in the treatment of acute gout. The risk/benefit ratio of colchicine use is high. The gastrointestinal side effects, such as nausea, diarrhea, vomiting, and stomach upset, occur in 80% of patients with therapeutic doses of colchicine (6). Oral colchicine cannot be given for 1 week because its accumulation in the body leads to risk of bone marrow suppression (7). Accumulation of colchicine in patients with renal dysfunction is a risk factor for neuromyopathy (8). Colchicine can be administered intravenously; however, this route of administration is associated with potentially serious adverse effects such as tissue necrosis cytopenias, disseminated intravascular coagulation, and death (7).
Oral administration of colchicine has several dose-dependent side effects, which suggest the need for developing alternative dosage form that selectively deliver colchicine topically to affected joints. Colchicine has also been shown to have beneficial effects on different dermatological conditions, including leukocytoclastic vasculitis, psoriasis, and Sweet’s syndrome on topical administration, thereby suggesting that topical administration of colchicine could be beneficial in the treatment of gout along with increase its site-specificity.
Vesicular systems have been widely studied as vehicles for dermal and transdermal drug delivery (9). Their benefit in enhancing drug permeation is well established. However, the effectiveness of a vesicular system is strongly dependent on its physicochemical characteristics, in particular its thermodynamic state. Deformable vesicles like elastic liposomes have been found to be more effective in enhancing drug transport as compared to rigid vesicles like conventional liposomes for number of drug molecules like dexamethasone (10), diclofenac (11), zidovudine (12), lamivudine (13), propranolol (14), and rizatriptan (15). It was found that high elasticity of these vesicles could facilitate drug transport across the skin as compared to vesicles with rigid membranes.
In the literature, only few studies have been reported for sustained delivery of colchicine using microspheres and vesicular formulations as carrier system (16,17). However, all these attempts have been limited to in vitro characterization of formulation. In the present study, an attempt has been made for the formulation development and its in vivo characterization using well-known monosodium urate (MSU) induced air pouch model for sustained and site-specific delivery of colchicine.
MATERIALS AND METHODS
Materials
Colchicine was received as a gift sample from Cepham Pharmaceuticals Pvt. Ltd. Soya phosphatidylcholine (PC), Sephadex G-50, picrosirius red and 6-carboxyfluorescein (6-CF) were purchased from Sigma Chemicals USA. Span 60, Span 80, and cholesterol were purchased from Hi Media Ltd., India. Ethanol, acetonitrile, acetic acid, hematoxylin, eosin, and phosphomolybidic acid were procured from E. Merck, India. All reagents used in this study were of analytical grade.
Preparation of Formulations
The elastic liposomal formulation was prepared by conventional rotary evaporation sonication method (18). Briefly, phospholipid and surfactant were taken in a clean, dry round bottom flask. This lipid mixture was dissolved in chloroform/methanol mixture (2:1). The organic solvent was removed by rotary evaporation above the lipid transition temperature (Rotary Evaporator, Superfit, India). Final traces of solvent were removed under vacuum. The deposited lipid film was hydrated for 1 h with drug or fluorescence marker solution in ethanol (7% v/v) by rotating at 60 rpm at 40 ± 1.0°C. The resulting vesicles were swollen for 2 h at room temperature to get large multilamellar vesicles (LMLVs). To prepare smaller vesicles, LMLVs were probe-sonicated at 4°C for 20 min at 40 W (Probe Ultrasonicator, Imeco Ultrasonics, India). The sonicated vesicles were extruded through a sandwich of 100 and 200 nm polycarbonate membranes (Millipore, USA). The final lipid and drug concentrations in vesicular formulations were 5% w/v and 0.2% w/v, respectively.
The same method was used for preparing conventional liposomal and niosomal formulations that were used for comparison purpose.
Characterization of Elastic Liposomal Formulations
The vesicle size and distribution were determined by dynamic light scattering (DLS) method (CILAS, 1064L, France). For morphological characterization, transmission electron microscopic (TEM) studies using phosphotungastic acid (PTA) as a negative stain were performed (Moragagni 268D FEI, The Netherlands). A drop of the sample was placed on a carbon-coated copper grid to leave a thin film on the grid. Before the film dried on the grid, it was negatively stained with 1% PTA. A drop of the staining solution was added to the film, and the excess of the solution was drained off with a filter paper. The grid was allowed to thoroughly dry in air, and samples were viewed under a transmission electron microscope. Vesicles without sonication were also visualized using optical microscope (Olympus, DX31, Japan).
The entrapment efficiency was determined after separating unentrapped drug using Sephadex G-50 column. The eluted vesicles were lysed using Triton-X 100 (0.1% v/v) and subsequently analyzed for drug content. Elastic liposomal formulation (without sonication) was diluted five times with 0.9% NaCl solution, and the number of elastic liposomes per cubic millimeter was counted by optical microscopy using hemocytometer (10).
Elasticity Measurement
For the measurement of elasticity of vesicular membrane, elastic liposomal formulations were extruded at 2.5 bars through the polycarbonate filter membrane having a pore diameter of 60 to 200 nm using a stainless steel pressure holder for 25-mm diameter filters with 200-ml capacity barrel. The amount of vesicle suspension that was extruded during 5 min was measured, and the vesicle shape (by TEM) and size (by DLS) were monitored before and after filtration. The elasticity of vesicle membrane was calculated by using the following formula (19):
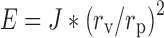 |
Where, E = elasticity of vesicle membrane; J = amount of suspension, which was extruded during 10 min; rv = vesicles size (after extrusion); and rp = pore size of the barrier
Skin Permeation Study
The in vitro skin permeation of colchicine from different formulations was studied using Franz glass diffusion cell maintained at 37 ± 1°C under non-occlusive conditions. The effective permeation area of the diffusion cell was 2.303 cm2. The receptor compartment contained 22.5 ml phosphate-buffered saline (PBS, 6.4) and was constantly stirred at 100 rpm. Excised albino abdomen rat skin was mounted between the donor and the receptor compartment. Elastic liposomal formulation (2.0 ml) was applied to the epidermal surface of skin. The samples (0.5 ml) were withdrawn through the sampling port of the diffusion cell at 1-, 2-, 4-, 6-, 12-, 16-, 20-, and 24-h time intervals and analyzed. An equal volume of fresh phosphate buffer maintained at 37 ± 1°C was replaced into the receptor compartment after each sampling.
At the end of the permeation experiments (6, 12, and 24 h), the surface of the skin was washed five times with 50% ethanol, then with water to remove excess drug from the surface. The washing protocol was verified and was found to remove more than 95% of the applied dose at zero time. The skin was then cut into small pieces. The tissue was further homogenized with 50% ethanol (10 ml) and left for 24 h at room temperature. After shaking for 5 min and centrifugation for 5 min at 3,000 rpm, the colchicine content in the upper phase was determined.
Vesicle-Skin Interaction Study
Scanning Electron Microscopy
Wistar rats, weighing 150–200 g were divided into four groups, each comprising of three rats. The first group served as control and received topical application of drug solution (1.0 mg/ml) prepared in PBS, pH 6.4. The second, third, and fourth groups received 1.0 ml of conventional liposomes, niosomes, and optimized elastic liposomal formulation, respectively. The formulations were applied non-occlusively to the abdomen side of the rat over an area of 1 cm2. The treated rats were caged and killed after 6 h of treatment. The skin was removed immediately and fixed at 4°C in Karnvosky’s fixative overnight, followed by 1% w/v osmium tetraoxide for 2 h, and finally in ruthenium tetraoxide 0.2% w/v and K3Fe(CN)6 0.25% w/v for 1 h. After fixation, the samples were dehydrated in a range of ethanolic solutions 70, 90, 95, and 100% v/v and coated with gold coater. The coated samples were visualized under scanning electron microscope (SEM, LEO43 SVP, Cambridge). All investigations were performed after approval of the Institutional Animal Ethics Committee of the Department of Pharmaceutical Sciences and Drug Research, Punjabi University, Patiala and in accordance with the disciplinary principles and guidelines of CPCSEA.
Confocal Laser Scanning Microscopy
Wistar rats, weighing 150–200 g, were divided into four groups each consisting of three animals. The first group served as control and received topical application of 1.0 ml of 0.16% w/v solution of marker 6-CF in PBS (pH 6.4). The second, third, and fourth groups received topical application of 1.0 ml of conventional liposomes, niosomes, and elastic liposomes loaded with 6-CF (0.16% w/v) as fluorescence marker, respectively. The formulations were applied topically on the abdomen of the rat at a marked area of 1 cm2. The animals were caged individually after application of formulation and were killed after 6 h of application. The skin was removed immediately, cut into pieces, and washed with PBS. The skin was blotted and fixed in Carny’s fluid (absolute alcohol/chloroform, 3:1) for 3 h and sectioned into the pieces of 1-mm2 size and evaluated for depth of penetration of 6-CF. The full skin thickness was optically scanned at different increments through the z-axis of a CLS microscope (Leica, DMIRE2, Germany). Optical excitations were carried out with a 489-nm Argon laser beam and fluorescence emission was detected above 515 nm for 6-CF.
In Vivo Evaluation
Evaluation of Anti-gout Activity
To perform biological evaluation of anti-gout activity of elastic liposomal formulation of colchicine, the MSU crystals were synthesized using established procedure (20). The crystals were suspended in PBS at a concentration of 10 mg/ml. The crystals were sonicated to obtain rod-shaped crystals of uniform length (5–25 μm) and sterilized by autoclaving at 121°C for 30 min. The subcutaneous air pouch model was used for the evaluation of anti-gout activity (21). Briefly, the rats were anesthetized, the dorsal area was shaved, and 10 ml sterile air was injected subcutaneously. Sterile air was re-injected in air pouch every 2 days to maintain pseudo-gout conditions. After 6 days, the rats were randomly divided into two groups. Group1 [GP1 (sham control)] composed nine animals that received 10 ml normal saline solution into subcutaneous air pouch. The group 2 (GP2) comprised of 45 animals that received 10 ml MSU (1 mg/ml) into subcutaneous air pouch. Furthermore, second group was again subdivided into five subgroups each containing nine rats. After 24 h of MSU administration, rats in the first subgroup [GP2a (control group)] received no treatment, and second subgroup (GP2b) rats were treated with elastic liposomal formulation (ELP20) on the air pouch. Rats in the third subgroup were treated by applying conventional liposomes (GP2c), the fourth subgroup received niosomal (GP2d) formulation, and the fifth subgroup was treated using drug solution (GP2e) on the air pouch. Rats (n = 3) from GP1 and subgroups of GP2 were killed at 6, 12, and 24 h, respectively, by spinal dislocation method, and various parameters were employed to check the extent of anti-gout activity.
Measurement of Exudate Volume of Air Pouch and Leukocytes Count
The pouch exudate was collected from all groups using glass syringe just before killing animals. The exudate volume was measured immediately after collection using graduated centrifuge tubes. Inflammatory exudate harvested from each animal was placed into heparinized saline. An aliquot of the diluted exudate was used to count leukocytes.
Histopathological Studies
The skin from air pouch was excised and immediately immersed in 10% buffered formalin, dehydrated in graded concentrations of ethanol, immersed in xylene, and then embedded in paraffin. The 5-µm thick sections of skin were cut using microtome and were mounted on slide using commercial Baker’s mounting fluid. The paraffin wax was removed by warming the slide gently, until the wax melted, and then was washed with xylene followed by washings with absolute alcohol and water. The sections were stained with hematoxylin–eosin and picrosirius red stains to determine gross histopathology and collagen deposition, respectively. The slides were analyzed at 100-fold magnification using optical microscope.
Statistical Analysis
Data is expressed as the mean ± SD of obtained results. The statistical analysis of data was performed using analysis of variance (Graphpad, Version 2.01, San Diego, CA, USA). A value of p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Table I shows the composition of different formulations of elastic liposomes, conventional liposomes, and niosomes. Different batches of elastic liposomes were prepared with Span 80 and PC using conventional rotary evaporation sonication method (18). Span 80 was selected as an edge activator because it is biocompatible, pharmaceutically acceptable (22), and provide optimum elasticity to vesicle membrane as optimized in our previous study (11). PC was used as bilayer-forming agent. Liposomes and niosomes were used as control and were prepared by optimized composition as reported (23,24). These elastic liposomal formulations were colloidal dispersions having average diameter in range of 100–200 nm.
Table I.
Composition of Different Vesicular Formulations
| S. no. | Formulation code | PC (mg) | Span 80 (mg) | Span 60 (mg) | Cholesterol | Drug (mg) |
|---|---|---|---|---|---|---|
| 1 | ELP10 | 85 | 15 | – | – | 10 |
| 2 | ELP15 | 85 | 15 | – | – | 15 |
| 3 | ELP20 | 85 | 15 | – | – | 20 |
| 4 | ELP25 | 85 | 15 | – | – | 25 |
| 5 | Liposomes | 70 | – | – | 30 | 20 |
| 6 | Niosomes | 10 | – | 60 | 30 | 20 |
PC phosphatidylcholine, ELP elastic liposomal formulation
Characteristics of Elastic Liposomes of Colchicine
Different characteristic parameters of vesicular formulations are summarized in Table II. Electron microscopy was used for the initial characterization of the formulations, where samples were negatively stained by using PTA and the elastic liposomes appeared as spherical multilamellar vesicles (photomicrograph not shown). The size of the elastic liposomes was measured for formulations extruded through polycarbonate membranes, and the results are expressed as the average vesicle size for vesicles. These elastic liposomal formulations were colloidal dispersions having average diameter in range of 100–200 nm.
Table II.
Characterization of Colchicines Loaded Formulations
| Formulation code | Shape | Particle size | Entrapment efficiency (%) | Elasticity | No. of vesicles per cubic millimeter × 1,000 |
|---|---|---|---|---|---|
| ELP10 | Multilammelar vesicular | 132 ± 11 | 64.2 ± 1.3 | 58.2 ± 3.1 | 49 ± 2.4 |
| ELP15 | Multilamellar vesicular | 134 ± 12 | 63.5 ± 2.4 | 56.8 ± 2.9 | 53 ± 1.4 |
| ELP20 | Multilamellar vesicular | 135 ± 11 | 66.3 ± 2.2 | 56.9 ± 2.8 | 51 ± 2.1 |
| ELP25 | Multilamellar vesicular | 178 ± 15 | 45.2 ± 1.5 | 60.0 ± 2.8 | 47 ± 2.5 |
| Liposomes | Multilamellar vesicular | 112 ± 13 | 33.4 ± 2.8 | 2.1 ± 0.6 | 46 ± 1.2 |
| Niosomes | Multilamellar vesicular | 155 ± 16 | 31.6 ± 1.4 | 4.2 ± 0.6 | 50 ± 1.7 |
Value represented as mean ± SD (n = 3)
To optimize the maximum amount of drug that could be loaded in elastic liposomal formulation, increasing amounts of colchicine ranging from 10 to 25 mg was added in the vesicular formulations (Table II). Maximum amount of drug that could be incorporated in formulations was 20 mg. The entrapment efficiency data shows that elastic liposomal formulation prepared with 20 mg drug have maximum entrapment (66.3 ± 2.2%) of drug. The skin permeation studies also showed the maximum permeation (53.5 ± 2.0% cumulative amount of drug permeated) with elastic liposomal formulation prepared with 20 mg drug (ELP20). Therefore, all the batches of elastic liposomal formulations for further studies were prepared using 20 mg colchicine; the drug loading in the case of liposomes and niosomes was also done using 20 mg of the drug to meet comparison purposes.
The entrapment efficiency of colchicine in elastic liposomes was calculated as percentage of total drug entrapped into the vesicular formulation and determined using Sephadex G-50 mini-column centrifugation method. Results are summarized in Table II. The maximum entrapment efficiency obtained was 66.3 ± 2.2% for elastic liposomes (ELP20), 33.4 ± 2.8% for liposomes, and 31.6 ± 1.4% for niosome formulation, respectively. Colchicine is a highly hydrophilic drug, and its entrapment efficiency has been reported to be only 30% in conventional liposomal formulation (17). The higher entrapment efficiency in elastic liposomes can be attributed to the presence of higher lipid content and surfactant Span 80 in these vesicles. Lipid composition of elastic liposomes was higher in comparison to that of conventional liposomes, and this may result in higher entrapment efficiency (Table I). Similarly, higher entrapment efficiency of protein tetanus toxoid in elastic liposomes in comparison to liposomes was reported by Gupta el al. (25). It was also reported that Span forms hydrogen bond with the amido group of colchicines (17). This might also lead to better retention in elastic liposomes.
Skin Permeation and Deposition Study
The in vitro skin permeation studies of different formulations of colchicine across excised rat abdominal skin were conducted using Franz-diffusion cell, and results are summarized in Table III. The value of transdermal flux for different elastic liposomal formulations was observed to range between 32.8 ± 1.2 to 44.4 ± 1.9 µg h−1 cm−2. The maximum flux was shown by ELP20 formulation (44.4 ± 1.9 µg h−1 cm−2). The flux obtained by elastic liposomal formulation (ELP20) was about 10.2-fold higher than that obtained from drug solution and near about twofold higher than that obtained with niosomal and liposomal formulation. The elastic liposomes showed maximum permeation, whereas liposomes and niosomes relatively showed less skin permeation possibly because of cholesterol affecting their membrane properties. Incorporation of cholesterol in gel state bilayer can induce a continuous and permanent transition to an ordered crystalline state that may be the possible reason for poor skin permeation in liposomes and niosomes. The elastic characteristics of vesicle membrane and permeation enhancement effects of elastic liposomes support their better skin permeation potential (12,18). Elasticity measurement studies support this hypothesis. Elastic liposomal formulation showed significantly (p < 0.05) higher elasticity of vesicle membrane in comparison to conventional liposomes and niosomes (Table II).
Table III.
Transdermal Permeation Parameters of Different Colchicine Formulations across Rat Skin
| S. no. | Formulation code | Jss1 (μg cm−2 h−1) | Amount of drug deposited (μg) | ER2 | ER3 |
|---|---|---|---|---|---|
| 1 | ELP10 | 32.8 ± 1.2 | 212 ± 5.8 | 4.65 | 7.6 |
| 2 | ELP15 | 34.9 ± 1.5 | 235 ± 3.2 | 5.14 | 8.0 |
| 3 | ELP20 | 44.4 ± 1.9 | 383 ± 2.4 | 8.38 | 10.2 |
| 4 | ELP25 | 35.9 ± 1.8 | 246 ± 2.8 | 5.39 | 8.3 |
| 5 | Liposomes | 28.2 ± 2.1 | 198 ± 2.7 | 4.34 | 6.5 |
| 6 | Niosomes | 25.9 ± 2.3 | 159 ± 2.8 | 3.47 | 5.5 |
| 7 | Drug solution | 4.3 ± 0.6 | 45.7 ± 1.1 | – | – |
Value represented as mean ± SD (n = 3)
Jss 1 transdermal flux, calculated from the slop of Cartestan plot of cumulative amount of drug present in receptor compartment versus time;
ER 2 enhancement ratio, it is the ratio of amount of drug deposited from formulation to drug solution; ER 3 enhancement ratio, it is the ratio of transdermal flux from formulation to drug solution
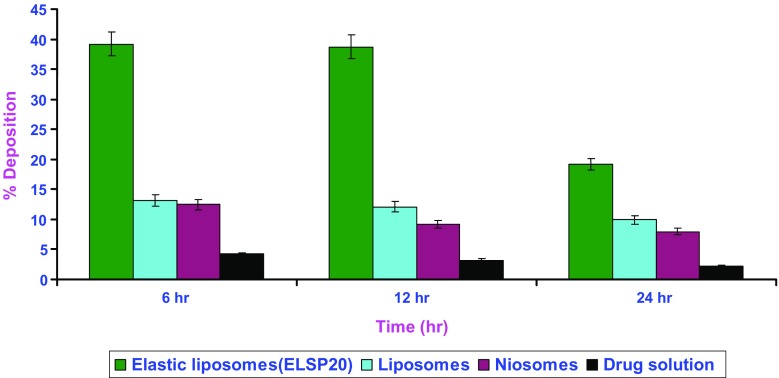
Skin retention studies were carried out with the objective of determining the depot effect of elastic liposomes in the deeper layers of skin. For the effective management of inflammation in gout and therapeutic effect in other dermatological conditions like psoriasis and leukocytoclastic vasculitis, dosage form should accumulate the drug to mainly in the deeper layer of skin. Figure 1 compares the percentage skin deposition of colchicine after topical application of elastic liposomal formulation, liposomes, niosomes, and drug solution. The amount of drug deposited in the skin was found to be ten times higher after 6, 12, and 24 h administration of elastic liposomes as compared to the drug solution, three- and fivefold higher than liposomal and niosomal formulations. The observed better skin accumulation of elastic liposomes could be attributed to the difference in the mechanism of drug transport across the skin from elastic liposomes and drug solution (12,15). In contrast to colchicine molecule, elastic liposome is too large to enter into the cutaneous blood circulation; they directly bypass the cutaneous capillary bed, cross over to the subcutaneous capillary bed and then to the subcutaneous tissue where they act as depot and sustain the drug release. As a consequence, the drug concentration is increased locally in the effected area, and the other body organs are spared from dose-related side effects of colchicine. The reason for high permeation and deposition potential of elastic liposomal formulations is their deformable nature and depot-forming ability in deeper layers of skin.
Fig. 1.
Skin deposition of colchicine from different formulations as function of time. All values shown as mean ± standard deviation (n = 3)
The low penetration of colchicine after administration as drug solution (19.2-fold less in comparison to optimized elastic liposomes) suggested that the drug has difficulties in penetrating across the skin, probably due to its hydrophilic nature. Skin retention of drug was also observed to be low. Since the amount of drug in the tissues increased as drug percutaneous absorption increased, it can be said that the accumulation of the drug in the skin is directly correlated with the flux values obtained in each case. Thus, the higher the flux values obtained, the more is the retention of the drug in the skin. The results of the present study are well correlated with earlier reports, in which the skin accumulation of several NSAIDs has been well correlated to their flux values across the epidermis (26). El Maghraby et al. (22) also reported similar correlation for the delivery of oestradiol using similar elastic liposomal formulation.
Vesicle Skin Interaction Study
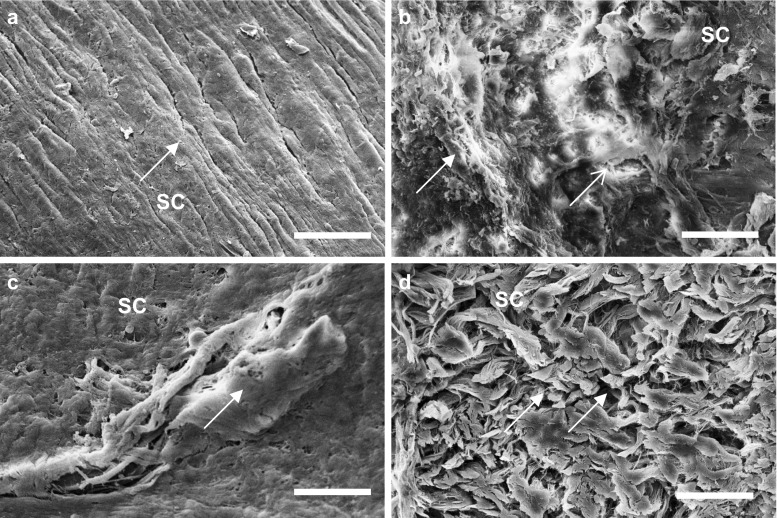
Results of the skin permeation study showed that elastic liposomal formulation exhibited nearly tenfold better skin permeation as compared to the drug solution. To further verify the better skin permeation and deposition potential of elastic liposomal formulation, SEM and confocal laser scanning microscopy (CLSM) studies of skin sections were carried out. Figure 2a–d shows the SEM photomicrograph of rat skin treated with PBS (pH 6.4) containing drug considered as control, liposomal, niosomal, and elastic liposomal formulation. In rat skin incubated with PBS containing drug, there was absence of intracellular vesicular structures in stratum corneum (Fig. 2a). However, elastic liposomes were visualized on the surface of stratum corneum. The vesicular suspension formed networks and stacks of lipid bi-layers at the interface of the stratum corneum. Intracellular vesicular structures were observed in superficial layers of the stratum corneum, and their appearance might be explained by desquamating corneocytes with a leaky membrane, through which elastic liposomes penetrate (Fig. 2d). These vesicular suspensions are more flexible and can easily pass through skin pores. In comparison to skin treated with liposomes (Fig. 2b) and niosomes (Fig. 2c), surface accumulation of lipids occurred due to fusion with skin surface that is probably responsible for their poor skin permeation.
Fig. 2.
Scanning electron microscopy micrograph of viable rat skin treated with phosphate-buffered solution of drug (a) and drug-loaded liposomes (b), niosomes (c), and optimized elastic liposomes [ELP20] (d)] after 6 h of treatment. SC stratum corneum. Arrow indicates the effect of treatment on surface morphology of skin. IL inter-lamellar space. Scale bar = 100 nm. Magnification, ×15,000
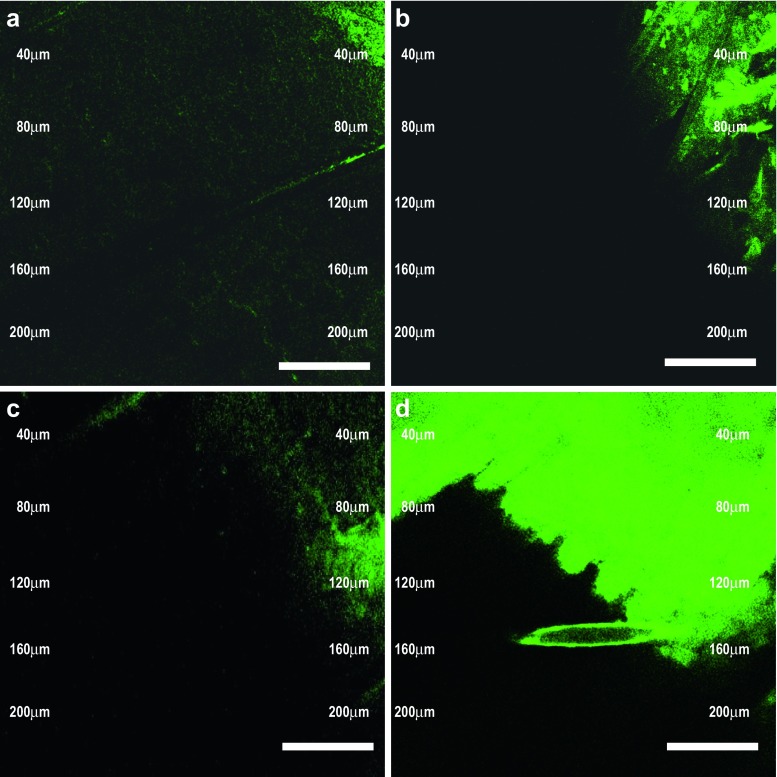
The superior skin penetration and deposition potential of developed vesicular carrier was further confirmed by CLSM studies. The untreated rat skin does not show any fluorescence. Figure 3a–d shows the CLSM photomicrograph of rat skin treated with PBS solution of marker, liposomal, niosomal, and elastic liposomal formulation loaded with 6-CF. The florescence markers 6-CF do not normally get into deeper layer of skin, when applied in the form of aqueous solution (Fig. 3a) (10). However, this dye reaches the deeper layer of skin in significantly higher quantity when applied with the elastic liposomal formulations (Fig. 3d). Elastic liposomal formulation-treated skin showed fivefold deeper skin penetration (up to 200 μm) of 6-CF as compared to liposomes (up to 40 μm) and niosomes (up to 35 μm) loaded with 6-CF and 13-fold higher skin penetration as compared to PBS solution of marker (up to 15 μm). This showed the better skin penetration potential and depot-forming properties of investigated elastic liposomal formulations. These results are well correlated with our previous study, in which we have evaluated the better skin permeation ability using fluorescence microscopy (10) and TEM (12) and found better skin permeation profile of elastic liposomes that is necessary for the effective treatment of gout, as higher drug accumulation is required in deeper layers of skin (4). The CLSM study indicated that the elastic liposomal formulations are able to deliver the drug to deeper layer of skin.
Fig. 3.
CSLM photomicrograph of viable rat skin treated with 6-CF marker solution in PBS (a) and 6-CF-loaded liposomes (b), niosomes (c), and optimized elastic liposomes [ELP20] (d)] after 6 h of treatment. Scale bar = 100 nm. Magnification, ×10,000
Evaluation of Biological Anti-gout Activity
In vivo performance of different colchicine formulation was evaluated using MSU-induced rat air pouch model. This model is well established to evaluate activity of anti-gout agents (27). Edema is a characteristic feature of inflammation, which is measured in terms of exudate volume in the present study. The exudate collected was measured before rats were killed at 6, 12, and 24 h, respectively, in various groups employed in the present study (Table IV), and leukocytes quantification was done as summarized in Table V. There was continuous increase in exudate volume in MSU-treated control group (GP2a, 3.0 ± 0.2, 5.1 ± 0.6, and 6.5 ± 0.9 ml) measured at 6, 12, and 24 h than the saline-treated sham group (GP1, 2.0 ± 0.1, 2.1 ± 0.2, and 2.0 ± 0.2 ml). Moreover, the exudate collected in the control group (GP2a) was observed to be turbid than the exudates collected from the sham control group 1 (GP1), which indicates the enhanced inflammatory cell infiltration. Effect of different colchicine formulations on percentage reduction in exudates volume and leukocytes count was observed as compared to sham control group (GP1). Colchicine formulations were applied after 24 h administration of MSU, as pseudo-gout conditions were fully developed at this time, evidenced by exudate volume and leukocyte count measurements. The elastic-liposome-treated group (GP2b) showed significant (p < 0.05) higher reduction in exudate volume as compared to colchicines-loaded liposomes, niosomes, and drug solution after 6, 12, and 24 h of treatment (Table V). The result showed nearly fivefold higher reduction in exudate volume as compared to drug-solution-treated group. The better skin penetration and depot-forming property of elastic liposomes may account for their better activity than other formulations.
Table IV.
In Vivo Study Frame for Rat Air Pouch Model for Evaluation of Anti-gout Activity
| S. no. | Group | Normal saline | MSU (1 mg/ ml) solution | Treatment |
|---|---|---|---|---|
| 1 | GP1 (sham group) | 10 ml | NO | NO |
| 2 | GP2a (control group) | NO | 10 ml | NO |
| 3 | GP2b (treated group) | NO | 10 ml | ELP20 |
| 4 | GP2c (treated group) | NO | 10 ml | Liposomes |
| 3 | GP2d (treated group) | NO | 10 ml | Niosomes |
| 3 | GP2e (treated group) | NO | 10 ml | Drug solution |
Table V.
Evaluation of Anti-gout Activity
| Group | % Reduction in volume of exudatea | % Reduction in leukocyte counta | ||||
|---|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 6 h | 12 h | 24 h | |
| Gp2a, control group | – | – | – | – | – | – |
| Gp2b (ELP20-treated group) | 30.0 ± 1.5 | 41.2 ± 3.6 | 63.1 ± 5.7 | 29.1 ± 2.2 | 59.8 ± 3.2 | 74.2 ± 6.0 |
| Gp2c (liposomes-treated group) | 13.3 ± 0.5 | 33.3 ± 2.1 | 26.2 ± 1.8 | 13.9 ± 1.1 | 44.7 ± 2.8 | 30.1 ± 2.1 |
| Gp2d (niosomes-treated group) | 16.7 ± 0.8 | 21.6 ± 1.2 | 20.0 ± 1.3 | 10.0 ± 0.3 | 39.4 ± 1.8 | 24.1 ± 1.2 |
| Gp2e (drug-solution-treated group) | 7.1 ± 0.4 | 17.6 ± 0.8 | 9.6 ± 0.5 | 6.9 ± 0.3 | 14.1 ± 1.8 | 4.1 ± 0.3 |
Value represented as mean ± SD (n = 3)
aValues indicate the percentage reduction of volume of exudates and leukocyte count compared to control group (V control − V treated)/V control × 100)
Results of leukocytes count are indicator of inflammation in joint spaces, and their increase is proportional to severity of inflammatory condition. The exudate was collected, and leukocytes were quantified before rats were killed at 6, 12, and 24 h, respectively, in various groups employed in the present study. There was continuous increase in leukocytes count in MSU-treated group (GP2a, 230 ± 12, 284 ± 14, and 302 ± 15 cells/mm3) quantified at 6, 12, and 24 h than saline-treated sham group (GP1, 140 ± 8, 129 ± 7, 131 ± 9 mean of cell/mm3), which indicates the enhanced inflammatory cell infiltration and development of gout conditions in MSU-treated group. The effect of different colchicine formulations on leukocyte count in exudates was observed and compared with the control group (GP2a) in various treatment groups employed in present study (Table V). The elastic-liposome-treated group (GP2b) reduced leukocyte count 4.2 times higher as compared to drug-solution-treated group (GP2eb). In comparison, the group treated with conventional liposomes showed only twofold and niosomal treatment observed 1.5-fold reductions in leukocyte count after 6 h. Similar better reduction in leukocyte count was observed after 12 h of administration. The reduction in leukocyte count was sustained to 24 h, and 18-fold higher inhibitions were observed in comparison to drug solution (Table V).
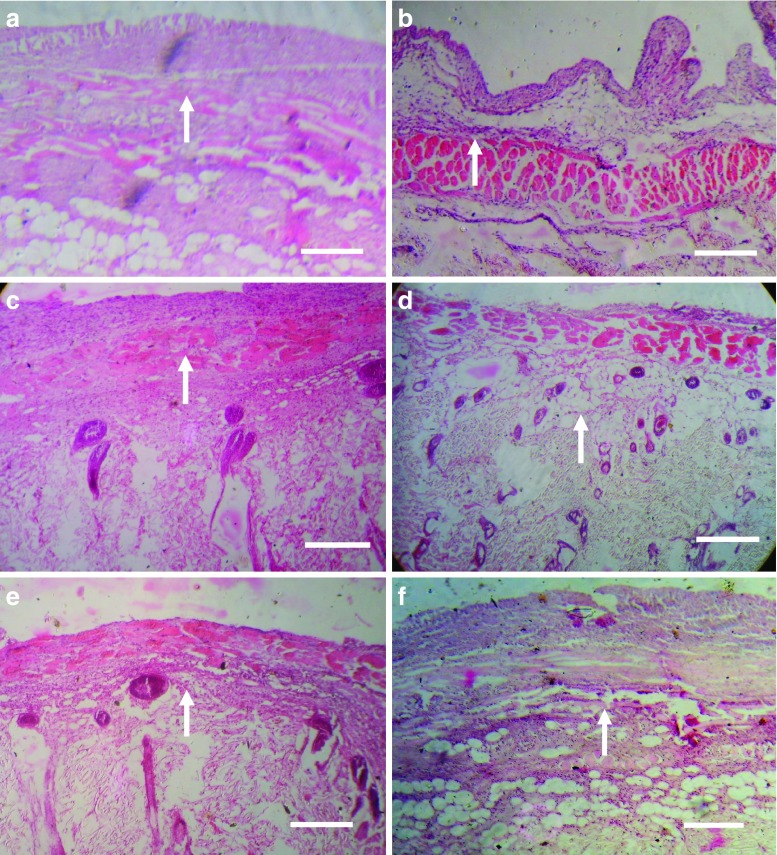
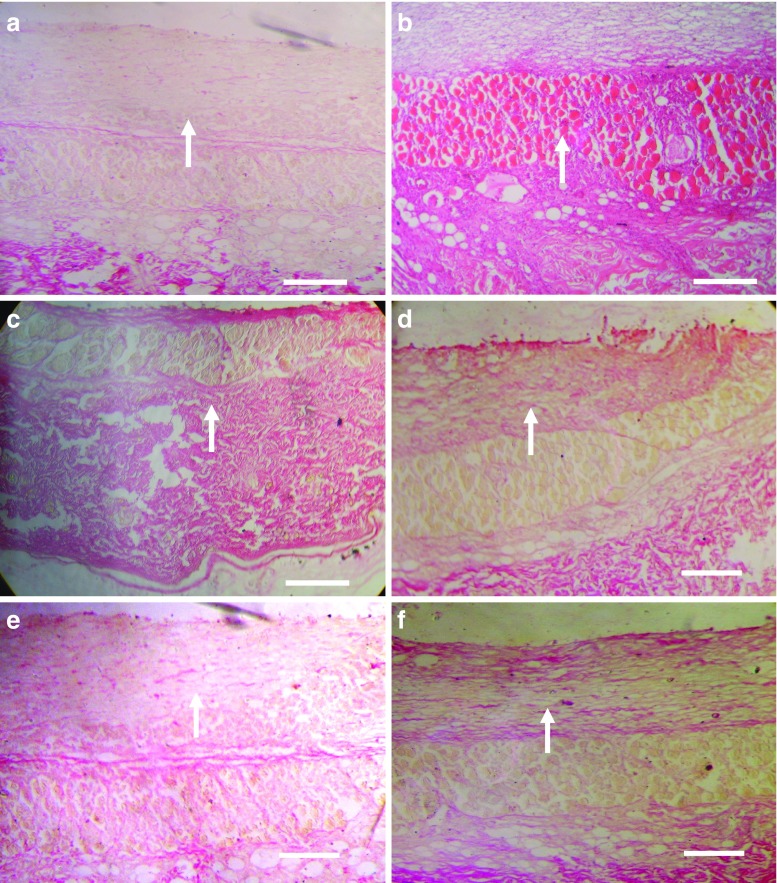
Better anti-gout activity of developed formulation was further confirmed by the staining of air pouch membrane with eosin–hematoxylin using established procedure for checking extent of infiltration of inflammatory cells (28). Figure 4a–f shows the eosin–hematoxylin-stained photomicrograph of saline-treated sham control group, control group, drug solution, conventional liposomes, niosomes, and elastic-liposome-treated group, respectively. When we compare the effects of different formulations, the MSU-treated control group (GP2a, Fig. 4b) shows high level of infiltrating cells. The level of infiltrating inflammatory cells was low in elastic-liposome-treated group (Fig. 4f) as compared to liposomes (Fig. 4d), niosomes (Fig. 4e), and drug solution (Fig. 4c) treated groups. Eosin–hematoxylin-stained air pouch membrane injected with MSU crystals alone showed the intense histological evidence of inflammation and had large numbers of lining cells, infiltrating neutrophil, and vessels (Fig. 4b). Elastic-liposome-treated group (Fig. 4f) showed significant decrease in lining cells, infiltrating neutrophil, and vessels in comparison to control MSU-treated group. The extent of treatment on air pouch membrane of different colchicines formulation was found to be elastic liposomes > liposomes > niosomes > drug solution. Elastic-liposome-treated pouche membranes were the only ones to have a significant decrease in lining cells and interstitial inflammation. In contrast, the group treated with liposomes, niosomes, and drug solution had areas with significantly higher number of inflammatory cells in comparison to elastic-liposome-treated group.
Fig. 4.
Eosin–hematoxylin-stained photomicrograph of skin of rat pouch model of sham control (a), control group treated with phosphate-buffered saline (b), drug solution (c), liposomes (d), niosomes (e), and optimized elastic liposomes (ELP20) (f) after 6 h of treatment. Arrow indicates presence of inflammatory cells. Scale bar = 500 μm. Magnification, ×100
Collagen deposition is also an important indicator for the development of fibrosis associated with gout. An increase in collagen deposition reflects the severity of the disease. In the present study, collagen-specific picrosirius red staining was employed to evaluate reduction in extent of fibrosis using different colchicine formulations using established procedure (29). Figure 5a–f shows the picrosirius red-stained photomicrograph of rat pouch skin of saline-treated sham control group, control group, drug solution, conventional liposomes, niosomes, and elastic-liposomes-treated group, respectively. While studying the deposition of collagen in response to MSU in rat skin, the elastic liposome treatment group (GP2b) showed better effect than liposomes, niosomes, and drug-solution-treated group to decrease the deposition of collagen after 24 h. This study further confirmed the better in vivo performance of developed elastic liposomal formulation for sustained and better effect for the management of gout.
Fig. 5.
Picrosirius red-stained photomicrograph of skin of rat pouch model of sham control (a), control group treated with phosphate-buffered saline (b), drug solution (c), liposomes (d), niosomes (e), and optimized elastic liposomes (ELP20) (f) after 6 h of treatment. Arrow indicates collagen deposition. Scale bar = 500 μm. Magnification, ×100
Colchicine has been used for long in treatment of acute gouty arthritis, though its use is associated with several side effects such as gastrointestinal abnormalities, renal dysfunction, neuropathy, watery or bloody stool, and bone marrow suppression. The results of the present study suggest that topical drug delivery systems especially elastic liposomes are better option for sustained delivery of colchicine for the management of gouty arthritis and other dermatological conditions. Elastic liposomes are known for the reduction in free drug movement in blood and increased drug accumulation in deeper layer of skin (10,18). Colchicine elastic liposomal formulation would decrease the free movement of drug and increase its accumulation at target site, resulting in reduction of dose-dependent side effects. In the present study, results of skin deposition and CLSM study revealed the skin deposition potential of elastic liposomes. Our previous study using elastic liposomal formulations of diclofenac (11) and rizatriptan (15) also indicates the increase in skin deposition and reduction in systemic absorption of these molecules. Colchicine medication is inexpensive than most immunosuppressive agents; however, present therapy associated with toxic effects limited its use as first line therapy in different dermatological conditions. Developed topical formulation will provide a better alternative for delivering this drug in more efficient manner.
CONCLUSION
Results of the present study revealed that elastic liposomal formulation has great potential as an effective and safe way to administer colchicine as an anti-gout agent. The results of skin permeation, deposition, and CLSM studies showed the depot-forming ability of elastic liposomal formulation that shall be beneficial to localize the colchicine to diseased tissue and minimize its systemic side effects. The measurement of anti-gout activity in rats showed many-fold increase in biological activity measured in terms of percentage reduction in exudate volume, leukocytes count, and collagen deposition. Hence, the present study reveals that elastic liposomal formulation of colchicine possesses greater potential to enhance skin accumulation, prolong drug release, and improve the site specificity of colchicine.
ACKNOWLEDGEMENTS
Authors are grateful to Cepham Pharmaceuticals Pvt. Ltd., India for providing colchicine as a gift sample. The Director, Electron Microscopy Section, AIIMS, New Delhi, India is acknowledged for providing the facilities for SEM, TEM, and confocal microscopic studies. The authors acknowledge the University Grants Commission, New Delhi for providing financial assistance [Sanction no. 32-141/2006(SR)].
References
- 1.Terkeltaub R. Gout in 2006: the perfect storm. Bull. NYU. Hosp. Jt. Dis. 2006;64:82–86. [PubMed] [Google Scholar]
- 2.Choi H. K., Atkinson K., Karlson E. W., Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch. Intern. Med. 2005;165:742–748. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 3.Segasothy M., Chin G. L., Sia K. K., Zulfiqar A., Samad S. A. Chronic nephrotoxicity of anti-inflammatory drugs used in the treatment of arthritis. Rheumatology. 1995;34:162–165. doi: 10.1093/rheumatology/34.2.162. [DOI] [PubMed] [Google Scholar]
- 4.Kim K. Y., Schumacher H. R., Hunsche E., Pol D., Wertheimer A. I., Kong S. X. A literature review of the epidemiology and treatment of acute gout. Clin. Ther. 2003;25:1593–1617. doi: 10.1016/S0149-2918(03)80158-3. [DOI] [PubMed] [Google Scholar]
- 5.Nuki G. Gout. Medicine. 2006;34:417–423. doi: 10.1053/j.mpmed.2006.07.012. [DOI] [Google Scholar]
- 6.Harris M. D., Siegel L. B., Alloway J. A. Gout and hyperuricemia. Am. Fam. Phys. 1999;59:925–934. [PubMed] [Google Scholar]
- 7.Evans T. I., Wheeler M. T., Small R. E., Breitbach S. A., Sanders K. M., Roberts W. N. A comprehensive investigation of inpatient intravenous colchicine use shows more education is needed. J. Rheumatol. 1996;23:143–148. [PubMed] [Google Scholar]
- 8.Kuncl R. W., Duncan G., Watson D., Alderson K., Rogawski M. A., Peper M. Colchicine myopathy and neuropathy. N. Engl. J. Med. 1987;316:1562–1568. doi: 10.1056/NEJM198706183162502. [DOI] [PubMed] [Google Scholar]
- 9.Honeywell-Nguyen P. L., Bouwstra J. A. Vesicles as a tool for transdermal and dermal delivery. Drug Discov. Today. 2005;2:67–74. doi: 10.1016/j.ddtec.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Jain S., Jain P., Umamaheswari R. B., Jain N. K. Transfersomes—a novel vesicular carrier for enhanced transdermal delivery: Development, characterization and performance evaluation. Drug Devel. Ind. Pharm. 2003;29:1013–1026. doi: 10.1081/DDC-120025458. [DOI] [PubMed] [Google Scholar]
- 11.Jain S., Jain N., Bhadra D., Tiwary A. K., Jain N. K. Transdermal delivery of an analgesic agent using elastic liposomes: preparation, characterization and performance evaluation. Curr. Drug Deliv. 2005;2:1–11. doi: 10.2174/1567201054368020. [DOI] [PubMed] [Google Scholar]
- 12.Jain S., Tiwary A. K., Jain N. K. Sustained and targeted delivery of an anti-HIV agent using elastic liposomal formulation: mechanism of action. Curr. Drug Deliv. 2006;3:157–166. doi: 10.2174/156720106776359221. [DOI] [PubMed] [Google Scholar]
- 13.Jain S., Tiwary A. K., Sapra B., Jain N. K. Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS Pharm. Sci. Tech. 2007;8:E1–E9. doi: 10.1208/pt0804111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra D., Garg M., Dubey V., Jain S., Jain N. K. Elastic liposomes mediated transdermal delivery of an anti-hypertensive agent: Propranolol hydrochloride. J. Pharm. Sci. 2007;96:145–155. doi: 10.1002/jps.20737. [DOI] [PubMed] [Google Scholar]
- 15.Garg T., Jain S., Singh H. P., Sharma A., Tiwary A. K. Elastic liposomal formulation for sustained delivery of anti-migraine drug: in vitro characterization and biological evaluation. Drug Devel. Ind. Pharm. 2008;34:1100–1110. doi: 10.1080/03639040801965079. [DOI] [PubMed] [Google Scholar]
- 16.Das G. S., Rao G. H. R., Wilson R. F., Chandy T. Colchicine encapsulation within poly(ethylene glycol)-coated poly(lactic acid)/poly(ɛ-caprolactone) microspheres-controlled release studies. Drug. Deliv. 2000;7:129–138. doi: 10.1080/10717540050120160. [DOI] [PubMed] [Google Scholar]
- 17.Hao Y., Zhao F., Li N., Yang Y., Li K. Studies on a high encapsulation of colchicine by a noisome system. Int. J. Pharm. 2002;244:73–80. doi: 10.1016/S0378-5173(02)00301-0. [DOI] [PubMed] [Google Scholar]
- 18.Cevc G., Blume G., Schatzlein A. Transfersomes mediated transepidermal delivery improves the regio-specificity and biological activity of corticosteroids in vivo. J. Control. Release. 1997;45:211–226. doi: 10.1016/S0168-3659(96)01566-0. [DOI] [Google Scholar]
- 19.van den Bergh B. A. I., Wertz P. W., Junginger H. E., Bouwstra J. A. Elasticity of vesicles assessed by electron spin resonance, electron microscopy and extrusion measurements. Int. J. Pharm. 2001;217:13–24. doi: 10.1016/S0378-5173(01)00576-2. [DOI] [PubMed] [Google Scholar]
- 20.Murakami Y., Akahoshi T., Kawai S., Inoue M., Kitasato H. Anti-inflammatory effect of retrovirally transfected interleukin-10 on monosodium urate monohydrate crystal-induced acute inflammation in murine air pouches. Arthritis Rheum. 2002;46:2504–2513. doi: 10.1002/art.10468. [DOI] [PubMed] [Google Scholar]
- 21.Edwards J. C. W., Sedgwick A. D., Willoughby D. A. The formation of a structure with the features of synovial lining by subcutaneous injection of air: An in vivo tissue culture system. J. Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- 22.El Maghraby G. M. M., Williams A. C., Barry B. W. Skin delivery from ultradeformable liposomes: refinement of surfactant concentration. J. Pharm. Pharmacol. 1999;51:1123–1134. doi: 10.1211/0022357991776813. [DOI] [PubMed] [Google Scholar]
- 23.Bangham D., Standish M. M., Watkins J. C. Diffusion of univalent ion across the lamellae of swollen phospholipids. J. Mol. Biol. 1965;13:238–252. doi: 10.1016/S0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 24.Liu T., Guo R., Hua W., Qiu J. Structure behaviors of hemoglobin in PEG 6000/Tween 80/Span 80/H2O niosome system. Colloids Surf. A Physicochem. Eng. Aspects. 2007;293:255–261. doi: 10.1016/j.colsurfa.2006.07.053. [DOI] [Google Scholar]
- 25.Gupta P. N., Mishra V., Rawat A., Dubey P., Mahor S., Jain S., Chatterji D. P., Vyas S. P. Non-invasive vaccine delivery in tranfersomes, niosomes and liposomes: a comparative study. Int. J. Pharm. 2005;293:73–82. doi: 10.1016/j.ijpharm.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Singh P., Roberts M. S. Skin permeability and local tissue concentration of nonsteroidal anti-inflammatory drugs after topical application. J. Pharmacol. Exp. Ther. 1994;268:144–151. [PubMed] [Google Scholar]
- 27.Schiltz C., Lioté F., Prudhommeaux F., Meunier A., Champy R., Callebert J., Bardin T. Monosodium urate monohydrate crystal-induced inflammation in vivo: quantitative histomorphometric analysis of cellular events. Arthritis Rheum. 2002;46:1643–1650. doi: 10.1002/art.10326. [DOI] [PubMed] [Google Scholar]
- 28.Clayden E. C. Practical section cutting and staining. London: Churchill Livingstone; 1971. [Google Scholar]
- 29.Dolber P. C., Spach M. S. Picrosirius red staining of cardiac muscle following phosphomolybdic acid treatment. Stain. Technol. 1987;62:23–26. doi: 10.3109/10520298709107961. [DOI] [PubMed] [Google Scholar]