Abstract
Tissue inhibitor of matrix metalloproteinase-3 (TIMP-3) is an important regulator of matrix metalloproteinase activity in many types of disease, including atherosclerosis, neoplasia, and inflammatory conditions. Among TIMPs, TIMP-3 uniquely binds the extracellular matrix (ECM). We performed IHC staining on 17 tissue microarrays containing >1500 samples to determine the location of ECM TIMP-3 staining in a variety of predominantly vascular tissues. We found a unique pattern of TIMP-3 staining in the ECM of renal arterioles, small pulmonary vessels and parenchyma, and Bruch's membrane in the retina. There was no staining in larger caliber arteries including coronary and internal mammary arteries. TIMP-3 protein accumulation was found to be an age-dependent phenomenon, with staining appearing in all three tissues in early adulthood and becoming more robust among the elderly. These findings may help to explain the late onset of the TIMP-3–associated ocular diseases Sorsby fundus dystrophy and age-related macular degeneration and suggest a similar phenomenon could be at work in other age-related conditions. (J Histochem Cytochem 57:207–213, 2009)
Keywords: tissue inhibitor of matrix metalloproteinase-3, extracellular matrix, immunohistochemistry
Normal turnover of the extracellular matrix (ECM) is regulated in large part by the balance between matrix metalloproteinases (MMPs) and the tissue inhibitors of metalloproteinases (TIMPs) (Visse and Nagase 2003; Malemud 2006). The MMPs are a family of >20 secreted proteinases. Four TIMPs exist that regulate the MMP activity. In vascular tissues, the MMP/TIMP activity ratio has been suggested to control aspects of vascular remodeling and angiogenesis (Raffetto and Khalil 2008). Fluctuations in the MMP–TIMP equilibrium contribute to diseases including atherosclerosis and vascular aneurysm (Hobeika et al. 2007).
The TIMPs are small (21–28 kDa) secreted proteins that bind to and inhibit MMP active sites. In addition to their role in ECM turnover, TIMPs are important in apoptosis and cell growth (Gomez et al. 1997; Baker et al. 1998).
TIMP-3 is unique among TIMPs because it tightly binds to the ECM (Leco et al. 1994). Both the N- and C-terminal domains of the TIMP-3 protein bind to the ECM to promote MMP inhibition (Lee et al. 2007). TIMP-3 is an effective inhibitor of membrane-type MMPs (MT-MMPs) and the tumor necrosis factor α–converting enzyme (TACE) (Amour et al. 1998; Brew et al. 2000). TIMP-3 gene expression is variable by organ with the highest RNA levels in murine lung and kidney (Leco et al. 1994). TIMP-3 knockout mice show spontaneous lung air space enlargement as a result of impaired bronchiole branching morphogenesis (Leco et al. 2001; Gill et al. 2003). In humans, mutations in the TIMP-3 gene cause Sorsby's fundus dystrophy (Weber et al. 1994), an autosomal dominant disorder characterized by abnormal Bruch's membrane thickening, choroidal neovascularization, retinal degeneration, and loss of vision usually by middle age (Sorsby and Mason 1949). Mice lacking TIMP-3 in their eyes develop abnormal choroidal vessels (Janssen et al. 2008)
The goal of this study was to characterize the distribution of TIMP-3 in human vascular tissues, using tissue microarrays (TMAs) containing samples taken from 100 subjects undergoing autopsy (Halushka et al. in press). We hypothesized that TIMP-3 would be differentially expressed in the various tissues on these TMAs.
Materials and Methods
Harvesting and Processing of Donor Tissues
Tissue samples from 100 autopsies, with ages ranging from 20 to 101 years, were collected at The Johns Hopkins Hospital and Johns Hopkins Bayview Medical Center as described in detail (Halushka et al. in press). Briefly, 59% of the cohort was male, 76% were white, 30% had diabetes, 62% had hypertension, 38% had a history of smoking, and the average age of the group was 64 years. The Johns Hopkins University School of Medicine Institutional Review Board granted an exemption for this study, and permission for tissue harvesting was obtained from the hospital's autopsy consent form. The postmortem interval for all autopsies was <28 hr. Thirteen tissue or blood vessel samples were taken from each subject (carotid artery, coronary artery, dorsalis pedis artery, iliac artery, internal mammary artery, mesenteric artery, pulmonary artery, pulmonary parenchyma, renal arcuate artery, renal artery, renal cortex, retina, and skin). Only the renal cortex, retina, and pulmonary parenchyma had ECM staining; thus, the other vascular tissues on the TMAs are not reported on in this study. Tissue samples 1–2 cm in size were placed in 10% buffered formalin (Cardinal Health; Dublin, OH) for at least 24 hr before processing and paraffin embedding.
TMAs
TMAs were produced as previously described (Halushka et al. in press). Briefly, 1.5-mm tissue segments were used to construct 17 99-spot arrays with a manual tissue arrayer (Beecher Instruments; Silver Spring, MD). Each array consisted of 9 controls and 90 autopsy tissues. Duplicate renal parenchyma and coronary artery features were incorporated in tandem on each TMA. Additional pulmonary parenchyma, coronary artery, carotid artery, and renal cortex from 17 subjects were placed across each TMA for between-slide controls. Four-μm-thick sections were placed on slides and stored at −20C before IHC staining. An additional 18 unstained slides of lung, kidney, or retina were obtained from the autopsy and surgical pathology case files of The Johns Hopkins Hospital to augment the study with younger individuals (22 weeks to 23 years of age).
IHC Detection of TIMP-3
All IHC staining was performed using a polyclonal rabbit anti-human TIMP-3 antibody (LabVision; Fremont, CA). This antibody has previously been validated in autolyzed vascular tissues (Maleszewski et al. 2007). Staining was performed per the manufacturer's instructions. Briefly, after deparaffinization and rehydration, slides were incubated with 3% H2O2 (Labvision) to block endogenous peroxidase activity. Antigen retrieval was done using 0.01 M sodium citrate buffer (JT Baker Chemical; Phillipsburg, NJ) in a microwave oven for 10 min. Slides were blocked with Ultra V Block (LabVision) and incubated with the rabbit anti-human TIMP-3 antibody (1:400 dilution) at 4C overnight. Slides were incubated with biotinylated anti-rabbit secondary antibody for 10 min at room temperature and subsequently incubated with streptavidin peroxidase for 10 min (LabVision). Slides were incubated with DAB chromagen (Labvision) and counterstained with hematoxylin (Sigma; St. Louis, MO). To determine background and nonspecific staining, a negative control was performed with each run in which the primary TIMP-3 antibody was omitted, which resulted in no ECM staining. Staining with the TIMP-3 antibody was compared with prior reports of the pattern of positive ECM TIMP-3 staining to confirm the robustness of staining (Selman et al. 2000).
Scoring Scale
All tissues were scored by pathologists (MKH and CGE) blinded to demographic information. A 0–3 scale was used: 0 for no staining; 1 for mild staining; 2 for moderate staining; and 3 for strong staining. Staining was evaluated only in the extracellular matrix of small vessels, the renal parenchyma, or in Bruch's membrane. When tissue was missing on a slide or was not able to be evaluated, it was removed from analysis.
Statistical Analysis of Clinical Variables and Staining
Staining values for the eye, lung, and kidney were compared with the clinical and pathological variables of sex, ethnicity, hypertension, smoking, and diabetes by t-test (Stata 10.0; StataCorp, College Station, TX). Comparisons of staining between age groups for each tissue were also performed by t-test. There was insufficient data on eye disease in this population for use in statistical analysis.
Results
TIMP-3 Staining in the Lung
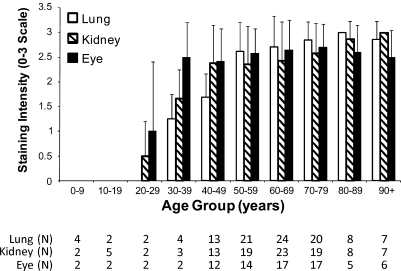
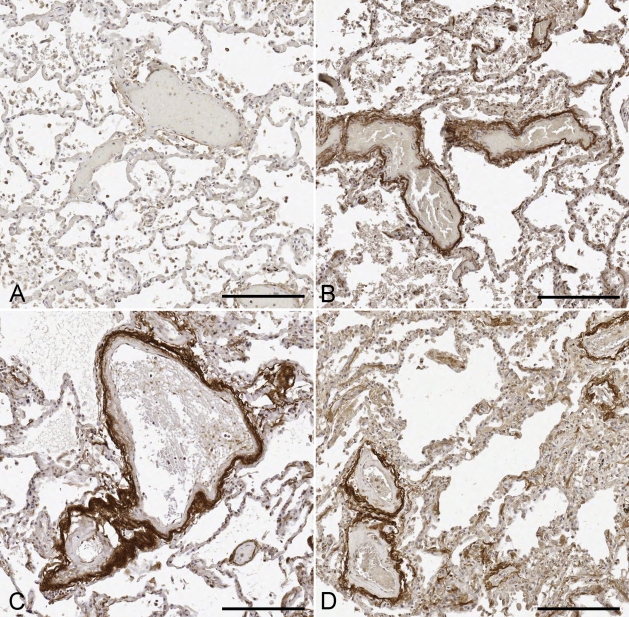
TIMP-3 staining intensity was evaluated for 105 pulmonary parenchymal tissue samples from subjects ranging from 22 weeks to 101 years (Figure 1). Staining was most consistently observed around vessels (arteries and veins) within the pulmonary parenchymal tissue (Figure 2). Occasionally, staining of the basement membrane was also found to extend into the alveoli walls. In addition, staining intensity increased with age. TIMP-3 staining was essentially absent in all subjects <30 years of age (Figures 1 and 2A). Mild perivascular staining was present in the 30- to 39- year age group, with staining intensity increasing through the deciles of middle-aged subjects. By 50 years of age, strong TIMP-3 perivascular staining was observed in all subjects (Figures 2B–2D). Moreover, 96% (77/80) of subjects >50 years of age exhibited moderate to strong TIMP-3 staining in the pulmonary parenchyma. By univariate analysis, smoking was associated with increased pulmonary TIMP-3 staining (2.7 ± 0.1 vs 2.3 ± 0.1, p<0.01, t-test), but ethnicity, sex, hypertension, and diabetes were not.
Figure 1.
Age-related extracellular matrix (ECM) tissue inhibitor of matrix metalloproteinase-3 (TIMP-3) staining in lung parenchyma, renal microvasculature, and Bruch's membrane. In all three tissues, all age deciles >30 years had significantly increased staining compared with the youngest decile (t-test, p<0.05). n=number of tissues evaluated for each age decile.
Figure 2.
Representative images of TIMP-3 staining in the lung parenchyma. TIMP-3 staining was perivascular in an age-related fashion. (A) Twenty-eight-year-old man with no TIMP-3 staining. (B) Fifty-nine-year-old man with moderate TIMP-3 staining. (C) Sixty-one-year-old man with strong TIMP-3 staining. (D) Seventy-year-old woman with strong TIMP-3 staining. Bar = 200 μm.
TIMP-3 Staining in the Kidney
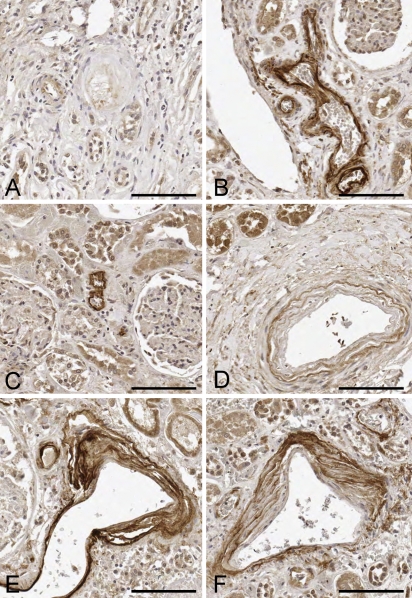
Renal parenchymal tissues for 101 subjects, with an age range of 22 weeks to 101 years, were evaluated for TIMP-3 staining. ECM TIMP-3 staining was generally observed around small and intermediate-sized arterioles (Figure 3). Staining was occasionally seen in the afferent arteriole of the glomerulus, extending up to Bowman's capsule; however, the glomerulus itself did not stain for TIMP-3. Staining often had an onion skin appearance within the larger arterioles. Once again, staining intensity correlated with increasing age. TIMP-3 staining was absent in all subjects <20 years of age. Mild TIMP-3 staining was first seen in the 20- to 29-year-old age group and became stronger in the 30- to 39-year-old age group (Figure 1). Strong staining was present in most subjects in their 40s, and this staining persisted through all older age groups. Ninety-two percent (70/76) of all tissue samples >50 years of age stained moderately or strongly for TIMP-3. Renal TIMP-3 staining was unrelated to sex, ethnicity, diabetes, hypertension, or smoking status by univariate analysis.
Figure 3.
Representative images of TIMP-3 staining in the renal parenchyma. (A) Twenty-eight-year-old man with no TIMP-3 staining. (B) Seventy-two-year-old woman with strong TIMP-3 staining of a small arteriole. (C) Fifty-four-year-old woman with strong arteriolar TIMP-3 staining. (D) Fifty-one-year-old man with moderate TIMP-3 staining in an “onion skin” pattern. (E) Seventy-six-year-old man with strong multilayered TIMP-3 staining. (F) One-hundred-one-year-old woman with strong TIMP-3 staining. Bar = 100 μm.
TIMP-3 Staining in the Retina
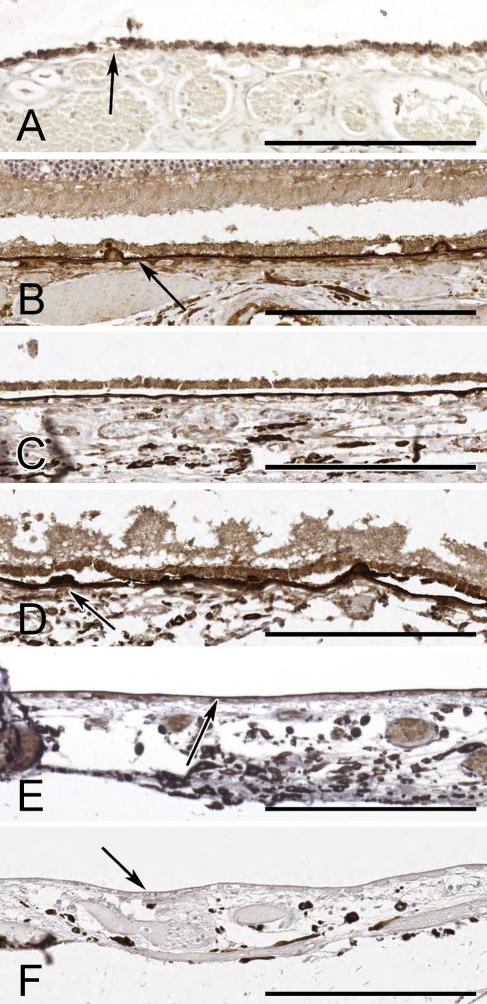
Seventy-nine subjects from 18 months to 101 years were evaluated for TIMP-3 staining in the retina (Figure 1). ECM TIMP-3 staining was strongest in Bruch's membrane (Figure 4). Cellular ECM staining was also present in the ganglion cell layer of some retinas. TIMP-3 also strongly stained drusen deposits below the retinal pigment epithelium, as shown in Figure 4D. As observed in the other tissues, TIMP-3 staining increased with advancing age. Staining was absent in the youngest population (<20 years of age), and weak staining was first observed in subjects 20–30 years of age. Strong staining first appeared in the 30- to 39-year-old age group (Figures 1 and 4). Staining remained strong for all of the remaining deciles. More than 98% (58/59) of subjects >50 years of age were found to have moderate to strong TIMP-3 staining in the eye. Retinal TIMP-3 staining was unrelated to sex, ethnicity, diabetes, hypertension, or smoking status by univariate analysis.
Figure 4.
Representative images of TIMP-3 staining of Bruch's membrane in the eye. (A) Thirteen-year-old boy with no TIMP-3 staining. Arrow identifies Bruch's membrane below the pigmented retinal pigment epithelium (RPE) cells. (B) Eighty-six-year-old man with moderate TIMP-3 staining. Arrow identifies Bruch's membrane. (C) Sixty-nine-year-old man with strong TIMP-3 staining. (D) Ninety-five-year-old man with strong TIMP-3 staining. Arrow shows strongly stained drusen deposits on Bruch's membrane. (E) Forty-three-year-old woman with moderate TIMP-3 staining. Arrow identifies Bruch's membrane. The RPE layer is artifactually detached. (F) Unstained control tissue from the same 43-year-old woman. Arrow denotes the Bruch's membrane. The RPE layer is artifactually detached. Bar = 200 μm.
Discussion
TIMP-3 plays an integral role in the regulation and turnover of the ECM (Visse and Nagase 2003; Malemud 2006). TIMP-3 is unique in its binding and localization to the ECM. In this study, we fully characterized the ECM staining patterns of TIMP-3 in multiple organs and a variety of vascular tissues.
We found extracellular TIMP-3 protein to be present in the kidney, lung, and eye by IHC, which was consistent with previous RNA findings (Leco et al. 1994). We found no significant ECM TIMP-3 staining in any of the large- to medium-sized elastic or muscular blood vessels present on the TMAs (data not shown). We also did not observe staining of TIMP-3 in small hepatic vessels performed on additional material (data not shown). Pulmonary TIMP-3 staining was increased in patients with a history of smoking, but all other univariate analyses were negative. This finding was counterintuitive because increased pulmonary remodeling has been related to smoking (Santos et al. 2002).
ECM TIMP-3 staining has not previously been described in renal parenchyma. We found it localized to small- and intermediate-sized arterioles but not present in interlobar arteries, arcuate arteries, or glomeruli. Airola et al. (1998) has shown TIMP-3 mRNA in adult kidney glomeruli and tubules. Such gene and protein expression discrepancies are to be expected with a protein that is localized to the ECM. In the eye, TIMP-3 protein is present on Bruch's membrane, whereas TIMP-3 mRNA is found in the retinal pigment epithelium (Della et al. 1996; Ruiz et al. 1996; Fariss et al. 1997; Kamei et al. 1997).
The other two places we identified TIMP-3 have been reported. TIMP-3 protein is known to localize to Bruch's membrane in the human eye (Fariss et al. 1997), as well as the elastic lamina and ECM of the pulmonary parenchyma (Selman et al. 2000). Interestingly, Selman et al. (2000) reported TIMP-3 staining in lungs from patients with idiopathic pulmonary fibrosis (IPF) but not in control lung tissues. However, the mean age of the healthy donors was 43.2 ± 11.1 years, whereas the mean age of the IPF patients was 58.1 ±13.2 years, which may better explain their findings.
TIMP-3 has been implicated in a variety of disease processes including atherosclerosis, tumor metastasis, and tissue inflammation (Baker et al. 1999; Mohammed et al. 2003; Hobeika et al. 2007). The relationship between its vascular location in kidney and lung and these pathologies is not clear. In patients with TIMP-3 mutations, there have been very few non-ocular progressive pathological findings reported. There are no reports of progressive pulmonary or renal disease in these patients, although the original Sorsby kindred had a patient with unilateral renal agenesis (Thompson and Baraitser 1988). Although there are several diseases such as Goodpasture's disease that share renal and pulmonary pathologies, we can find no reported diseases that share pathologies of small pulmonary and renal arteries.
It has previously been suggested that TIMP-3 is a senescence-related protein (Kamei and Hollyfield 1999). Their study showed elevations in TIMP-3 protein levels in Bruch's membrane with age-related macular degeneration (AMD) and with normal aging. In our study, we showed that TIMP-3 protein increases with age in lung and kidney concurrent with our Bruch's membrane findings (Figure 1). TIMP-3 staining was absent in all tissues for all subjects <20 years of age. Mild to moderate staining was observed in middle-aged subjects, and nearly all subjects >50 years of age exhibited strong TIMP-3 staining.
We can only speculate on the reasons for this unusual age-related appearance of TIMP-3. One possible explanation for this finding is that it simply takes a long time for TIMP-3 protein to accumulate to levels detectable by IHC. Another possibility is that the age-related TIMP-3 protein deposition is the result of an age-related increase in TIMP-3 gene expression. Ryu et al. (2005) have shown that TIMP-3 gene and protein expression both increase with age in the human lung. However, Bailey et al. (2001) found that TIMP-3 gene expression did not increase with age in the human eye. A third possibility is that ECM content is increased or altered by events such as glycosylation as we age, and these changes facilitate TIMP-3 binding and accumulation (Monnier and Sell 2006). Perhaps the most intriguing explanation is that ECM TIMP-3 levels increase at the end of full developmental organogenesis to prevent further organ growth. For example, a typical human stops growing by their third decade of life. At that time, TIMP-3 levels increase to prevent further MMP-mediated remodeling and growth.
Our finding of TIMP-3 expression only in later life is consistent with the pathogenesis of Sorsby fundus dystrophy (SFD). Manifestation of SFD usually occurs in the third to fourth decade of life. Similarly, patients with AMD experience the onset of symptoms in their 50s and 60s. Although no genetic mutation in TIMP-3 has been found to cause AMD, Kamei and Hollyfield (1999) have found that patients with AMD have more TIMP-3 protein in Bruch's membrane than age-matched controls. Our finding that ocular TIMP-3 is absent in young individuals may help to explain the late onset of these diseases.
In summary, in a global survey of organs and vascular tissues using TMAs, we found ECM TIMP-3 staining only in lung parenchyma, renal arterioles, and Bruch's membrane. In all three tissues, TIMP-3 staining was not present until early adulthood and gained steadily in intensity in an age-dependent fashion.
Acknowledgments
This study was supported by a junior faculty award from the American Diabetes Association (1-05-JF-20 to MKH).
The authors thank Sonny Dike, MD, for assistance with IHC.
References
- Airola K, Ahonen M, Johansson N, Heikkilä P, Kere J, Kähäri V-M, Saarialho-Kere UK (1998) Human TIMP-3 is expressed during fetal development, hair growth cycle, and cancer progression. J Histochem Cytochem 46:437–447 [DOI] [PubMed] [Google Scholar]
- Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith BJ, Stephens PE, et al. (1998) TNF-alpha converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett 435:39–44 [DOI] [PubMed] [Google Scholar]
- Bailey TA, Alexander RA, Dubovy SR, Luthert PJ, Chong NHV (2001) Measurement of TIMP-3 expression and Bruch's membrane thickness in human macula. Exp Eye Res 73:851–858 [DOI] [PubMed] [Google Scholar]
- Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC (1999) Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer 79:1347–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AH, Zaltsman AB, George SJ, Newby AC (1998) Divergent effects of tissue inhibitor of metalloproteinase-1, -2, or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest 101:1478–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H (2000) Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta 1477:267–283 [DOI] [PubMed] [Google Scholar]
- Della N, Campochiaro P, Zack D (1996) Localization of TIMP-3 mRNA expression to the retinal pigment epithelium. Invest Ophthalmol Vis Sci 37:1921–1924 [PubMed] [Google Scholar]
- Fariss R, Apte S, Olsen B, Iwata K, Milam A (1997) Tissue inhibitor of metalloproteinases-3 is a component of Bruch's membrane of the eye. Am J Pathol 150:323–328 [PMC free article] [PubMed] [Google Scholar]
- Gill S, Pape M, Khokha R, Watson A, Leco K (2003) A null mutation for tissue inhibitor of metalloproteinases-3 (TIMP-3) impairs murine bronchiole branching morphogenesis. Dev Biol 261:313–323 [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP (1997) Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 74:111–122 [PubMed] [Google Scholar]
- Halushka MK, Cornish TC, Lu J, Selvin S, Selvin E (In Press) Creation, validation, and quantitative analysis of protein expression in vascular tissue microarrays. Cardiovasc Pathol [DOI] [PMC free article] [PubMed]
- Hobeika M, Thompson R, Muhs B, Brooks P, Gagne P (2007) Matrix metalloproteinases in peripheral vascular disease. J Vasc Surg 45:849–857 [DOI] [PubMed] [Google Scholar]
- Janssen A, Hoellenriegel J, Fogarasi M, Schrewe H, Seeliger M, Tamm E, Ohlmann A, et al. (2008) Abnormal vessel formation in the choroid of mice lacking tissue inhibitor of metalloprotease-3. Invest Ophthalmol Vis Sci 49:2812–2822 [DOI] [PubMed] [Google Scholar]
- Kamei M, Apte S, Lewis H, Hollyfield JG (1997) TIMP-3 accumulation in Bruch's membrane and drusen in eyes from normal and age-related macular degeneration donors. In LaVail MM, Hollyfield JG, Anderson RE, eds. Degenerative Retinal Diseases. New York, Plenum Press, 11–15
- Kamei M, Hollyfield JG (1999) TIMP-3 in Bruch's membrane: changes during aging and in age-related macular degeneration. Invest Ophthalmol Vis Sci 40:2367–2375 [PubMed] [Google Scholar]
- Leco K, Khokha R, Pavloff N, Hawkes S, Edwards D (1994) Tissue inhibitor of metalloproteinases-3 (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem 269:9352–9360 [PubMed] [Google Scholar]
- Leco K, Waterhouse P, Sanchez O, Gowing K, Poole A, Wakeham A, Mak T, et al. (2001) Spontaneous air space enlargement in the lungs of mice lacking tissue inhibitor of metalloproteinases-3 (TIMP-3). J Clin Invest 108:817–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-H, Atkinson S, Murphy G (2007) Identification of the extracellular matrix (ECM) binding motifs of tissue inhibitor of metalloproteinases (TIMP)-3 and effective transfer to TIMP-1. J Biol Chem 282:6887–6898 [DOI] [PubMed] [Google Scholar]
- Malemud CJ (2006) Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci 11:1696–1701 [DOI] [PubMed] [Google Scholar]
- Maleszewski J, Lu J, Fox-Talbot K, Halushka MK (2007) Robust immunohistochemical staining of several classes of proteins in tissues subjected to autolysis. J Histochem Cytochem 55:597–606 [DOI] [PubMed] [Google Scholar]
- Mohammed FF, Smookler DS, Khokha R (2003) Metalloproteinases, inflammation, and rheumatoid arthritis. Ann Rheum Dis 62(suppl 2):ii43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM, Sell DR (2006) Prevention and repair of protein damage by the Maillard reaction in vivo. Rejuvenation Res 9:264–273 [DOI] [PubMed] [Google Scholar]
- Raffetto J, Khalil R (2008) Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75:346–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Brett P, Bok D (1996) TIMP-3 is expressed in human retinal pigment epithelium. Biochem Biophys Res Commun 226:467–474 [DOI] [PubMed] [Google Scholar]
- Ryu J, Vicencio AG, Yeager ME, Kashgarian M, Haddad GG, Eickelberg O (2005) Differential expression of matrix metalloproteinases and their inhibitors in human and mouse lung development. Thromb Haemost 94:175–183 [DOI] [PubMed] [Google Scholar]
- Santos S, Peinado VI, Ramírez J, Melgosa T, Roca J, Rodriguez-Roisin R, Barberà JA (2002) Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J 19:632–638 [DOI] [PubMed] [Google Scholar]
- Selman M, Ruiz V, Cabrera S, Segura L, Ramírez R, Barrios R, Pardo A (2000) TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol 279:L562–574 [DOI] [PubMed] [Google Scholar]
- Sorsby A, Mason M (1949) A fundus dystrophy with unusual features. Br J Ophthalmol 33:67–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EM, Baraitser M (1988) Sorsby syndrome: a report on further generations of the original family. J Med Genet 25:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 92:827–839 [DOI] [PubMed] [Google Scholar]
- Weber B, Vogt G, Pruett R, Stöhr H, Felbor U (1994) Mutations in the tissue inhibitor of metalloproteinases-3 (TIMP3) in patients with Sorsby's fundus dystrophy. Nat Genet 8:352–356 [DOI] [PubMed] [Google Scholar]