Abstract
Various imaging techniques have been used to assess degeneration of the intervertebral disc, including many histological methods, but cartilage-oriented histological stains do not clearly show the comparatively complex structures of the disc. In addition, there is no integrated method to assess efficiently both the compartmental organization and matrix composition in disc samples. In this study, a novel histological method, termed FAST staining, has been developed to investigate disc growth and degeneration by sequential staining with fast green, Alcian blue, Safranin-O, and tartrazine to generate multichromatic histological profiles (FAST profiles). This identifies the major compartments of the vertebra-disc region, including the cartilaginous endplate and multiple zones of the annulus fibrosus, by specific FAST profile patterns. A disc degeneration model in rabbit established using a previously described puncture method showed gradual but profound alteration of the FAST profile during disc degeneration, supporting continual alteration of glycosaminoglycan. Changes of the FAST profile pattern in the nucleus pulposus and annulus fibrosus of the postnatal mouse spine suggested matrix remodeling activity during the growth of intervertebral discs. In summary, we developed an effective staining method capable of defining intervertebral disc compartments in detail and showing matrix remodeling events within the disc. The FAST staining method may be used to develop a histopathological grading system to evaluate disc degeneration or malformation. (J Histochem Cytochem 57:249–256, 2009)
Keywords: intervertebral disc, growth, degeneration, remodeling, matrix, staining and labeling
The intervertebral disc (IVD) comprises three main structures: the endplates that contain chondrocytes, the nucleus pulposus (NP) that contains vacuolated notochordal cells and chondrocyte-like cells, and the annulus fibrosus (AF) that contains cells phenotypically resembling both fibroblasts and chondrocytes. Collagens and proteoglycans, the main extracellular matrix (ECM) constituents of the IVD, are present in different proportions in the NP and AF (Walker and Anderson 2004). IVD degeneration can occur because of aging, trauma, or genetic predisposition. The NP is highly gelatinous and translucent in young humans but becomes more opaque and fibrous with aging, because of changes in its matrix that start with the breakdown of proteoglycans. Previous in vivo studies have suggested that dynamic compression can modulate expression of anabolic and catabolic genes in IVD, suggesting the IVD has the ability to maintain structural integrity and adapt to mechanical loading through ECM remodeling (MacLean et al. 2004,2005).
Various imaging techniques have been widely used to assess IVD degeneration, among which histological methods are most common. A number of histological grading systems have been established to evaluate the severity of degeneration based on macroscopic structure and cellularity changes in humans (Boos et al. 2002) and animal models (Nishimura and Mochida 1998; Masuda et al. 2005).
Several histological staining methods have been used to study the normal biology and degeneration of IVDs. Alcian blue–fast red (Smits and Lefebvre 2003; Semba et al. 2006) and Safranin-O–fast green (Lu et al. 1997; Sahlman et al. 2001; Chubinskaya et al. 2007) are popular combinations, in conjunction with the standard hematoxylin–eosin staining (Kim et al. 2003; Semba et al. 2006). These methods, although useful, have limitations in delineating the structures of the IVD compared with their use for articular cartilage, because of the greater complexity of the compartments and matrix composition of the disc. Triple dye methods, including picrosirius red–Alcian blue–hematoxylin (Gruber et al. 2002,2005) and Masson's trichrome (Gruber et al. 2005; Ho et al. 2008) have recently been used to increase the histological resolution of the IVD with some success.
Because proteoglycan is a major component of the IVD, we hypothesized that synergistic staining with dyes with affinity for glycoproteins might generate a structural image of the disc with enhanced resolution. Safranin-O has been widely used for general detection of glycosaminoglycans. Alcian blue at acidic pH detects strongly sulfated glycoproteins. Fast green, which has been used as a counterstain in Safranin-O and Masson's trichrome protocols, preferentially stains glycoprotein-rich rigid structures such as cellulose, cell wall, and bone. Tartrazine has also been shown to interact preferentially with mucin-associated ECM of fibroblastic origin such as woven bone (Lee et al. 2001), as well as intestine (Jones and Bevins 1992). We therefore studied whether a combination of these dyes could provide a unique histological tool to study glycoprotein- or proteoglycan-rich tissues.
In this study, we report the development of a multichromatic staining protocol for histological assessment of the IVD using a combination of fast green, Alcian blue, Safranin-O, and tartrazine stains (FAST staining). Using mouse and rabbit disc samples, we asked whether this technique can show the fine structure of the IVD, as well as provide information regarding matrix remodeling events, such as during disc growth and degeneration.
Materials and Methods
Disc Degeneration Model
Four female New Zealand White rabbits at 6 months old were anesthetized, and disc degeneration was induced at levels L2/L3 and L4/L5 by annulus puncture at 5 mm depth with a 19-gauge needle (BD; Singapore) through an anterolateral retroperitoneal approach as previously described (Masuda et al. 2005; Ho et al. 2008). Level L3/L4 was untreated and served as a control. A titanium clip was placed in the psoas muscle at the level of the L3/L4 discs to mark the operated levels. The operated animals were closely monitored but not restricted from movement after the operation. Degeneration of the punctured discs was allowed to progress up to 12 months before the animals were killed by intravenous injection of 100 mg/kg pentobarbitone sodium (Abbott Laboratories; Chicago, IL). All animal experiments were performed according to protocols approved by the local health department and institutional ethics committee.
Tissue Histology
To study changes in the IVD during postnatal growth, we harvested coccygeal intervertebral discs from C57BL mice at birth, 10 days, 4 weeks, and 8 weeks of age. Four discs were evaluated for each time point. To study the degenerative patterns, we harvested the control and the punctured lumbar discs from the rabbits at 3–12 months after surgery and from non-operated 6-month-old rabbits. All tissues were fixed in 4% paraformaldehyde and were decalcified at 4C in 0.5 M EDTA (pH 7.5) for the mouse discs or in Morse's solution (Greep et al. 1947) (prepared by mixing 22.5% formic acid and 10% sodium citrate) for the rabbit discs (all reagents from Sigma-Aldrich; St. Louis, MO). They were dehydrated stepwise in 30%, 70%, and 100% ethanol (Merck; Darmstadt, Germany), cleared in xylene (Merck), and embedded in paraffin wax (Paraplast Plus; McCormick Scientific, St. Louis, MO). The embedded samples were cut by a Leica RM2135 microtome (Leica Microsystems; Wetzlar, Germany) into 7-μm-thick sagittal sections.
FAST Staining
FAST staining refers to a multidye staining procedure using fast green, Alcian blue, Safranin-O, and tartrazine. All dyes were obtained from Sigma-Aldrich. After dewaxing in xylene and stepwise rehydration in 70% ethanol, 30% ethanol, and distilled water, the sections were first stained with 1% Alcian blue 8GX (pH 1.0) for 2.5 min and then 0.1% Safranin-O for 3 min, followed by color differentiation in 50% ethanol for 1 min. The sections were stained with 0.25% tartrazine (supplied in 0.25% acetic acid) for 10 sec and finally counterstained in 0.001% fast green for 5 min. A brief rinse in distilled water was applied after each of the above staining and differentiation steps. Sections were finally air-dried, mounted in DePeX (BDH Laboratory; Poole, UK), and examined under a Nikon Eclipse 80i microscope (Tokyo, Japan). In some experiments, the Alcian blue staining step was prolonged to 10 min. The staining procedures were carried out at room temperature.
Results
Defining Intervertebral Disc Structures by FAST Staining
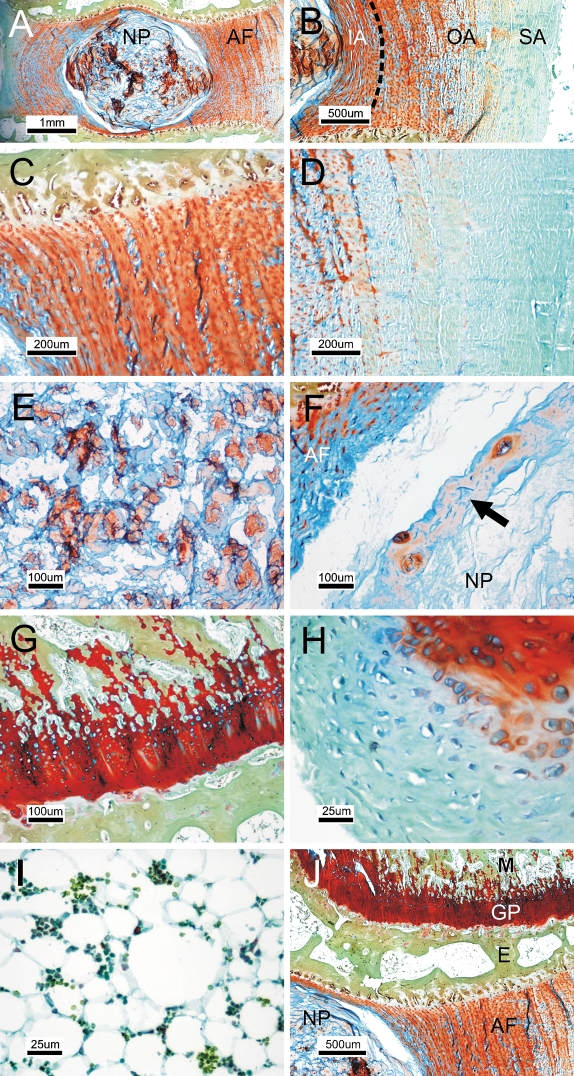
We first tested whether the FAST staining method can deliver unique information about the IVD structure. FAST staining of lumbar discs of 6-month-old rabbits gave different patterns for the AF and the NP (Figure 1A). Alcian blue stain was most intense in the NP matrix and in the innermost layers of the AF (Figure 1B). The inner annulus and part of the outer annulus showed a similar staining pattern, with a transition of color in the periphery of the outer annulus, which we defined as the superficial annulus. Under higher magnification, both the inner and outer annulus were seen to be preferentially stained orange by Safranin-O, with intercalated Alcian blue–positive matrix (Figure 1C), whereas the superficial annulus was stained greenish-blue because of domination of fast green (Figure 1D). In contrast, the NP was Alcian blue positive, whereas the pericellular matrix was Safranin-O positive (Figure 1E). The periphery of the NP was lined by a thin sheath of cell-containing matrix (Figure 1F). Apart from the disc, the cartilage of the vertebral growth plate was stained red by Safranin-O (Figure 1G). Bone was stained yellowish green because of dominant costaining by tartrazine/fast green. The perichondrium of the vertebral physis was negative for Safranin-O but slightly positive for Alcian blue (Figure 1H). The stroma of epiphyseal bone marrow was largely composed of adipocytes and showed no preferential uptake of stain (Figure 1I). Contrasting cytoplasmic staining was observed between cells in the AF/growth plate (Alcian blue positive; Figures 1C and 1G) and in the NP (Safranin-O positive; Figure 1E). Thus, the FAST protocol was able to differentiate all major vertebral and intervertebral compartments with a distinctive color (Figure 1J). The FAST staining pattern, also called the FAST profile, is summarized in Table 1.
Figure 1.
Differentiation of disc compartments by FAST staining. (A) The sagittal section of lumbar discs of a 6-month-old rabbit was stained to visualize the annulus fibrosus (AF) and nucleus pulposus (NP). (B) Inner annulus (IA), outer annulus (OA), and superficial annulus (SA) at the periphery of OA. (C) Magnified view of IA and OA. (D) Magnified view of SA. (E) Central region of NP. (F) Periphery of NP composed of a sheath of cell-adhered matrix (arrow). (G) Vertebra growth plate. (H) Perichondrium of the vertebral physis. (I) Epiphyseal bone marrow. (J) An overview of vertebra-disc compartments. GP, growth plate; M, metaphysis of vertebra; E, epiphysis of vertebra.
Table 1.
FAST profile of intervertebral disc under standard protocol
| Compartment | Subdivision | Matrix color | Cell color (pericellular) |
|---|---|---|---|
| Growth plate | Red | Blue | |
| Bone | Yellowish green | Nil | |
| Annulus fibrosus | Outer/inner | Orange, with blue patches | Blue |
| Superficial | Greenish blue | Blue | |
| Nucleus pulposus | Blue | Orange (orange halo) | |
| Cartilaginous endplate | Orange | Orange |
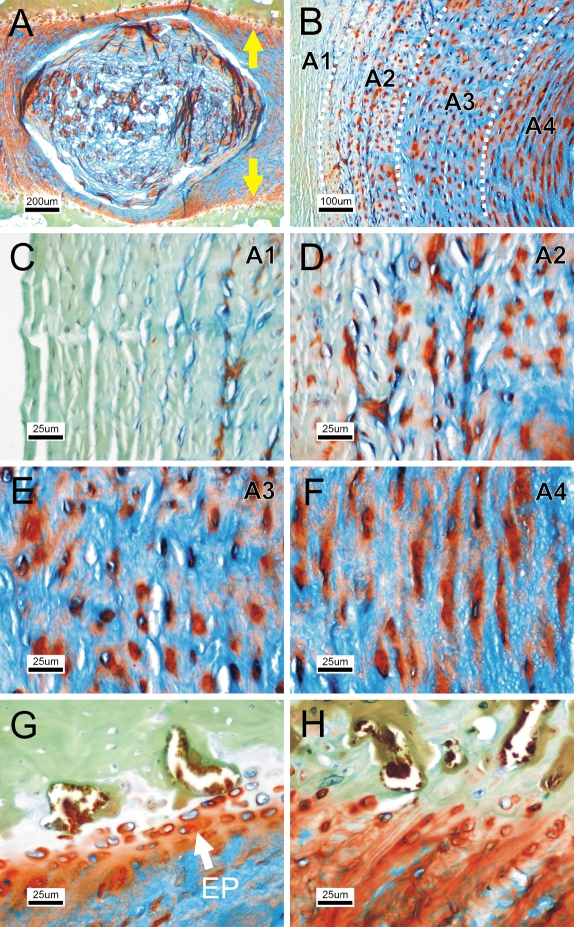
We further tested whether different staining times would show additional disc structures. After prolonged staining with Alcian blue, the contrast between NP and AF decreased (Figure 2A); however, four distinct AF zones became distinguishable (Figure 2B). The outermost zone of AF (A1) was negative for Alcian blue/Safranin-O (Figure 2C). The second zone (A2) was slightly positive for Alcian blue, whereas the cells were surrounded by a Safranin-O–positive pericellular halo (Figure 2D). The third zone (A3) was similar to A2 but was strongly positive for Alcian blue (Figure 2E). In the fourth zone (A4), cells were embedded in an extended orange halo parallel to the lamella (Figure 2F). Under this extended staining protocol, the endplate was shown as distinct orange bands in the adjacent vertebral bodies flanking the AF (Figure 2A, yellow arrows), consisting of a few layers of chondrocytes positive for Alcian blue (Figure 2G). The endplate structure was indiscernible under the standard protocol (Figure 2H). The staining profile is summarized in Table 2.
Figure 2.
Additional disc structure shown using an extended FAST protocol. (A) Prolonged staining with Alcian blue in FAST staining of rabbit disc, showing the endplate contour (arrows). (B) Overview of annulus fibrosus patterns. (C) First zone of annulus A1. (D) Second zone A2. (E) Third zone A3. (F) Fourth zone A4. (G) Cartilaginous endplate structure (EP). (H) The endplate is less distinguishable using the standard protocol.
Table 2.
FAST profile of intervertebral disc under extended protocol
| Compartment | Subdivision | Matrix color | Cell color (pericellular) |
|---|---|---|---|
| Growth plate | Red | Blue | |
| Bone | Yellowish green | Nil | |
| Annulus fibrosus | A1 | Greenish blue | Blue |
| A2 | Greenish blue with blue patches | Blue (orange halo) | |
| A3 | Blue | Blue (orange halo) | |
| A4 | Blue | Blue (orange stretch) | |
| Nucleus pulposus | Blue | Orange (orange halo) | |
| Cartilaginous endplate | Orange | Blue |
Sign of Disc Degeneration Shown by FAST Staining
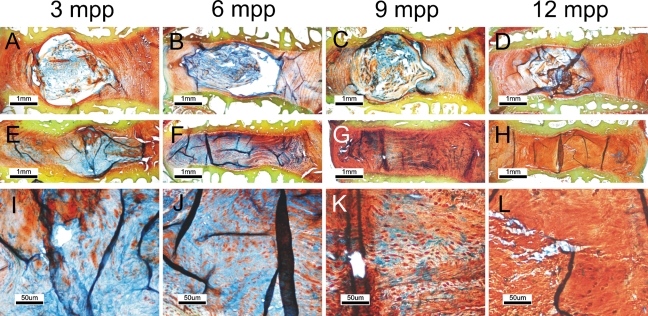
Because the FAST staining was able to differentiate different disc compartments based on staining patterns of the extracellular matrix, we tested whether the progression of disc degeneration, which is associated with a change of matrix components, was reflected in the FAST profile. We used a previously established disc degeneration model (Masuda et al. 2005; Ho et al. 2008) and examined the FAST profiles of the discs at 3–12 months postpuncture. Control discs showed well-defined compartments of NP and AF, with the Alcian blue stain confined in the NP (Figures 3A and 3B). As the rabbit matured, the boundary between the NP and AF became blurred (Figures 3C and 3D). In puncture-induced disc degeneration, the boundary between NP and AF deteriorated progressively at the early stages of 3 and 6 months postpuncture, with enhanced Alcian blue staining in the AF (Figures 3E and 3I). At 6 months, the NP became indistinguishable, and the disc center became occupied by fibrous tissue and a serpentine structure similar to the annulus (Figures 3F and 3J). Notably, the disc matrix became uniformly positive for Alcian blue. At 9 months postpuncture, the Alcian blue staining was significantly reduced (Figures 3G and 3K), and by 12 months, the matrix became predominantly positive for Safranin-O, with no Alcian blue reactivity (Figures 3H and 3I). The disc also showed a decrease in cellularity with an apparent reduction of intracellular Alcian blue stain in the remnant AF cells.
Figure 3.
Disc degeneration is associated with a change in the FAST profile. Degeneration was induced in rabbit lumbar disc by annulus puncture and examined using the standard FAST protocol. (A–D) Normal discs 3 to 12 months postpuncture (mpp). (E–H) Degenerated discs at the same times. (I–L) Nucleus pulposus region of the degenerated discs under a higher magnification.
Continual Change of FAST Profile During Disc Growth
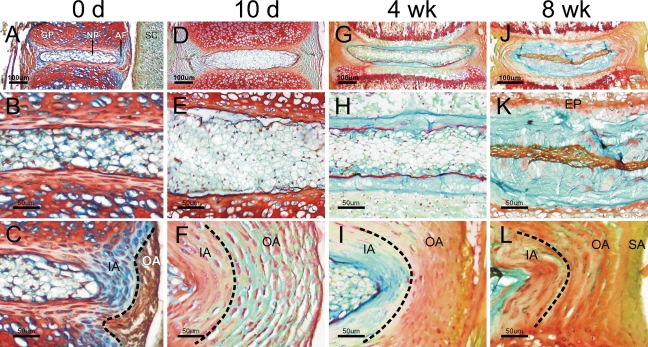
To test whether the growth of the IVD after birth also involved changes or reorganization of matrix, IVDs of 0- to 8-week-old mice were harvested and processed for FAST staining. From birth to 8 weeks, the mouse disc was found to grow with continual changes in matrix volume and staining pattern (Figures 4A, 4D, 4G, and 4J). At birth, the NP was slightly positive for Alcian blue and was composed of cells of various sizes (Figure 4B). The inner annulus contained ellipsoidal cells in an Alcian blue–positive matrix, whereas the outer annulus was predominantly positive for Safranin-O (Figure 4C). The vertebral growth plate was stained red by Safranin-O and the spinal cord grayish green. At 10 days old, the NP enlarged with an apparent increase of cell number (Figure 4E), whereas the AF became thickened and composed of flattened cells embedded in a fast-green–positive lamella structure (Figure 4F). At 4 weeks old, the epiphysis started to ossify to form the secondary ossification center (Figure 4G). An Alcian blue–positive matrix was deposited at the periphery of the NP, with the NP cell cluster becoming fattened and wrapped within a thin sheath of Safranin-O–positive matrix (Figure 4H). The AF became a multicolored lamella structure, from Alcian blue dominant to Safranin-O dominant to tartrazine/fast-green dominant (Figure 4I). By 8 weeks old, the Alcian blue–positive NP matrix had expanded further, and the cells became highly compressed within the Safranin-O–positive matrix (Figure 4K). The AF matrix became Safranin-O–positive in general, with the superficial annulus stained yellowish green (Figure 4L). The AF cells were not Alcian blue positive until 8 weeks old (Figure 4L), suggesting phenotypic difference between AF cells in immature and mature discs.
Figure 4.
FAST profiles of growing mouse intervertebral discs. The coccygeal intervertebral discs of newborn mice (A–C) and 10-day-old (D–F), 4-week-old (G–I), and 8-week-old (J–L) mice were stained using the standard FAST protocol. The nucleus pulposus (B,E,H,K) and annulus fibrosus (C,E,I,L) are also shown under a higher magnification. GP, growth plate; NP, nucleus pulposus; AF, annulus fibrosus; SC, spinal cord; EP, cartilaginous endplate; IA, inner annulus; OA, outer annulus; SA, superficial annulus.
Discussion
Rationale of FAST Staining
In this study, we reported a novel staining protocol using a combination of different dyes with affinity for glycoproteins to assess the structural and matrix properties of the IVD. The rationale of the technique is that staining the acidic glycoproteins first with Alcian blue blocks them chemically so that they cannot react with Safranin-O. The neutral glycoproteins are subsequently stained with Safranin-O. Where there is a mixture of acidic and neutral glycoproteins, the resultant color will reflect the dominant moiety. The Safranin-O staining of the disc, mainly in the AF, is differentiated from the growth plate cartilage by subsequent alcohol treatment. The remaining mucin and collagen moieties in bone and fibrous structures are finally stained by tartrazine and fast green. Because of a strong reaction with tartrazine and trace reactivity to Safranin-O, bone becomes yellowish green, which can be distinguished from the superficial AF that is stained greenish blue.
Unprecedented Resolution of Disc Structure
With this integrated staining method, the disc compartments can be labeled with a spectrum of colors, which, for the first time to our knowledge, enables differentiation of the NP and AF matrix, categorization of different AF zones, clear visualization of the endplate, and distinct differentiation of the disc compartments (AF/NP/endplate) from hyaline cartilage and bone. Furthermore, this method enables differentiation of the superficial AF from the outer AF and with an extended protocol further defined the AF as four discrete zones based on staining of the pericellular and interterritorial matrix. Bruehlmann and colleagues have reported three different types of annulus cells according to the appearance of cellular processes (Bruehlmann et al. 2002). We postulate that the zones we see may be associated with different composition of specific annulus cell types. Negative Alcian blue staining in the pericellular lacunae of AF cells (Postacchini et al. 1984) suggests that undersulfated glycosaminoglycan may modulate the local mechanical environment and hence mechanobiological response of annulus cells (Setton and Chen 2006).
Detection of Degenerative Changes by FAST Staining
The NP matrix shows a preferential reaction with Alcian blue compared with other disc compartments. This characteristic enables the tracing of NP matrix using FAST profiling, which showed an interesting phenomenon during disc degeneration. Our analysis of the puncture-induced rabbit disc degeneration model has shown gradual but profound alteration of matrix in the NP and AF during progressive disc degeneration. The FAST profiles showed that the intensity and extent of the distribution of Alcian blue stain in the NP are related to the severity of the degeneration. We propose that these changes are related to a progressive infiltration of NP matrix into the AF, followed by complete breakdown, or a dynamic matrix remodeling, of the disc initiated by the degeneration. The reduction in glycosaminoglycan and change of matrix components in degenerating disc reported in human and animal models may favor either or both notions. We suggest that the overall content and distribution of strongly sulfated glycosaminoglycan, which is the preferred interacting moiety of Alcian blue at low pH, can provide an additional cue to the severity of disc degeneration. The apparent reduction of intracellular Alcian blue reactivity in the annulus suggests the degeneration is associated with an alteration of GAG content or metabolism in AF cells. Although this puncture model of disc degeneration has been widely adapted, whether these phenomena apply to other models of degeneration or degenerative disc disease in humans needs to be examined.
Matrix Remodeling During Disc Growth
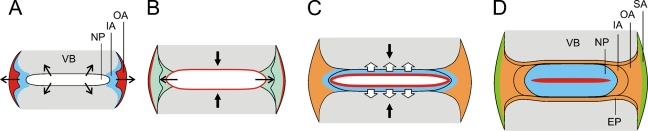
Analysis of the FAST profiles of the mouse spine at different postnatal stages showed dynamic changes in the ECM correlated with potential active anabolic processes during the growth of the IVD. The mouse IVD, which has limited ECM content and a relatively primitive annulus structure at birth, seemed to undergo two stages of significant changes during growth. At the early stage, the disc enlarged rapidly through an increase in the cell population (Figures 5A and 5B). Later, the disc appeared to enlarge through an increase in the deposition of extracellular matrix, likely to be mostly proteoglycan as indicated by positive staining with Alcian blue and Safranin-O, leading to an apparent reduced cell density in the disc (Figure 5C). Flattening of the NP cell cluster may be caused by the robust matrix deposition inside the limited space of the NP (Figure 5D). We propose that mechanical forces may stimulate matrix deposition during the postnatal growth of the IVD, as the NP and AF matrices start to accumulate at the time when the IVD is subject to loading and movement. This notion is in line with the evidence from mechanobiology studies in rodent IVD (Iatridis et al. 1999; Ching et al. 2004).
Figure 5.
A model of disc growth. (A) At birth, intervertebral disc grows through proliferation and differentiation of the cell population (thin arrows) in the nucleus pulposus (NP) and annulus fibrosus (AF). (B) Under mechanical loading (direction indicated by the thick black arrows), NP cell clusters continue to expand in the lateral direction. Remodeling of extracellular matrix (ECM) begins in AF. (C) At a later stage, ECM deposition increases in both the NP and AF, leading to a considerable reduction of cell density in the disc. The more proteoglycan-rich matrix increases the swelling pressure in the NP (hollow arrows) against the mechanical loading (black arrows), with the NP cell population regressing in the matrix. Matrix remodeling continues in AF. (D) As the disc matures, the cartilaginous endplates are formed after completion of secondary ossification in the epiphysis of vertebra. The colors in the panels depict the FAST staining patterns observed at different stages of growth in the mouse. NP, nucleus pulposus; IA, inner annulus; OA, outer annulus; SA, superficial annulus; EP, cartilaginous endplate; VB, vertebral body.
Notably, the FAST profiles also showed qualitative changes in the IVD matrix during growth, suggesting that, along with active matrix synthesis, the composition of the existing matrix is modified in the growing disc. We propose that this is related to continual matrix remodeling in which the existing matrix, such as proteoglycans and collagens, is catabolized and replaced with a new matrix or that the existing molecular structure of the matrix is extensively modified. The continual change in the FAST staining pattern observed in the AF implies that the annulus architecture is constructed through vigorous remodeling events rather than solely by matrix deposition. Whether this remodeling is related to progressive cellular differentiation, biosynthetic modulation by mechanotransduction, or other mechanisms awaits to be elucidated. Overall, because the ECM plays an essential role in the structural integrity and mechanical properties of the IVD, our findings imply that deregulation of matrix biosynthesis or remodeling during growth might compromise IVD function and lead to degenerative disc disease.
Conclusion
The FAST staining method is capable of profiling intervertebral disc structures in detail, which may facilitate quick retrieval of information from samples to generate biological insights and hypotheses regarding the malformation or degeneration status of the disc. In this study, the staining showed that matrix remodeling is involved in the growth and degeneration of intervertebral disc and that the progression of disc degeneration is associated with an altered distribution of the highly sulfated glycosaminoglycan. This method may be used in the future to develop histopathological grading systems to assess IVD specimens of experimental or clinical origin.
Acknowledgments
This work was partially supported by a grant from the University Grants Committee of the Hong Kong Special Administrative Region of China (HKU7496/05M and AoE 04/04) and AOSPINE (AOSBRC-07-02).
We thank Prof. Koichi Masuda and his team in Rush Medical Center, Chicago, IL, for excellent technical advice on the generation of rabbit disc degeneration model.
References
- Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG (2002) Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 27:2631–2644 [DOI] [PubMed] [Google Scholar]
- Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA (2002) Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J Anat 201:159–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching CT, Chow DH, Yao FY, Holmes AD (2004) Changes in nuclear composition following cyclic compression of the intervertebral disc in an in vivo rat-tail model. Med Eng Phys 26:587–594 [DOI] [PubMed] [Google Scholar]
- Chubinskaya S, Kawakami M, Rappoport L, Matsumoto T, Migita N, Rueger DC (2007) Anti-catabolic effect of OP-1 in chronically compressed intervertebral discs. J Orthop Res 25:517–530 [DOI] [PubMed] [Google Scholar]
- Greep RO, Fischer CJ, Morse A (1947) Histochemical demonstration of alkaline phosphatase in decalcified dental and osseous tissues. Science 105:666. [DOI] [PubMed] [Google Scholar]
- Gruber HE, Ingram J, Hanley EN Jr (2002) An improved staining method for intervertebral disc tissue. Biotech Histochem 77:81–83 [PubMed] [Google Scholar]
- Gruber HE, Sage EH, Norton HJ, Funk S, Ingram J, Hanley EN Jr (2005) Targeted deletion of the SPARC gene accelerates disc degeneration in the aging mouse. J Histochem Cytochem 53:1131–1138 [DOI] [PubMed] [Google Scholar]
- Ho G, Leung VY, Cheung KM, Chan D (2008) Effect of severity of intervertebral disc injury on mesenchymal stem cell-based regeneration. Connect Tissue Res 49:15–21 [DOI] [PubMed] [Google Scholar]
- Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M (1999) Compression-induced changes in intervertebral disc properties in a rat tail model. Spine 24:996–1002 [DOI] [PubMed] [Google Scholar]
- Jones DE, Bevins CL (1992) Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 267:23216–23225 [PubMed] [Google Scholar]
- Kim KW, Lim TH, Kim JG, Jeong ST, Masuda K, An HS (2003) The origin of chondrocytes in the nucleus pulposus and histologic findings associated with the transition of a notochordal nucleus pulposus to a fibrocartilaginous nucleus pulposus in intact rabbit intervertebral discs. Spine 28:982–990 [DOI] [PubMed] [Google Scholar]
- Lee S, Stocks R, de Sa DJ (2001) Accidental modification of Attwood's stain on decalcified preparations, yielding improved differentiation between woven and lamellar bone. Pediatr Dev Pathol 4:32–36 [DOI] [PubMed] [Google Scholar]
- Lu DS, Shono Y, Oda I, Abumi K, Kaneda K (1997) Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine 22:1828–1834 [DOI] [PubMed] [Google Scholar]
- MacLean JJ, Lee CR, Alini M, Iatridis JC (2004) Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res 22:1193–1200 [DOI] [PubMed] [Google Scholar]
- MacLean JJ, Lee CR, Alini M, Iatridis JC (2005) The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res 23:1120–1127 [DOI] [PubMed] [Google Scholar]
- Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, et al. (2005) A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine 30:5–14 [DOI] [PubMed] [Google Scholar]
- Nishimura K, Mochida J (1998) Percutaneous reinsertion of the nucleus pulposus. An experimental study. Spine 23:1531–1538 [DOI] [PubMed] [Google Scholar]
- Postacchini F, Bellocci M, Massobrio M (1984) Morphologic changes in annulus fibrosus during aging. An ultrastructural study in rats. Spine 9:596–603 [DOI] [PubMed] [Google Scholar]
- Sahlman J, Inkinen R, Hirvonen T, Lammi MJ, Lammi PE, Nieminen J, Lapvetelainen T, et al. (2001) Premature vertebral endplate ossification and mild disc degeneration in mice after inactivation of one allele belonging to the Col2a1 gene for Type II collagen. Spine 26:2558–2565 [DOI] [PubMed] [Google Scholar]
- Semba K, Araki K, Li Z, Matsumoto K, Suzuki M, Nakagata N, Takagi K, et al. (2006) A novel murine gene, Sickle tail, linked to the Danforth's short tail locus, is required for normal development of the intervertebral disc. Genetics 172:445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setton LA, Chen J (2006) Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am 88(suppl 2):52–57 [DOI] [PubMed] [Google Scholar]
- Smits P, Lefebvre V (2003) Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development 130:1135–1148 [DOI] [PubMed] [Google Scholar]
- Walker MH, Anderson DG (2004) Molecular basis of intervertebral disc degeneration. Spine J 4:158S–166S [DOI] [PubMed] [Google Scholar]