Abstract
Despite wide clinical application, the efficacy of platelet-rich plasma (PRP) for repairing bone defects and enhancing osseointegration of metal implants is still subject of debate. This study aimed to evaluate the effects of a well-defined PRP-like mixture containing platelet-derived growth factor-BB, transforming growth factor (TGF)-β1, TGF-β2, albumin, fibronectin, and thrombospondin [growth factors (GFs) + proteins] on the development of the osteogenic phenotype on titanium (Ti) in vitro. Human alveolar bone-derived osteoblastic cells were subcultured on Ti discs and exposed during the first 7 days to osteogenic medium supplemented with GFs + proteins and to osteogenic medium alone thereafter up to 14 days. Control cultures were exposed to only osteogenic medium. Dose–response experiments were carried out using rat primary calvarial cells exposed to GFs + proteins and 1:10 or 1:100 dilutions of the mixture. Treated human-derived cell cultures exhibited a significantly higher number of cycling cells at days 1 and 4 and of total cells at days 4 and 7, significantly reduced alkaline phosphatase (ALP) activity at days 4, 7, and 10, and no Alizarin red–stained areas (calcium deposits) at day 14, indicating an impairment in osteoblast differentiation. Although the 1:10 and 1:100 dilutions of the mixture restored the proliferative activity of rat-derived osteogenic cells to control levels and promoted a significant increase in ALP activity at day 10 compared with GFs + proteins, mineralized nodule formation was only observed with the 1:100 dilution (∼50% of the control). These results showed that a PRP-like protein mixture inhibits development of the osteogenic phenotype in both human and rat osteoblastic cell cultures grown on Ti. (J Histochem Cytochem 57:265–276, 2009)
Keywords: cell culture, osteoblasts, growth factors, cell proliferation, cell differentiation, mineralization, titanium
The ability to induce new bone formation around endosseous implants is of critical importance for improving healing, increasing stability, and accelerating functional loading. Various regenerative therapies with growth factors (GFs) have been applied to promote and, in some cases, to induce new bone formation in bone defects, at sites of fracture healing, and around metal implant devices (Bessho et al. 1999; Ramoshebi et al. 2002; Schliephake 2002; Kloen et al. 2003). Recombinant human bone morphogenetic proteins (rhBMPs) and transforming growth factor β (rhTGF-β) have been shown in various experimental models to improve key parameters associated with successful osseointegration (Bessho et al. 1999; Clokie and Bell 2003; De Ranieri et al. 2005; Jones et al. 2006; Hall et al. 2007; Liu et al. 2007).
During bone tissue healing, cells are exposed to various GFs simultaneously, particularly at early response intervals when platelets from the blood clot liberate multiple constituents (Bolander 1992). In this context, concentrates of plasma platelets have been exploited for repair of bone defects (Marx et al. 1998; Dugrillon et al. 2002; Anitua et al. 2007). These so-called platelet-rich plasma (PRP) preparations are rich in platelet-derived growth factor (PDGF; ranging from 1 to 150 ng/ml) and TGF-β (ranging from 10 to 400 ng/ml) and also contain various plasma proteins (Venne et al. 1999; Gruber et al. 2003; Lacoste et al. 2003; Lucarelli et al. 2003; Martineau et al. 2004; Borzini and Mazzucco 2005; Graziani et al. 2006; van den Dolder et al. 2006; Borzini and Mazzucco 2007; Roussy et al. 2007). Although beneficial clinical results have been reported, some studies have shown that PRP does not promote bone formation (Ferreira et al. 2005; Hokugo et al. 2005; Gerard et al. 2006; Graziani et al. 2006; Sarkar et al. 2006; Anitua et al. 2007; Hokugo et al. 2007; Mooren et al. 2007; Ranly et al. 2007; Roussy et al. 2007). These divergent outcomes have been attributed to significant intra- and interspecies variations in the relative proportions of PRP components (Lacoste et al. 2003; van den Dolder et al. 2006). Thus, the effectiveness of PRP in enhancing osseointegration of metal implants and of bone grafts is still a subject of debate (Fuerst et al. 2003; Weibrich et al. 2004; Nikolidakis et al. 2008). Importantly, an attempt to establish guidelines for the production of PRP has been made (Borzini et al. 2006).
This study aimed to investigate the effects of a mixture of GFs and proteins (hereafter referred to as GFs + proteins) on the development of the osteogenic phenotype on titanium (Ti), using both human and rat osteoblastic cell cultures. To avoid the inherent variations in PRP preparations, we opted to experiment with a well-defined PRP-like mixture formulated to contain the major components of PRP extracts.
Materials and Methods
Culture of Osteogenic Cells Derived From Human Alveolar Bone
Human alveolar bone fragments were obtained from adult healthy donors, using the research protocols approved by the Committee of Ethics in Research of the School of Dentistry of Ribeirão Preto of the University of São Paulo. Osteogenic cells were isolated by enzymatic digestion of the explants using type II collagenase (Gibco–Life Technologies; Grand Island, NY) as previously described (Beloti et al. 2006). They were cultured in an osteogenic medium consisting of Gibco α-MEM (Invitrogen; Carlsbad, CA) supplemented with 10% FBS (Gibco), 50 μg/ml gentamicin (Gibco), 0.3 μg/ml Fungizone (Gibco), 10−7 M dexamethasone (Sigma; St. Louis, MO), 5 μg/ml ascorbic acid (Gibco), and 7 mM β-glycerophosphate (Sigma). Subconfluent cells in primary cultures were harvested after treatment with 1 mM EDTA (Gibco) and 0.25% trypsin (Gibco) and subcultured on polished, machined Ti discs (prepared as described in de Oliveira et al. 2007). The discs were placed in 24-well polystyrene plates (Falcon; Franklin Lakes, NJ), and cells were seeded at a density of 2 × 104 cells/well. Cultures were grown at 37C in a humidified atmosphere of 5% CO2 and 95% air, and the medium was changed every 3 days.
Composition of the GF and Protein Mixture and Treatment Protocol
The GFs and proteins of the mixture were selected from major components found in porcine (Venne et al. 1999) and human (Lacoste et al. 2003; Martineau et al. 2004) platelet preparations (referred to as extracts or concentrates). The GFs and proteins were diluted in osteogenic medium in the following amounts: 27 ng/ml recombinant human (rh)PDGF-BB, 22 ng/ml rhTGF-β1, 15 ng/ml rhTGF-β2, 3.7 μg/ml serum-derived human albumin, 2 μg/ml plasma-derived human fibronectin, and 0.5 μg/ml platelet-derived thrombospondin, all purchased from Sigma. Treated cultures were exposed during the first 7 days to the GFs + proteins mixture, which was added at time 0 and at day 3, and to osteogenic medium alone thereafter. The timing of exposure to the GFs + proteins mixture was defined based on the life span of platelets in wounds and the period of the direct influence of its GFs, which is <7 days (Marx et al. 1998; Marx 2004). Control cultures were exposed to only osteogenic medium throughout the culture period.
Fluorescence Labeling
On days 1, 4, 7, 10, and 14, cells were fixed for 10 min at room temperature using 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB), pH 7.2. For dual staining with Alizarin red (Sigma) and immunolabeling with bone sialoprotein (BSP), cells were fixed in 70% ethanol for 60 min at 4C (see below). After washing in PB, cultures were processed for immunofluorescence labeling as described previously (de Oliveira et al. 2007). Briefly, they were permeabilized with 0.5% Triton X-100 in PB for 10 min, followed by blocking with 5% skimmed milk in PB for 30 min. Primary antibodies to human bone alkaline phosphatase [ALP; monoclonal B4-78, 1:100; Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA], human Ki-67 (polyclonal, 1:70; Diagnostic Biosystems, Pleasanton, CA), human BSP (polyclonal LF-100, 1:1000, kindly provided by Dr. Larry W. Fisher; NIH, Bethesda, MD) (Mintz et al. 1993), rat BSP (monoclonal WVID1-9C5, 1:200; DSHB), and human vinculin (monoclonal hVIN-1, 1:400; Sigma) were used, followed by Alexa Fluor 488 (green fluorescence) or 594 (red fluorescence)-conjugated goat anti-mouse or anti-rabbit secondary antibody (1:200; Molecular Probes, Invitrogen, Eugene, OR). Alexa Fluor 488 (green fluorescence)-conjugated phalloidin (1:200; Molecular Probes) was used to label actin cytoskeleton. Replacement of the primary antibodies with PB was used as control. All antibody incubations were performed in a humidified environment for 60 min at room temperature. Between each incubation step, the samples were washed three times (5 min each) in PB. Before mounting for microscope observation, cell nuclei were stained with 300 nM 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes) for 5 min, and samples were briefly washed with dH2O. Titanium discs were placed face up on glass slides and mounted with a Fisherbrand 12-mm-round glass coverslip (Fisher Scientific; Suwanee, GA) using an antifade mounting medium (Vectashield; Vector Laboratories, Burlingame, CA).
The samples were examined under epifluorescence using a Leica DMLB light microscope (Leica; Bensheim, Germany), with N Plan (×10/0.25, ×20/0.40) and HCX PL Fluotar (×40/0.75) objectives, outfitted with a Leica DC 300F digital camera. The fluorescence filter cubes used were as follows: A (blue fluorescence; bandpass excitation filter, 340–380; longpass suppression filter, 425), I3 (green fluorescence; bandpass excitation filter, 450–490; longpass suppression filter, 515), and N2.1 (red fluorescence; bandpass excitation filter, 515–560; longpass suppression filter, 590). The acquired digital images were processed with Adobe Photoshop (Adobe Systems; San Jose, CA).
Scanning Electron Microscope (SEM) Imaging
At days 1 and 4, cells were fixed for 60 min at room temperature using 4% paraformaldehyde in PB, pH 7.2, washed in PB, followed by dH2O. Samples were examined wet in a JEOL JSM-6460LV variable pressure scanning electron microscope (Tokyo, Japan) operated at 7 kV and 50–70 Pa. Digital images were processed with Adobe Photoshop.
Growth Curve and Cell Viability
Cells grown for periods of 1, 4, and 7 d were enzymatically detached from Ti discs using 1 mM EDTA, 1.3 mg/ml collagenase, and 0.25% trypsin solution (Gibco; Invitrogen). Total number of cells per well and percentage of viable and non-viable cells were determined after Trypan blue (Sigma) staining using a hemacytometer.
Proportion of Cycling Cells
The proportion of cycling cells at days 1 and 4 was determined by means of double Ki-67/DAPI labeling. The total number of nuclei (DAPI stained) and of Ki-67–expressing cycling cells, that is, cells strictly associated with proliferation (Scholzen and Gerdes 2000) on control and treated cultures, was calculated by light microscope counting under epifluorescence at ×40 objective. A minimum total of 200 cells were counted on three Ti discs at each time point.
Total Protein Content
Total protein content was calculated at days 4, 7, and 10, according to the method described by Lowry et al. (1951). The wells were filled with 2 ml of deionized water. After five cycles of thermal-shock (alternating temperature between 15 min at 37C and 20 min at −20C), 1 ml of the sample from each well was mixed with 1 ml of Lowry solution (Sigma) and left for 20 min at room temperature. Subsequently, 0.5 ml of phenol reagent of Folin and Ciocalteau (Sigma) was added. This was left for 30 min at room temperature, after which absorbance was measured using a spectrophotometer (CE3021; Cecil, Cambridge, UK) at 680 nm.
Total protein content was calculated from a standard curve using BSA (Sigma), giving a range of 25–400 μg protein/ml, and data were expressed as micrograms protein per milliliter.
ALP Activity
ALP activity has been correlated with the development of the osteogenic phenotype in osteoblastic cell cultures (discussed in de Oliveira et al. 2008) and was assayed in the same lysates used for determining total protein content as the release of thymolphthalein from thymolphthalein monophosphate using a commercial kit (Labtest Diagnóstica; Lagoa Santa, Brazil). Briefly, 50 μl of thymolphthalein monophosphate was mixed with 0.5 ml of 0.3 M diethanolamine buffer, pH 10.1, and left for 2 min at 37C. The solution was added to 50 μl of the lysates obtained from each well for 10 min at 37C. For color development, 2 ml of 0.09 M Na2CO3 and 0.25 M NaOH was added. After 30 min, absorbance was measured at 590 nm, and ALP activity was calculated from a standard curve using thymolphthalein to give a range of 0.012–0.4 μmol thymolphthalein/hr/ml. Data were expressed as micromoles thymolphthalein per hour per milligram protein.
Mineralized Bone-like Nodule Formation
For the histochemical detection of calcium deposits in mineralized bone-like nodule formation (de Oliveira et al. 2007), cultures at day 14 were washed in Hanks' balanced salt solution (Sigma) and fixed in 70% ethanol for 60 min at 4C. The samples were washed in PBS and dH2O and stained with 2% Alizarin red, pH 4.2, for 15 min at room temperature. After being profusely washed in dH2O, cultures were also processed for triple labeling with anti-BSP antibody (WVID1-9C5 or LF-100) and DAPI and observed under epifluorescence using the filter cubes N2.1, I3, and A.
Dose–Response Experiments With Calvarial Cell Cultures
To test the effects of serial dilutions of the GFs + proteins mixture on the development of the osteogenic phenotype, we used rat osteogenic cells obtained by enzymatic digestion of newborn calvaria and cultured under the same conditions as for human cells (Nanci et al. 1996; Irie et al. 1998; de Oliveira and Nanci 2004; de Oliveira et al. 2007). This cell culture model was selected because of its high and well-defined osteogenic activity, especially in the presence of dexamethasone (Bellows and Aubin 1989; Bellows et al. 1990). During the first 7 days of culture, calvarial cells were exposed to three different dilutions of GFs + proteins mixture (1:1, 1:10, and 1:100 referred to as GFs + proteins, GFs + proteins/10, and GFs + proteins/100, respectively). Because many of the PRP effects on cells do not reflect a simple combination of its major GFs (Marx 2004; Kawase et al. 2005), cells were also exposed to the mixture containing only the protein constituents. The parameters evaluated for calvarial cell cultures were (1) at days 7 and 14, BSP labeling (as described above); (2) at day 7, mitochondrial tetrazolium test (MTT); (3) at days 7 and 10, ALP activity and total protein content; and (4) at day 14, mineralized bone-like nodule formation (as described above).
The MTT assay for cell viability/proliferation (Mosmann 1983) is based on the reductive cleavage of yellow tetrazolium salt to a purple formazan compound by the dehydrogenase activity of intact mitochondria. Therefore, this conversion only occurs in living cells. At the end of the proliferative phase/exposure time (day 7), cells were incubated with 100 μl of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (5 mg/ml) in PBS at 37C for 4 hr. The medium was aspirated from the well, and 1 ml of acid isopropanol (0.04 N HCl in isopropanol) was added to each well. The plates were agitated on a plate shaker for 5 min, and 100 μl of this solution was transferred to a 96-well format using opaque-walled transparent-bottomed plates (Fisher Scientific; Pittsburgh, PA). The optical density was read at 570–650 nm on the plate reader (μQuant; Biotek, Winooski, VT), and data were expressed as absorbance.
Alizarin red–stained cultures were photographed with a high-resolution digital camera (Canon EOS Digital Rebel Camera, 6.3 Megapixel CMOS sensor, with a Canon EF 100 mm f/2.8 macro lens; Canon, Lake Success, NY) and were also imaged by epifluorescence using the N2.1 filter cube (red fluorescence). The percentage of the substrate area occupied by Alizarin red–stained mineralized nodules was determined according to de Oliveira et al. (2007) by analyzing the macroscopic images using Image Tool software (University of Texas Health Science Center; San Antonio, TX).
Statistical Analysis
Data are presented as mean ± SD. The χ2 test was used to determine normality of data sets. Comparisons were carried out using the non-parametrical Mann-Whitney test for two independent samples or the Kruskal-Wallis test for multiple comparisons, followed by the Fischer test based on rank, where appropriate. The level of significance was set at 5%. The results described below are representative of a minimum of two sets of human and rat osteogenic cell cultures.
Results
Human Alveolar Bone-derived Cells
Important differences were clearly noticed in terms of cell morphology and tissue architecture beginning from day 1 between control and GFs + proteins–treated cultures as shown by epifluorescence and SEM imaging (Figures 1 and 2, respectively). At day 1, although both control and treated cells were polygonal in shape, the latter exhibited a higher proportion of thin cytoplasmic extensions (Figures 1A, 1E, 2A, and 2C). For both groups, vinculin labeling was localized perinuclearly and in focal adhesions sites (Figures 1A and 1E, insets). At days 4 and 7, treated cultures exhibited a higher cell density and abundant mitoses (Figures 1F, 1G, and 2, compare D with B), with the majority of cells showing smaller nuclear dimensions compared with control cells (compare Figures 1F with 1B and 1G with 1C). Noticeably, areas of calcified matrix were only detected in control cultures at day 14 (compare Figure 1D with 1H), and these exhibited a higher proportion of cells showing morphological aspects of apoptosis (Figure 1D, right inset). Calcified nodular areas stained with Alizarin red and labeled for BSP (Figure 1D, left inset). At this culture interval, treated cultures showed cell alignment (Figure 1H), no BSP labeling, and reduced amounts of apoptotic cells (Figure 1H, left and right insets, respectively).
Figure 1.
Progression of control (A–D) and growth factors (GFs) + proteins–treated (E–H) human alveolar bone-derived osteoblastic cell cultures grown on titanium discs at days 1 (A,E), 4 (B,F), 7 (C,G), and 14 (D,H). Green fluorescence represents actin cytoskeleton labeling for A–H (whitish for A,E insets), whereas blue fluorescence indicates cell nuclei. At day 1, control and treated cells were polygonal in shape, with treated cells showing thinner cytoplasmic extensions (compare E with A). No differences were detected between control and treated cells in terms of vinculin labeling, which was mostly perinuclear and associated with focal adhesions sites (A,E, insets; red fluorescence). At days 4, 7, and 14, GFs + proteins–treated cultures exhibited a higher number of cells compared with control (compare F–H with B–D). At day 14, control cultures showed focal areas of calcified matrix (D, red autofluorescence) and cell nuclei with typical morphological aspects of apoptosis (D, right inset), whereas treated cultures exhibited no signs of osteogenic phenotype (H) and only occasional apoptotic cells (H, right inset). Only control cultures exhibited bone sialoprotein (BSP) labeling at day 14 (green fluorescence; compare D, left inset, with H, left inset), which in some areas circumscribed Alizarin red (AR; red fluorescence)-stained nodule formation (D, left inset). Bars: A–H = 100 μm; A,E insets = 50 μm; D,H left insets = 200 μm; D,H right insets = 25 μm.
Figure 2.
Scanning electron micrographs of control (A,B) and GFs + proteins–treated (C,D) human alveolar bone-derived osteoblastic cell cultures grown on titanium discs, at days 1 (A,C) and 4 (B,D). At day 1, GFs + proteins–treated cultures exhibited cells with thinner cytoplasmic extensions (C and inset) compared with control cells (A). At day 4, a higher number of cells was clearly noticed for treated cultures (compare D with B). Bars: A,C = 20 μm; C inset = 25 μm; B,D = 100 μm.
Cell proliferation/viability analyses showed that treated cultures supported the growth of significantly more cells at days 4 and 7 (Figure 3; Table 1) and a higher number of viable cells at day 4 (Table 1). The proportion of cycling cells was significantly higher for treated cultures both at days 1 and 4 (Figures 4 and 5; Table 1) compared with the control.
Figure 3.
Total cell number of control and GFs + proteins–treated human alveolar bone-derived osteoblastic cell cultures grown on titanium discs. At days 4 and 7, total cell number was significantly higher (p<0.01) for the treated group. Data are reported as mean ± SD (n=5).
Table 1.
Quantitative analysis [mean ± SD (n)] of total cell number (×104), cell viability (%), cycling cells (% Ki-67–positive cells), total protein content (μg/ml), and ALP activity (μmol thymolphthalein/hr/mg protein) of control and GFs + proteins–treated human alveolar bone-derived osteogenic cell cultures grown on titanium discs
| Parameters | Time points (days) | Control | GFs + proteins | Mann-Whitney test |
|---|---|---|---|---|
| Total cell number | 1 | 1.6 ± 0.4 (5) | 1.5 ± 0.5 (5) | NSa |
| 4 | 6.9 ± 0.4 (5) | 11.1 ± 0.5 (5) | Sb | |
| 7 | 14.3 ± 2.6 (5) | 37.4 ± 6.7 (5) | Sb | |
| Cell viability | 4 | 95.2 ± 1.6 (5) | 97.0 ± 1.6 (5) | Sc |
| 7 | 95.3 ± 2.0 (5) | 97.3 ± 0.8 (5) | NSa | |
| Cycling cells | 1 | 71.9 ± 1.7 (3) | 83.8 ± 8.6 (3) | Sd |
| 4 | 66.2 ± 10.9 (3) | 81.7 ± 1.6 (3) | Sd | |
| Total protein content | 4 | 16.8 ± 4.5 (5) | 33.9 ± 9.3 (5) | Sb |
| 7 | 34.6 ± 5.6 (5) | 101.5 ± 15.2 (5) | Sb | |
| 10 | 90.0 ± 6.4 (5) | 150.4 ± 24.5 (3) | Sc | |
| ALP activity | 4 | 29.8 ± 10.1 (5) | 9.6 ± 2.1 (5) | Sb |
| 7 | 43.6 ± 9.1 (5) | 2.8 ± 0.2 (5) | Sb | |
| 10 | 31.4 ± 2.4 (5) | 2.6 ± 0.3 (5) | Sb |
Non-significant (p>0.05).
Significant (p<0.01).
Significant (p<0.05).
Significant (p=0.05).
ALP, alkaline phosphatase; GF, growth factor.
Figure 4.
Double labeling Ki-67/4',6-diamidino-2-phenylindole dihydrochloride (DAPI) (red and blue fluorescence, respectively) to determine the proportions of cycling cells of control (A,B) and GFs + proteins–treated (C,D) human alveolar bone-derived osteoblastic cell cultures grown on titanium discs at days 1 (A,C) and 4 (B,D). Bar = 50 μm.
Figure 5.
Proportions of cycling cells of control and GFs + proteins–treated human alveolar bone-derived osteoblastic cell cultures grown on titanium discs. A significant enhancement (p = 0.05) in the proportion of cycling cells was observed for GFs + proteins–treated cultures. Data are reported as mean ± SD (n=3).
Total protein content was significantly higher in treated cultures at all time points and the amount of ALP, as determined by activity measurements and immunolabeling of the protein, was reduced (Figures 6 and 7; Table 1). Notably, at days 7 and 10, ALP-specific activity levels were reduced >90% compared with control (15- and 12-fold, respectively). Although the profile of ALP activity over time showed peak levels at day 7 for control cultures, the GFs + proteins group exhibited a significant decrease from day 4 to days 7 and 10, despite the significant increase in the total of protein content compared with control (2-, 2.9-, and 1.7-fold, respectively).
Figure 6.
Double labeling alkaline phosphatase (ALP)/DAPI (red and blue fluorescence, respectively) of control (A–C) and GFs + proteins–treated (D–F) human alveolar bone-derived osteoblastic cell cultures grown on titanium discs, at days 4 (A,D), 7 (B,E), and 10 (C,F). Weak ALP labeling was clearly observed for treated cultures. Bar = 200 μm.
Figure 7.
ALP activity (μmol thymolphthalein/hr/mg protein) of control and GFs + proteins–treated human alveolar bone-derived osteoblastic cell cultures grown on titanium discs at days 4, 7, and 10. Cultures exposed to GFs + proteins exhibited significantly (p<0.01) reduced ALP activity levels. Data are reported as mean ± SD (n=5).
Rat Calvarial Cells: Dose–Response Experiments
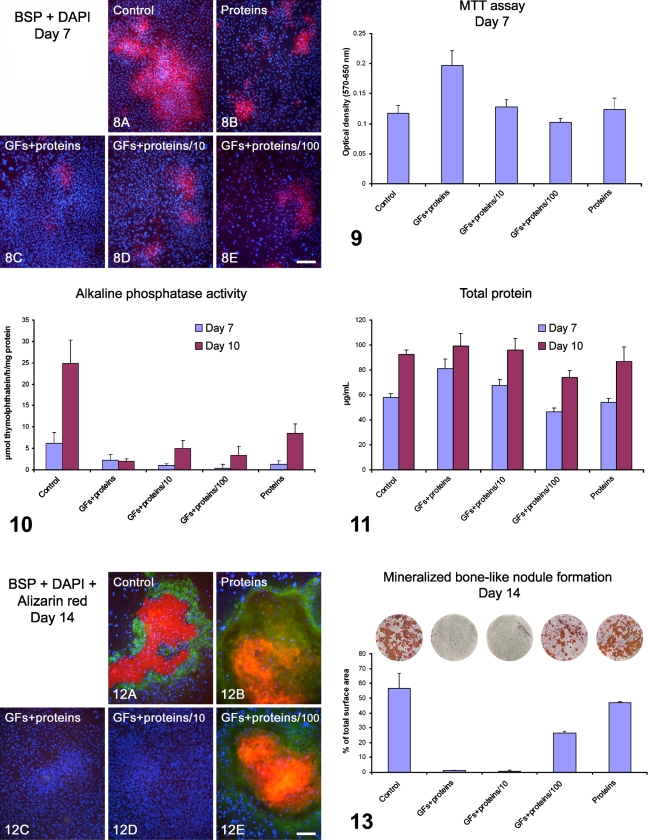
Dose–response experiments showed that only the GFs + proteins/100 group supported the development of the osteogenic phenotype, although in reduced levels compared with control and proteins groups (Figures 8–13). At day 7, control cultures exhibited large areas of BSP-positive cells, mostly in sites of initial cell multilayering (Figure 8A), whereas treated cultures showed only focal sites of weak BSP labeling (Figures 8B–8E). Cell viability/proliferation assay showed that mitochondrial activity was significantly higher for GFs + proteins cultures, reducing to control levels for the GFs + proteins/10 and GFs + proteins/100 groups (Figure 9; Tables 2 and 3). At day 7, there was a significant difference in ALP activity between the control and the GFs + proteins–treated groups, which exhibited low levels. At day 10, ALP-specific activity increased in all experimental groups, except for GFs + proteins, which remained unaltered, but there was no significant GFs + proteins dose–response relationship. The highest ALP activity was observed with the control, followed by the proteins-only group (Figure 10; Tables 2 and 3). Total protein content profiles at days 7 and 10 correlated with the MTT results at day 7 (compare Figure 11 with Figure 9), with significantly higher values for GFs + proteins cultures (Tables 2 and 3). At day 14, qualitative and quantitative analyses showed that the control, proteins, and GFs + proteins/100 groups supported the development of mineralized bone-like nodule formation (Figures 12 and 13); nodules stained with Alizarin red and were BSP immunoreactive (Figures 12A, 12B, and 12E). Proportions of Alizarin red–stained areas ranked as follows: control = proteins > GFs + proteins/100 > GFs + proteins/10 = GFs + proteins (Figure 13; Tables 2 and 3). Strikingly, a complete lack of bone-like nodule formation was noticed for GFs + proteins– and GFs + proteins/10–treated cultures (Figures 12C, 12D, and 13).
Figure 8.
Dual-labeled BSP (red fluorescence)/DAPI (blue fluorescence) of control (A), proteins- (B), GFs + proteins– (C), GFs + proteins/10– (D), and GFs + proteins/100– (E) treated calvaria-derived osteogenic cultures grown on titanium discs for 7 days. Although control cultures showed larger areas of BSP labeling mostly associated with initial cell multilayering (A), treated cultures exhibited fewer areas of BSP labeling and of weaker intensity (B–E). Note a higher cell density for GFs + proteins–treated cultures (C). Bar = 200 μm.
Figure 9.
Viability/proliferation analysis (MTT assay) of control, GFs + proteins–, GFs + proteins/10–, GFs + proteins/100–, and proteins-treated calvaria-derived osteogenic cultures grown on titanium discs at day 7. Cultures exposed to GFs + proteins exhibited a higher number of viable cells. The optical density was read at 570–650 nm, and data were expressed as absorbance.
Figure 10.
ALP activity (μmol thymolphthalein/hr/mg protein) of control, GFs + proteins–, GFs + proteins/10–, GFs + proteins/100–, and proteins-treated calvaria-derived osteogenic cultures grown on titanium discs at days 7 and 10. Note that there was no dose–response relationship for ALP activity at day 10. The lowest levels were observed for the GFs + proteins–treated cultures, whereas no differences were detected between the 1:10 and 1:100 dilutions.
Figure 11.
Total protein content (μg/ml) of control, GFs + proteins–, GFs + proteins/10–, GFs + proteins/100–, and proteins-treated calvaria-derived osteogenic cultures grown on titanium discs at days 7 and 10. The graph pattern/profile matches the one for MTT assay at day 7 (compare with Figure 9).
Figure 12.
Triple labeling BSP (green fluorescence)/DAPI (blue fluorescence)/Alizarin red (red fluorescence) of control (A), proteins- (B), GFs + proteins– (C), GFs + proteins/10– (D), and GFs + proteins/100– (E) treated calvaria-derived osteogenic cultures grown on titanium discs at day 14. Calcified bone-like nodules (A,B,E; Alizarin red stained) were only observed for control, proteins, and GFs + proteins/100 groups, which also exhibited BSP labeling (green fluorescence). No Alizarin red staining and BSP labeling were detected for GFs + proteins and GFs + proteins/10 groups (C and D, respectively). Bars: A,B,E = 100 μm; C,D = 200 μm.
Figure 13.
Macroscopic imaging and quantitative analysis of Alizarin red–stained areas for control, GFs + proteins–, GFs + proteins/10–, GFs + proteins/100–, and proteins-treated calvaria-derived osteogenic cultures grown on titanium discs at day 14. The percentage of total area occupied by stained regions was significantly higher for control and proteins group. Although a complete lack of bone-like nodule formation was clearly noticed for the GFs + proteins and GFs + proteins/10 groups, GFs + proteins/100 supported the development of the osteogenic phenotype. Titanium disc diameter = 12 mm.
Table 2.
Quantitative analysis [mean ± SD (n)] of MTT assay (optical density, 570–650 nm), ALP activity (μmol thymolphthalein/hr/mg protein), total protein content (μg/ml), and proportion of bone-like nodule formation (%) of control, GFs + proteins–, GFs + proteins/10–, GFs + proteins/100–, and protein-treated rat calvarial osteogenic cell cultures grown on titanium discs
| MTT assay
|
ALP activity
|
Total protein content
|
Bone-like nodule formation
|
|||
|---|---|---|---|---|---|---|
| Groups | Day 7 | Day 7 | Day 10 | Day 7 | Day 10 | Day 14 |
| Control | 0.12 ± 0.01 (4) | 6.2 ± 2.6 (5) | 24.9 ± 5.4 (5) | 58.0 ± 3.2 (5) | 92.6 ± 3.4 (5) | 56.6 ± 10.1 (4) |
| GFs + proteins | 0.20 ± 0.02 (4) | 2.2 ± 1.4 (4) | 2.0 ± 0.6 (5) | 81.3 ± 7.4 (4) | 99.0 ± 10.1 (5) | 0.9 ± 0.3 (4) |
| GFs + proteins/10 | 0.13 ± 0.01 (4) | 1.0 ± 0.4 (5) | 5.0 ± 1.9 (5) | 67.5 ± 4.9 (5) | 96.0 ± 9.1 (5) | 0.7 ± 0.7 (4) |
| GFs + proteins/100 | 0.10 ± 0.01 (4) | 0.4 ± 0.9 (5) | 3.4 ± 2.0 (5) | 46.5 ± 3.1 (5) | 73.9 ± 5.8 (5) | 26.4 ± 1.4 (4) |
| Proteins | 0.12 ± 0.02 (4) | 1.2 ± 0.9 (4) | 8.6 ± 2.1 (4) | 53.9 ± 3.5 (4) | 87.0 ± 11.4 (4) | 46.8 ± 0.7 (4) |
| Kruskal-Wallis test | Sa | Sa | Sa | Sa | Sa | Sa |
Significant (p<0.01).
MTT, mitochondrial tetrazolium test; ALP, alkaline phosphatase; GF, growth factor.
Table 3.
Comparative analysis (Fischer post-test) for MTT, ALP activity, total protein content, and bone-like nodule formation of control, GFs + proteins–, GFs + proteins/10–, GFs + proteins/100–, and protein-treated rat calvarial osteogenic cell cultures grown on titanium discs
| MTT assay
|
ALP activity
|
Total protein content
|
Bone-like nodule formation
|
|||
|---|---|---|---|---|---|---|
| Comparisons (Fischer post-test) | Day 7 | Day 7 | Day 10 | Day 7 | Day 10 | Day 14 |
| Control × GFs + proteins | 1% | 5% | 0.1% | 0.1% | NSa | 0.1% |
| Control × GFs + proteins/10 | NSa | 0.1% | 0.1% | 0.1% | NSa | 0.1% |
| Control × GFs + proteins/100 | NSa | 0.1% | 0.1% | 0.1% | 1% | 0.1% |
| Control × proteins | NSa | 1% | 5% | 5% | NSa | NSa |
| GFs + proteins × GFs + proteins/10 | 5% | NSa | 0.1% | 1% | NSa | NSa |
| GFs + proteins × GFs + proteins/100 | 0.1% | 1% | 5% | 0.1% | 0.1% | 1% |
| GFs + proteins × proteins | 1% | NSa | 0.1% | 0.1% | 5% | 0.1% |
| GFs + proteins/10 × GFs + proteins/100 | 1% | NSa | NSa | 0.1% | 0.1% | 0.1% |
| GFs + proteins/10 × proteins | NSa | NSa | 5% | 0.1% | NSa | 0.1% |
| GFs + proteins/100 × proteins | 5% | NSa | 0.1% | 1% | NSa | 1% |
Non-significant (>0.05).
MTT, mitochondrial tetrazolium test; ALP, alkaline phosphatase; GF, growth factor.
Discussion
The results of this study showed that a mixture of major GFs and proteins found in platelet extracts dramatically inhibits development of the osteogenic phenotype in both human alveolar bone and rat calvarial cell cultures grown on Ti. Remarkably, despite interspecies variations, complete lack of bone-like nodule formation took place in both cultures. In addition, dose–response experiments with calvarial cells showed that dilution of the mixture can eventually partially restore bone-like nodule formation.
It has been well established that there is an inverse relationship between proliferation and differentiation of osteoblastic cells, including for cells cultured on Ti (van den Dolder et al. 2003). Indeed, full expression of the osteoblast phenotype leads to terminal cell cycle exit (Stein et al. 1996; Thomas et al. 2004). To make bone repair more predictable, there have been attempts to balance proliferation/differentiation by using serial dilutions of PRP preparations (Weibrich et al. 2004; Choi et al. 2005; Ferreira et al. 2005; Graziani et al. 2006; Gruber et al. 2006; Tomoyasu et al. 2007; Uggeri et al. 2007). Our GFs + proteins mixture is less concentrated than typical PRP preparations, and although a 1:10 dilution significantly reduced cell proliferation, it did not enhance osteoblastic differentiation; a dilution of 1:100 was necessary to restore bone-like nodule formation. Clearly, further studies are needed to optimize the relative proportions of PRP components and duration and timing of exposure to achieve a functional balance between mitogenic activity and osteoblast differentiation effects of PRP-related GF and protein mixtures.
The inhibition of ALP activity in both human and rat osteogenic cultures treated with GFs + proteins is consistent with an effect on osteogenic differentiation. In a previous study by our group, it was shown that BMP-7 and growth and differentiation factor 5 (GDF-5) could, in part, influence osteogenic differentiation in GFs + proteins–treated cultures by upregulating ALP mRNA expression (de Oliveira et al. 2008). ALP activity and mineralized nodule formation were, however, not significantly affected, a finding interpreted as reflecting an early differentiation status of the cells. In this study, the dose–response experiments showed that ALP activity increases with dilution of the mixture, and although there is no significant difference in activity between the 1:10 or 1:100 dilutions, only the latter supported mineral deposition. This suggests that, in addition to ALP activity, other parameters must be taken in consideration for initiating and sustaining the mineralization process. Indeed, both GFs + proteins and GFs + proteins/10 showed no BSP labeling at day 14. This matricellular protein is considered an early marker of differentiating osteoblasts and is crucial for promoting hydroxyapatite nucleation on collagen (Tye et al. 2003,2005; Baht et al. 2008). However, it cannot be ruled out that the difference in mineralization observed between the 1:10 and 1:100 dilutions relates to ALP activity. It has been shown that even a moderate reduction in ALP expression levels and enzyme activity could be sufficient to impair the mineralization process (Wennberg et al. 2000). The procedure used herein to assay ALP activity does not allow determining small differences that may exist between the two dosages. In addition, difficulties in extraction of proteins/enzymes from calcified matrices could in some cases result in ALP-specific activity values that do not accurately correlate with total protein content and cell number (Simão et al. 2007). In bone, ALP is confined to the cell surface of osteoblasts, including the membranes of the matrix vesicle they shed, where the enzyme is particularly enriched compared with the plasma membrane of the cell. It has been proposed that the main role of ALP is to generate the phosphate needed for hydroxyapatite formation. However, ALP has also been hypothesized to hydrolyze pyrophosphate, a mineralization inhibitor, to facilitate mineral precipitation and growth (Millán 2006). Therefore, treatment with GFs + proteins may also affect the phosphate/pyrophosphate equilibrium, which per se is crucial for the regulation of matrix mineralization.
In conclusion, this study showed that a GFs + proteins mixture, which contains the major components of PRP preparations, impairs the progression of both human and rat osteogenic cells grown on Ti. Dose–response experiments showed that the reduction in cell proliferation associated with higher ALP activity levels does not necessarily correlate with production of mineralized matrix. Thus, our data lend support to previous studies showing that PRP preparations do not promote bone repair under various experimental conditions. They also raise the question of whether PRP-based multiple component molecular therapy can benefit osseointegration of titanium implants.
Acknowledgments
This study was supported by The State of São Paulo Research Foundation (FAPESP, Brazil), National Council of Scientific and Technological Development (CNPq, Brazil), Canadian Institutes of Health Research, and Natural Sciences and Engineering Research Council of Canada.
We thank Sylvia Francis Zalzal (Université de Montréal, Montréal, QC, Canada) for SEM analysis and imaging and Dr. Larry W. Fisher (National Institutes of Health, Bethesda, MD) for providing the anti-human BSP (LF-100) antibody. M.A.O. and L.M.S.C. are recipients of Masters Scholarships from FAPESP. W.M.A.M. and P.E.S. are recipients of Internships Scholarships from FAPESP and CNPq, respectively. The mouse monoclonal antibodies anti-human bone ALP (B4-78), developed by Jerry A. Katzmann, and anti-rat BSP (WVID1-9C5), developed by Michael Solursh and Ahnders Franzen, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biological Sciences of the University of Iowa (Iowa City, IA).
References
- Anitua E, Sánchez M, Orive G, Andia I (2007) The potential impact of the preparation rich in growth factors (PRGF) in different medical fields. Biomaterials 28:4551–4560 [DOI] [PubMed] [Google Scholar]
- Baht GS, Hunter GK, Goldberg HA (2008) Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol 27:600–608 [DOI] [PubMed] [Google Scholar]
- Bellows CG, Aubin JE (1989) Determination of numbers of osteoprogenitors present in isolated fetal rat calvaria cells in vitro. Dev Biol 133:8–13 [DOI] [PubMed] [Google Scholar]
- Bellows CG, Heersche JN, Aubin JE (1990) Determination of the capacity for proliferation and differentiation of osteoprogenitor cells in the presence and absence of dexamethasone. Dev Biol 140:132–138 [DOI] [PubMed] [Google Scholar]
- Beloti MM, Oliveira PT, Gimenes R, Zaghete MA, Bertolini MJ, Rosa AL (2006) In vitro biocompatibility of a novel membrane of the composite poly(vinylidene-trifluoroethylene)/barium titanate. J Biomed Mater Res A 79:282–288 [DOI] [PubMed] [Google Scholar]
- Bessho K, Carnes DL, Cavin R, Chen HY, Ong JL (1999) BMP stimulation of bone response adjacent to titanium implants in vivo. Clin Oral Implants Res 10:212–218 [DOI] [PubMed] [Google Scholar]
- Bolander ME (1992) Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med 200:165–170 [DOI] [PubMed] [Google Scholar]
- Borzini P, Mazzucco L (2005) Platelet gels and releasates. Curr Opin Hematol 12:473–479 [DOI] [PubMed] [Google Scholar]
- Borzini P, Mazzucco L (2007) Platelet-rich plasma (PRP) and platelet derivatives for topical therapy. What is true from the biologic view point? ISBT Science Series 2:272–281 [Google Scholar]
- Borzini P, Mazzucco L, Giampaolo A, Hassan HJ (2006) Platelet gel: the Italian way: a call for procedure standardization and quality control. Transfus Med 16:303–304 [DOI] [PubMed] [Google Scholar]
- Choi BH, Zhu SJ, Kim BY, Huh JY, Lee SH, Jung JH (2005) Effect of platelet-rich plasma (PRP) concentration on the viability and proliferation of alveolar bone cells: an in vitro study. Int J Oral Maxillofac Surg 34:420–424 [DOI] [PubMed] [Google Scholar]
- Clokie CM, Bell RC (2003) Recombinant human transforming growth factor beta-1 and its effects on osseointegration. J Craniofac Surg 14:268–277 [DOI] [PubMed] [Google Scholar]
- de Oliveira PT, de Oliva MA, Maximiano WM, Sebastião KE, Crippa GE, Ciancaglini P, Beloti MM, et al. (2008) Effects of a mixture of growth factors and proteins on the development of the osteogenic phenotype in human alveolar bone cell cultures. J Histochem Cytochem 56:629–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira PT, Nanci A (2004) Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials 25:403–413 [DOI] [PubMed] [Google Scholar]
- de Oliveira PT, Zalzal SF, Beloti MM, Rosa AL, Nanci A (2007) Enhancement of in vitro osteogenesis on titanium by chemically produced nanotopography. J Biomed Mater Res A 80:554–564 [DOI] [PubMed] [Google Scholar]
- De Ranieri A, Virdi AS, Kuroda S, Shott S, Leven RM, Hallab NJ, Sumner DR (2005) Local application of rhTGF-beta2 enhances peri-implant bone volume and bone-implant contact in a rat model. Bone 37:55–62 [DOI] [PubMed] [Google Scholar]
- Dugrillon A, Eichler H, Kern S, Kluter H (2002) Autologous concentrated platelet-rich plasma (cPRP) for local application in bone regeneration. Int J Oral Maxillofac Surg 31:615–619 [DOI] [PubMed] [Google Scholar]
- Ferreira CF, Gomes MCC, Filho JS, Granjeiro JM, Simões CMO, Magini RS (2005) Platelet-rich plasma influence on human osteoblasts growth. Clin Oral Implants Res 16:456–460 [DOI] [PubMed] [Google Scholar]
- Fuerst G, Gruber R, Tangl S, Sanroman F, Watzek G (2003) Enhanced bone-to-implant contact by platelet-released growth factors in mandibular cortical bone: a histomorphometric study in minipigs. Int J Oral Maxillofac Implants 18:685–690 [PubMed] [Google Scholar]
- Gerard D, Carlson ER, Gotcher JE, Jacobs M (2006) Effects of platelet-rich plasma on the healing of autologous bone grafted mandibular defects in dogs. J Oral Maxillofac Surg 64:443–451 [DOI] [PubMed] [Google Scholar]
- Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M (2006) The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin Oral Implants Res 17:212–219 [DOI] [PubMed] [Google Scholar]
- Gruber R, Kandler B, Fischer MB, Watzek G (2006) Osteogenic differentiation induced by bone morphogenetic proteins can be suppressed by platelet-released supernatant in vitro. Clin Oral Implants Res 17:188–193 [DOI] [PubMed] [Google Scholar]
- Gruber R, Karreth F, Frommlet F, Fischer MB, Watzek G (2003) Platelets are mitogenic for periosteum-derived cells. J Orthop Res 21:941–948 [DOI] [PubMed] [Google Scholar]
- Hall J, Sorensen RG, Wozney JM, Wikesjö UM (2007) Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J Clin Periodontol 34:444–451 [DOI] [PubMed] [Google Scholar]
- Hokugo A, Ozeki M, Kawakami O, Sugimoto K, Mushimoto K, Morita S, Tabata Y (2005) Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng 11:1224–1233 [DOI] [PubMed] [Google Scholar]
- Hokugo A, Sawada Y, Hokugo R, Iwamura H, Kobuchi M, Kambara T, Morita S, et al. (2007) Controlled release of platelet growth factors enhances bone regeneration at rabbit calvaria. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 104:44–48 [DOI] [PubMed] [Google Scholar]
- Irie K, Zalzal S, Ozawa H, McKee MD, Nanci A (1998) Morphological and immunocytochemical characterization of primary osteogenic cell cultures derived from fetal rat cranial tissue. Anat Rec 252:554–567 [DOI] [PubMed] [Google Scholar]
- Jones AA, Buser D, Schenk R, Wozney J, Cochran DL (2006) The effect of rhBMP-2 around endosseous implants with and without membranes in the canine model. J Periodontol 77:1184–1193 [DOI] [PubMed] [Google Scholar]
- Kawase T, Okuda K, Saito Y, Yoshie H (2005) In vitro evidence that the biological effects of platelet-rich plasma on periodontal ligament cells is not mediated solely by constituent transforming-growth factor-beta or platelet-derived growth factor. J Periodontol 76:760–767 [DOI] [PubMed] [Google Scholar]
- Kloen P, Di Paola M, Borens O, Richmond J, Perino G, Helfet DL, Goumans MJ (2003) BMP signaling components are expressed in human fracture callus. Bone 33:362–371 [DOI] [PubMed] [Google Scholar]
- Lacoste E, Martineau I, Gagnon G (2003) Platelet concentrates: effects of calcium and thrombin on endothelial cell proliferation and growth factor release. J Periodontol 74:1498–1507 [DOI] [PubMed] [Google Scholar]
- Liu Y, Enggist L, Kuffer AF, Buser D, Hunziker EB (2007) The influence of BMP-2 and its mode of delivery on the osteoconductivity of implant surfaces during the early phase of osseointegration. Biomaterials 28:2677–2686 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, et al. (2003) Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials 24:3095–3100 [DOI] [PubMed] [Google Scholar]
- Martineau I, Lacoste E, Gagnon G (2004) Effects of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials 25:4489–4502 [DOI] [PubMed] [Google Scholar]
- Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62:489–496 [DOI] [PubMed] [Google Scholar]
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:638–646 [DOI] [PubMed] [Google Scholar]
- Millán JL (2006) Mammalian Alkaline Phosphatases: From Biology to Applications in Medicine and Biotechnology. Weinhein, Wiley VCH
- Mintz KP, Grzesik WJ, Midura RJ, Robey PG, Termine JD, Fisher LW (1993) Purification and fragmentation of nondenatured bone sialoprotein: evidence for a cryptic, RGD-resistant cell attachment domain. J Bone Miner Res 8:985–995 [DOI] [PubMed] [Google Scholar]
- Mooren RE, Merkx MA, Bronkhorst EM, Jansen JA, Stoelinga PJ (2007) The effect of platelet-rich plasma on early and late bone healing: an experimental study in goats. Int J Oral Maxillofac Surg 36:626–631 [DOI] [PubMed] [Google Scholar]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63 [DOI] [PubMed] [Google Scholar]
- Nanci A, Zalzal S, Gotoh Y, McKee MD (1996) Ultrastructural characterization and immunolocalization of osteopontin in rat calvarial osteoblast primary cultures. Microsc Res Tech 33:214–231 [DOI] [PubMed] [Google Scholar]
- Nikolidakis D, van den Dolder J, Wolke JG, Jansen JA (2008) Effect of platelet-rich plasma on the early bone formation around Ca-P-coated and non-coated oral implants in cortical bone. Clin Oral Implants Res 19:207–213 [DOI] [PubMed] [Google Scholar]
- Ramoshebi LN, Matsaba TN, Teare J, Renton L, Patton J, Ripamonti U (2002) Tissue engineering: TGF-beta superfamily members and delivery systems in bone regeneration. Expert Rev Mol Med 4:1–11 [DOI] [PubMed] [Google Scholar]
- Ranly DM, Lohmann CH, Andreacchio D, Boyan BD, Schwartz Z (2007) Platelet-rich plasma inhibits demineralized bone matrix-induced bone formation in nude mice. J Bone Joint Surg Am 89:139–147 [DOI] [PubMed] [Google Scholar]
- Roussy Y, Bertrand Duchesne MP, Gagnon G (2007) Activation of human platelet-rich plasmas: effect on growth factors release, cell division and in vivo bone formation. Clin Oral Implants Res 18:639–648 [DOI] [PubMed] [Google Scholar]
- Sarkar MR, Augat P, Shefelbine SJ, Schorlemmer S, Huber-Lang M, Claes L, Kinzl L, et al. (2006) Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials 27:1817–1823 [DOI] [PubMed] [Google Scholar]
- Schliephake H (2002) Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg 31:469–484 [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J (2000) The Ki-67 protein: from the known and the unknown. J Cell Physiol 182:311–322 [DOI] [PubMed] [Google Scholar]
- Simão AM, Beloti MM, Rosa AL, de Oliveira PT, Granjeiro JM, Pizauro JM, Ciancaglini P (2007) Culture of osteogenic cells from human alveolar bone: a useful source of alkaline phosphatase. Cell Biol Int 31:1405–1413 [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Stein JL, Van Wijnen AJ, Montecino M (1996) Transcriptional control of osteoblast growth and differentiation. Physiol Rev 76:593–629 [DOI] [PubMed] [Google Scholar]
- Thomas DM, Johnson SA, Sims NA, Trivett MK, Slavin JL, Rubin BP, Waring P, et al. (2004) Terminal osteoblast differentiation, mediated by runx2 and p27KIP1, is disrupted in osteosarcoma. J Cell Biol 167:925–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu A, Higashio K, Kanomata K, Goto M, Kodaira K, Serizawa H, Suda T, et al. (2007) Platelet-rich plasma stimulates osteoblastic differentiation in the presence of BMPs. Biochem Biophys Res Commun 361:62–67 [DOI] [PubMed] [Google Scholar]
- Tye CE, Hunter GK, Goldberg HA (2005) Identification of the type I collagen-binding domain of bone sialoprotein and characterization of the mechanism of interaction. J Biol Chem 280:13487–13492 [DOI] [PubMed] [Google Scholar]
- Tye CE, Rattray KR, Warner KJ, Gordon JA, Sodek J, Hunter GK, Goldberg HA (2003) Delineation of the hydroxyapatite-nucleating domains of bone sialoprotein. J Biol Chem 278:7949–7955 [DOI] [PubMed] [Google Scholar]
- Uggeri J, Belletti S, Guizzardi S, Poli T, Cantarelli S, Scandroglio R, Gatti R (2007) Dose-dependent effects of platelet gel releasate on activities of human osteoblasts. J Periodontol 78:1985–1991 [DOI] [PubMed] [Google Scholar]
- van den Dolder J, Mooren R, Vloon AP, Stoelinga PJ, Jansen JA (2006) Platelet-rich plasma: quantification of growth factor levels and the effect on growth and differentiation of rat bone marrow cells. Tissue Eng 12:3067–3073 [DOI] [PubMed] [Google Scholar]
- van den Dolder J, Spauwen PH, Jansen JA (2003) Evaluation of various seeding techniques for culturing osteogenic cells on titanium fiber mesh. Tissue Eng 9:315–325 [DOI] [PubMed] [Google Scholar]
- Venne D, Raymond J, Allas S, Roy D, Leclerc G, Boushira M, Brazeau P (1999) Healing of experimental aneurysms. II: platelet extracts can increase the thickness of the neointima at the neck of treated aneurysms. J Neuroradiol 26:92–100 [PubMed] [Google Scholar]
- Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE (2004) Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 34:665–671 [DOI] [PubMed] [Google Scholar]
- Wennberg C, Hessle L, Lundberg P, Mauro S, Narisawa S, Lerner UH, Millán JL (2000) Functional characterization of osteoblasts and osteoclasts from alkaline phosphatase knockout mice. J Bone Miner Res 15:1879–1888 [DOI] [PubMed] [Google Scholar]