INTRODUCTION
The pre-administration of the anti-inflammatory drugs dexamethasone (DEX) and cortisone acetate reduces toxicity and enhances efficacy of anticancer agents in murine models and in human clinical trials (1–5). We previously reported on the formulation of the lipophilic dexamethasone palmitate ester (DEX-P) in nanoparticles (NPs) employing a microemulsion template engineering technique to achieve tumor-specific delivery of dexamethasone (6). The nanoparticles exhibited significantly enhanced stealth properties as indicated by reduced macrophage uptake and decreased adsorption of opsonin proteins in in vitro assays (6). Unexpectedly, preliminary biodistribution studies of NPs containing [3H]-DEX-P in tumor-bearing mice showed that the radiolabel was cleared from the circulation rapidly and exhibited high liver uptake. Our previous in vitro release studies demonstrated that rapid release of the radiolabel from the NPs was observed when 10% mouse plasma was used as the medium, while nominal release was observed in phosphate-buffered saline (PBS) buffer (6). Esterolysis of NP-associated DEX-P was presumed to be the main cause for the rapid drug release in plasma, as most of the released radioactivity was in the form of DEX and not DEX-P. High degradation rates of ester prodrugs in rodent plasma has been attributed to increased esterase activity, while only minimal degradation in human plasma has been observed (7–9). Based on our observation of the release of [3H]-DEX from NPs in mouse plasma, we studied the release of DEX from nanoparticles in various plasma sources as a guide for the design of future in vivo experiments.
MATERIALS AND METHODS
Materials
Tritiated dexamethasone-([6,7-3H(N)]; specific activity = 35–50 Ci/mmol) was purchased from American Radiolabeled Chemicals (Saint Louis, MO, USA). Bis(p-nitrophenyl)phosphate (BNPP) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse and human plasma containing Na heparin were purchased from Innovative Research (Novi, MI, USA). Plasma from nu/nu mice and Sprague–Dawley rats were obtained from animals housed at the University of Kentucky and from carboxylesterase-deficient mice (Es1e(−/−)/SCID) maintained at St. Jude Children’s Research Hospital.
Preparation of Radiolabeled Nanoparticles
The procedure to prepare radiolabeled DEX-P and the method to incorporate it into solid lipid nanoparticles by the nanotemplate engineering approach have been previously described (6). Briefly, [3H]-DEX-P was synthesized by the reaction of [3H]-DEX and palmitoyl chloride. Nanoparticles were derived from a microemulsion comprising stearyl alcohol (1.6 mg/mL), [3H]-DEX-P (20% of the weight of the stearyl alcohol), polysorbate 60 (0.4 mg/mL), Brij78® (2.8 mg/mL), and PEG6000 MS (3 mg/mL) that was prepared by adding warm PBS buffer (pH 7.4, 70°C) to the melted mixture of oil, drug, and surfactants. After stirring in a 70°C water bath for 1 h, the warm microemulsion was cooled to 25°C, resulting in the formation of nanoparticles. Nanoparticle suspensions were diluted 1:30 (v/v) with filtered PBS buffer (0.22 μm filter, Nalgene International) and then incubated at 37°C for 0–20 h. Particle size distributions were measured at 37°C by photon correlation spectroscopy using a Coulter N4 Plus Submicron Particle Sizer (Beckman Coulter Corporation, Miami, FL, USA), n = 3.
Release of Drug from Nanoparticles in Various Media
The media used in the in vitro [3H] release study included PBS buffer, plasma from nu/nu and carboxylesterase-deficient mice, rats, and humans. Human tumor (A549) homogenate grown in nu/nu mice and A549 cell lysate from cells grown in vitro were also used. Heat-denatured plasma (70°C for 30 min) or BNPP-treated plasma were used as controls. A suspension of NPs containing [3H]-DEX-P was added to 10% media (diluted with PBS buffer) at a ratio of 1:14 (v/v) and incubated at 37°C for up to 24 h. Ultrafiltration (Millipore Ultracel YM-100) was used to separate the particles from the medium, and the radioactivity that was released into the filtrate was quantified by liquid scintillation counting. Thin-layer chromatography (TLC) was used to quantify the amount of [3H]-DEX-P and [3H]-DEX that had been released into the medium.
Statistical Analysis
Data are presented as mean ± standard deviation. Groups were compared using analysis of variance, one-way or two-way tests as appropriate, with SigmaStat 3.11 software (Systat, San Jose, CA, USA). Differences were considered statistically significant when P < 0.05, and the Holm–Sidak method was used to perform pair-wise multiple comparisons on significant effects and interactions.
RESULTS AND DISCUSSION
The size stability of [3H]-DEX-P loaded NPs was initially assessed in PBS buffer at 37°C to rule out the release of drug from the NPs due to particle degradation. As seen in Fig. 1, the size of the particles remained constant during the first 3 h in which NP particle size was measured. A slight but not statistically significant increase in the average particle size from 125.7 (±16.3) nm to 164.7 (±24.3) nm was observed after 20 h of incubation at 37°C. At all times, the suspension remained clear with no evidence of particle aggregation or flocculation. These studies confirmed our previous results in which we used [14C]-stearyl alcohol, a primary matrix component, to demonstrate that the particles remained intact (6).
Fig. 1.
[3H]-DEX-P nanoparticle size following incubation in PBS at 37°C. Each value represents the mean ± SD (n = 3)
Here, initial release studies showed that only 7.3 ± 0.4% release of [3H] from NPs was detected after 7 days when PBS buffer was used as the release media. In contrast, when the radiolabeled NPs were suspended in 10% mouse plasma, a burst release (5.0 ± 0.3%) of the radiolabel was observed, and after 3 h, approximately 55.7 ± 0.4% of the radiolabel was detected in the filtrate. TLC analysis demonstrated that most of the radioactivity (3H) in the retentate (i.e., in the NPs) was present as intact DEX-P, while analysis of the filtrates revealed that 100% of the radioactivity was present as DEX indicating that de-esterification of DEX-P had occurred.
The palmitate ester of DEX is nearly insoluble in water and has been reported to be an amphipathic molecule that can insert into phospholipid monolayers (10). As such, during the preparation of the NPs, DEX-P is expected to align itself in the microemulsion precursor with the DEX group directed toward the aqueous phase and the hydrophobic palmitate chain embedded in the oil phase. After cooling to form the NPs, this orientation may allow plasma esterases easy access to the ester bond of DEX-P resulting in the release of DEX, which has a water solubility of 100 μg/mL, from the nanoparticle matrix.
We anticipated that the addition of polyethylene glycol (PEG) derivatives to the NP formulations might impede degradation of DEX-P by shielding the molecule from exposure to enzymes. While increased addition of PEG6000 MS to NP formulations was able to reduce macrophage uptake in vitro (6), the addition of this PEGylating agent and others, including DSPE-PEG5000, did not change the release profile of radioactivity when the [3H]-DEX-NPs were incubated in 10% mouse plasma. The results showed that incorporation of amphipathic esters into PEGylated nanoparticles may not necessarily protect the compound from enzymatic degradation. This has implications for studies conducted in mice that employ various nanoparticle carriers containing esters, including PEGylating agents bound to nanocarriers via ester linkages.
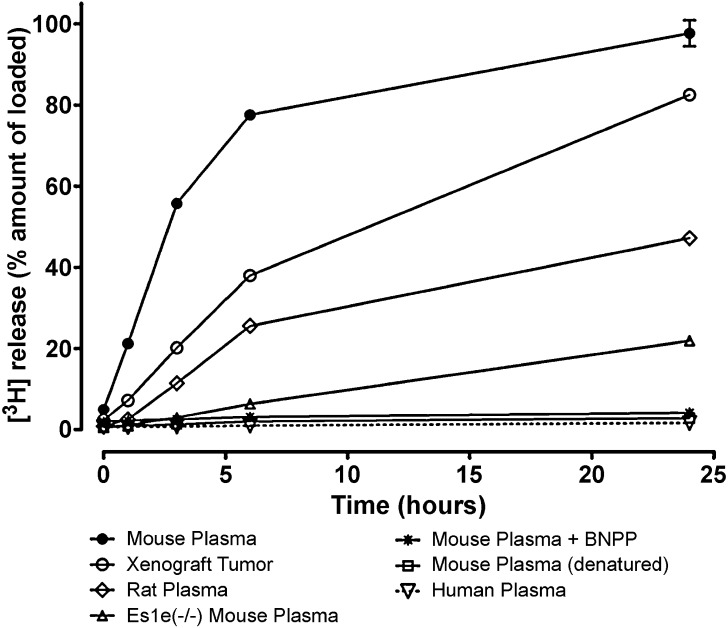
Results from preliminary in vivo studies revealed a difference in the disposition of the radiolabeled drug in mouse and rat. Thus, additional studies measuring the release of radioactivity from [3H]-DEX-NPs in 10% heparinized plasma from different species were conducted (Fig. 2). The time-dependent release over 24 h is depicted in the figure with denatured 10% mouse plasma as the control, showing negligible release (2.8 ± 0.1%) after 24 h. Following 24 h of incubation, extensive release was observed from NPs suspended in nu/nu mouse plasma (97.7 ± 3.2%) and rat plasma (47.2 ± 0.2%). In contrast, much lower release (22.0 ± 1.0%) was observed when the NPs were suspended in plasma obtained from carboxylesterase-deficient mice (Es1e(−/−)/SCID). Multiple comparisons versus the denatured plasma control group were done using the Holm–Sidak method. The release of the radiolabel increased with time in mouse, rat, and Es1e(−/−)/SCID mouse plasma and was significantly different from that of the control group (P < 0.001). The role of esterases in the release of DEX from the nanoparticles was confirmed as mouse plasma containing the esterase inhibitor BNPP (1 mM) showed low release (4.1 ± 0.3%). Interestingly, nominal release (1.6 ± 0.1%) was observed from NPs suspended in human plasma, showing no significant difference from the denatured plasma control group. NPs incubated in a human tumor homogenate showed a much greater extent of release than human plasma. Subsequently, to confirm that DEX-P hydrolysis in human tumor xenograft homogenate was not the result of any residual mouse plasma activity, we prepared cell lysates from the same tumor type (A549) grown in vitro. Extensive release was also observed in 10% tumor cell lysate (88.9 ± 5.5%) after 24 h.
Fig. 2.
Release of [3H] from nanoparticles containing [3H]-DEX-P in 10% plasma from different sources and in 10% human tumor xenograft homogenate. Each value represents the mean ± SD (n = 3)
Carboxylesterase and butyrylcholinesterase are two major esterases known to degrade prodrugs (11), and their activities in a variety of animal species have been thoroughly characterized (9). The release of radiolabeled drug from the NPs in 10% plasma from a carboxylesterase-deficient mouse was significantly lower than that observed in mouse and rat plasma, although an increase in the release of the radiolabel was observed with time. It has been reported that liver homogenates have greater esterase-specific activities compared to plasma in both rodents and humans (9). In our study, minimal drug release from the NPs was observed in human plasma, but DEX-P might be de-esterified in human liver because of its high esterase activity. Previous studies by us and others on opsonin protein adsorption and macrophage uptake suggest that PEGylated nanoparticles can avoid rapid uptake by the reticuloendothelial system leading to increased residence times in the circulation (6,12). The rapid release of the radiolabel in human tumor homogenate indicated the potential of esterases to serve as a trigger to release DEX in tumors after passive accumulation of DEX-P NPs via the enhanced permeability and retention (EPR) effect.
CONCLUSION
Studies with nanoparticles containing amphipathic esters should be conducted with caution in rodents. Our studies clearly demonstrate that their esterase-rich plasma will alter biodistribution. This limitation may be overcome, to some extent, by using a carboxylesterase-deficient mouse model. In humans, it is speculated that PEGylated dexamethasone palmitate delivered in a solid lipid nanoparticle will not be de-esterified in plasma, but rapid de-esterification to dexamethasone will occur in the tumor after accumulation via the EPR effect. The released dexamethasone can then act to reduce interstitial hydrostatic pressure resulting in the enhancement of the uptake of systemically administered chemotherapeutic agents.
Acknowledgments
The authors are grateful for financial support from the Markey Cancer Center Experimental Therapeutics Program (ML), the Buck-Kentucky Lung Cancer Research Chair (JJR), the Benedict Cassen Postdoctoral Fellowship from the Education and Research Foundation for the Society of Nuclear Medicine (XL), the Lyman T. Johnson Postdoctoral Fellowship (DT), and grant DGE-0653710 from the NSF IGERT Program (MDH).
References
- 1.Rinehart J., Keville L., Neiddhart J., Wong L., DiNunno L., Kinney P., Aberle M., Tadlock L., Cloud G. Hematopoietic protection by dexamethasone or granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients treated with carboplatin and ifosfamide. Am. J. Clin. Oncol. 2003;26:448–458. doi: 10.1097/01.coc.0000027268.23258.7D. [DOI] [PubMed] [Google Scholar]
- 2.Rinehart J. J., Keville L. R. Reduction in carboplatin hematopoietic toxicity in tumor bearing mice: comparative mechanisms and effects of interleukin-1 beta and corticosteroids. Cancer Biother. Radiopharm. 1997;12:101–109. doi: 10.1089/cbr.1997.12.101. [DOI] [PubMed] [Google Scholar]
- 3.Wang H., Li M., Rinehart J. J., Zhang R. Dexamethasone as a chemoprotectant in cancer chemotherapy: hematoprotective effects and altered pharmacokinetics and tissue distribution of carboplatin and gemcitabine. Cancer Chemother Pharmacol. 2004;53:459–467. doi: 10.1007/s00280-003-0759-9. [DOI] [PubMed] [Google Scholar]
- 4.Wang H., Li M., Rinehart J. J., Zhang R. Pretreatment with dexamethasone increases anti-tumor activity of carboplatin and gemcitabine in mice bearing human cancer xenografts: In vivo activity, pharmacokinetics and clinical implications for cancer chemotherapy. Clin. Cancer Res. 2004;10:1633–1644. doi: 10.1158/1078-0432.CCR-0829-3. [DOI] [PubMed] [Google Scholar]
- 5.M. Leggas, K. Kuo, F. Robert, G. Cloud, M. DeShazo, R. Zhang, M. Li, H. Wang, S. Davidson, and J. Rinehart. Intensive anti-inflammatory therapy with dexamethasone in patients with non-small cell lung cancer: effect on chemotherapy toxicity and efficacy. Cancer Chemother Pharmacol. 63:731–743. doi:10.1007/s00280-008-0767-x. [DOI] [PubMed]
- 6.Lu X., Howard M. D., Mazik M., Eldridge J., Rinehart J.J., Jay M., Leggas M. Nanoparticles containing anti-inflammatory agents as chemotherapy adjuvants: optimization and characterization. AAPS J. 2008;10:133–140. doi: 10.1208/s12248-008-9013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liederer B. M., Borchardt R. T. Stability of oxymethyl-modified coumarinic acid cyclic prodrugs of diastereomeric opioid peptides in biological media from various animal species including human. J. Pharm. Sci. 2005;94:2198–2206. doi: 10.1002/jps.20452. [DOI] [PubMed] [Google Scholar]
- 8.Pan H., Kopeckova P., Liu J., Wang D., Miller S. C., Kopecek J. Stability in plasmas of various species of HPMA copolymer–PGE1 conjugates. Pharm. Res. 2007;24:2270–2280. doi: 10.1007/s11095-007-9449-3. [DOI] [PubMed] [Google Scholar]
- 9.Liederer B. M., Borchardt R. T. Enzymes involved in the bioconversion of ester-based prodrugs. J. Pharm. Sci. 2006;95:1177–1195. doi: 10.1002/jps.20542. [DOI] [PubMed] [Google Scholar]
- 10.Benameur H., De Gand G., Brasseur R., Van Vooren J. P., Legros F. J. Liposome-incorporated dexamethasone palmitate: Chemical and physical properties. Int. J. Pharmaceutics. 1993;89:157–167. doi: 10.1016/0378-5173(93)90239-C. [DOI] [Google Scholar]
- 11.Morton C. L., Wadkins R. M., Danks M. K., Potter P. M. The anticancer prodrug CPT-11 is a potent inhibitor of acetylcholinesterase but is rapidly catalyzed to SN-38 by butyrylcholinesterase. Cancer Res. 1999;59:1458–1463. [PubMed] [Google Scholar]
- 12.Moghimi S. M., Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42:463–478. doi: 10.1016/S0163-7827(03)00033-X. [DOI] [PubMed] [Google Scholar]