Abstract
The aim of this study was to study the expression of various claudins in sarcoidosis, usual interstitial pneumonia (UIP), and normal human lung. The expression and cell-specific localization of claudin-1, -2, -3, -4, -5, and -7 was analyzed by IHC. Bronchiolar epithelial cells showed mostly strong expression for claudin-1, -2, -3, -4, and -7 and mainly weak expression for claudin-5 in UIP, sarcoidosis, and normal lung. Three claudins, claudin-3, -4, and -7, were expressed in normal alveolar epithelium, mainly in type II pneumocytes. Claudin-5 was expressed strongly in endothelium of normal lung, and its staining was extremely intense in endothelium of UIP. Moderate or strong expression for claudin-1, -2, -3, -4, and -7 was observed in metaplastic alveolar- and bronchiolar-type epithelium in UIP and also in metaplastic alveolar-type epithelium in sarcoidosis. Expression of claudin-5 was mainly weak in metaplastic alveolar- and bronchiolar-type epithelium in UIP. We conclude that claudin-1, -2, -3, -4, -5, and -7 are expressed in UIP and sarcoidosis, and furthermore, the most prominent enhancement of staining is localized in metaplastic alveolar- and bronchiolar-type epithelium in UIP compared with the healthy lung. (J Histochem Cytochem 57:187–195, 2009)
Keywords: epithelium, metaplasia, tight junction
In normal lung, flat (type I pneumocytes) and cuboidal (type II pneumocytes) epithelial cells line the alveoli, the former covering 93% and the latter 7% of the alveolar surface (Crapo et al. 1982). In fibrotic pulmonary disorders, alterations in alveolar epithelium have been shown to participate in the remodeling process; in fact, several decades ago, it was proposed that type II pneumocytes and cells of bronchiolar origin might serve as sources of epithelial regeneration, and squamous-type metaplasia and bronchiolization have been observed in histological samples of the fibrotic human lung (Kawanami et al. 1982). In previous studies, three types of metaplastic epithelium at the alveolar level have been observed in usual interstitial pneumonia [i.e., idiopathic interstitial pneumonia (UIP/IPF)]: metaplastic alveolar-type epithelium, metaplastic squamous-type epithelium, and metaplastic bronchiolar-type epithelium (Kawanami et al. 1982; Hinata et al 2003;Lappi-Blanco et al. 2006).
Epithelial and endothelial cells communicate with each other by many kinds of cell–cell interactions, such as tight and gap junctions. Both types of junctions have been shown to exist in alveolar epithelium of lung, but there is no detailed knowledge of their function, and even their distribution in normal and diseased human lung is poorly understood. The tight junctions vary in the different epithelia, and they are often divided into leaky and tight types (Van Itallie and Anderson 2004). Less than a decade ago, it was observed that claudins are the main family of proteins that make up the tight junctions (Tsukita et al. 2001). In addition to the large family of claudins, which now consists of >20 members, other junction adhesion molecules such as occludin, tricellulin-α, ZO1, ZO2, and ZO3 also are involved in tight junctional structures (Martin-Padura et al. 1998; Pummi et al. 2001; Langbein et al. 2002; Tebbe et al. 2002; Morita et al. 2004; Schlüter et al. 2007). Epithelial cells often express multiple claudin types, and moreover, they have been shown to have distinctive expression profiles (Rahner et al. 2001). There are also human phenotypes of mutations in claudin-14 and -16. A mutation of claudin-16 is associated with hypomagnesemia (Simon et al. 1999) and a mutation of claudin-14 with deafness (Wilcox et al. 2001).
Recently, several studies on the expression of various claudins in different kinds of lung carcinomas have been published (Moldvay et al. 2007; Paschoud et al. 2007). However, thus far, there are no published studies on expression of claudins in normal human peripheral lung or interstitial lung disorders. Therefore, our aim was to study the expression and cell-specific localization of six different types of claudins in UIP, sarcoidosis, and normal human lung. The pathogenetic mechanisms of both UIP and sarcoidosis are unknown; however, recently it was proposed that idiopathic UIP may result partly from epithelial cell injury (Selman and Pardo 2006). We hypothesized that there might be some alteration in the expression of claudins in these disorders of unknown etiology and for which there is no efficient treatment. The results of this study have been previously reported in abstract form (Kaarteenaho-Wiik and Soini 2007).
Materials and Methods
Patients and Handling of Specimens
Histopathologically typical cases of UIP and sarcoidosis were retrieved from the files of the Department of Pathology, Oulu University Hospital, by re-evaluating lung biopsies taken either by open or thoracoscopic operations between 1993 and 2002. Twenty patients with either sarcoidosis (n=10) or UIP (n=10) were included in the study. Diagnoses in all patients were based on light microscopic evaluations fulfilling the histologic criteria presented by Katzenstein (2006) and Travis et al. (2002). The clinical follow-up information from the patients was obtained from the patient records at the University Central Hospital, at the different central hospitals, and from the local health centers. Uninvolved peripheral lung tissue, used as a control, was obtained from seven patients operated on for malignant lung tumors. The study protocol was approved by the ethical committee of Oulu University Hospital.
Lung samples were taken from different parts of the left or right lung. Material was fixed in 10% formalin under vacuum to expand the tissue and to remove air bubbles or they were perfused by injecting the fixative, using a small syringe, into the bronchioles (Wagenvoort 1980; Dail and Hammar 1994). The specimens were dehydrated and embedded in paraffin. Sections (5 μm thick) were stained with hematoxylin–eosin, with staining for acid-fast bacilli being done in every case. In selected cases, stainings with Giemsa, Verhoeff, van Gieson, Perls' blue, periodic acid-Schiff Alcian blue, and periodic acid-Schiff were also conducted. All material was evaluated, and one representative tissue block from each case was selected for IHC studies.
Antibodies and IHC Staining
The primary antibodies used for IHC were all purchased from Zymed Laboratories (South San Francisco, CA) and designed to be used in formalin-fixed, paraffin-embedded tissues. They were polyclonal rabbit anticlaudin 1 (clone JAY.8), monoclonal mouse anticlaudin 2 antibody (clone 12H12), polyclonal rabbit anticlaudin 3 (clone Z23.JM), monoclonal mouse anticlaudin 4 (clone 3E2C1), monoclonal mouse anticlaudin 5 (clone 4C3C2), and polyclonal rabbit anticlaudin 7 (clone ZMD.241). The specificity of the claudin antibodies has been characterized previously in other studies (Tokés et al. 2005; Bello et al. 2008; Kim et al. 2008). Before application of the primary antibodies, the sections were heated in a microwave oven in 10 mM citrate buffer, pH 6.0, for 10 min. After a 60-min incubation with the primary antibody (dilution 1:50 for anticlaudin-1, -2, -3, -4, -5, and -7), a biotinylated secondary anti-rabbit or anti-mouse antibody and Histostain-SP kit (Zymed Laboratories) were used. In all IHC experiments, the color was developed by diaminobenzidine and, subsequently, the sections were lightly counterstained with hematoxylin and mounted with Eukitt (Kindler; Freiburg, Germany).
Negative controls were obtained by substituting non-immune rabbit or mouse serum and PBS for the primary antibodies.
Scoring of Immunoreactivity
The extent and intensity of various claudins was evaluated semiquantitatively as negative (0), weak (+), moderate (++), or strong (+++) in different types of pulmonary cells, such as epithelial cells of bronchioli, alveolar epithelium (i.e., pneumocytes), endothelial cells, interstitial cells (fibroblasts and myofibroblasts), and mesothelium. In the evaluation, membrane-bound positivity was considered significant.
Analysis of types of metaplastic alveolar epithelium was based oprevious studies of ours and other investigators, in which it was classified on the basis of morphologic features (Kawanami et al. 1982; Hinata et al 2003;Lappi-Blanco et al. 2006). In these studies, alveolar-type, bronchiolar-type, and squamous-type metaplastic alveolar epithelium was observed in UIP on the basis of the localization and morphology by light and electron microscopic methods.
Results
Normal Lung
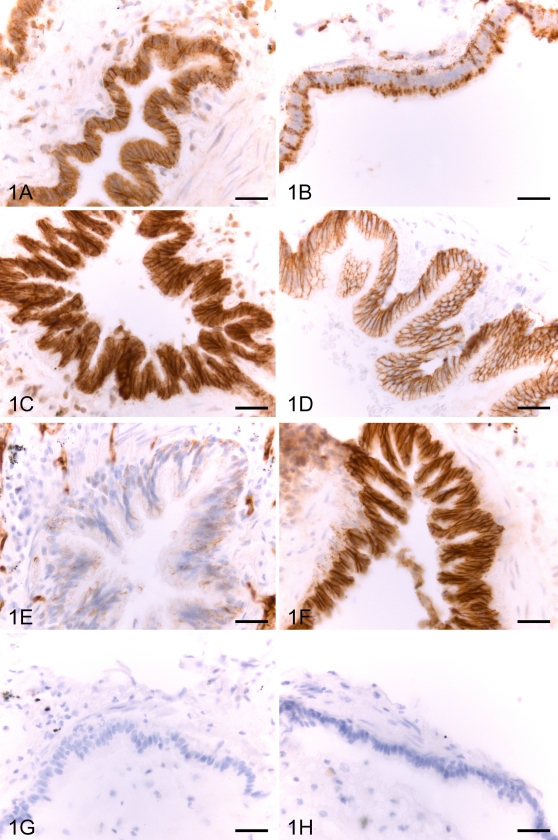
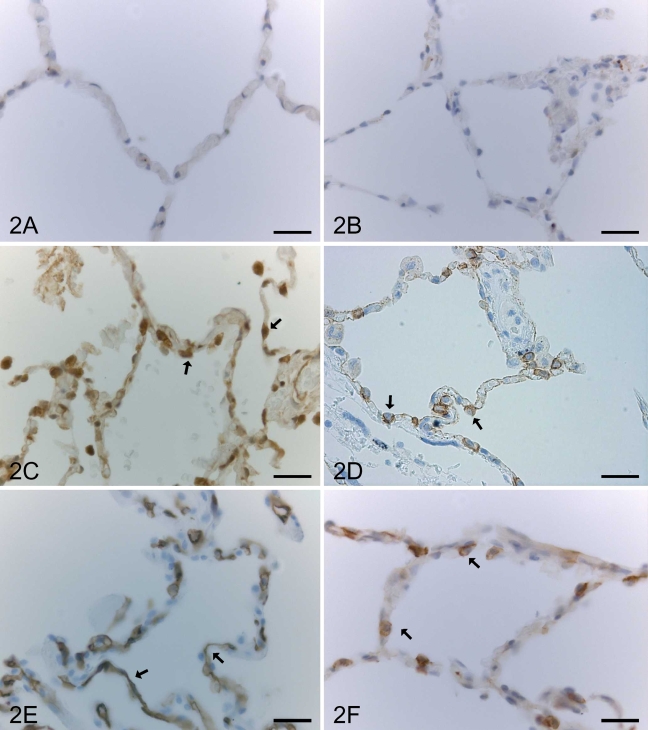
In normal lung, claudin-1 and claudin-2 were expressed moderately or strongly in bronchiolar epithelium (Figures 1A and 1B), whereas alveolar epithelium (Figures 2A and 2B), fibroblasts, and mesothelium were negative. Type II pneumocytes of alveolar epithelium and bronchiolar epithelium of normal lung were positive for claudin-3 and claudin-4 (Figures 1C, 1D, 2C, and 2D). Claudin-5 was strongly positive in endothelial cells of alveolar capillaries, arteries, veins, and lymphatic vessels (Figure 2E). The expression for claudin-5 was weak in bronchiolar epithelium (Figure 1E). Type II pneumocytes of alveolar epithelium and bronchiolar epithelial cells were also positive for claudin-7 (Figures 1F and 2F).
Figure 1.
IHC staining for claudin-1 (A), claudin-2 (B), claudin-3 (C), claudin-4 (D), claudin-5 (E), and claudin-7 (F) in an epithelium of a bronchiole of a normal lung. The IHC expression for claudin-1, -2, -3, -4, and -7 is intense, whereas that of claudin-5 is weak (E). Control sections (G,H) were treated with non-immune rabbit serum (G) and PBS with hematoxylin counterstain (H). Bar = 60 μm.
Figure 2.
IHC staining for claudin-1, -2, -3, -4, -5, and -7 in an alveolar section of a normal lung. Alveolar epithelium of normal lung is negative for claudin-1 (A) and claudin-2 (B). Type II pneumocytes (arrows) are positive for claudin-3 (C) and claudin-4 (D). (E) A positive staining for claudin-5 is seen in alveolar capillaries (arrows). (F) Type II pneumocytes (arrows) are positive for claudin-7. Bar = 50 μm.
UIP
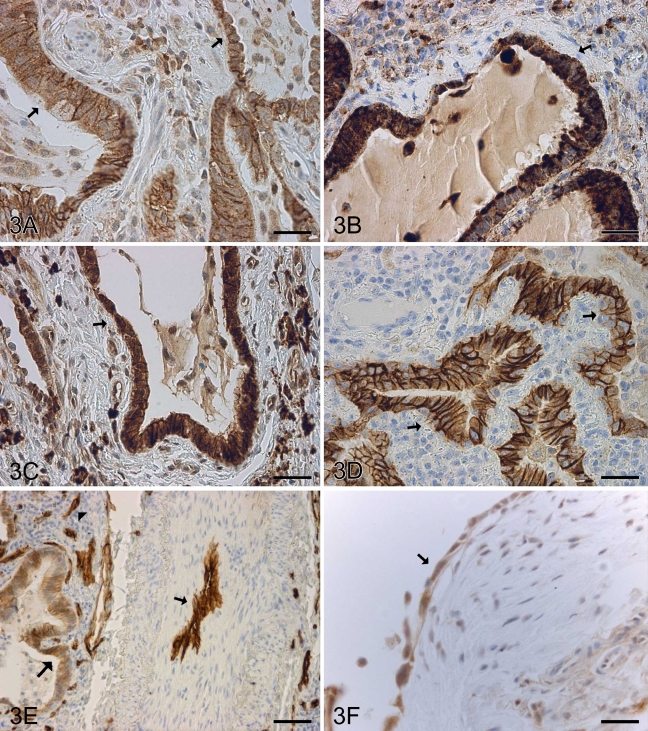
In UIP, claudin-1, claudin-2, claudin-3, claudin-4, and claudin-7 were highly expressed and scored as moderate or strong in most cases in metaplastic alveolar epithelium of every type including alveolar-, bronchiolar-, and squamous-type metaplastic epithelium (Figures 3A–3D and 3F). Claudin-5 was strongly positive in endothelial cells of alveolar capillaries, arteries, veins, and lymphatic vessels, with its expression being particularly strong in those arteries of UIP that were remodeled (Figure 3E). Weak positive staining for claudin-5 was also observed in bronchiolar epithelium and metaplastic alveolar epithelium (Figure 3E).
Figure 3.
IHC staining for claudin-1, -2, -3, -4, -5, and -7 in a patient with usual interstitial pneumonia (UIP). Metaplastic epithelium overlying remodeled and fibrotic alveolar walls (arrows) are positive for claudin-1 (A), claudin-2 (B), claudin-3 (C), and claudin-4 (D). (E) Claudin-5 is strongly positive in the endothelium of a remodeled artery (arrow) and capillaries of alveolar wall (arrowhead). Claudin-5 also shows a weak positive staining in the metaplastic epithelium of fibrotic alveolar wall (large arrow). (F) Metaplastic epithelium overlying a fibroblast focus within alveolar wall is positive for claudin-7 (arrow). Bar = 50 μm.
Sarcoidosis
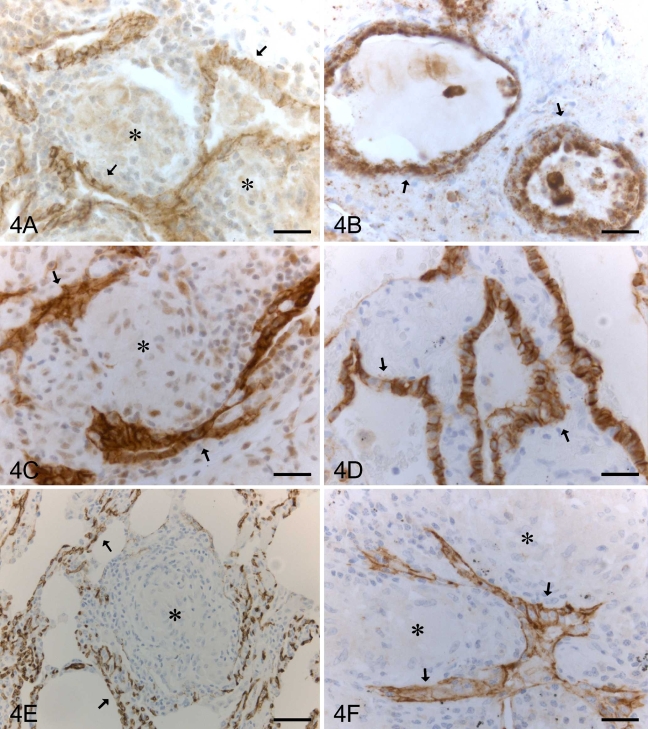
Metaplastic alveolar-type epithelium in sarcoidosis was positive for claudin-1, -2, -3, -4, -5, and -7 (Figures 4A–4F). Claudin-5 was strongly positive in endothelial cells of alveolar capillaries, arteries, veins, and lymphatic vessels (Figure 4E).
Figure 4.
IHC staining for claudin-1, -2, -3, -4, -5, and -7 in a patient with sarcoidosis. Metaplastic alveolar epithelium (arrows), which is often located beside granulomas (asterisks), is positive for claudin-1 (A), claudin-2 (B), claudin-3 (C), claudin-4 (D), and claudin-7 (F). (E) Endothelial cells of capillaries of alveolar walls are positive for claudin-5 (arrows) surrounding a granuloma (asterisk). Bar = 50 μm.
Alveolar-type metaplastic epithelium within alveoli was seen in every case of UIP. Most cases of UIP also showed bronchiolar-type alveolar metaplastic epithelium, whereas squamous-type metaplasia at the alveolar level was observed in only four cases. In general, the expression of various claudins in bronchiolar- and squamous-type metaplastic alveolar epithelium was similar to that encountered in alveolar-type metaplastic epithelium. In sarcoidosis, only alveolar-type alveolar metaplastic epithelium was observed in most cases. The amount of metaplastic alveolar-type epithelium within the alveoli in sarcoidosis was considerably less than that seen with UIP. All claudins were expressed in submucosal glands, whereas they were absent in fibroblasts and myofibroblasts. In addition to the membrane-bound positivity, there was also cytoplasmic positivity in some cells. In particular, claudin-2 was expressed in macrophages and granulomas. The significance of cytoplasmic staining remains to be studied, because this study considered only the membrane-bound positivity. The results are compiled in Tables 1–3.
Table 1.
Expression of claudin-1, -2, -3, -4, -5, and -7 in normal lung
| Localization | Claudin-1 | Claudin-2 | Claudin-3 | Claudin-4 | Claudin-5 | Claudin-7 |
|---|---|---|---|---|---|---|
| Alveolar epithelium | 0 (7) | 0 (7) | + (7) | + (7) | 0 (7) | + (7) |
| Bronchiolar epithelium | ++ (3) +++ (4) | ++ (2) +++ (5) | +++ (7) | ++ (4) +++ (3) | + (7) | +++ (7) |
| Endothelium | (+)a (7) | 0 (7) | 0 (7) | 0 (7) | +++ (7) | 0 (7) |
Very weak expression.
Total numbers of cases are shown in parentheses.
Table 2.
Expression of claudin-1, -2, -3, -4, -5, and -7 in patients with UIP
| Localization | Claudin-1 | Claudin-2 | Claudin-3 | Claudin-4 | Claudin-5 | Claudin-7 |
|---|---|---|---|---|---|---|
| Alveolar epitheliuma | 0 (10) | 0 (10) | + (10) | + (10) | 0 (10) | + (10) |
| Bronchiolar epithelium | ++ (2) +++ (8) | ++ (3) +++ (7) | +++ (10) | +++ (10) | + (10) | +++ (10) |
| Metaplastic alveolar epitheliumb | ++ (2) +++ (8) | ++ (2) +++ (8) | ++ (5) +++ (5) | ++ (2) +++ (8) | + (10) | +++ (10) |
| Endothelium | (+)c (10) | 0 (10) | 0 (10) | 0 (10) | +++d (10) | 0 (10) |
Type II pneumocytes.
Metaplastic alveolar-type, metaplastic squamous-type, and metaplastic bronchiolar-type epithelium.
Very weak expression.
Very strong in remodeled arteries.
Total numbers of cases are shown in parentheses. UIP, usual interstitial pneumonia.
Table 3.
Expression of claudin-1, -2, -3, -4, -5, and -7 in patients with sarcoidosis
| Localization | Claudin-1 | Claudin-2 | Claudin-3 | Claudin-4 | Claudin-5 | Claudin-7 |
|---|---|---|---|---|---|---|
| Alveolar epitheliuma | 0 (10) | 0 (10) | + (10) | + (10) | 0 (10) | + (10) |
| Bronchiolar epithelium | ++ (2) +++ (8) | ++ (4) +++ (6) | +++ (10) | ++ (3) +++ (7) | + (10) | +++ (10) |
| Metaplastic alveolar epitheliumb | ++ (10) | + (10) | + (3) ++ (7) | ++ (10) | + (10) | ++ (6) +++ (4) |
| Endothelium | (+)c (10) | 0 (10) | 0 (10) | 0 (10) | +++ (10) | 0 (10) |
Type II pneumocytes.
Metaplastic alveolar-type epithelium.
Very weak expression.
Total numbers of cases are shown in parentheses.
Discussion
In this study, we observed that claudin-1, -2, -3, -4, and-7 are strongly and claudin-5 weakly expressed in normal bronchiolar epithelium. There are no previous studies examining the expression of various claudins at the bronchiolar level (i.e., in the small airways). However, in a recent study by Moldvay et al. (2007), it was shown that five of these proteins, claudin-1, -2, -3, -4, and -7, were expressed in normal bronchial epithelial cells, which suggests that at least these claudins are similarly present in the large and small airways of human lung. Claudin-5 is classically thought to be mainly expressed in endothelial cells (Morita et al. 1999), but its epithelial expression has also been observed in tumor cells (Paschoud et al. 2007).
Claudin-3, -4, and -7 are expressed in alveolar epithelium of normal human lung in type II pneumocytes, whereas there was no evidence for expression of claudin-1, -2, and -5 in normal alveoli. There are no previous studies on the expression of claudins in normal human peripheral lung, and thus, this study provides novel in vivo information on this topic. A recent in vitro study reported that alveolar epithelial cell transdifferentiation and/or epidermal growth factor exposure are mediated partly by changes in the pattern of expression of specific claudin isoforms in rat lung (Chen et al. 2005). In a study using human fetal lungs, it was proposed that, during differentiation of the alveolar epithelial cell to a type II cell phenotype, fetal alveolar epithelial cells make use of differential claudin expression and localization in the plasma membrane to help regulate tight junction permeability (Daugherty et al. 2004). An IHC study of adult rat lung sections indicated that claudin-3, -4, and -5 were found to be coexpressed by type II alveolar epithelial cells (Wang et al. 2003). We also observed that claudin-3 and -4 were expressed in type II pneumocytes, whereas claudin-5 was expressed at the alveolar level in endothelial cells of the capillaries of alveoli, a finding in line with the study of Morita et al. (1999), showing that claudin-5 was concentrated at cell–cell borders of endothelial cells of all segments of blood vessels of lung.
We observed that claudin-5 was widely expressed in endothelial cells of arteries, veins, and capillaries of both normal and diseased lung. In UIP, staining for claudin-5 was very intense in those arteries that were remodeled. We also observed weak staining for claudin-5 in bronchiolar epithelium of normal and diseased lung and also in metaplastic alveolar epithelium of sarcoidosis and UIP, suggesting that, in addition to being present in lung tumors, claudin-5 may also be induced in epithelial cells in non-neoplastic lung disease.
All of the claudins assessed in this study are expressed in the metaplastic alveolar epithelium, especially in UIP and to a lesser extent in sarcoidosis. The expression of claudins in sarcoidosis is less than that in UIP, which is understandable given that the metaplastic adjustive response is a function of claudin expression, because the damage in the alveolar epithelium is not as comprehensive in sarcoidosis as in UIP. Claudins are increased in all kinds of metaplastic alveolar epithelium including the alveolar, bronchiolar, and squamous types. It would therefore be tempting to postulate that claudins might have a role in the differentiation process of epithelial cells or alternatively that their expression seems to change in sarcoidosis and UIP at the alveolar level. It seems that in UIP, the alveolar epithelial cells are changing their phenotype with respect to claudins into that resembling bronchiolar cells, which is an interesting finding because the regeneration process of damaged alveolar epithelium in diseased lung is not well understood. It was surprising that the levels of all claudins studied are increased in metaplastic epithelium and are quite similarly expressed in bronchiolar epithelium of both normal and diseased lung. Further research will be needed to determine whether there are some specific claudins that associate with a specific lung disorder.
In conclusion, claudin-1, -2, -3, -4, -5, and -7 are expressed in normal human lung bronchial epithelial cells. Claudin-3, -4, and -7 are expressed in type II pneumocytes. In sarcoidosis and UIP, enhancement of claudin expression is detected in all types of metaplastic alveolar epithelial cells, with no clear distinction between metaplastic cell types. However, in UIP, claudin expression seems stronger, which could reflect the differences in the extent and nature of the metaplastic process and the underlying cellular damage.
Acknowledgments
This study was supported by the Academy of Finland, the Jalmari and Rauha Ahokas Foundation, and the Finnish Anti-Tuberculosis Association Foundation.
The technical assistance of Mirja Vahera, Erja Tomperi, and Hannu Wäänänen is kindly acknowledged.
References
- Bello IO, Vilen ST, Niinimaa A, Kantola S, Soini Y, Salo T (2008) Expression of claudins 1, 4, 5, and 7 and occludin, and relationship with prognosis in squamous cell carcinoma of the tongue. Hum Pathol 39:1212–1220 [DOI] [PubMed] [Google Scholar]
- Chen SP, Zhou B, Willis BC, Sandoval AJ, Liebler JM, Kim KJ, Ann DK, et al. (2005) Effects of transdifferentiation and EGF on claudin isoform expression in alveolar epithelial cells. J Appl Physiol 98:322–328 [DOI] [PubMed] [Google Scholar]
- Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER (1982) Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis 125:740–745 [DOI] [PubMed] [Google Scholar]
- Dail DH, Hammar SP (1994). Pulmonary Pathology. New York: Springer-Verlag
- Daugherty BL, Mateescu M, Patel AS, Wade K, Kimura S, Gonzales LW, Guttentag S, et al. (2004) Developmental regulation of claudin localization by fetal alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 287:L1266–1273 [DOI] [PubMed] [Google Scholar]
- Hinata N, Takemura T, Ikushima S, Yanagawa T, Ando T, Okada J, Oritsu M, et al. (2003) Phenotype of regenerative epithelium in idiopathic interstitial pneumonias. J Med Dent Sci 50:213–224 [PubMed] [Google Scholar]
- Kaarteenaho-Wiik R, Soini Y (2007) Expression of claudins 1, 2, 3, 4, 5 and 7 in sarcoidosis and usual interstitial pneumonia. Eur Respir J Suppl 30:2954 [Google Scholar]
- Katzenstein A (2006) Katzenstein and Askin's Surgical Pathology of Non-Neoplastic Lung Diseases. Philadelphia, Saunders
- Kawanami O, Ferrans VJ, Crystal RG (1982) Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest 46:39–53 [PubMed] [Google Scholar]
- Kim TH, Huh JH, Lee S, Kang H, Kim GI, An HJ (2008) Down-regulation of claudin-2 in breast carcinomas is associated with advanced disease. Histopathology 53:48–55 [DOI] [PubMed] [Google Scholar]
- Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, et al. (2002) Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol 81:419–435 [DOI] [PubMed] [Google Scholar]
- Lappi-Blanco E, Kaarteenaho-Wiik R, Maasilta PK, Anttila S, Paakko P, Wolff HJ (2006) COX-2 is widely expressed in metaplastic epithelium in pulmonary fibrous disorders. Am J Clin Pathol 126:717–724 [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, et al. (1998) Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 142:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldvay J, Jackel M, Paska C, Soltesz I, Schaff Z, Kiss A (2007) Distinct claudin expression profile in histologic subtypes of lung cancer. Lung Cancer 57:159–167 [DOI] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S (1999) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Tsukita S, Miyachi Y (2004) Tight junction-associated proteins (occludin, ZO-1, claudin-1, claudin-4) in squamous cell carcinoma and Bowen's disease. Br J Dermatol 151:328–334 [DOI] [PubMed] [Google Scholar]
- Paschoud S, Bongiovanni M, Pache JC, Citi S (2007) Claudin-1 and claudin-5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol 20:947–954 [DOI] [PubMed] [Google Scholar]
- Pummi K, Malminen M, Aho H, Karvonen SL, Peltonen J, Peltonen S (2001) Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J Invest Dermatol 117:1050–1058 [DOI] [PubMed] [Google Scholar]
- Rahner C, Mitic LL, Anderson JM (2001) Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120:411–422 [DOI] [PubMed] [Google Scholar]
- Schlüter H, Moll I, Wolburg H, Franke WW (2007) The different structures containing tight junction proteins in epidermal and other stratified epithelial cells, including squamous cell metaplasia. Eur J Cell Biol 86:645–655 [DOI] [PubMed] [Google Scholar]
- Selman M, Pardo A (2006) Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc 3:364–372 [DOI] [PubMed] [Google Scholar]
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, et al. (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103–106 [DOI] [PubMed] [Google Scholar]
- Tebbe B, Mankertz J, Schwarz C, Amasheh S, Fromm M, Assaf C, Schultz-Ehrenburg U, et al. (2002) Tight junction proteins: a novel class of integral membrane proteins. Expression in human epidermis and in HaCaT keratinocytes. Arch Dermatol Res 294:14–18 [DOI] [PubMed] [Google Scholar]
- Tokés AM, Kulka J, Paku S, Szik A, Páska C, Novák PK, Szilák L, et al. (2005) Claudin-1, -3 and -4 proteins and mRNA expression in benign and malignant breast lesions: a research study. Breast Cancer Res 7:R296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Colby TV, Koss MN, de Rosado-Christenson ML, Muller NL, King TE (2002) Atlas of Nontumor Pathology. Non-Neoplastic Disorder of Lower Respiratory Tract. Washington, DC: The American Registry of Pathology and Armed Forces Institute of Pathology
- Tsukita S, Furuse M, Itoh M (2001) Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293 [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM (2004) The role of claudins in determining paracellular charge selectivity. Proc Am Thorac Soc 1:38–41 [DOI] [PubMed] [Google Scholar]
- Wagenvoort CA (1980) Lung biopsy specimens in the evaluation of pulmonary vascular disease. Chest 77:614–625 [DOI] [PubMed] [Google Scholar]
- Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M (2003) Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 29:62–70 [DOI] [PubMed] [Google Scholar]
- Wilcox ER, Burton QL, Naz S, Riazuddin S, Smith TN, Ploplis B, Belyantseva I, et al. (2001) Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell 104:165–172 [DOI] [PubMed] [Google Scholar]