Abstract
The reduced injection frequency and more nearly constant serum concentrations afforded by sustained release devices have been exploited for the chronic delivery of several therapeutic peptides via poly(lactide-co-glycolide) (PLG) microspheres. The clinical success of these formulations has motivated the exploration of similar depot systems for chronic protein delivery; however, this application has not been fully realized in practice. Problems with the delivery of unmodified proteins in PLG depot systems include high initial “burst” release and irreversible adsorption of protein to the biodegradable polymer. Further, protein activity may be lost due to the damaging effects of protein-interface and protein-surface interactions that occur during both microsphere formation and release. Several techniques are discussed in this review that may improve the performance of PLG depot delivery systems for proteins. One promising approach is the covalent attachment of poly(ethylene glycol) (PEG) to the protein prior to encapsulation in the PLG microspheres. The combination of the extended circulation time of PEGylated proteins and the shielding and potential stabilizing effects of the attached PEG may lead to improved release kinetics from PLG microsphere system and more complete release of the active conjugate.
Key words: PEGylation, protein delivery, protein denaturation, microencapsulation, sustained release
INTRODUCTION
With increased understanding of diseases at a molecular level, proteins have become prime candidates as therapeutic drugs due to their high activity and specificity. Additionally, recombinant DNA technology enables almost any protein to be mass-produced, in principle.
However, proteins are limited in their current application as biopharmaceuticals as routes of delivery are limited and they can be difficult to maintain in an active state due to their frequent high sensitivity to their environment. Protein drugs are typically given by injection or intravenous infusion, since simple oral administration is ineffective due to degradation in the digestive tract (1). Effective regimens for the injection- and infusion-based delivery of protein drugs are bracketed by the clearance and immune responses of the body. Proteins, particularly those smaller than 50 kDa, are quickly removed from circulation by renal excretion, necessitating either frequent dosing or larger individual doses. The cutoff in molecular weight is related to the size of the pores of the glomerulus, which are about 7.5 nm in diameter (2). Large systemic doses can induce an immune response (1). Thus, a regimen of frequent injections is typically used to maintain therapeutic levels. This is not only inconvenient for the patient but also expensive and, most importantly, increases the possibility of patient noncompliance.
The main aim of a systemic drug delivery system for chronic treatment is to maintain therapeutic blood or tissue levels of the drug for an extended period of time, also known as zero-order release (see Fig. 1 for an example of this release profile). However, many drug delivery modes, including conventional oral tablets and injections, do not attain this type of release. Instead, the drug is released immediately, giving a fast first-order kinetic systemic concentration profile. Sustained-release systems attempt to approach zero-order release kinetics for at least some period of time before the loaded drug is completely released. The advantages of sustained-release delivery devices are that they can be taken less frequently than instant-release formulations of the same drug and that they maintain more nearly constant concentrations of the drug in the bloodstream. A common class of sustained-release devices that can be used with proteins are depot systems wherein the therapeutic drug is dispersed throughout a gel-like carrier matrix and the loaded gel is injected subcutaneously or intramuscularly. The gel breaks down, by chemical and/or physical processes, releasing the drug into the surrounding tissue and its vasculature at a nearly constant rate.
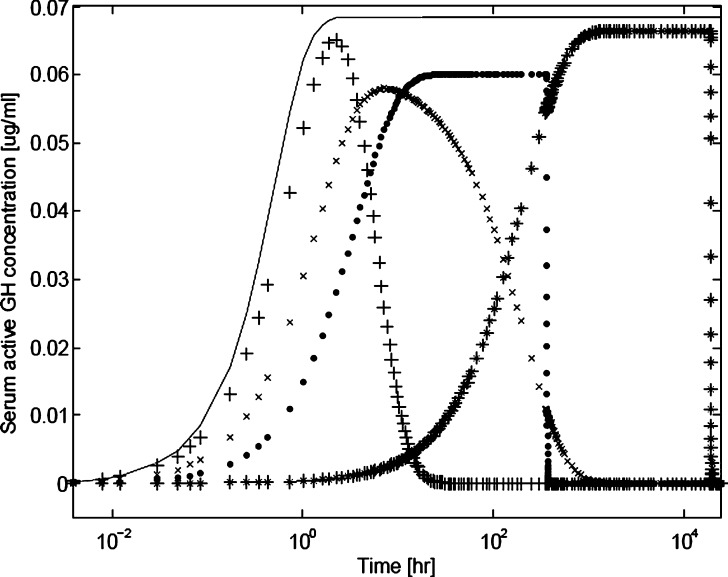
Fig. 1.
Simulated serum hGH level versus time profiles showing differences between subcutaneous injections of hGH (plus signs), PEG-modified hGH (crosses), and hGH encapsulated in a PLG depot device (dots). A possible profile for PEG-hGH encapsulated in a PLG depot device (stars) is also presented for illustrative purposes. The profile for an ideal zero-order release is also shown for comparison (solid line). Profiles were generated using a single compartmental kinetic model, with the following kinetic expression: d[hGH]/dt = −(d(hGH)dose/dt)/v − k c[hGH], where [hGH], (hGH)dose, v, and k c represent the serum hGH concentration at time t, the hGH dose mass at time t, the serum volume, and the clearance rate, respectively. Assumptions made were that the body (serum) is a single compartment, a subcutaneous dose form supplies drug to the serum compartment by either a first- or zero-order process beginning at time zero, and the serum volume is 5 l; the serum concentration is assumed to be negligible at time t = 0. The model for ideal first-order release was −d(hGH)dose/dt = k r(hGH)dose; that for zero-order release had the expression −d(hGH)dose/dt = k r. The serum half-lives specified were 2.5 h and 6 days for hGH and PEG-hGH species, respectively (3,5); the relationship between serum half-life and the clearance rate constant is t 1/2 = ln2/k c. The release rate constants used were 1 h−1, 1 h−1, 0.0832 μg/h, and 0.0016 μg/h for hGH, PEG-hGH, hGH depot, and PEG-hGH depot, respectively. The rates of release were selected in order to achieve therapeutic serum concentrations of ∼0.1 μg/ml, which are scaled from clinical data (3–5). Dose mass loads used were 0.6, 0.3, 30, and 30 μg for hGH, PEG-hGH, hGH in depot, and PEG-hGH in depot, respectively
Representative simulated serum concentration profiles of human growth hormone (hGH), a protein drug intended for chronic therapy, administered via injection and via a depot delivery system based on the biodegradable polymer poly(lactide-co-glycolide) (PLG) are shown in Fig. 1. Also shown in Fig. 1 is an idealized zero-order release profile, assuming an arbitrary therapeutic window. These profiles were generated using a basic one-compartment pharmacokinetic model with dosages and order-of-magnitude release and clearance kinetic parameters estimated from clinical data reported by Genentech for a subcutaneous hGH (Nutropin AQ®) injection and a subcutaneous PLG depot system containing hGH (Nutropin Depot®; 3,4). It is expected and obvious that a depot-based delivery system can deliver much more protein over much longer time scales than does subcutaneous injection. As seen in the figure, the profile for injected hGH follows fast first-order kinetics. The simulated profile of the hGH in the depot system approaches the idealized sustained-release system in that it shows a zero-order delivery profile until the dose runs out. The mean terminal half-life after subcutaneous injection of hGH in a healthy adult is on the order of hours (3). hGH administered through a PLG depot system has a potential duration of delivery on the order of weeks. For example, Nutropin Depot® had mean duration of delivery of 2.5 weeks in Rhesus monkeys (4).
Benefits and Drawbacks of Protein Delivery from PLG Depots
The reduced injection frequency and more nearly constant serum concentrations afforded by sustained release devices have been exploited for the chronic delivery of several therapeutic peptides via PLG and related depots. Table I summarizes the current US Food and Drug Administration (FDA)-approved PLG-based small peptide formulations. The clinical success of these formulations has motivated the exploration of similar depot systems for chronic protein delivery.
Table I.
Marketed PLG Microparticle-Based Small Peptide Drug Delivery Products [extracted from (6)]
| Product name | Active ingredient | Distributor | Indication | Dosing interval |
|---|---|---|---|---|
| Parlodel® LA | Bromcriptine | Sandoz | Pituitary tumors | 1 month |
| Suprecur® MP | Buserelin | Aventis | Endometriosis | 1 month |
| Somatuline® LA | Lanreotide | Ipsen | Acromegaly | 1 month |
| Lupron® Depot | Leuprolide acetate | TAP | Prostate cancer, endometriosis | 1, 3, 4 months |
| Arestin® | Minocycline | OraPharma | Periodontal disease | 2 weeks |
| Sandostatin® LAR | Octreotide | Novartis | Acomegaly, carcinod syndrome | 1 month |
| Risperdal® Consta | Risperidone | Jannsen/Alkermes | Schizophrenia | 2 weeks |
| Decapeptyl® | Triptorelin | Ferring | Prostate cancer | 1, 3 months |
| Decapeptyl® LP | Triptorelin | Ipsen | Prostate cancer | 1, 3 months |
| Pamorelin® LA | Triptorelin | Ipsen | Prostate cancer | 1, 3 months |
| Trelstar® Depot | Triptorelin | Pfizer | Prostate cancer | 1, 3 months |
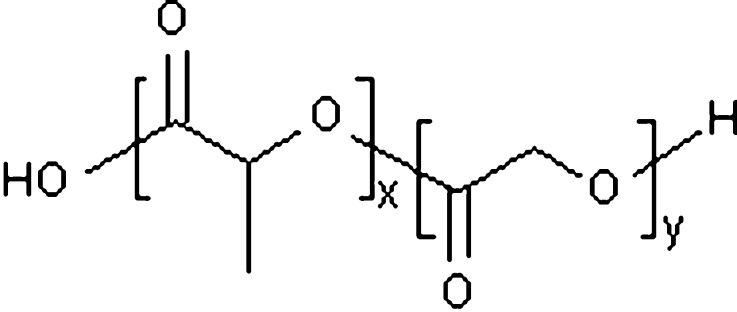
The use of polylactide, polyglycolide, and their copolymers poly(lactide-co-glycolide) to form microspheres as sustained-release protein delivery systems has been studied extensively (7–9) and comprise perhaps the most compelling biodegradable polymers for depot systems due to their inclusion in the FDA’s Generally Recognized as Safe (GRAS) list for use in medical devices and drug formulations (10). Lactic acid and glycolic acid have homologous molecular structures, as shown in Fig. 2, with lactic acid bearing an additional methylene group at the beta carbon position that makes it somewhat more hydrophobic than glycolic acid. The copolymer degrades by bulk erosion through hydrolysis of the ester bonds. It has been shown that the time required for degradation of PLG is related to the lactic acid/glycolic acid ratio used in the production of microspheres: the higher the content of lactide units and therefore the more hydrophobic the polymer, the slower the rate of degradation due to decreased water imbibition (11). Thus, the degradation rate can be tailored according to the desired release pattern of the incorporated protein by controlling the monomer ratio during synthesis. The size of the biodegradable polymer microspheres is also a primary determinant of polymer degradation and drug release rates. As particle size increases, surface area/volume ratio decreases, which decreases both water penetration and release of degradation products. Because the mechanism of drug release is typically diffusion through the polymer phase of aqueous-filled pores in the polymer matrix, the decrease in surface area/volume ratio with increasing particle diameter translates into a decrease in drug release rate (12). Finally, PLG average molecular weight is found to impact release rates with increasing molecular weights slowing the erosion process (13). Typical molecular weights for the drug formulations listed in Table I range from 25 to 100 kDa (14).
Fig. 2.
Chemical structure of poly(lactide-co-glycolide). Indices x and y refer to the relative amounts of lactide and glycolide units, respectively, in a specific PLG copolymer. These indices are incorporated in PLG nomenclature; e.g. an x = 85% lactide, y = 15% glycolide is indicated as 85:15 PLG. x and y can be manipulated to alter the degradation rate of the PLG. An increase in x results in a slower degradation rate
Release of the protein from the microsphere is controlled by two mechanisms: diffusion of the protein out of the microsphere and erosion of the polymer matrix. Typically, the diffusion process consists of an initial “burst” release of protein at or near the surface of the microsphere, followed by the additional release of protein by diffusion from the pores of the microsphere. Erosion occurs by hydrolysis of the polymer backbone, generating pores that expose interior pockets of protein to the bathing liquid. For continuous release, the diffusion and erosion processes must balance each other to allow the protein to diffuse out of the microspheres at a constant rate (14).
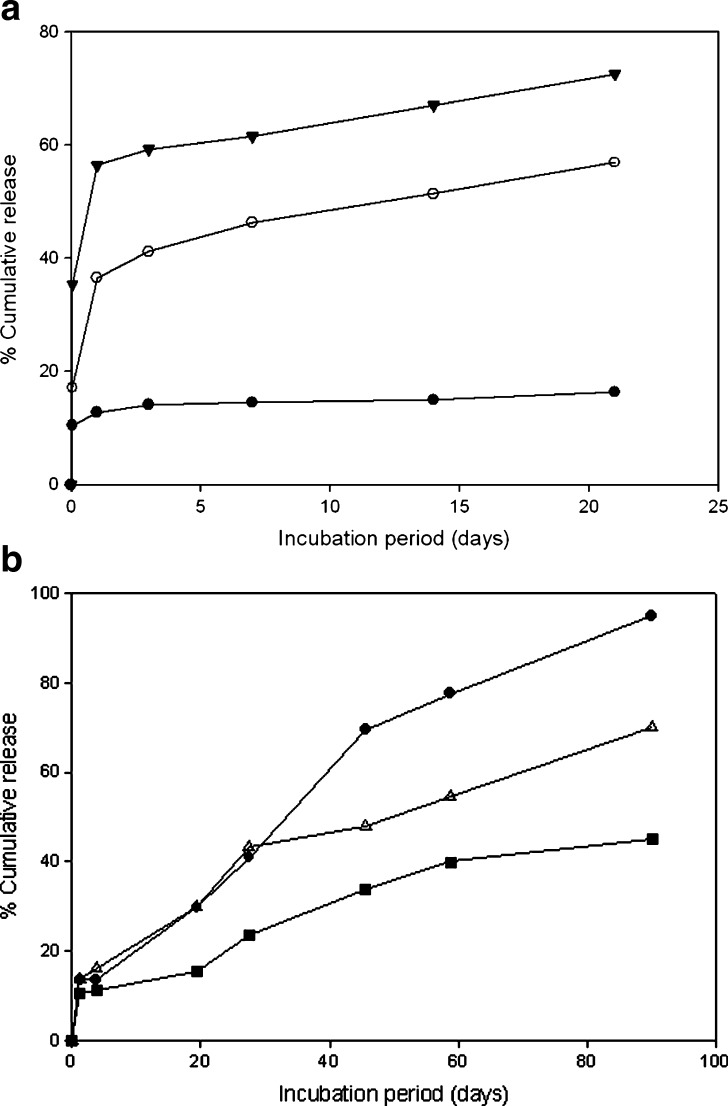
Figure 3 shows in vitro release profiles of interferon-α 2a (IFN) and poly(ethylene glycol)-modified IFN (IFN-mPEG2000 and IFN-mPEG5000) as well as in vitro release profiles of ribonuclease A (RNase A) and poly(ethylene glycol)-modified RNase A (mono-PEG-RNase A and di-PEG-RNase A) from PLG microspheres (15,16). The details of these experiments are described later in the review; however, the figure serves to illustrate typical release profiles from PLG microspheres. As seen, IFN microspheres exhibit an initial “burst” release within the first hour of study, followed by negligible additional release. PEGylated IFN microspheres also exhibit an initial burst release, followed by a more desirable continuous release profile for the same period of observation (15). Similarly, the release profiles for unmodified RNase A, mono-PEG-RNase A, and di-PEG-RNase A all exhibited an initial burst of approximately 15% release from the PLG microspheres, followed by additional release over 3 months (16).
Fig. 3.
a In vitro release profile of protein from PLG microspheres carrying native or PEGylated IFN (IFN-mPEG2000 and IFN-mPEG5000) as shown with closed circle, inverted closed triangle, and open circle, respectively [replotted from (15), p. 111, Copyright (2003), with permission from Elsevier]. For the IFN study, 50:50 PLG microspheres had an average size of 1.8, 1.2, and 1.5 μm for those containing IFN, IFN-mPEG2000, and IFN-mPEG5000, respectively (15). b RNase A (filled squares), mono-PEG-RNase A (open triangles), and di-PEG-RNase A (filled circles) release from 85:15 PLG microspheres [replotted from (16), p. 866, Copyright (2005), with permission from John Wiley & Sons, Inc.]. In the RNase A study, 85:15 PLG microspheres, with a broad distribution of diameters ranging from 5 to 50 μm, were used (16)
Proteins can be entrapped in the PLG microsphere depot by a number of methods, including formation of a water-in-oil emulsion with water-borne protein and organic solvent-borne polymer (emulsion method), formation of a solid-in-oil suspension with solid protein dispersed in a solvent-based polymer solution (suspension method), or by dissolving the protein in a solvent-based polymer solution (dissolution method). The details of these methods are discussed further below. After introduction of the protein in the PLG solution, further processing is required to form microparticles. In many cases, a new emulsion is created for this purpose by adding a nonsolvent for the PLG, usually water, together with an emulsifier such as poly(vinyl alcohol) or silicone oil. While the mixture is stirred, the microparticles are formed as the PLG phase separates. During and after this emulsification step, the organic solvent in which the PLG was originally dissolved is either extracted or allowed to evaporate. This causes the PLG to phase separate as spherical particles entrapping the protein (17).
An alternative method to produce protein-loaded microparticles is spray drying (18,19). The protein/PLG dispersion is sprayed through a heated nozzle, and the organic solvent is rapidly evaporated by a hot gas flow. While the high temperatures required for spray drying may adversely affect protein integrity, the duration of exposure to these elevated temperatures is often very short (18,19). A variation of the conventional spray-drying method is a cryogenic method in which a protein/PLG dispersion is ultrasonically sprayed into liquid nitrogen over solid ethanol. During evaporation of the liquid nitrogen, the ethanol melts, resulting in extraction of the organic solvent from the microparticles formed by the spraying process (18).
Protein delivery from PLG microspheres has not been fully realized in practice. Only one protein-releasing injectable sustained release system has reached the market thus far: Genentech’s Nutropin® Depot for delivery of recombinant human growth hormone, marketed in 1998 in the USA (4). Slow degradation of the PLG matrix resulted in a slow release of the protein, sustaining therapeutic concentrations for weeks. However, in 2004, the product was withdrawn from the market by Genentech due to production cost issues; these costs were presumed to be associated with the cryogenic spray-drying microsphere production process (17) used to mitigate activity losses during depot formulation (20).
There are several other reports of PLG microsphere release studies with proteins and peptides in the literature as summarized in Table II (15,16,21–27). As noted, each example exhibited an initial burst release, followed by relatively little subsequent release. Several of these studies also note significant activity losses in the released protein. For example, Pérez et al. measured a decrease in lysozyme activity from 95% to 43% after 24 h of in vitro release from 50:50 PLG microspheres (24). Daly et al. also noted that the mean intrinsic activity of ribonuclease A was diminished by ∼60% upon release from 50:50 PLG microspheres after 60 days (16). This points out a critical drawback of PLG microsphere delivery systems for proteins: Activity losses during formulation and/or release can be significant. The fact that a far greater number of small molecule drugs have been incorporated into approved PLG microsphere delivery systems than protein drugs is a direct reflection of the difficulty in maintaining bioactivity when proteins are encapsulated, subsequently stored, and ultimately released from the device.
Table II.
Examples of PLG Microsphere Formulations Studied for Controlled Release of Proteins and Larger Peptides
| Protein or peptide | Polymer | Summary of results |
|---|---|---|
| α-Chymotrypsin | 50:50 PLG (60 kDa) | 70% burst, additional 10% over 50 days (21) |
| Cyclosporin A | 50:50 PLG (0.44 and 0.80 dl/g) | No drug–polymer interaction biphasic release; 1–40% burst, >28-day release (22) |
| Interferon-α | 50:50 PLG | 11.0% burst w/in 1 h, additional 16.3% released over 21 days (15) |
| Lysozyme | 50:50 PLG | 50% burst, additional 10% over 50 days (23) |
| Lysozyme | 50:50 PLG (10 kDa) | 40% burst, additional 40% over 6 days; reduced activity upon encapsulation (24) |
| Ribonuclease A | 85:15 PLG | 15% burst, additional 50% over 3 months; reduced activity upon encapsulation (16) |
| Somatostatin analog | 55:45 PLG (23–76 kDa) | Release decreased with media ionic strength; <20% burst, continuous or triphasic release for >30 days (25) |
| Tetanus toxoid | 50:50 PLG (100 kDa) | <30% burst, continuous or triphasic for >30 days (26) |
| Thyrotropin | 75:25 PLG (11 kDa) | <20% burst, 30-day continuous release (27) |
Protein conformation—and hence biological activity—is very sensitive to local environment, particularly to the damaging effects of protein interactions with interfaces. Interfacial adsorption has been found to limit the performance of sustained protein release from biodegradable depots (28). Adsorption processes at liquid/liquid interfaces during protein encapsulation and solid/liquid interfaces during release are both relevant. Protein adsorption to interfaces decreases the amount of protein released and may also cause conformational changes and/or aggregation. In addition, proteins are exposed to a variety of detrimental stresses during microsphere generation including heat, shear, and organic solvent exposure. Denatured or aggregated protein species are therapeutically inactive and may also cause unpredictable side effects, such as immunogenicity or toxicity (29).
Improved Efficacy of PEG-Modified Protein Therapeutics
Covalent attachment of poly(ethylene glycol) to proteins (PEGylation), a technology proven to increase the in vivo half-life of circulating therapeutic proteins and decrease immune response (30,31), can potentially address some of the problems associated with the delivery of proteins via PLG depot systems. For example, Somavert®, a PEGylated form of hGH (PEG-rhGH) designed for subcutaneous injection that is marketed by Pfizer, is eliminated from serum with a mean half-life of approximately 6 days following either single or multiple doses (5) as opposed to the half-life of hours observed for unmodified rhGH (3).
The protective effects of PEGylation have been attributed to steric and hydration repulsions between PEGylated proteins and other molecules (32). In aqueous solutions, the PEG chains are highly hydrated. This allows the PEG to sweep out an exclusion volume, thereby acting as a steric barrier to other molecules. In this manner, the immune response is reduced by blocking antibodies’ access to epitopes on the protein molecule. Similarly, PEG decreases proteolytic degradation by repelling proteases and decreases the first-pass clearance in the glomerulus by adding to the overall size of the protein (2,32). Depending on the number, molecular weight, and location of the attached PEG chains, these protective effects can accrue without loss of protein biological activity. PEGylation may also confer additional thermodynamic stability to proteins, making some conjugates more resistant to unfolding than their unmodified counterparts (33).
One particularly enticing application of PEGylated proteins is in anticancer therapies. It has been shown that PEGylated proteins accumulate more extensively in tumors than the unmodified proteins. This enhanced permeation and retention effect has been attributed to the increased circulation time of the proteins that allows more passages through the leaky tumor vasculature (32,34,35). Examples of PEGylated proteins that have been approved for therapeutic use by the FDA include PEGasys® (Roche; interferon α-2a), PEG-Intron® (Schering-Plough; interferon α-2b), Neulasta® (Amgen; granulocyte colony-stimulating factor), Adagen (Enzon; adenosine deaminase), Oncaspar® (Enzon; l-asparaginase), and Somavert® (Pfizer; growth hormone; 36).
Could protein PEGylation be the route to successful delivery of proteins via PLG delivery systems? Although water is a good solvent for PEG, this polymer is somewhat amphiphilic and tends to adsorb on hydrophobic surfaces as well as acidic surfaces. The interfacial activity of PEG leads to the hypothesis that PEGylation may enhance the release of active proteins from PLG microsphere delivery systems. It is anticipated that the PEG portion of PEGylated conjugates may preferentially populate the liquid–liquid and solid–liquid interfaces present during microsphere generation and erosion, protecting the protein portion from denaturing adsorption phenomena. Further, any thermodynamic stabilization realized by conjugates should improve activity by increasing resistance to denaturing stresses experienced during formulation. Lastly, steric repulsions between PEG chains may also make PEGylated proteins less vulnerable to adsorption-induced aggregation.
The potential impact of PEGylation on the delivery of a protein such as hGH from PLG microspheres is illustrated in Fig. 1 using the release kinetics of the depot together with the increased circulating half-life of the conjugate. Note that the purpose of the incorporation of the PEGylated protein conjugate is not to extend the delivery time of the depot system; the time scales of the depot release and clearance reduction are too different for the impact on the delivery kinetics to be significant. Rather, the potential benefit is anticipated as a greater release of active conjugate from the PLG depot at similar protein and conjugate loadings. The simple pharmacokinetic model in Fig. 1 suggests that the extended circulation time of the conjugates would also require much slower depot release rates and lower loadings than that for the unmodified protein to maintain the serum concentration in the therapeutic window. In this review, we summarize the problems associated with PLG microsphere delivery systems for proteins, the potential for the synergistic use of protein PEGylation technology with PLG microsphere delivery, and the molecular basis for this expected synergy.
PROTEIN INSTABILITY DURING PLG DEPOT LOADING
The most straightforward and therefore most popular method of forming microspheres is the water-in-oil-in-water (W/O/W) double emulsion solvent evaporation technique (34). In this method, an aqueous protein solution is dispersed in a PLG-organic solvent solution. This primary emulsion (W1/O) is then reemulsified in a large volume of an aqueous polymer solution, typically poly(vinyl alcohol), to generate a stable (W1/O) W2 double emulsion. Precipitation of the PLG by an extraction/evaporation process entraps and immobilizes the aqueous protein-containing droplets within a PLG matrix (37). The major disadvantage of this method is the presence of aqueous/organic solvent interfaces. The type of organic solvent used may also play a critical role. Compared to the more hydrophobic methylene chloride, ethyl acetate usually results in less emulsification-induced denaturation of proteins (38–40). However, poor encapsulation efficiencies for recombinant human growth hormone were also observed when preparing microspheres using a mixture of methylene chloride and ethyl acetate in varying volume ratios (41). In this study, the authors stated that the addition of ethyl acetate, a water-miscible solvent, to a water-immiscible methylene chloride phase (O) might result in a slightly less porous internal structure due to a preferential escape tendency of ethyl acetate over methylene chloride into the aqueous outer phase (W2) from embryonic microspheres during the solvent evaporation process (42).
The suspension method involves fine dispersion of a solid protein in an organic solvent to form a solid-in-oil (S/O) primary suspension (18,38). This suspension is emulsified in an aqueous solution containing an emulsifier (S/O/W). Microspheres are then hardened by dissolving or evaporating the organic solvent, after which they are finally washed and lyophilized. A drawback of this method is that prior to encapsulation, the protein must be freeze-dried, usually in the presence of a lyoprotectant, which may induce some protein damage (43).
Unlike the suspension method, the dissolution method, also known as the single emulsion method, involves the direct addition of protein and polymer together to the organic solvent, which is then emulsified into an aqueous phase to form an oil-in-water (O/W) emulsion. One major disadvantage of the O/W method is poor encapsulation efficiencies of water-soluble drugs, such as proteins and peptides (44,45). Proteins readily diffuse out or partition from the dispersed oil phase into the aqueous continuous phase. Then, microcrystalline fragments of the hydrophilic drug get deposited on the outer surface of the microspheres and dispersed in the PLG matrix. A major concern of this method is the potential for intimate protein-PLG contact.
Although several methods exist for microencapsulation, all have been shown to be detrimental to protein stability in some way (30,37,46–48). Depending on the method used to form the emulsions, proteins are exposed to cavitation, heat, shear, and/or organic solvent during the microencapsulation process. Sonication, the most popular method for creating the primary emulsion, produces all of the above conditions with the additional generation of free radicals (49,50). Zambaux et al. showed that a reduction in sonication time during PLG nanoparticle preparation lowered the activity loss of encapsulated protein C (51). Morlock et al. showed that the use of sonicators and vortex mixers in the first emulsion step increased aggregation of recombinant human erythropoietin compared to the use of homogenizers (52). However, for several proteins, it was demonstrated that the effects of cavitation, shear, and continual creation of a new air/water interface were not the primary causes of protein instability. Instead, exposure of the protein to the organic solvent was identified as a major cause of instability, aggregation, and denaturation (40,48,53,54).
For this review, we focus on the W/O/W emulsion technique. This technique is viewed as perhaps the least cost-intensive means for formulating PLG depot systems and thus, if associated activity losses can be mitigated, the route with the most commercial potential. One approach to minimize average protein exposure to the O/W interface was to increase protein concentration during emulsification while maintaining similar interfacial area. The idea here was to sacrifice some hopefully small fraction of the protein to the interface. While a self-protecting effect was observed for lysozyme, it was difficult to prevent aggregation at such high concentrations. An observed loss in specific enzyme activity of soluble lysozyme was caused by the irreversible formation of an unfolded lysozyme species, which was found to be monomeric, and was able to leave the water/methylene chloride interface and accumulate in the aqueous phase (55).
Another approach is to use an amphipathic excipient to compete with the therapeutic protein for the interface. Experiments with bovine serum albumin (BSA), S-carboxymethylated BSA, and reduced S-carboxymethylated BSA demonstrated that a free thiol group and/or disulfide bond participated in the methylene chloride/water interface-associated dimerization and polymerization of proteins. Interfacial reactions that led to the aggregation of ovalbumin and lysozyme were inhibited by adding hydroxypropyl-β-cyclodextrin or BSA into their aqueous solution (48). One potential disadvantage of using a protein for this purpose is that the additional protein itself could aggregate under these conditions, possibly making the approach unattractive in medical applications. Furthermore, the use of two medical grade recombinant proteins in a given formulation could add significantly to production costs.
Finally, another approach is the ion pairing of proteins with oppositely charged polyelectrolytes to form ionic complexes capable of protecting the protein from degrading conditions. Encapsulation of complexes of cationic lysozyme with variable amounts of anionic polyelectrolyte chondroitin sulfate (CsA) by a W/O/W method resulted in microspheres with enhanced encapsulation efficiency compared to native lysozyme, with simultaneous reduction in the amount of insoluble lysozyme aggregates (56). The authors concluded that the numerous carboxylate termini of CsA may mediate a hyperstructural shielding (complexation) of the labile protein, thereby reducing the tendency of lysozyme to interact with the water/organic solvent interface and preventing protein degradation. These results showed that chondroitin sulfate is a viable candidate for protein drug delivery; however, its use with other proteins should be investigated. Food manufacturers recently applied to the FDA to list chondroitin sulfate as GRAS for use in food and beverages (10). In the meantime, work must be done to determine the proper usage levels for formulating with chondroitin sulfate for the drug delivery purposes.
PROTEIN INSTABILITY DURING PLG DEPOT RELEASE
Release from the PLG microspheres is characterized by several steps (46,47,57) which include an initial “burst” wherein protein adsorbed on or encapsulated near the surface of the microsphere is released. Contrary to the ideal zero-order depot delivery profiles depicted in Fig. 1, an initial burst release of hGH is seen in practice for the Nutropin® Depot system during the first few days after injection where serum GH levels increase rapidly before maintaining more constant levels. As the PLG undergoes bulk degradation by hydrolysis of the ester bonds, protein is released at a slower, more uniform rate. Finally, the entire microsphere erodes. The nonlinear release kinetics are a limitation of PLG microspheres that remains to be addressed. This initial large release of protein is undesirable. Following the initial burst, release rates are nearly constant for an extended period of time, which is highly desirable. Often, one observes an asymptotic approach to a limiting extent of release that is less than 100%, i.e., a final “nonrelease period”. Protein adsorption to the PLG/aqueous interface during release has been demonstrated to be a critical factor contributing to both incomplete protein release and loss of bioactivity (28,46,47).
Park and coworkers (29,58,59) investigated the mechanism of incomplete release by extracting nonreleased protein with three media that disrupt different types of interactions. By separately adding 0.5 M sodium chloride (NaCl), 5 M guanidine hydrochloride (GdnHCl), and 5 mM sodium dodecyl sulfate (SDS) into the release medium during the nonrelease period, it was possible to selectively identify a specific protein nonrelease mechanism: ionic interaction, noncovalent aggregation, and/or surface adsorption, respectively. An increase in ionic strength in the medium reduces the extent of ionic interaction by shielding the charged ionic groups. A faster rate of release in the presence of a higher NaCl concentration would indicate that ionic interaction played a pivotal role. The denaturant GdnHCl will dissolve noncovalent aggregates and disrupt electrostatic and hydrogen bonding interactions with carboxylate/carboxyl PLG end groups. SDS will release both noncovalent aggregates and proteins adsorbed to PLG by hydrophobic interactions. The difference in protein amount extracted by SDS and GdnHCl is a measure of the relative contribution of end-group interactions and hydrophobic adsorption to PLG to the incomplete release. Using this approach, Park et al. (58) showed that the initial release of lysozyme from uncapped 50:50 PLG microspheres was mainly controlled by electrostatic interactions between lysozyme and free carboxylate end groups of PLG chains, whereas noncovalent aggregation and hydrophobic PLG-protein contacts were responsible for the incomplete release later on (58). Similar studies with rhGH and BSA showed that noncovalent aggregation was the main cause of incomplete release after the burst. At a later stage, nonspecific adsorption also contributed to the incomplete release (41,59).
Butler et al. measured the extent and kinetics of BSA adsorption to films of PLG with different end groups, including a hydrophilic carboxylate end group and a hydrophobic ester end group (60). BSA adsorbed readily to both native PLG films and the amount adsorbed was insensitive to the type of end group. Aging of the films in water prior to exposure to BSA decreased the hydrophobicity of the films as the ester linkages were hydrolyzed and in turn correlated with a significant decrease in the initial BSA adsorption rate. This behavior is consistent with the expected trend that decreased surface hydrophobicity favors decreased protein adsorption (61).
IMPROVING DELIVERY FROM PLG DEPOTS VIA PROTEIN PEGYLATION
The combination of PEGylation and microsphere encapsulation of proteins has been shown to have positive effects on both protein stability and release. As mentioned previously, the potential benefit is a greater release of active conjugate from the PLG depot at similar protein and conjugate loadings. Several groups have investigated these effects in more detail and their findings are summarized below.
Diwan and Park prepared PEGylated lysozyme for 50:50 PLG encapsulation by the W/O/W double emulsion method using a mixture of mono-, di-, and tri-PEGylated species as well as unmodified protein (23); in this study, a succinimidyl succinate derivative of methoxy polyethylene glycol (Mw 5,000 Da) was used in the PEGylation reaction. After 1 h of incubation, microspheres released 40 ± 10.1% of the encapsulated lysozyme while only 6.4 ± 0.7% was released from those carrying PEGylated lysozyme. After 83 days of incubation, about 30% of lysozyme still remained unreleased. In contrast, the release of PEGylated lysozyme continued from microspheres and more than 90% release was measured in the same period of time (23). Diwan and Park provided an explanation for the possible role played by PEG as a built-in protein stabilizer. The authors stated that when the protein solution is emulsified with an organic phase, a large interfacial area between aqueous and organic phases is generated. The protein adsorbed onto the interface undergoes unfolding and subsequently aggregates. For PEGylated lysozyme, the authors suggested that the PEG chain prevents the adsorption of protein on the interface, thereby reducing the loss of soluble protein fraction (23); however, this hypothesis was not directly tested.
Diwan and Park also investigated in vitro release profiles of interferon-α 2a and PEGylated IFN using the W/O/W method; in this study, succinimidyl succinate and succinimidyl propionate derivatives of methoxy polyethylene glycol (Mw 5,000 and 2,000 Da, respectively) were used in the PEGylation reaction (15). The IFN microspheres showed an initial burst release followed by nearly no release. In contrast, PEGylated IFN showed continuous release for the same period of observation. IFN-mPEG2000 and IFN-mPEG5000 exhibited net IFN release of 72.5 ± 2.1% and 56.8 ± 2.5%, respectively, in a 3-week incubation period, compared to 16.3 ± 0.4% for unmodified IFN. Release profiles of IFN microspheres appeared to level off after 3 weeks while PEGylated IFN microspheres continued to release (Fig 3a). The authors speculated that the difference in the relative release amounts of protein between the two types of encapsulated PEGylated IFN may be due to the different PEG chain lengths. The increased hydrodynamic size associated with the longer PEG chain would offer more resistance to PEGylated IFN diffusion out of the micropores of microspheres than the shorter one. Additionally, a larger burst release was observed for IFN-mPEG5000 compared to that of lysozyme-mPEG5000 (15). The authors attributed the discrepancy between the different behaviors of PEGylated IFN and lysozyme to be caused by the physicochemically different characters of the two PEGylated proteins, although the underlying reasons were still obscure (15). The authors noted that freeze-dried PEGylated IFN, when dissolved in a small amount of water for microencapsulation, was not readily dissolved but became finely dispersed particles. This may have resulted in an uneven distribution of PEGylated IFN in the polymer matrix (15). The differences in behavior may also be attributed to the inherent properties of the proteins. Compared to lysozyme, interferon-α 2a has a higher molecular weight and a lower isoelectric point. IFN also bears a higher Bigelow hydrophobicity than lysozyme, which may be a critical parameter when the protein is exposed to a somewhat hydrophobic surface such as PLG (62).
Castellanos et al. (21) prepared PEGylated-α-chymotrypsin for encapsulation in 50:50 PLG microspheres (Mw = 60 kDa) by the S/O/W emulsification method; in this study, methoxy-PEG-succinimidyl propionate (Mw = 5,000 Da) was used in the PEGylation reaction. The authors showed that even the lowest level of modification drastically reduced the amount of insoluble aggregates from 18% for the unmodified α-chymotrypsin to 4% (21). In vitro release profiles of unmodified and PEGylated-α-chymotrypsin were investigated and all formulations showed an initial burst within the first few days of incubation. For unmodified α-chymotrypsin microspheres, approximately 70% of the protein was released by burst and only 80% of the encapsulated protein was ultimately released over 30 days. For PEG-α-chymotrypsin microspheres, a lower initial release was observed with near complete release over 2 months. The authors concluded that the encapsulation process was the main cause for the formation of protein aggregates under the conditions used (21).
Hinds and coworkers (63) developed a novel controlled release formulation with PEGylated human insulin encapsulated in 50:50 PLG microspheres that produced multiday release in vivo. In this study, PEGylated insulin conjugates were prepared having a single methoxy-PEG-succinimidyl propionate (5,000 Da) polymer chain selectively attached to the amino terminus of insulin’s B chain (PheB1) via a hydrolytically stable amide bond; the desired PEGylated conjugate was separated from other reaction species by ion-exchange chromatography. After an initial release of <0.5% in the first day and a subsequent lag period, the microparticles released their contents almost completely (93%) in a nearly continuous fashion over the next 16 days (63). The authors postulated that a phase mixed monolithic polymer-drug internal structure made possible by the solubility properties of the PEGylated protein promoted these results. The combination of PEGylation and microencapsulation offered potential for sustained delivery of basal insulin with a single weekly dose (63).
Our group noted a similar increase in the extent of PEGylated RNase A release from 85:15 PLG microspheres compared to that for the unmodified protein (16). RNase A and 20 kDa nominal molecular mass mPEG-propionaldehyde (1:6 mole ratio) were reacted under conditions that favor N-terminal modification of the protein (64). The conjugate species were purified via size exclusion chromatography (SEC). The release profiles for unmodified RNase A, mono-PEG-RNase A, and di-PEG-RNase A all exhibited an initial burst of approximately 15% release from the PLG microspheres. After the burst, the release of unmodified RNase A from the PLG microspheres was incomplete over the 3-month duration of the study, with only approximately 50% of the total encapsulated protein released into solution (Fig. 3b). Two possible explanations for the incomplete release were protein adsorption onto the PLG matrix and the formation of water-insoluble aggregates during the encapsulation process, although these issues were not directly addressed in the study. Upon PEGylation, approximately 65% of mono-PEG-RNase A and 95% di-PEG-RNase A were released from the PLG microspheres over 3 months. Furthermore, activity assays showed that the released protein remained active, with significantly higher mean intrinsic activity values for PEGylated RNase A compared to the unmodified form (16). While the mean intrinsic activity (kcat/KM) of unmodified RNase A after release had decreased by a factor of 3 relative to a freshly prepared protein solution, mono-PEG RNAse A and di-PEG-RNase A suffered decreases of a factor of 2 and 1.3, respectively (16).
UNDERSTANDING PEG-PROTEIN CONJUGATE ADSORPTION AT THE MOLECULAR LEVEL
The therapeutic impact of PEGylation depends upon the location and extent of the PEG modification, PEG molecular weight, and the structure of the conjugates. The beneficial effect of PEGylation has been described as originating from a shell of PEG chains surrounding the protein (32). However, previous work in this group to measure conjugate dimensions suggests that the grafted PEG chains exist as relatively unperturbed random-coil domains adjacent to the protein molecule rather than as a protein shroud, for both RNase A and lysozyme (16,65). The morphology of the PEG-protein conjugates must be further explored to determine if the conjugates take on a dumbbell shape as our data suggests (16,65). This knowledge would aid in the interpretation of adsorption behavior and associated possible reorientation effects to form a firm molecular basis for any conferred protective effect as described below.
PEGylation Has a Pronounced Effect on Lysozyme Adsorption Mechanisms
Previous work in this group has contrasted adsorption mechanisms for PEGylated proteins and their unmodified protein counterparts. This group has studied the conformation, orientation, and clustering of a layer of lysozyme adsorbed to a silica surface. While not intended as a model for PLG, it should be noted that silica and PLG are both negatively charged surfaces. A multistage reorientation was observed in the adsorption process, whereby lateral repulsions between adjacent proteins triggered an abrupt reorientation at a critical surface coverage, followed by a more gradual reorientation of proteins as the adsorbed layer approached saturation. The occurrence of a molecular reorientation at a critical threshold coverage was consistent with the shape of the adsorption isotherm (66).
Lysozyme and 20 kDa nominal molecular mass mPEG-propionaldehyde (1:6 mole ratio) were reacted under conditions that favor N-terminal modification of the protein and the conjugates were purified via SEC (64). It was shown that as the extent of PEGylation of lysozyme was increased, the surface concentration of proteins adsorbed to silica decreased over a wide range of solution concentrations. The adsorption isotherm of mono-PEG-lysozyme retained the same multistage isotherm shape as that of unmodified lysozyme, also suggesting a reorientation (65). Using atomic force microscopy, it was determined that the PEGylated lysozyme initially adsorbed in a disordered manner with both PEG chains and lysozyme molecules in contact with the surface. As the surface coverage increased, lateral repulsions triggered a reorientation such that the PEG chains were lifted off the surface and presented to the solution as a mushroom-like layer, to use the parlance of the polymer grafting literature. Whereas unmodified lysozyme adsorbed to silica with the N terminus in contact with the surface, the N terminus of PEG-lysozyme was occupied by the grafted PEG chains. As a result, PEGylated lysozyme could not adsorb in the orientation that maximizes the strength of lysozyme adsorption. Accordingly, the reversibility of PEG-lysozyme adsorption was greater than that of the unmodified lysozyme (65).
In addition, the effect of PEGylation on two-dimensional clustering of lysozyme via intermolecular beta sheet formation at the silica/water interface was investigated using a thioflavin T dye-binding assay. It was hypothesized that the steric barrier introduced by PEG grafting would decrease surface-induced protein clustering. While one PEG modification did not decrease the extent of surface-induced aggregation, a second PEG modification significantly reduced this aggregation, likely due to steric effects (67). If this protection against adsorption-induced aggregation is realized on PLG surfaces as well, it suggests an important mechanism by which PEGylation would preserve the activity of released proteins.
Altered RNase A Adsorption to PLG Surfaces upon PEGylation
The effect of PEGylation on RNase A adsorption to an 85:15 PLG film was also investigated (16). RNase A and 20 kDa nominal molecular mass mPEG-propionaldehyde (1:6 mole ratio) were reacted under conditions expected to favor N-terminal modification of the protein (64). It was shown that adsorption kinetics of PEG and PEG-RNase A conjugates achieve the transport-limited rate (rapid), while RNase adsorption is surface-limited (relatively slow), indicating that PEG anchors the conjugates to the surface during the transport-limited regime. PLG aging and the associated increase in the negative zeta potential as hydrolysis progresses resulted in an increased rate and extent of RNase A adsorption but a decreased rate and extent of PEG and PEG-RNase A adsorption. These results correlated well with an increase in the rate, total extent, and preservation of bioactivity of PEGylated RNase A released from PLG microspheres compared to unmodified RNase A (16).
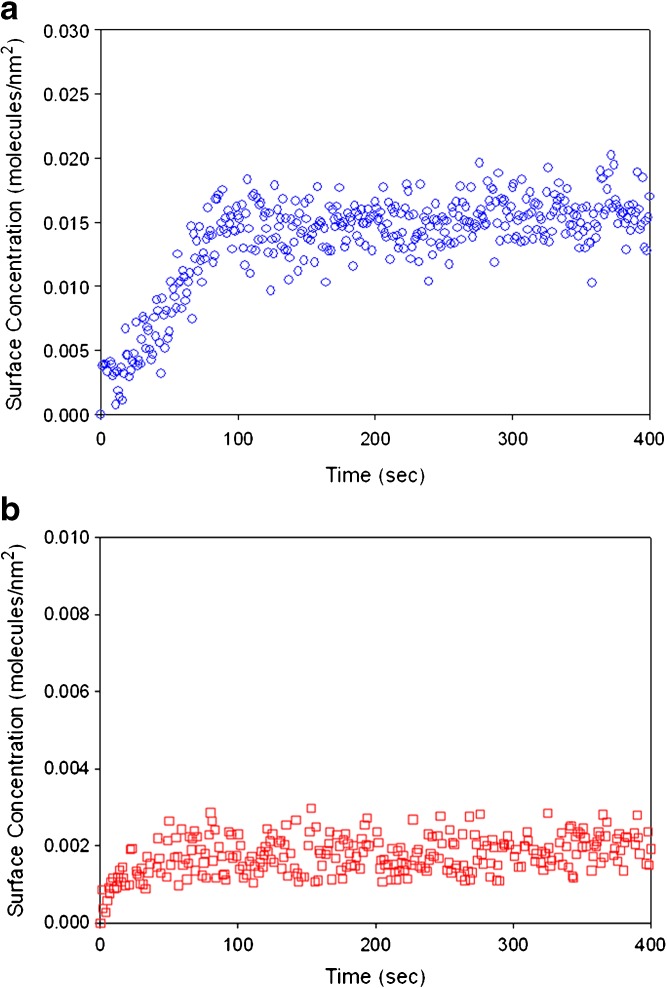
The lysozyme–silica system and the RNase A adsorption data on PLG motivated our current work on the lysozyme-PLG system to determine whether a similar multistage reorientation can be seen in the adsorption isotherms of lysozyme and PEGylated lysozyme on PLG and whether the adsorption process follows transport-limited or surface-limited kinetics. Initial data (Fig. 4) demonstrate that lysozyme adsorption on PLG is also reduced upon PEGylation.
Fig. 4.
Total internal reflection fluorescence-based adsorption kinetic profiles of a 0.685 μM lysozyme solution and b 0.289 μM monoPEG-lysozyme solution in pH 7.4, 5 mM triethanolamine buffer on 85:15 PLG. The average surface concentrations were 0.0146 ± 0.0022 and 0.0023 ± 0.0006 molecules/nm2 for unmodified and PEGylated lysozyme, respectively
CONCLUSIONS
Problems with the delivery of unmodified proteins in PLG depot systems include adsorption of protein to the biodegradable polymer, limiting the extent of release and high ‘burst’ release, which is wasteful and may be dangerous for narrow therapeutic window molecules. Several techniques have been discussed that may improve the performance of PLG depot delivery systems for proteins, particularly depot systems generated by the inexpensive W/O/W approach. The use of an amphipathic excipient or ion pairing with polyelectrolytes are two methods of protection for the protein; however, both methods have associated disadvantages. We have focused on the possibility that covalent attachment of poly(ethylene glycol) to the protein, PEGylation, can lead to a better product with better in vivo performance. As illustrated in Fig. 1, the extended circulation time of PEGylated protein and the release kinetics of PLG microsphere systems may lead to a greater release of active conjugate from the PLG depot at similar loadings compared to the unmodified protein. In addition, the PEG portion of PEGylated conjugates may protect the protein portion from denaturing adsorption phenomena.
Further work is still needed in order to fully understand the benefits of PEGylation. Most of the studies published on encapsulated PEGylated bioactive molecules used semirandom group-specific PEGylation schemes. Site-specific PEGylation may be the next approach to find the modified species with the highest activity that still produces the desired change in pharmacokinetics. There is also a need to understand the effects of PEGylation on the protein adsorption process and the evolution of the adsorbed layer structure and the dynamics of PEG-protein conjugates at the solid/water interface. Such knowledge can aid in the design of therapeutic protein drug delivery devices. Further protein adsorption studies are necessary in order to establish a basis for the prediction, generalization, and control of protein behavior on depot surfaces.
Acknowledgements
This work was supported in part by a grant from the National Science Foundation (grant CBET 0755284).
References
- 1.Harris J. M., Chess R. B. Effect of PEGylation on pharmaceuticals. Nat. Rev. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 2.Hutton P., Cooper G., James F. M., James F. M., III, Butterworth J. F., IV . Anaesthesia: Fundamental principles and practice. London: Informa Health Care; 2002. [Google Scholar]
- 3.Genentech, http://www.gene.com/gene/products/information/opportunistic/nutropin-aq/insert.jsp. Accessed various dates.
- 4.FDA, http://www.fda.gov/MEDWATCH/SAFETY/2004/oct_PI/Nutropin_PI.pdf. Accessed various dates.
- 5.Pfizer, http://www.pfizer.com/files/products/uspi_somavert.pdf. Accessed various dates.
- 6.Tice T. Delivering with depot formulations. Drug Deliv. Technol. 2004;4:44–47. [Google Scholar]
- 7.Wise D. L., Trantolo D. J., Marino R. T., Kitchell J. P. Opportunities and challenges in the design of implantable biodegradable polymeric systems for the delivery of antimicrobial agents and vaccines. Adv. Drug Deliv. Rev. 1988;1(3):269–269. doi: 10.1016/0169-409X(88)90023-3. [DOI] [Google Scholar]
- 8.Cohen S., Yoshioka T., Lucarelli M., Hwang L. H., Langer R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 1991;8:713–720. doi: 10.1023/A:1015841715384. [DOI] [PubMed] [Google Scholar]
- 9.O’Hagan D. T., Singh M., Gupta R. K. Poly(lactide-co-glycolide) microparticles for the development of single-dose controlled-release vaccines. Adv. Drug Deliv. Rev. 1998;32:225–246. doi: 10.1016/S0169-409X(98)00012-X. [DOI] [PubMed] [Google Scholar]
- 10.FDA, http://vm.cfsan.fda.gov/%7Edms/eafus.html. Accessed various dates.
- 11.Smith K. L., Schimpf M. E., Thompson K. E. Bioerodible polymers for delivery of macromolecules. Adv. Drug Deliv. Rev. 1990;4:343–357. doi: 10.1016/0169-409X(90)90026-O. [DOI] [Google Scholar]
- 12.Cleland J. Protein delivery from biodegradable microspheres. In: Sanders L. M., Henderen R. W., editors. Protein Delivery: Physical Systems. New York: Plenum; 1997. pp. 1–43. [Google Scholar]
- 13.Tracy M. A., Ward K. L., Firouzabadian L., Wang Y., Dong N., Qian R., Zhang Y. Factors affecting the degradation rate of poly(lactide-co-glycolde) microspheres in vivo and in vitro. Biomaterials. 1999;20:1057–1062. doi: 10.1016/S0142-9612(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 14.M. Ahlheim, M. Ausborn, D. Bodmer, C. Schoch. Ophthalmic depot formulations for periocular or subconjunctival administration. European patent EP1429725.
- 15.Diwan M., Park T. G. Stabilization of recombinant interferon-a by PEGylation for encapsulation in PLGA microspheres. Int. J. Pharm. 2003;252:111–122. doi: 10.1016/S0378-5173(02)00636-1. [DOI] [PubMed] [Google Scholar]
- 16.Daly S. M., Przybycien T. M., Tilton R. D. Adsorption of poly(ethylene glycol)-modified ribonuclease A to a poly(lactide-co-glycolide) surface. Biotechnol. Bioeng. 2005;90(7):856–868. doi: 10.1002/bit.20481. [DOI] [PubMed] [Google Scholar]
- 17.Van de Weert M., Hennink W. E., Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm. Res. 2000;17:1159–1167. doi: 10.1023/A:1026498209874. [DOI] [PubMed] [Google Scholar]
- 18.Johnson O. L., Jaworowicz W., Cleland J. L., Bailey L., Charnis M., Duenas E., Wu C., Shepard D., Magil S., Last T., Jones A. J. S., Putney S. D. The stabilization and encapsulation of human growth hormone into biodegradable microspheres. Pharm. Res. 1997;14:730–735. doi: 10.1023/A:1012142204132. [DOI] [PubMed] [Google Scholar]
- 19.Tracy M. A. Development and scale-up of a microsphere protein delivery system. Biotechnol. Prog. 1998;14:108–115. doi: 10.1021/bp9701271. [DOI] [PubMed] [Google Scholar]
- 20.Alkermes, http://www.alkermes.com/newsroom/showArticle.aspx?id = 278. Access Aug 13, 2007.
- 21.Castellanos I. J., Al-Azzam W., Griebenow K. Effect of the covalent modification with poly(ethylene glycol) on α-chymotrypsin stability upon encapsulation in poly(lactic-co-glycolic) microspheres. J. Pharm. Sci. 2005;94(2):327–340. doi: 10.1002/jps.20243. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez A., Vila-Jato J. L., J Alonso M. Development of biodegradable microspheres and nanospheres for the controlled release of cyclosporine A. Int. J. Pharm. 1993;99:263–273. doi: 10.1016/0378-5173(93)90369-Q. [DOI] [Google Scholar]
- 23.Diwan M., Park T. G. Pegylation enhances protein stability during encapsulation in PLGA microspheres. J. Control. Release. 2001;73:233–244. doi: 10.1016/S0168-3659(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 24.Pérez C., Jesús P.D., Griebenow K. Preservation of lysozyme structure and function upon encapsulation and release from poly(lactic-co-glycolic) acid microspheres prepared by the water-in-oil-in-water method. Int. J. Pharm. 2002;248:193–206. doi: 10.1016/S0378-5173(02)00435-0. [DOI] [PubMed] [Google Scholar]
- 25.Bodmer D., Kissel T., Traechslin E. Factors influencing the release of peptides and proteins from biodegradable parenteral depot systems. J. Control. Release. 1992;21:129–138. doi: 10.1016/0168-3659(92)90014-I. [DOI] [Google Scholar]
- 26.Alonso M. J., Cohen S., Park T. G., Gupta R. K., Siber G. R., Langer R. Determinants of release rate of tetanus vaccine from polyester microspheres. Pharm. Res. 1993;10:945–953. doi: 10.1023/A:1018942118148. [DOI] [PubMed] [Google Scholar]
- 27.Heya T., Okada H., Ogawa Y., Toguchi H. In vitro and in vivo evaluation of thyrotropin releasing hormone release from copoly(dl-lactic/glycolic acid) microspheres. Int. J. Pharm. 1991;72:199–205. doi: 10.1016/0378-5173(91)90108-Z. [DOI] [PubMed] [Google Scholar]
- 28.Crotts G., Sah H., Park T. G. Adsorption determines in vitro protein release rate from biodegradable microspheres: quantitative analysis of surface area during degradation. J. Control. Release. 1997;47:101–111. doi: 10.1016/S0168-3659(96)01624-0. [DOI] [Google Scholar]
- 29.Cleland J. L., Powell M. F., Shire S. J. The development of stable protein formulations: A close look at protein aggregation, deamidation, and oxidation. Crit. Rev. Ther. Drug Carr. Syst. 1993;10:307–377. [PubMed] [Google Scholar]
- 30.Harris J. M., Martin N. E., Modi M. Pegylation: a novel process for modifying pharmacokinetics. Clin. Pharmacokinet. 2001;40:539. doi: 10.2165/00003088-200140070-00005. [DOI] [PubMed] [Google Scholar]
- 31.Michaelis M., Cinati J., Cinati J., Pouckova P., Langer K., Kreuler J., Matousek J. Coupling of the antitumoral enzyme bovine seminal ribonuclease to polyethylene glycol chains increases its systemic efficacy in mice. Anticancer Drugs. 2002;13:149–154. doi: 10.1097/00001813-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Greenwald R. B., Choe Y. H., McGuire J., Conover C. D. Effective drug delivery by PEGylated drug conjugates. Adv. Drug Deliv. Rev. 2003;55:217–250. doi: 10.1016/S0169-409X(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 33.Shu J. Y., Tan C., DeGrado W. D., Xu T. New design of helix bundle peptide-polymer conjugates. Biomacromolecules. 2008;9:2111–2117. doi: 10.1021/bm800113g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguchi Y., Wun J., Duncan R., Strohalm J., Ulbrish K., Akaike T., Maeda H. Early phase tumor accumulation of macromolecules: A great difference in clearance rate between tumor and normal tissues. Jpn. J. Cancer Res. 1998;89:307–314. doi: 10.1111/j.1349-7006.1998.tb00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami Y., Tabata Y., Ikada Y. Tumor accumulation of poly(ethylene glycol) with different molecular weights after intravenous injection. Drug Delivery. 1997;4:23–32. doi: 10.3109/10717549709033184. [DOI] [Google Scholar]
- 36.Morar A.S., Schrimsher J. L., Chavez M. D. PEGylation of proteins: a structural approach: structural properties of PEGylated proteins could play an increasingly important role in developing optimal therapeutic protein drugs. (Protein PEGylation) Biopharm International. 2006;19(4):34. [Google Scholar]
- 37.Perez C., Castellanos I. J., Costantino H. R., Al-Azzam W., Griebenow K. Recent trends in stabilizing protein structure upon encapsulation and release from bioerodible polymers. J. Pharm. Pharmacol. 2001;54(3):310–313. doi: 10.1211/0022357021778448. [DOI] [PubMed] [Google Scholar]
- 38.Cleland J. L., Jones A. J. S. Stable formulations of recombinant human growth hormone and interferon-gamma for microencapsulation in biodegradable microspheres. Pharm. Res. 1996;13:1464–1475. doi: 10.1023/A:1016063109373. [DOI] [PubMed] [Google Scholar]
- 39.Alonso M. J., Gupta R. K., Min C., R Siber G., Langer R. Biodegradable microspheres as controlled-release tetanus toxoid delivery systems. Vaccine. 1994;12:299–306. doi: 10.1016/0264-410X(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 40.Sah H. Protein instability toward organic solvent/water emulsification: Implications for protein microencapsulation into microspheres. PDA J. Pharm. Sci. Technol. 1999;53:3–10. [PubMed] [Google Scholar]
- 41.Kim H. K., Park T. G. Microencapsulation of human growth hormone within biodegradable polyester microspheres: protein aggregation stability and incomplete release mechanism. Biotechnol. Bioeng. 1999;65:659–667. doi: 10.1002/(SICI)1097-0290(19991220)65:6<659::AID-BIT6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Crotts G., Park T. G. Preparation of porous and nonporous biodegradable polymeric hollow microspheres. J. Control. Release. 1995;35:91–105. doi: 10.1016/0168-3659(95)00010-6. [DOI] [Google Scholar]
- 43.Knubovets T., Osterhout J. J., Klibanov A. M. Structure of lysozyme dissolved in neat organic solvents as assessed by NMR and CD spectroscopies. Biotechnol. Bioeng. 1999;63:242–248. doi: 10.1002/(SICI)1097-0290(19990420)63:2<242::AID-BIT13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 44.Jain R., Shah N. H., Malick A. W., Rhodes C. T. Controlled drug delivery by biodegradable poly(ester) devices: Different preparative approaches. Drug Devel. Ind. Pharm. 1998;24(8):703–727. doi: 10.3109/03639049809082719. [DOI] [PubMed] [Google Scholar]
- 45.Arshady R. Preparation of biodegradable microspheres and microcapsules: 2. Polylactides and related polyesters. J. Control. Release. 1999;17:1–21. doi: 10.1016/0168-3659(91)90126-X. [DOI] [Google Scholar]
- 46.Péan J.-M., Venier-Julienne M.-C., Boury F., Menei P., Denizot B., Benoit J.-P. NGF release from poly(D,L-lactide-co-glycolide) microspheres. Effect of some formulation parameters on encapsulated NGF stability. J. Control. Release. 1998;56:175–187. doi: 10.1016/S0168-3659(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 47.Rojas J., Pinto-Alphandary H., Leo E., Pecquet S., Couvreur P., Fattal E. Optimization of the encapsulation and release of beta-lactoglobulin entrapped poly(D,L-lactide-co-glycolide) microspheres. Int. J. Pharm. 1999;183:67–71. doi: 10.1016/S0378-5173(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 48.Sah H. Stabilization of proteins against methylene chloride/water interface induced denaturation and aggregation. J. Control. Release. 1999;58:143–151. doi: 10.1016/S0168-3659(98)00148-5. [DOI] [PubMed] [Google Scholar]
- 49.Suslick K.S., Hammerton D. A., Cline R. E. The sono-chemical hot spot. J. Am. Chem. Soc. 1986;108:5641–5642. doi: 10.1021/ja00278a055. [DOI] [Google Scholar]
- 50.Reisz P., Kondo T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992;13:247–270. doi: 10.1016/0891-5849(92)90021-8. [DOI] [PubMed] [Google Scholar]
- 51.Zambaux M. F., Bonneaux F., Gref R., Dellacherie E., Vigneron C. Preparation and characterization of protein C-loaded PLA nanoparticles. J. Control. Release. 1999;60:179–188. doi: 10.1016/S0168-3659(99)00073-5. [DOI] [PubMed] [Google Scholar]
- 52.Morlock M., Koll H., Winter G., Kissel T. Microencapsulation of rh-erythropoietin using biodegradable poly(D,L-lactide-co-glycolide): Protein stability and the effect of stabilizing excipients. Eur. J. Pharm. Biopharm. 1997;43:29–36. doi: 10.1016/S0939-6411(96)00017-3. [DOI] [Google Scholar]
- 53.Uchida T., Shiosaki K., Nakada Y., Fukada K., Eda Y., Tokiyoshi S., Nagareya N., Matsuyama K. Microencapsulation of hepatitis B core antigen for vaccine preparation. Pharm. Res. 1998;15:1708–1713. doi: 10.1023/A:1011904627929. [DOI] [PubMed] [Google Scholar]
- 54.Sah H. Protein behavior at the water/methylene chloride interface. J. Pharm. Sci. 1999;88:1320–1325. doi: 10.1021/js9900654. [DOI] [PubMed] [Google Scholar]
- 55.Perez C., Griebenow K. Improved activity and stability of lysozyme at the water/CH2Cl2 interface: enzyme unfolding and aggregation and its prevention by polyols. J. Pharm. Sci. 2001;53:1217–1226. doi: 10.1211/0022357011776667. [DOI] [PubMed] [Google Scholar]
- 56.Lee E. S., Park K. H., Kim D. M., Park I. S., Min H. Y., Lee D. H., Kim S., Kim J. H., Na K. Protein complexed with chondroitin sulfate in poly(lactide-co-glycolide) microspheres. Biomaterials. 2007;28(17):2754–2762. doi: 10.1016/j.biomaterials.2007.01.049. [DOI] [PubMed] [Google Scholar]
- 57.Bittner B., Witt C., Mader K., Kissel T. Degradation and protein release properties of microspheres prepared from biodegradable poly(lactide-co-glycolide) and ABA triblock copolymers: influence of buffer media on polymer erosion and bovine serum albumin release. J. Control. Release. 1999;60:297–309. doi: 10.1016/S0168-3659(99)00085-1. [DOI] [PubMed] [Google Scholar]
- 58.Park T. G., Lee H. Y., Nam Y. S. A new preparation method for protein loaded poly (D,L-lactic-co-glycolic acid) microspheres and protein release mechanism study. J. Control. Release. 1998;55:181–191. doi: 10.1016/S0168-3659(98)00050-9. [DOI] [PubMed] [Google Scholar]
- 59.Crotts G., Park T. G. Stability and release of bovine serum albumin encapsulated within poly(D,L-lactide-co-glycolide) microparticles. J. Control. Release. 1997;44:123–134. doi: 10.1016/S0168-3659(96)01511-8. [DOI] [Google Scholar]
- 60.Butler S. M., Tracy M. A., Tilton R. D. Adsorption of serum albumin to thin films of poly(lactide-co-glycolide) J. Control. Release. 1999;58:335–347. doi: 10.1016/S0168-3659(98)00173-4. [DOI] [PubMed] [Google Scholar]
- 61.Bouillot P., Ubrich N., Sommer F., Duc T. M., Loeffler J. P., Dellacherie E. Protein encapsulation in biodegradable amphiphilic microspheres. Int. J. Pharm. 1999;181:159–172. doi: 10.1016/S0378-5173(99)00023-X. [DOI] [PubMed] [Google Scholar]
- 62.A. Klibanov and R. S. Langer. Methods of decreasing the hydrophobicity of fibroblast and other interferons. United States Patent 4,414,147.
- 63.Hinds K. D., Campbell K. M., Holland K. M., Lewis D. H., Piche C. A., Schmidt P. G. PEGylated insulin in PLGA microparticles. In vivo and in vitro analysis. J. Control. Release. 2005;104:447–460. doi: 10.1016/j.jconrel.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 64.O. B. Kinstler, N. E. Gabriel, C. E. Farrar, R. B. DePrince. N-Terminally chemically modified protein compositions and methods. United States Patent 5, 824, 784.
- 65.Daly S. M., Przybycien T. M., Tilton R. D. Adsorption of poly(ethylene glycol)-modified lysozyme to silica. Langmuir. 2005;21:1328–1337. doi: 10.1021/la048316y. [DOI] [PubMed] [Google Scholar]
- 66.Daly S. M., Przybycien T. M., Tilton R. D. Coverage dependent orientation of lysozyme adsorbed on silica. Langmuir. 2003;19:3848–3857. doi: 10.1021/la026690x. [DOI] [Google Scholar]
- 67.Daly S. M., Przybycien T. M., Tilton R. D. Aggregation of lysozyme and poly(ethylene glycol)-modified lysozyme after adsorption to silica. Colloids Surf., B Biointerfaces. 2007;57(1):81–88. doi: 10.1016/j.colsurfb.2007.01.007. [DOI] [PubMed] [Google Scholar]