Abstract
Among neuroscientists, astrocytes have for long played Cinderella to their neuron stepsisters. While the importance of glia in regulating brain activity was predicted by Ramon y Cajal more than a century ago (Garcia-Marin et al., Trends. Neurosci. 30:479–787, 2007), these cells, until recently, have been thought to play mainly a passive part in synaptic signaling. Results obtained over the last decade have begun to suggest otherwise. Experiments carried out in a number of labs have shown that glial cells, especially astrocytes, directly participate in synaptic signaling and potentially regulate synaptic plasticity and network excitability. The presence of signaling pathways on astrocytes that are analogous to those at presynaptic terminals suggests a role for these cells in network plasticity. Findings that the same signaling pathways can be activated by receptors for drugs of abuse present on astrocytes suggest a role for these cells in the addictive process. In this review, we summarize current understanding of astrocytic role in synaptic signaling and suggest that a complete understanding of the process of addiction requires a better understanding of the functional role of these cells.
Key words: astrocyte, calcium, gliotransmission, nicotinic, synapse, tripartite synapse
WHAT IS A GLIAL CELL?
The earlier notion that glial cells can be classified into multiple categories based on the presence of biochemical markers has been challenged by the realization that different cell types in the brain have common precursors (2) and can often be phenotypically interconvertible. ‘Glial’ cells in the sub-ventricular region identified by markers such as the glial fibrillary acidic protein (GFAP) act as neuronal precursors. Similarly, transient expression of the glial marker S100β marks granule neuron precursors in the cerebellum (3). Conversely, many protoplasmic astrocytes in the brain do not express GFAP (4). Radial glia, long thought to provide a scaffold for neuronal migration, can also act as neuronal stem cells (5–7). An interesting aspect of glial precursors of neurogenesis is that it is both neuron and region specific in the brain. Projection neurons in the cortex are derived from radial glia but interneurons are not. Unlike neurons in the cortex that are radial glial in origin, neurons in the basal ganglion do not arise from glial-like precursors (8). These studies on radial glia add to their already established role as astrocyte precursors (9,10). It appears that, depending on induction of specific transcriptional factors, glial cells can differentiate into neurons and/or astrocytes (11). Further contradicting the long-lived dogma, radial glia do not have an exclusive role during development but also might play an important role in adult neuro- and glio-genesis (12). These studies indicate that we need to re-define what a glial cell is in order to understand their role in CNS function.
CURRENT UNDERSTANDING OF COMMUNICATION BETWEEN ASTROCYTES AND NEURONS
The idea that astrocytes are important for neuronal function and plasticity is now widely accepted. Normal functioning of synapses is controlled by astrocytic mechanisms. In an elegant study, Barres and coworkers demonstrated that the number of mature and functional synapses between neurons are controlled by surrounding glia (13,14). Interestingly, these effects are mediated by glycoproteins known as thrombospondins, previously thought to be only involved in blood coagulation. Thrombospondins are secreted in the CNS by astrocytes and act as putative paracrine effectors of synapse function (15).
Astrocytes and Brain Microcirculation: An Example of Neuron–Astrocyte Signaling
Blood circulation in the brain is entirely under local control. The advantage of such an organization is to divert blood flow specifically to areas of activity in the brain. This allows local synaptic activity to control the supply of nutrients to that specific area. The role of astrocytes in the control of brain microcirculation is now well accepted.
Specialized astrocytes, with characteristic endfeet that abut blood vessels in various brain areas (16–18), control the elasticity of local arterioles. In addition, a single astrocyte has numerous processes enveloping a large number of synapses (Fig. 1a). Anatomical studies have invoked numbers in the order of 6,000 synapses contacted by a single Bergmann glial cell in the cerebellum (19). In the CA1 region of the mammalian hippocampus, almost all adjacent synapses are separated by astrocytes, although not all might be in close enough proximity to be considered a part of the synaptic complex (20).
Fig. 1.
Signaling at a tripartite glutamatergic synapse. a A single astrocyte (cyan, labeled A) sends out processes that envelop or are in close proximity to a number of synapses. Postsynaptic neuron and its dendrite (red, labeled N); presynaptic axon and bouton (green). b Both astrocytes and the postsynaptic neuron respond to released glutamate at a synapse. Glutamate release from the presynaptic terminal acts on the ionotropic glutamate receptors (blue rectangles) at the postsynaptic membrane (red) and on mGluRs (blue cylinders) on the astrocytic membrane (cyan). Activation of mGluRs on astrocytes initiates an intracellular calcium wave that propagates down the length of the astrocytic processes. c Astrocytes have a form of excitability mediated by calcium signals. An example of a mechanism for intracellular propagation of calcium waves. Activation of astrocytic P2Y receptors (blue rectangles) activates the phospholipase C signaling cascade to produce IP3 as a second messenger. Released IP3 acts on its receptors on the ER (green ovals) to release calcium from stores. Calcium thus released can either modulate the activation of IP3Rs by ambient level of IP3, or activate Phospholipase C to increase production of the second messenger. At the same time, release of ATP from the astrocyte acts as a regenerative mechanism for the propagation of calcium signals via progressive activations of P2YRs down the astrocytic soma and processes. d Astrocytes can release transmitters to talk back to neurons. An example of astrocyte to neuron communication. Activation of an astrocyte by synaptically released glutamate can, in turn, trigger release of the transmitter from astrocytic vesicles (cyan circles) back on the postsynaptic neuron. One mechanism by which this communication can occur is via the activation of extrasynaptic NMDA receptors (blue cylinders) on the postsynaptic dendrite. This would be a distinct mechanism from the presynaptic release of glutamate (green vesicles) acting on synaptic ionotropic glutamate receptors (blue rectangles)
Synaptic activity can trigger astrocytic calcium signaling, leading to the release of both vasodilatory and constrictive agents. The mechanisms underlying such synapse–neuron communication is described in detail in the next section. At the endfeet of the astrocyte, the arrival of calcium signals triggers a second messenger cascade starting with the activation of phospholipase A2. Activation of the enzyme leads to the production of downstream prostaglandins, predominantly PGE2, resulting in vasodilation (21,22). In addition, activation of cytochrome P450 enzymes (CYP)-dependent pathways led to the production of eicosatrienoic acids (EETs), another set of vasodilatory compounds, from endfeet (23). Nitric oxide (NO) produced by neurons and astrocytes in an activity-dependent manner can inhibit the activity of CYP2, a key enzyme in the production of epoxyeicosatrienoic acids (24).
Increased potassium concentrations around vascular smooth muscle cells can activate an inwardly rectifying potassium channel (Kir) leading to their relaxation. This process can be linked to neuronal stimulation and astrocytic calcium signals as astrocytic endfeet contain large calcium-dependent potassium conductances (BK channels), activation of which leads to this form of potassium channel–potassium channel-mediated vasodilation (25).
Surprisingly, astrocytic calcium signaling can also result in vasoconstriction (26–28). A separate arm of the phospholipase A2 pathway appears to be involved in astrocyte-mediated vasoconstriction. One pathway for this apparently paradoxical effect is thought to be the diffusion of arachidonic acid across the endfoot into the smooth muscle cells. The metabolite is acted upon by cytochrome P450 enzymes in the smooth muscle cells, the key among these being CYP4A, resulting in the generation of vasoconstrictive eicosanoids like 20-hydroxyeicosatetraenoic acid (20-HETE; 26).
This contradictory effect of astrocytes on vascular tone suggests that the balance between vasoconstriction and vasodilation can be actively manipulated by perivascular astrocytes. One regulator of such a balance between vasodilation or vasoconstriction could be NO (18). However, it is as yet unclear how astrocytes respond to neuronal energy requirements in order to drive the production of arachidonic acid metabolites to one or the other end process. This remains an active area of investigation.
Astrocytes—A Component of Tripartite Signaling at CNS Synapses
The idea that astrocytes, like neurons, might have diverse, and region-specific, roles to play in development and function of the central nervous system is slowly gaining recognition. This implies that it might not be useful to talk about astrocytic function in general anymore than with neurons. Examining neurons, their synapses, and astrocytes as a single local functional unit involving mutual interactions will help in the elucidation of local network modulation by these glial cells. That astrocytes form an integral and active part of synaptic transmission is a currently accepted concept.
The physiological role of astrocytes in maintaining and modulating synaptic function is yet to be fully understood. Current evidence suggests that astrocytes might act as local controllers of synaptic function determined by the extent of influence for a single astrocyte. As one astrocyte can contact, or be in proximity, of multiple synapses, the idea that these cells might act as integrators of local network activity is tempting.
Such an idea requires the astrocyte to fulfill three requirements (Fig. 1): (1) They must be able to respond to synaptic activity. (2) They need to transmit this information to relatively distant synapses. (3) They need to have mechanisms for communicating back to synapses.
Neuron-to-Astrocyte Communication
The idea that astrocytes can actively respond to neuronal activity is now quite well accepted. A number of functional receptors for various neurotransmitters that might be released at a synapse have been reported (29). These include purinergic receptors (29,30), AMPA receptors (AMPARs; 31,32), metabotropic glutamate receptors (mGluRs; 33), GABAA receptors (GABARs; 34), nicotinic acetylcholine receptors (nAChRs; 35–38), muscarinic receptors (mAChRs; 39), and α1- as well as β2-noradrenergic receptors (40,41), possibly among others. In some cases where ultrastructural evidence exists, the astrocytic receptors have been localized close to synaptic release sites (42,43). In oligodendrocyte progenitor cells, ‘synaptic’ AMPAR currents have been seen in response to vesicular release of glutamate (44,45).
While each of these receptor types is likely to have specific functional roles, for the purpose of this review, it suffices to state that there is clear evidence that astrocytes can respond to synaptic release of various neurotransmitters in the brain.
Propagation of Signals by Astrocytes
While it is known that astrocytes possess low densities of sodium channels and, therefore, do not propagate signals via action potentials (APs), it is now recognized that these cells have a form of excitability based on sophisticated calcium-signaling mechanisms. This signal manifests in the form of intra- and intercellular calcium waves that can mediate communication across significant distances (Fig. 1c).
Complex calcium dynamics in astrocytes
The existence of propagating calcium signals along the length of an astrocyte provides credence to the idea of chemical propagation of signaling. From our current understanding of published literature, we can postulate one operative mechanism. Activation of surface metabotropic receptors (mGluRs, for instance) activates the phospholipase C (PLC) signaling cascade leading to the production of inositol trisphosphates (IP3) and the activation of their receptors (IP3Rs) on the endoplasmic reticulum (ER). Activation of IP3Rs triggers a regenerative propagation of calcium changes along an astrocyte (Fig. 1c). This propagation involves the balance of a number of finely tuned processes. IP3Rs are regulated in a biphasic manner by calcium, where higher local concentration of the ion inhibits the receptor channel open probability (46,47). Therefore, local accumulation of calcium around the channel would inhibit further release of ER calcium from that microdomain. In addition, there is tuning of the receptor opening by calcium binding to the luminal side of the IP3R within the ER (48). This tight positive and negative control of the receptor opening accounts for the dynamics of local calcium changes in the cytosol around this microdomain. In addition, diffusion of IP3 itself and calcium-dependent modulation of PLC also serve as effective means of signal propagation.
The control of calcium levels around these release sites, in turn, is dependent on the clearance of the ion. There is rapid calcium buffering in all cell types which in turn maintains a tight spatial and temporal control over calcium signal transduction. The domain of influence of calcium signals is thus determined by these buffers as well as the affinity of downstream calcium-signaling pathways for the ion. At synapses, where transmitter release requires micromolar calcium concentrations, incoming calcium flux via voltage-gated calcium channels (VGCCS) would exert a sphere of influence in the 100–200-nm range from influx sites (49,50).
Both mobile and immobile calcium buffering mechanisms play a significant role in these processes. Around the ER, calcium is pumped backed into the stores by the family of sarcoplasmic reticulum Ca/Mg ATPases (SERCA pumps). Another potentially high capacity buffer as well as a source of calcium release is the astrocytic mitochondria. Potential spatial organization of the ER and mitochondria (51) as well as the crosstalk of calcium signals between the two organelles (52) affects downstream consequences of astrocytic calcium waves, e.g., glutamate release. In addition, significant concentrations of high affinity diffusible calcium buffers (53) in astrocytes would serve to rapidly attenuate local calcium increases as well as increase diffusion and sphere of influence of calcium signals (54).
Signals via IP3Rs can thus be modulated via complex local and global calcium-signaling mechanisms in order to propagate signals down astrocytic processes as an effective form of signal propagation in response to synaptic activity.
In addition, astrocytes also possess ER ryanodine receptors (RyRs), which might play a role in astrocytic calcium signaling (51,55,56) both by linking calcium influx (e.g., via ionotropic receptor activation) to store calcium release via calcium-induced calcium release (CICR) as well as by spatial and temporal control of IP3R signaling.
Controlled changes in calcium levels in the astrocytic cytosol, therefore, have a distinct physiological role to play. Consistent with this view is the finding that astrocytes in more intact preparations show spontaneous, IP3-mediated, and well-defined calcium oscillations (21,57,58). These oscillations, via downstream signaling mechanisms, might play a role in modulating synaptic functions. Current evidence suggests that the frequency of calcium oscillations in astrocytes is controlled within each cell and not across astrocytes. The data suggest that these calcium oscillations might be involved in local regulation of synaptic activity rather than having a large field of influence (58). However, our data (Grybko and Vijayaraghavan, unpublished results) suggest that, under certain conditions, like that in the presence of nicotine, calcium oscillations across astrocytes can be synchronized, suggesting that these cells might function as ‘pacemakers’ for regulating short- and long-term excitability of local neuronal networks over a larger area than currently assumed (Fig. 2). The discovery that changes in astrocytic calcium lead to corresponding alterations in calcium signals from proximal neurons (also see below) is consistent with this view (59).
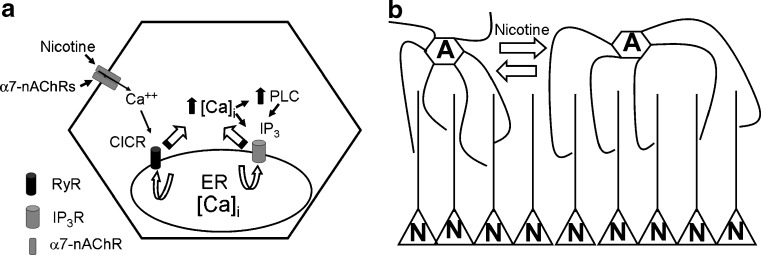
Fig. 2.
Putative mechanism for the action of nicotine via astrocytes. a Activation of calcium signaling by nicotine. Astrocytes possess functional α7-nAChRs. Activation of these receptors by nicotine results in calcium influx into the astrocyte which, in turn, triggers calcium-induced calcium release via the opening of RyRs on the ER calcium stores. These signals can further be amplified by the subsequent activation of IP3Rs and release of ATP as shown in Fig. 1c. b Hypothetical modulation of neuronal networks by nAChRs on astrocytes. Astrocytes (denoted by A), via their numerous processes and proximity to synapses, exert local influence on neuronal activity, possibly by synchronizing the activity of a small groups of neurons (denoted by N). Activation of nAChRs on astrocytes coordinates calcium oscillations across a group of astrocytes, in turn modulating network activity across a larger area
Propagation of calcium signals by extracellular mechanisms: a role for ATP
In addition to the complex control of calcium release and diffusion discussed above, astrocytes can also use ATP for an effective extracellular propagation of synaptic signals. Astrocytes are known to release ATP as a second messenger in response to activity (see below). While the mechanism of ATP release from astrocytes are not yet fully understood, nor is its extent of influence in intact brain tissue, the nucleotide remains an attractive candidate for extracellular propagation of calcium signals along the length of an astrocyte and, perhaps, across a limited population of glia and neurons.
Intercellular calcium waves: a relevant mechanism for astrocytic communication?
Evidence for communication across astrocytes has long been known in purified cell culture preparations (60–62) and in brain slice cultures (63,64). Both gap junction-mediate mechanisms (65–67) as well as propagation via external purinergic mechanisms (62,68) have been postulated to contribute to these intercellular waves. The initial idea that calcium released via IP3R activation directly spreads by diffusion through glial gap junctions appears unlikely due to the limited diffusion of the ion, as discussed above. A likely candidate for such a propagation across gap junctions is the generated IP3 itself (65,69) which not only has a longer range of diffusion but can also be regenerative due to its production by local calcium-dependent mechanisms.
The idea that gap junctions play a significant role in propagating calcium waves is undercut by the fact that: (1) most gap junction blockers used in these studies are notoriously non-specific; (2) significant reduction in the number of gap junctions in connexin-43 knockout mice does not show dramatic effects on wave propagation (70), even though it has been suggested that expression of connexin hemichannels plays a role in ATP-mediated wave propagation (68).
Lastly, while intercellular calcium waves have been demonstrated in more intact preparations like the retina (60,71), occurrences of long distance communication in brain tissue appear to be rare (for review see 72). Intercellular signaling by astrocytes may have other potential roles like long distance transport of metabolites (73), but its function as a direct means for modulation of calcium-dependent synaptic plasticity might be limited. Instead, astrocyte neuron communication might be restricted to more local domains and intercellular calcium waves might have a role to play only in certain pathological conditions. However, under these pathological conditions, when astrocytes become reactive and undergo significant changes in their properties, such long distance communication may exacerbate symptoms (74,75).
While the extent of propagation of calcium signals by astrocytes might be debated, one conclusion can be derived from the two sections described above. Astrocytes possess both an ability to detect synaptic activity and to transmit these signals across significant distances, albeit restricted in space.
Communicating Back to Neurons: Gliotransmitters
A third requirement to the idea of an active role for astrocytes in modulating synaptic plasticity is the existence of mechanisms in these cells to talk back to the synapse (Fig. 1d). The evidence, obtained over the last decade, for the existence and release of potential transmitters from astrocytes clearly points to their ability to directly modulate neuronal activity (59,76,77). A few of the known neurotransmitters shown to be released by astrocytes in the brain are discussed below.
ATP
The release of ATP from astrocytes has been known for a long time and was initially proposed as a mechanism for the propagation of intercellular calcium waves (see above). Equally documented is ability of ATP acting via the ionotropic P2X and the metabotropic P2Y receptors to mediate and modulate synaptic transmission. ATP receptors are ubiquitous in the nervous system and act as fast transmitters in the CNS (78), postsynaptic modulators (79,80), as well as powerful modulators of transmitter release affecting the release of glutamate (81), dopamine (82), norepinephrine (83), and GABA (84,85), to name a few. The receptors have been implicated in a number of CNS functions ranging from modulation of learning and memory to roles in CNS pathology (reviewed in 86).
There is a significant debate on the mechanisms underlying ATP release from astrocytes. A number of mechanisms have been proposed. ATP has been suggested to be released via volume-regulated anion channels (87,88). Another potential mechanism is that ATP is released via connexin hemi-junctions expressed on the astrocytic plasma membrane (68,89–91). More recent evidence also points to vesicular exocytic mechanisms for the release of ATP (92). As at least some of these mechanisms are calcium dependent, release of ATP from astrocytes is regulated by neuronal activity and consequent changes in both extra- and intracellular calcium levels. Release of the nucleotide can be further regenerated by ATP-mediated ATP release via the activation of astrocytic P2X7 class of purinergic receptors (93).
The presence of high levels of ectonucleotidases also raises the possibility of astrocytic signaling byproducts of ATP hydrolysis. Adenosine, the end product of this process, is known to be a powerful modulator of synaptic activity via its actions on the A1, A2, and A3 adenosine receptors. ATP release from glia has been shown to inhibit synaptic activity via adenosine production (94,95).
Whatever the mechanism for its release, the consensus is that ATP is an important component of astrocytic signaling and plays an important role in glial–neuronal interaction.
Glutamate
In astrocytes, the presence of all components for exocytic release (96,97) has generated a lot of interest in release of transmitters in a manner analogous to the presynaptic terminals. Considerable attention has been focused over the last few years on the vesicular release of glutamate from astrocytes. The existence of calcium- and SNARE protein-dependent glutamate release from astrocytes is well documented (for review see 98–100). Further, the released transmitter produces slow and large glutamatergic currents on proximal neurons, mainly via the activation of extrasynaptic NMDA receptors, which then synchronizes neuronal activity (101). This large extrasynaptic current raises the possibility that glutamate release from astrocytes might involve larger vesicles than those at nerve terminals (102).
d-Serine
The demonstration of the presence of serine racemase in astrocytes resulting in the production of d-serine has led to the idea that the amino acid might be a significant player in the regulation of NMDA receptors by acting as the natural substrate for the glycine binding site on the receptor (103,104). Though the trigger for its release and mechanisms underlying its control are largely unknown, recent evidence suggests that d-serine might be released by calcium-dependent exocytosis (105).
The existence of glial-mediated transmitter release provides evidence that astrocytes can signal back to neurons thus fulfilling all criteria necessary for glia-mediated transmission in the CNS. The presence of astrocyte-dependent NMDA receptor currents on neurons is intriguing. How these receptors might be activated in the absence of a depolarization component to relieve the magnesium block at NMDA receptor-only regions is still unclear. The promiscuity of individual astrocytes in their transmitter release capabilities might provide a clue for how this might be achieved, e.g., by the co-release of ATP and glutamate at local sites.
Astrocyte-driven modulation of postsynaptic activity by the extrasynaptic release of gliotransmitters might be an important means of regulating synaptic strength and plasticity in the CNS (106).
A Potential Role for Astrocyte–Neuron Interactions in Drug Addiction
Though sparse in details at present, the role of astrocytes in the actions of drugs of abuse is a rapidly emerging area of research. There is accumulating evidence that astrocytes respond to addictive drugs and that altering astrocytic mechanisms changes addictive behaviors. It is the author’s opinion that recognition of the possibility that these drugs act more globally on brain processes will require a paradigm shift that includes a role for these cells.
Homeostatic view of drug addiction
Much of addiction research is centered on the mesolimbic reward areas of the brain. While it is true that these areas participate in self-administration of drugs of abuse in animal systems, addiction in humans is clearly predicated by a number of additional factors, cognitive, emotional, and contextual, as well as by individual perceptions of the world. Combined with the fact that targets for drugs of abuse exist throughout the brain, it is not clear where the appropriate emphasis of addiction research should be placed. Conceptually, the idea that addiction is a form of learning that is driven by an individual desire for hedonic homeostasis as proposed a few years ago (107,108) is appealing. In this view, compulsive drug taking would be a form of self-medication by an individual in order to achieve an acceptable level of balance between positive and negative emotional states. Such an idea would imply that these drugs allow an individual to ‘tune’ the brain in order to achieve a new level of affective homeostasis. The problem is that such a state (the term ‘allostasis’ is used to describe this state) would be dependent on the presence of the drug itself and its withdrawal would lead to emotional distress as the brain varies all parameters simultaneously in an effort to achieve a new affective balance. This would intuitively lead to a strong drive for reinstating the drug.
Proposing a role for astrocytes in the above view of synaptic plasticity in drug abuse is tempting. One interpretation of the discussion in the previous sections on astrocytic control of synaptic function is that these cells act as ‘pacemakers’ of neuronal activity by titrating the integral response of networks. This view is certainly an attractive one based on the above, more global, view of drug addiction. Such an idea would imply that astrocytes will respond to drugs of abuse and set activation thresholds for networks, altering synaptic plasticity over the short or long term. These effects on synaptic strength would also depend on the presence of the drug, contributing to its reinforcement as well as distress upon drug withdrawal.
Astrocytes and drugs of abuse
Clues to the involvement of astrocytes in mediating the effects of drugs of abuse come from studies in Drosophila. Hypomorphic mutants of a glial protein named Moody results in enhanced sensitivity to cocaine. Surprisingly, the same mutants show reduced sensitivity to the intoxicating effects of ethanol (109). Similarly, mutations or deletions of a number of glia-expressed proteins that participate in circadian control in flies alter or abolish rewarding effects of cocaine (for review see 110). The results provide genetic evidence for a role of astrocytic proteins in the addictive process.
This conclusion has been strengthened by studies in mammalian models that hint at a role for astrocytes in mediating effects of these drugs. Exposure to drugs of abuse like cocaine and morphine results in astrogliosis (111–113). The glial inhibitor, propentofylline, results in a reduction in the rewarding effects of both morphine and methamphetamine (114,115). Further, evidence exists that these glial changes might have a role to play in the development of tolerance to drugs of abuse. Administration of morphine increases GFAP expression in mice. At the same time, blocking astrocytic function with fluorocitrate partly attenuates tolerance to the analgesic effects of morphine (111). A recent report also indicates the presence of the functional cannabinoid receptor CB-1 on astrocytes which, upon activation, results in the triggering of IP3-dependent calcium signals in the astrocytes (116). Astrocytes exert significant control over the survival of midbrain dopaminergic neurons, which are involved in the rewarding effects of drugs of abuse (117).
The idea that astrocytes might determine activity threshold in the brain agrees well with a broader role for drugs of abuse in maintaining network homeostasis. Clearly, activation of receptors for drugs of abuse on astrocytes leads to changes in neuronal plasticity and consequently can result in the aberrant learning induced by drugs of abuse.
Nicotine, Synaptic Plasticity, and Astrocytes
Nicotine has been shown to have considerable effects on synaptic plasticity. The drug has short- and long-term potentiation effects (118–121) as well as alterations in behaviors involving learning and memory (118,122,123). In the mesolimbic reward areas, nicotine has significant effects on plasticity that might underlie some of its addictive properties (121,124). At the mossy fiber–CA3 pyramidal cell synapse, nicotine, acting via a nicotinic acetylcholine receptor (nAChRs) subtype containing the α7 gene product (α7-nAChR), mediates an unusual form of synaptic plasticity. It mediates a burst of glutamate release resulting in synaptic transmission in the absence of incoming presynaptic action potentials. This burst effectively ‘hijacks’ the synapse, making changes in synaptic strength independent of physiological contexts but dependent on the presence of the drug (125,126). The effects of nicotine on glutamate release at this synapse occur after a lag and are entirely dependent on calcium release from intracellular calcium stores. Whether such unusual mechanisms are employed by the drug at other synapses remains to be seen. A similar action potential-independent transmission has been shown at synapses in the caudal region of the nucleus of solitary tract, mediated by ATP and the activation of P2X receptors (81), suggesting that this effect might not be restricted to a specialized group of synapses.
One possible mechanism by which local nAChR activation can transmit information in the absence of incoming information down the presynaptic axon is by activating and modulating synapses via astrocytes. This would explain one unusual observation seen at the mossy fiber synapse. In spite of the fact that the proximal dendrite of the CA3 pyramidal neuron has a number of terminals clustered together, nicotine-induced burst of glutamate release has a very specifically timed onset. As this effect depends on endoplasmic reticulum store calcium release (125,126), the synchronization of transmitter release would depend on concerted release of calcium across boutons. Coupled with the observed lag, our findings suggest that there must be mechanisms allowing for crosstalk across a number of synapses in this region. A parsimonious explanation for these findings is that the synchrony comes from a single site of nicotine action, e.g., the astrocyte. Such a view is also supported by results showing that ATP (a potential candidate for the control of the mossy fiber-CA3 synapse mediated via nAChRs on astrocytes) can mediate presynaptic action potential-independent transmission at another synapse (81).
Functional nAChRs have been demonstrated from purified astrocytes (35,127). In these cells, activation of α7-nAChRs results in large calcium transients even in the presence of a very low density of receptors. These calcium transients result mainly from the release of the ion from calcium stores and involve both RyRs as well as IP3Rs in the astrocyte (35). Activation of α7-nAChRs on these cells triggers both intra- and intercellular calcium waves (128).
Presence of nAChRs has been demonstrated on astrocytes from more intact preparations. Mouse brain sections show immunoreactivity for a number of nAChR subtypes (37,38) and the expression patterns of these subtypes are differentially regulated with age (36). Further, our results, using calcium imaging, suggest the presence of functional α7-nAChRs on astrocytes from acute hippocampal slices (Grybko and Vijayaraghavan, unpublished results).
Taken together, the above findings provide sufficient evidence to implicate astrocytes in the actions of many drugs of abuse. The extent of astrocytic involvement and mechanistic bases underlying their effects on addiction remains to be determined.
CONCLUSIONS
Our understanding of astrocytic function has evolved dramatically over the last decade. Recognition of the active role played by these cells has broadened our perspective from a neuro-centric view of brain function to one that is more inclusive, dynamic, and interactive.
While it is well accepted that astrocytes have a form of excitability that is calcium driven and propagates at a slower rate than electrical signals, the exact purpose of such signals remains less clear. The idea that the astrocytic network functions as an integrator of neuronal network activity is currently gaining ground. Acceptance of this viewpoint will result in the revamping of our understanding of synaptic plasticity and behaviors involving learning and memory, among others.
A number of mechanistic features of astrocytic signaling involved in synaptic modulation remain to be worked out. A considerable confusion exists in the literature regarding the mechanics of ATP and glutamate release as well as the extent of propagation of astrocytic signals. Part of this confusion might result from a lack of appreciation of astrocytic diversity. If astrocytes are extensively involved in the modulation of normal brain functions, then it is not correct to treat them as a singular entity. Functionally, astrocytes are likely to be as diverse as neurons and treating them as a single population will be as uninformative as talking about neuronal function in the absence of their anatomical and physiological contexts.
An exciting development is the accumulating evidence for an astrocytic role in drug addiction. Yet in a nascent state, it is a field that is likely to burgeon over the next few years. A prediction is that factoring astrocytes into the equation will result in a better (and more realistic?) understanding of the process, one that moves away from interpretations based on defined loci, like the mesolimbic reward system, to an appreciation of a more global role played by these drugs in brain functions. Such a paradigm shift will be necessary for rational drug design to effectively combat the addictive process.
Acknowledgements
Funding was provided by the National Institute for Drug Abuse (RO1 DA 10266) and the National Institute of Deafness and Communication Disorders (RO1 DC 008855). The author thanks Geeta Sharma for comments on the manuscript.
References
- 1.Garcia-Marin V., Garcia-Lopez P., Freire M. Cajal’s contributions to glia research. Trends Neurosci. 2007;30:479–487. doi: 10.1016/j.tins.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla A., Garcia-Verdugo J. M., Tramontin A. D. A unified hypothesis on the lineage of neural stem cells. Nat. Rev. Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 3.Hachem S., Laurenson A. S., Hugnot J. P., Legraverend C. Expression of S100B during embryonic development of the mouse cerebellum. BMC Dev. Biol. 2007;7:17. doi: 10.1186/1471-213X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bushong E. A., Martone M. E., Jones Y. Z., Ellisman M. H. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyata T., Kawaguchi A., Okano H., Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 6.Noctor S. C., Flint A. C., Weissman T. A., Wong W. S. Dividing precursor cells of the embryonic cortical ventricular zone have morphological and molecular characteristics of radial glia. J. Neurosci. 2002;22:3161–3173. doi: 10.1523/JNEUROSCI.22-08-03161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noctor S. C., Flint A. C., Weissman T. A., Dammerman R. S. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 8.Malatesta P., Hack M. A., Hartfuss E., Kettenmann H. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 9.Schmechel D. E., Rakic P. A Golgi study of radial glial cells in developing monkey telencephalon: morphogenesis and transformation into astrocytes. Anat. Embryol. (Berl) 1979;156:115–152. doi: 10.1007/BF00300010. [DOI] [PubMed] [Google Scholar]
- 10.Chanas-Sacre G., Rogister B., Moonen G., Leprince P. Radial glia phenotype: origin, regulation, and transdifferentiation. J. Neurosci. Res. 2000;61:357–363. doi: 10.1002/1097-4547(20000815)61:4<357::AID-JNR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Colognato H., ffrench-Constant C. Mechanisms of glial development. Curr. Opin. Neurobiol. 2004;14:37–44. doi: 10.1016/j.conb.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Bonfanti L., Peretto P. Radial glial origin of the adult neural stem cells in the subventricular zone. Prog. Neurobiol. 2007;83:24–36. doi: 10.1016/j.pneurobio.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Ullian E. M., Sapperstein S. K., Christopherson K. S., Barres B. A. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 14.Ullian E. M., Barres B. A. The Schwann song of the glia-less synapse. Neuron. 1998;21:651–652. doi: 10.1016/s0896-6273(00)80579-6. [DOI] [PubMed] [Google Scholar]
- 15.Christopherson K. S., Ullian E. M., Stokes C. C., Mullowney C. E. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Iadecola C., Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 17.Straub S. V., Nelson M. T. Astrocytic calcium signaling: the information currency coupling neuronal activity to the cerebral microcirculation. Trends Cardiovasc. Med. 2007;17:183–190. doi: 10.1016/j.tcm.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon G. R., Mulligan S. J., MacVicar B. A. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- 19.Reichenbach A., Siegel A., Rickmann M., Wolff J. R. Distribution of Bergmann glial somata and processes: implications for function. J. Hirnforsch. 1995;36:509–517. [PubMed] [Google Scholar]
- 20.Ventura R., Harris K. M. Three-dimensional relationships between hippocampal synapses and astrocytes. J. Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zonta M., Sebelin A., Gobbo S., Fellin T. Glutamate-mediated cytosolic calcium oscillations regulate a pulsatile prostaglandin release from cultured rat astrocytes. J. Physiol. 2003;553:407–414. doi: 10.1113/jphysiol.2003.046706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takano T., Tian G. F., Peng W., Lou N. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 23.Blanco V. M., Stern J. E., Filosa J. A. Tone-dependent vascular responses to astrocyte-derived signals. Am. J. Physiol. Heart. Circ. Physiol. 2008;294:H2855–H2863. doi: 10.1152/ajpheart.91451.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udosen I. T., Jiang H., Hercule H. C., Oyekan A. O. Nitric oxide-epoxygenase interactions and arachidonate-induced dilation of rat renal microvessels. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2054–H2063. doi: 10.1152/ajpheart.00075.2003. [DOI] [PubMed] [Google Scholar]
- 25.Filosa J. A., Bonev A. D., Straub S. V., Meredith A. L. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 26.Mulligan S. J., MacVicar B. A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- 27.Metea M. R., Newman E. A. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J. Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chuquet J., Hollender L., Nimchinsky E. A. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J. Neurosci. 2007;27:4036–4044. doi: 10.1523/JNEUROSCI.0721-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter J. T., McCarthy K. D. Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 1997;51:439–455. doi: 10.1016/s0301-0082(96)00068-8. [DOI] [PubMed] [Google Scholar]
- 30.Porter J. T., McCarthy K. D. Adenosine receptors modulate [Ca2+]i in hippocampal astrocytes in situ. J. Neurochem. 1995;65:1515–1523. doi: 10.1046/j.1471-4159.1995.65041515.x. [DOI] [PubMed] [Google Scholar]
- 31.Seifert G., Zhou M., Steinhauser C. Analysis of AMPA receptor properties during postnatal development of mouse hippocampal astrocytes. J. Neurophysiol. 1997;78:2916–2923. doi: 10.1152/jn.1997.78.6.2916. [DOI] [PubMed] [Google Scholar]
- 32.Seifert G., Rehn L., Weber M., Steinhauser C. AMPA receptor subunits expressed by single astrocytes in the juvenile mouse hippocampus. Brain Res. Mol. Brain Res. 1997;47:286–294. doi: 10.1016/s0169-328x(97)00059-4. [DOI] [PubMed] [Google Scholar]
- 33.Shelton M. K., McCarthy K. D. Mature hippocampal astrocytes exhibit functional metabotropic and ionotropic glutamate receptors in situ. Glia. 1999;26:1–11. doi: 10.1002/(sici)1098-1136(199903)26:1<1::aid-glia1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 34.Fraser D. D., Duffy S., Angelides K. J., Perez-Velazquez J. L. GABAA/benzodiazepine receptors in acutely isolated hippocampal astrocytes. J. Neurosci. 1995;15:2720–2732. doi: 10.1523/JNEUROSCI.15-04-02720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma G., Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc. Natl. Acad. Sci. U. S. A. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gahring L. C., Persiyanov K., Rogers S. W. Mouse strain-specific changes in nicotinic receptor expression with age. Neurobiol. Aging. 2005;26:973–980. doi: 10.1016/j.neurobiolaging.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Gahring L. C., Persiyanov K., Dunn D., Weiss R. Mouse strain-specific nicotinic acetylcholine receptor expression by inhibitory interneurons and astrocytes in the dorsal hippocampus. J. Comp. Neurol. 2004;468:334–346. doi: 10.1002/cne.10943. [DOI] [PubMed] [Google Scholar]
- 38.Gahring L. C., Persiyanov K., Rogers S. W. Neuronal and astrocyte expression of nicotinic receptor subunit beta4 in the adult mouse brain. J. Comp. Neurol. 2004;468:322–333. doi: 10.1002/cne.10942. [DOI] [PubMed] [Google Scholar]
- 39.Araque A., Martin E. D., Perea G., Arellano J. I. Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J. Neurosci. 2002;22:2443–2450. doi: 10.1523/JNEUROSCI.22-07-02443.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Keyser J., Zeinstra E., Wilczak N. Astrocytic beta2-adrenergic receptors and multiple sclerosis. Neurobiol. Dis. 2004;15:331–339. doi: 10.1016/j.nbd.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Duffy S., MacVicar B. A. Adrenergic calcium signaling in astrocyte networks within the hippocampal slice. J. Neurosci. 1995;15:5535–5550. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aoki C. Beta-adrenergic receptors: astrocytic localization in the adult visual cortex and their relation to catecholamine axon terminals as revealed by electron microscopic immunocytochemistry. J. Neurosci. 1992;12:781–792. doi: 10.1523/JNEUROSCI.12-03-00781.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Douyard J., Shen L., Huganir R. L., Rubio M. E. Differential neuronal and glial expression of GluR1 AMPA receptor subunit and the scaffolding proteins SAP97 and 4.1N during rat cerebellar development. J. Comp. Neurol. 2007;502:141–156. doi: 10.1002/cne.21294. [DOI] [PubMed] [Google Scholar]
- 44.Bergles D. E., Roberts J. D., Somogyi P., Jahr C. E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 45.Ziskin J. L., Nishiyama A., Rubio M., Fukaya M. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 2007;10:321–330. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall I. C., Taylor C. W. Regulation of inositol 1,4,5-trisphosphate receptors. J. Exp. Biol. 1993;184:161–182. doi: 10.1242/jeb.184.1.161. [DOI] [PubMed] [Google Scholar]
- 47.Taylor C. W., Marshall I. C. Calcium and inositol 1,4,5-trisphosphate receptors: a complex relationship. Trends Biochem. Sci. 1992;17:403–407. doi: 10.1016/0968-0004(92)90009-x. [DOI] [PubMed] [Google Scholar]
- 48.Fraiman D., Dawson S. P. A model of IP3 receptor with a luminal calcium binding site: stochastic simulations and analysis. Cell Calcium. 2004;35:403–413. doi: 10.1016/j.ceca.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Oheim M., Kirchhoff F., Stuhmer W. Calcium microdomains in regulated exocytosis. Cell Calcium. 2006;40:423–439. doi: 10.1016/j.ceca.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 51.Simpson P. B., Mehotra S., Langley D., Sheppard C. A. Specialized distributions of mitochondria and endoplasmic reticulum proteins define Ca2+ wave amplification sites in cultured astrocytes. J. Neurosci. Res. 1998;52:672–683. doi: 10.1002/(SICI)1097-4547(19980615)52:6<672::AID-JNR6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 52.Reyes R. C., Parpura V. Mitochondria modulate Ca2+-dependent glutamate release from rat cortical astrocytes. J. Neurosci. 2008;28:9682–9691. doi: 10.1523/JNEUROSCI.3484-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wernyj R. P., Mattson M. P., Christakos S. Expression of calbindin-D28k in C6 glial cells stabilizes intracellular calcium levels and protects against apoptosis induced by calcium ionophore and amyloid beta-peptide. Brain Res. Mol. Brain Res. 1999;64:69–79. doi: 10.1016/s0169-328x(98)00307-6. [DOI] [PubMed] [Google Scholar]
- 54.Koster H. P., Hartog A., van Os C. H., Bindels R. J. Calbindin-D28K facilitates cytosolic calcium diffusion without interfering with calcium signaling. Cell Calcium. 1995;18:187–196. doi: 10.1016/0143-4160(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 55.Golovina V. A., Blaustein M. P. Unloading and refilling of two classes of spatially resolved endoplasmic reticulum Ca(2+) stores in astrocytes. Glia. 2000;31:15–28. doi: 10.1002/(sici)1098-1136(200007)31:1<15::aid-glia20>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 56.Simpson P. B., Holtzclaw L. A., Langley D. B., Russell J. T. Characterization of ryanodine receptors in oligodendrocytes, type 2 astrocytes, and O-2A progenitors. J. Neurosci. Res. 1998;52:468–482. doi: 10.1002/(SICI)1097-4547(19980515)52:4<468::AID-JNR11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 57.Nett W. J., Oloff S. H., McCarthy K. D. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]
- 58.Pasti L., Zonta M., Pozzan T., Vicini S. Cytosolic calcium oscillations in astrocytes may regulate exocytotic release of glutamate. J. Neurosci. 2001;21:477–484. doi: 10.1523/JNEUROSCI.21-02-00477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parpura V., Basarsky T. A., Liu F., Jeftinija K. Glutamate-mediated astrocyte–neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 60.Newman E. A. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J. Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Innocenti B., Parpura V., Haydon P. G. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J. Neurosci. 2000;20:1800–1808. doi: 10.1523/JNEUROSCI.20-05-01800.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guthrie P. B., Knappenberger J., Segal M., Bennett M. V. ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 1999;19:520–528. doi: 10.1523/JNEUROSCI.19-02-00520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris-White M. E., Zanotti S. A., Frautschy S. A., Charles A. C. Spiral intercellular calcium waves in hippocampal slice cultures. J. Neurophysiol. 1998;79:1045–1052. doi: 10.1152/jn.1998.79.2.1045. [DOI] [PubMed] [Google Scholar]
- 64.Dani J. W., Chernjavsky A., Smith S. J. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 65.Sneyd J., Wilkins M., Strahonja A., Sanderson M. J. Calcium waves and oscillations driven by an intercellular gradient of inositol (1,4,5)-trisphosphate. Biophys. Chem. 1998;72:101–109. doi: 10.1016/s0301-4622(98)00126-4. [DOI] [PubMed] [Google Scholar]
- 66.Zahs K. R., Newman E. A. Asymmetric gap junctional coupling between glial cells in the rat retina. Glia. 1997;20:10–22. [PubMed] [Google Scholar]
- 67.Verkhratsky A., Kettenmann H. Calcium signalling in glial cells. Trends Neurosci. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- 68.Cotrina M. L., Lin J. H., Lopez-Garcia J. C., Naus C. C. ATP-mediated glia signaling. J. Neurosci. 2000;20:2835–2844. doi: 10.1523/JNEUROSCI.20-08-02835.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barbara J. G. IP3-dependent calcium-induced calcium release mediates bidirectional calcium waves in neurones: functional implications for synaptic plasticity. Biochim. Biophys. Acta. 2002;1600:12–18. doi: 10.1016/s1570-9639(02)00439-9. [DOI] [PubMed] [Google Scholar]
- 70.Scemes E., Dermietzel R., Spray D. C. Calcium waves between astrocytes from Cx43 knockout mice. Glia. 1998;24:65–73. doi: 10.1002/(sici)1098-1136(199809)24:1<65::aid-glia7>3.0.co;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newman E. A., Zahs K. R. Calcium waves in retinal glial cells. Sci. 1997;275:844–847. doi: 10.1126/science.275.5301.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scemes E., Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernardinelli Y., Magistretti P. J., Chatton J. Y. Astrocytes generate Na+-mediated metabolic waves. Proc. Natl. Acad. Sci. U. S. A. 2004;101:14937–14942. doi: 10.1073/pnas.0405315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vesce S., Rossi D., Brambilla L., Volterra A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int. Rev. Neurobiol. 2007;82:57–71. doi: 10.1016/S0074-7742(07)82003-4. [DOI] [PubMed] [Google Scholar]
- 75.Wetherington J., Serrano G., Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Araque A., Sanzgiri R. P., Parpura V., Haydon P. G. Astrocyte-induced modulation of synaptic transmission. Can. J. Physiol. Pharmacol. 1999;77:699–706. [PubMed] [Google Scholar]
- 77.Pasti L., Volterra A., Pozzan T., Carmignoto G. Intracellular calcium oscillations in astrocytes: a highly plastic, bidirectional form of communication between neurons and astrocytes in situ. J. Neurosci. 1997;17:7817–7830. doi: 10.1523/JNEUROSCI.17-20-07817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edwards F. A., Gibb A. J. ATP—a fast neurotransmitter. FEBS Lett. 1993;325:86–89. doi: 10.1016/0014-5793(93)81419-z. [DOI] [PubMed] [Google Scholar]
- 79.Song Z., Vijayaraghavan S., Sladek C. D. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R423–R431. doi: 10.1152/ajpregu.00495.2006. [DOI] [PubMed] [Google Scholar]
- 80.Song Z., Vijayaraghavan S., Sladek C.D. Simultaneous exposure to ATP and phenylephrine induces a sustained elevation in the intracellular calcium concentration in supraoptic neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R37–R45. doi: 10.1152/ajpregu.00718.2005. [DOI] [PubMed] [Google Scholar]
- 81.Shigetomi E., Kato F. Action potential-independent release of glutamate by Ca2+ entry through presynaptic P2X receptors elicits postsynaptic firing in the brainstem autonomic network. J. Neurosci. 2004;24:3125–3135. doi: 10.1523/JNEUROSCI.0090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heine C., Wegner A., Grosche J., Allgaier C., et al. P2 receptor expression in the dopaminergic system of the rat brain during development. Neuroscience. 2007;149:165–181. doi: 10.1016/j.neuroscience.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 83.Csolle C., Heinrich A., Kittel A., Sperlagh B. P2Y receptor mediated inhibitory modulation of noradrenaline release in response to electrical field stimulation and ischemic conditions in superfused rat hippocampus slices. J. Neurochem. 2008;106:347–360. doi: 10.1111/j.1471-4159.2008.05391.x. [DOI] [PubMed] [Google Scholar]
- 84.Sperlagh B., Kofalvi A., Deuchars J., Atkinson L. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J. Neurochem. 2002;81:1196–1211. doi: 10.1046/j.1471-4159.2002.00920.x. [DOI] [PubMed] [Google Scholar]
- 85.Jameson H. S., Pinol R. A., Mendelowitz D. Purinergic P2X receptors facilitate inhibitory GABAergic and glycinergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain. Res. 2008;1224:53–62. doi: 10.1016/j.brainres.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Inoue K., Koizumi S., Ueno S. Implication of ATP receptors in brain functions. Prog. Neurobiol. 1996;50:483–492. doi: 10.1016/s0301-0082(96)00037-8. [DOI] [PubMed] [Google Scholar]
- 87.Queiroz G., Gebicke-Haerter P. J., Schobert A., Starke K. Release of ATP from cultured rat astrocytes elicited by glutamate receptor activation. Neuroscience. 1997;78:1203–1208. doi: 10.1016/s0306-4522(96)00637-9. [DOI] [PubMed] [Google Scholar]
- 88.Anderson C. M., Bergher J.P., Swanson R.A. ATP-induced ATP release from astrocytes. J. Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- 89.Kondo R. P., Wang S.Y., John S. A., Weiss J. N. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J. Mol. Cell. Cardiol. 2000;32:1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 90.Kang J., Kang N., Lovatt D., Torres A. Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin J. H., Lou N., Kang N., Takano T. A central role of connexin 43 in hypoxic preconditioning. J. Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pangrsic T., Potokar M., Stenovec M., Kreft M. Exocytotic release of ATP from cultured astrocytes. J. Biol. Chem. 2007;282:28749–28758. doi: 10.1074/jbc.M700290200. [DOI] [PubMed] [Google Scholar]
- 93.Suadicani S. O., Brosnan C. F., Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Newman E. A. Glial cell inhibition of neurons by release of ATP. J. Neurosci. 2003;23:1659–1666. doi: 10.1523/JNEUROSCI.23-05-01659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koizumi S., Fujishita K., Tsuda M., Shigemoto-Mogami Y. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11023–11028. doi: 10.1073/pnas.1834448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Q., Pangrsic T., Kreft M., Krzan M. Fusion-related release of glutamate from astrocytes. J. Biol. Chem. 2004;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- 97.Araque A., Li N., Doyle R. T., Haydon P. G. SNARE protein-dependent glutamate release from astrocytes. J. Neurosci. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fellin T., Pascual O., Haydon P. G. Astrocytes coordinate synaptic networks: balanced excitation and inhibition. Physiology (Bethesda) 2006;21:208–215. doi: 10.1152/physiol.00161.2005. [DOI] [PubMed] [Google Scholar]
- 99.Haydon P. G. GLIA: listening and talking to the synapse. Nat. Rev. Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- 100.Araque A., Carmignoto G., Haydon P. Dynamic signaling between astrocytes and neurons. Annu. Rev. Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- 101.Fellin T., Pascual O., Gobbo S., Pozzan T. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Chen X., Wang L., Zhou Y., Zheng L. H. “Kiss-and-run” glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J. Neurosci. 2005;25:9236–9243. doi: 10.1523/JNEUROSCI.1640-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mustafa A. K., Kim P. M., Snyder S. H. D-Serine as a putative glial neurotransmitter. Neuron. Glia. Biol. 2004;1:275–281. doi: 10.1017/S1740925X05000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schell M. J., Brady R. O., Molliver M. E., Snyder S. H. D-serine as a neuromodulator: regional and developmental localizations in rat brain glia resemble NMDA receptors. J. Neurosci. 1997;17:1604–1615. doi: 10.1523/JNEUROSCI.17-05-01604.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Martineau M., Galli T., Baux G., Mothet J.P. Confocal imaging and tracking of the exocytotic routes for D-serine-mediated gliotransmission. Glia. 2008;56:1271–1284. doi: 10.1002/glia.20696. [DOI] [PubMed] [Google Scholar]
- 106.Perea G., Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science. 2007;317:1083–1086. doi: 10.1126/science.1144640. [DOI] [PubMed] [Google Scholar]
- 107.Koob G. F., Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 108.Koob G. F. Drug addiction: the yin and yang of hedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 109.Bainton R. J., Tsai L. T., Schwabe T., DeSalvo M. moody encodes two GPCRs that regulate cocaine behaviors and blood–brain barrier permeability in Drosophila. Cell. 2005;123:145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 110.Haydon P. G., Blendy J., Moss S. J., Rob J. F. Astrocytic control of synaptic transmission and plasticity: a target for drugs of abuse. Neuropharmacology. 2009;56:83–90. doi: 10.1016/j.neuropharm.2008.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song P., Zhao Z. Q. The involvement of glial cells in the development of morphine tolerance. Neurosci. Res. 2001;39:281–286. doi: 10.1016/s0168-0102(00)00226-1. [DOI] [PubMed] [Google Scholar]
- 112.Narita M., Kuzumaki N., Narita M., Kaneko C. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J. Neurochem. 2006;97:1369–1378. doi: 10.1111/j.1471-4159.2006.03824.x. [DOI] [PubMed] [Google Scholar]
- 113.Miyatake M., Miyagawa K., Mizuo K., Narita M. Dynamic changes in dopaminergic neurotransmission induced by a low concentration of bisphenol-A in neurones and astrocytes. J. Neuroendocrinol. 2006;18:434–444. doi: 10.1111/j.1365-2826.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 114.Narita M., Miyatake M., Narita M., Shibasaki M. Direct evidence of astrocytic modulation in the development of rewarding effects induced by drugs of abuse. Neuropsychopharmacology. 2006;31:2476–2488. doi: 10.1038/sj.npp.1301007. [DOI] [PubMed] [Google Scholar]
- 115.Narita M., Suzuki M., Kuzumaki N., Miyatake M. Implication of activated astrocytes in the development of drug dependence: differences between methamphetamine and morphine. Ann. N.Y. Acad. Sci. 2008;1141:96–104. doi: 10.1196/annals.1441.032. [DOI] [PubMed] [Google Scholar]
- 116.Navarrete M., Araque A. Endocannabinoids mediate neuron–astrocyte communication. Neuron. 2008;57:883–893. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 117.Mena M. A., de Bernardo S., Casarejos M. J., Canals S. The role of astroglia on the survival of dopamine neurons. Mol. Neurobiol. 2002;25:245–263. doi: 10.1385/MN:25:3:245. [DOI] [PubMed] [Google Scholar]
- 118.Kenney J. W., Gould T. J. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol. Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dani J. A., Ji D., Zhou F. M. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349–352. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- 120.Mansvelder H. D., De Rover M., McGehee D. S., Brussaard A. B. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur. J. Pharmacol. 2003;480:117–123. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- 121.Mansvelder H. D., McGehee D. S. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 122.Kenney J. W., Gould T. J. Nicotine enhances context learning but not context-shock associative learning. Behav. Neurosci. 2008;122:1158–1165. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Portugal G. S., Kenney J. W., Gould T. J. Beta2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol. Learn. Mem. 2008;89:106–113. doi: 10.1016/j.nlm.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fagen Z. M., Mansvelder H. D., Keath J. R., McGehee D. S. Short- and long-term modulation of synaptic inputs to brain reward areas by nicotine. Ann. N.Y. Acad. Sci. 2003;1003:185–195. doi: 10.1196/annals.1300.011. [DOI] [PubMed] [Google Scholar]
- 125.Sharma G., Grybko M., Vijayaraghavan S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J. Neurosci. 2008;28:2563–2575. doi: 10.1523/JNEUROSCI.5407-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharma G., Vijayaraghavan S. Modulation of presynaptic store calcium induces release of glutamate and postsynaptic firing. Neuron. 2003;38:929–939. doi: 10.1016/s0896-6273(03)00322-2. [DOI] [PubMed] [Google Scholar]
- 127.Sharma G., Vijayaraghavan S. Nicotinic receptor signaling in nonexcitable cells. J. Neurobiol. 2002;53:524–534. doi: 10.1002/neu.10114. [DOI] [PubMed] [Google Scholar]
- 128.Rizzoli S., Sharma G., Vijayaraghavan S. Calcium rise in cultured neurons from medial septum elicits calcium waves in surrounding glial cells. Brain Res. 2002;957:287–297. doi: 10.1016/s0006-8993(02)03618-1. [DOI] [PubMed] [Google Scholar]