Abstract
Protein phosphorylation is frequently used as an indicator of cellular signaling activity. Elevated phosphorylation of tyrosine kinase receptors plays an important role in cancer pathogenesis. However, phosphoproteins are usually poorly preserved in clinical tissue samples that are routinely fixed in 10% formalin. Nonetheless, in oncology clinical trials, use of phosphoproteins as biomarkers has been considered to be of great value in evaluating the effectiveness of a given drug candidate. Therefore, it is worthy of investigating whether alternative fixatives would improve the preservation of phosphoproteins in tissue. We compared the IHC staining of a number of phosphoproteins in xenograft and human surgical tumor tissues fixed in three different fixatives: 10% formalin, 4% paraformaldehyde (PFA), and Streck’s tissue fixative (STF). We found that STF significantly enhanced the staining intensity of phosphoproteins compared with 10% formalin or 4% PFA. STF fixative also showed superiority of preservation of phosphoproteins in human surgical samples. Our results indicate that the choice of fixative could significantly affect the usability of clinical tissue samples for evaluating phosphoprotein by IHC. (J Histochem Cytochem 57:257–264, 2009)
Keywords: fixative, Streck’s tissue fixative, phosphoprotein, tumor tissues, paraffin tissue section
Cell-to-cell communication through signaling between growth factors, and their respective receptors, is essential for living tissues to maintain proper tissue structure and function (Weinberg 2007). Hyperactive signaling of growth factor receptors (generally because of overexpression or structural alterations) is thought to play an important role in cancer pathogenesis (Yarden and Sliwkowski 2001). Unusual signaling activities often result in elevated levels of protein phosphorylation in downstream signaling pathways, such as pAkt, and can be associated with poor prognosis in many types of human cancers (Massion et al. 2004; Nakanishi et al. 2004; Lim et al. 2005; Zhuang et al. 2005). Targeting overexpressed growth factor receptors with therapeutic monoclonal antibody can prevent phosphorylation and activation of cellular proteins. This approach has been shown to be effective in reducing cellular proliferation and promoting apoptosis in cancer cells, thus contributing to favorable outcomes for cancer patients. Herceptin, an anti-HER-2 receptor therapeutic monoclonal antibody, has proven to be very efficacious in breast cancer patients with overexpression of the HER-2 receptor. In oncology clinical trials, monitoring the expression levels of relevant phosphoproteins as biomarkers has been shown to be very informative in evaluating the effectiveness of drug candidates (Albanell et al. 2002; Esteva et al. 2003; Ma et al. 2005; Rojo et al. 2006; Serrels et al. 2006; Taillade et al. 2007; Felip et al. 2008; Guix et al. 2008).
Formalin, a commonly used fixative for tissue samples in pathology laboratories, is capable of forming cross-links with proteins, preventing cellular distortion and loss of chemical activity in the tissue, thus contributing to excellent preservation of cellular antigenicity (Warmington et al. 2000). Archival formalin-fixed, paraffin-embedded human tissue samples allow for the detection of protein expression by IHC for diagnosis and retrospective research. However, detection of phosphoproteins in formalin-fixed tissues is very difficult, if not impossible, especially for clinical surgical tissue samples (Baker et al. 2005; Jones et al. 2008). This may be partly because of rapid dephosphorylation of proteins in the tissues that quickly become oxygen deficient once the blood supply is tied off (Blow 2007). If tissue samples are not immediately fixed in 10% formalin after dissection, the majority of phosphoproteins are lost within 60 min (Baker et al. 2005; Jones et al. 2008). Routine procedures for surgical tissue removal, collection, and fixation pose a challenge for using phosphoproteins as biomarkers for clinical research and diagnostic applications. A fixative that is capable of better preservation of phosphoproteins than 10% formalin could enhance the clinical utility of phosphoproteins as biomarkers.
Many previous studies have looked at the effect of other fixatives on total protein in comparison to 10% formalin; however, these studies did not focus on phosphoproteins (Beckstead 1994; Chan et al. 1994; Prento and Lyon 1997; Williams et al. 1997; Warmington et al. 2000). To study whether other fixatives improve the preservation of tissue phosphoproteins, we sought to perform IHC on tumor xenograft tissues. This is simply because the time from tissue collection to fixation can be easily controlled and minimized. This study examined five different xenograft tumors (SKOV-3, Caco-2, Geo, HT-29, and A549), each fixed in 10% formalin, 4% paraformaldehyde (PFA), and Streck’s tissue fixative (STF). We found that STF had overwhelmingly higher staining intensity for nearly all antiphosphoprotein antibodies we tested compared with 10% formalin and 4% PFA. Remarkably, our results of human surgical tumor samples also showed the superiority of STF in preserving tissue phosphoproteins.
Materials and Methods
Xenograft Tumor Samples
Six-week-old, female NCr nude mice (Taconic; Germantown, NY) were implanted subcutaneously in the right flank with 1 × 107 SKOV-3, Geo, HT-29, and A549 cells suspended in 0.2 ml of Hanks buffered salt solution (Invitrogen; Carlsbad, CA); Caco-2 cells were suspended in Matrigel (BD Biosciences; San Jose, CA) and implanted similarly. Tumor development was monitored regularly. Tumor weight was estimated using a predefined formula of length × width × height, and tumors were harvested when the estimated weight reached 250 mg. All animal experiments were performed under an Institutional Animal Care and Use Committee (IACUC)-approved protocol at Merck and Co. (West Point, PA). Five types of xenograft tumor tissues (SKOV-3, Caco-2, Geo, HT-29, and A549) were harvested and divided into three equally sized pieces immediately after removal from the mouse. The samples were fixed in STF (Streck Laboratories; Omaha, NE), 10% neutral buffered formalin (10% formalin; ThermoFisher, Pittsburgh, PA), or 4% PFA (Boston BioProducts; Boston, MA) for 16–24 hr. The xenograft tumors were processed with 70% ethanol, 95% ethanol, 100% ethanol, xylenes, and paraffin in a tissue processor (Sakura; Torrance, CA). The xenograft tumors were embedded in paraffin, cut into 5-μm sections, and mounted onto Superfrost plus glass slides (ThermoFisher).
Human Surgical Tumor Samples
Five human postoperative surgical tumor samples (one lung, two colon, and two breast) were obtained from the National Disease Research Interchange (NDRI; Philadelphia, PA). Within 1 hr of surgical resection, the samples were divided into two pieces and fixed: one in STF and the other in 10% formalin for 1 hr/mm of tumor. The fixed samples were transferred to 70% ethanol for tissue processing following the procedures mentioned above for xenograft tumors.
IHC
The tissue sections were deparaffinized, rehydrated, and underwent heat-induced antigen retrieval using Target Retrieval (DakoCytomation; Carpinteria, CA) in a Decloaking Chamber (Biocare Medical; Concord, CA) at 125C for 30 sec. Endogenous peroxidase activity was blocked using Peroxidase Blocking Reagent (DakoCytomation) 10 min. Sections were washed with Tris-buffered saline (TBS; DakoCytomation) and incubated with multiple antiphosphoprotein antibodies (Table 1) for 16–24 hr at 4C. Sections were washed with TBS and incubated with labeled polymer-horseradish peroxidase (HRP) anti-rabbit or anti-mouse (DakoCytomation) for 30 min at room temperature and washed with TBS, and diaminobenzidine was used for development of a brown reaction product (DakoCytomation). The slides were washed with TBS and immersed in hematoxylin for 30 sec for counterstaining (Sigma; St. Louis, MO).
Table 1.
Antiphosphoprotein antibodies
| Antibody | Vendor | Phospho site | Host species | Concentration used |
|---|---|---|---|---|
| pMET | R&D (Minneapolis, MN) | Y1234/Y1235 | Rabbit | 0.25 pg/ml |
| pIGF-1R/IR | Cell Signaling (Danvers, MA) | Y1131/Y1 146 | Rabbit | 0.15 pg/ml |
| pEGFR | Cell Signaling | Y992 | Rabbit | 1.0 pg/ml |
| pHER2 | Cell Signaling | Y1221/Y1222 | Rabbit | 0.15 pg/ml |
| pHER3 | Cell Signaling | Y1289 | Rabbit | 1.92 pg/ml |
| pHER4 | Abgent (San Diego, CA) | Y1 162 | Rabbit | 0.62 pg/ml |
| pAkt | Cell Signaling | S473 | Rabbit | 0.1 pg/ml |
| pMAPK | Cell Signaling | T202/Y204 | Rabbit | 0.5 ng/ml |
| pTyr | R&D | pan T | Mouse | 2 pg/ml |
| pp53 | Abcam (Cambridge, MA) | S315 | Rabbit | 0.2 pg/ml |
To confirm the specificity of phosphoprotein staining, lambda phosphatase was applied on the sections before incubation with primary antibodies. Briefly, after peroxidase activity was blocked, the sections were washed with distilled water and incubated with lambda phosphatase (New England BioLabs; Ipswich, MA) diluted in reaction buffer plus MnCl2 at 10,000 U/ml and 50,000 U/ml and incubated at 37C for 60 min. The incubation was done in separate slide chambers to prevent cross-contamination of the phosphatase with the primary antibody and reaction buffer-only controls. Sections were washed with distilled water several times, and the procedure continued with the addition of primary antibody as above.
Scoring System and Statistics
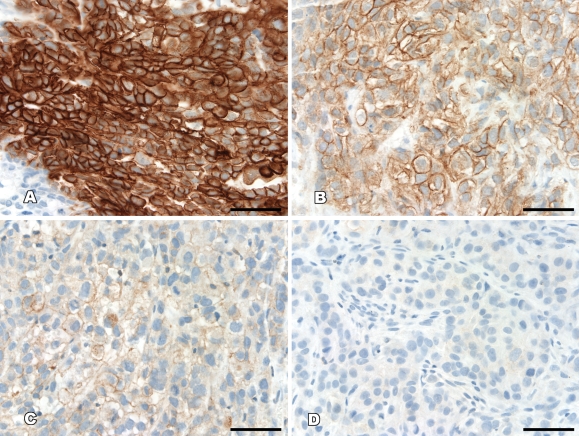
Staining intensity was directly compared and independently scored by two of the authors (JB and YL) according to a prespecified intensity scale, with discrepancies agreed on by consensus. Staining intensity was rated on a semiquantitative scale: −, negative with no staining; +, weak staining intensity in >10% of the tumor cells; ++, moderate staining intensity in >10% of the tumor cells; +++, strong staining intensity in >10% of the tumor cells (Figure 1).
Figure 1.
Examples of the scoring system used for the determination of phosphoprotein expression. (A) +++, strong staining in >10% of the tumor cells. (B) ++, moderate staining in >10% of the tumor cells. (C) +, weak staining in >10% of the tumor cells. (D) −, negative with no staining. Bar = 50 μm.
One-way ANOVA with Friedman’s test and Dunn’s multiple comparison post-test was used to compare the scoring results of xenograft tumor tissues fixed with STF, 10% formalin, and PFA. Wilcoxon matched-pairs test was used to compare the human surgical tumor samples fixed in STF and 10% formalin (GraphPad Prism version 5.01 for Windows; GraphPad Software, San Diego, CA). p<0.05 for both tests was considered significant.
Results
Effect of Different Fixatives on Preservation of Phosphoproteins in Xenograft and Human Surgical Tumor Samples
Overall, the staining intensity of nearly all antibodies was significantly higher in STF-fixed tissues than in 10% formalin– or 4% PFA–fixed tissues. The staining intensity of STF-fixed tissues was significantly higher in 60% (6/10) of the antiphosphoprotein antibodies tested for all types of xenograft tumor tissues (Table 2). For instance, pMET staining (Figure 2A) was scored as +++ for all STF-fixed tissue samples but was only scored as + for the majority of 10% formalin– or 4% PFA–fixed tissues (p=0.0008). Similar results were observed in pEGFR, pHER2, pHER3, pHER4, and pTyr (Table 2). Surprisingly, pEGFR was essentially undetectable in all 10% formalin– and 4% PFA–fixed tissues, except for SKOV-3. In contrast, all STF-fixed tissues had detectable pEGFR, with scores ranging from + to +++. For pIGF-1R/IR and pMAPK, there was a trend that STF-fixed tissues had stronger staining than 10% formalin– or 4% PFA–fixed tissues, although not statistically significant. Interestingly, the preservation of nuclear phosphoproteins, such as pp53 and pAkt, was not significantly affected by the selected fixatives. Overall, 10% formalin–fixed tissues had slightly higher staining intensities compared with 4% PFA–fixed tissues, although not statistically significant.
Table 2.
IHC staining of phosphoproteins in xenograft tumor tissues
| Xenograft tumor |
|||||||
|---|---|---|---|---|---|---|---|
| Antibody | Fixative | SKOV-3 | Caco-2 | Geo | HT-29 | A549 | p value |
| pMET | STF | +++ | +++ | +++ | +++ | +++ | 0.0008 |
| 10% formalin | ++ | + | + | + | — | ||
| 4% PFA | + | + | + | + | ++ | ||
| pIGF-1R/IR | STF | +++ | +++ | +++ | +++ | +++ | 0.0934 |
| 10% formalin | +++ | +++ | ++ | ++ | + | ||
| 4% PFA | + | ++ | ++ | ++ | +++ | ||
| pEGFR | STF | +++ | + | + | + | + | 0.0008 |
| 10% formalin | + | − | − | − | − | ||
| 4% PFA | + | − | − | − | − | ||
| pHER2 | STF | +++ | +++ | +++ | ++ | ++ | 0.0008 |
| 10% formalin | +++ | ++ | + | ++ | + | ||
| 4% PFA | ++ | + | − | + | + | ||
| pHER3 | STF | +++ | +++ | +++ | +++ | +++ | 0.0008 |
| 10% formalin | + | + | + | + | − | ||
| 4% PFA | + | + | − | + | + | ||
| pHER4 | STF | +++ | +++ | +++ | +++ | +++ | 0.0085 |
| 10% formalin | +++ | ++ | ++ | ++ | + | ||
| 4% PFA | + | + | + | + | ++ | ||
| pAkt | STF | +++ | + | + | + | − | 0.9537 |
| 10% formalin | + | + | + | + | + | ||
| 4% PFA | ++ | + | + | + | + | ||
| pMAPK | STF | +++ | +++ | + | ++ | + | 0.1242 |
| 10% formalin | ++ | + | + | + | − | ||
| 4% PFA | ++ | + | ++ | + | + | ||
| pTyr | STF | +++ | +++ | +++ | ++ | ++ | 0.0008 |
| 10% formalin | ++ | ++ | + | + | + | ||
| 4% PFA | ++ | ++ | + | + | + | ||
| pp53 | STF | +++ | +++ | +++ | +++ | +++ | 0.1242 |
| 10% formalin | +++ | +++ | +++ | +++ | +++ | ||
| 4% PFA | +++ | +++ | +++ | ++ | ++ | ||
STF, Streck’s tissue fixative; PFA, paraformaldehyde.
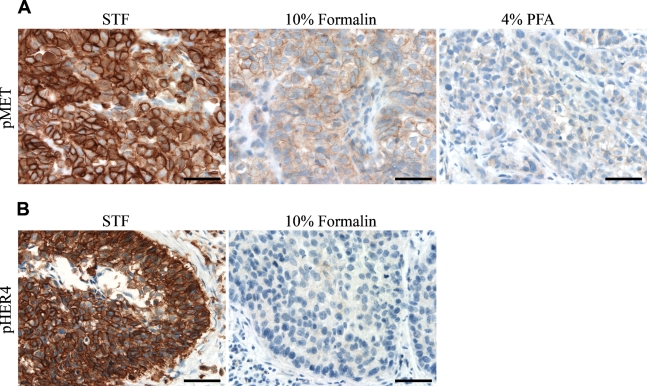
Figure 2.
IHC staining of phosphoproteins in xenograft and human clinical tumor tissues. (A) SKOV-3 xenograft tumor tissues fixed in Streck’s tissue fixative (STF), 10% formalin, and 4% paraformaldehyde (PFA) were stained with anti-pMet antibody. (B) Human lung tumor tissue fixed in 10% formalin and STF were stained with anti-pHER4 antibody. Bar = 50 μm.
In human postoperative surgical samples (Table 3; Figure 2B), the majority of phosphoproteins were undetectable in all five 10% formalin–fixed tumor tissue samples, with the exception of pMAPK and pp53. In contrast, STF-fixed lung tumor tissue was positive for nearly all phosphoproteins except for pAkt. Moreover, the staining intensity of pp53 and pHER4 (Figure 2B) was significantly higher in STF-fixed tissues, except one breast tumor tissue with +++ scores. Taken together, for preservation of phosphoproteins in xenograft tumor tissues as well as in clinical surgical tumor samples for IHC detection, STF is clearly shown as a better choice of fixative compared with 10% formalin and 4% PFA.
Table 3.
IHC staining of phosphoproteins in human clinical tumor tissues
| Tumor type |
||||||
|---|---|---|---|---|---|---|
| Antibody | Fixative | Lung squamous cell carcinoma | Breast ductal carcinoma | Breast lobular carcinoma | Colon adenocarcinoma | Colon mucin-producing adenocarcinoma |
| pMET | STF | +++ | − | − | − | − |
| 10% formalin | − | − | − | − | − | |
| pIGF-1R/IR | STF | +++ | ++ | − | − | − |
| 10% formalin | − | − | − | − | − | |
| pEGFR | STF | + | − | − | − | − |
| 10% formalin | − | − | − | − | − | |
| pHER2 | STF | + | − | − | − | − |
| 10% formalin | − | − | − | − | − | |
| pHER3 | STF | ++ | − | − | − | − |
| 10% formalin | − | − | − | − | − | |
| pHER4 | STF | +++ | − | − | − | − |
| 10% formalin | − | − | − | − | − | |
| pAkt | STF | − | − | − | − | − |
| 10% formalin | − | − | − | − | − | |
| pMAPK | STF | + | ++ | − | − | − |
| 10% formalin | + | + | − | − | − | |
| pTyr | STF | ++ | − | − | − | − |
| 10% formalin | − | − | − | − | − | |
| pp53 | STF | +++ | ++ | +++ | ++ | + |
| 10% formalin | ++ | − | +++ | − | − | |
STF, Streck’s tissue fixative.
Heterogeneous Staining Pattern of Phosphoproteins in Tissue Section
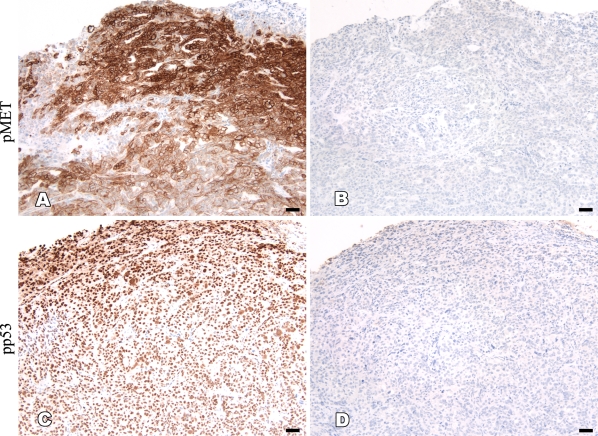
We observed a consistent heterogeneous staining pattern of the antiphosphoprotein antibodies in the xenograft and human surgical tumor samples used in this study, regardless of fixative. The staining intensity of phosphoproteins was generally higher in the area closer to the tissue surface and gradually decreased toward the center (Figure 3). The membranous phosphoproteins showed no staining in the center of tumor tissues, whereas a nuclear phosphoprotein (pp53) showed weaker staining in the same area. To confirm whether the staining pattern was phosphoprotein specific, sections of the SKOV-3 xenograft tumor were incubated with lambda phosphatase before addition of primary antibodies. We found that 10,000 or 50,000 U/ml of lambda phosphatase was sufficient to completely eliminate the positive staining in both STF- and formalin-fixed tissue sections. These results indicate that protein dephosphorylation after tissue removal from the body occurs so rapidly that penetration of fixative into the tissues can only preserve portions of phosphoproteins that are close to the tissue surface. Thus, it is likely that fixatives that are capable of faster and deeper penetration into tissue would be very helpful in uniformly preserving phosphoproteins in tissues.
Figure 3.
Heterogeneous staining of phosphoproteins in tumor tissues. (A,C) STF-fixed SKOV-3 xenograft tumor tissues were stained with anti-pMET and pp53 antibodies showing more staining in areas closer to the tissue surface and less staining in areas toward the center of tissue. (B,D) The specificity of these uneven staining patterns of phosphoproteins was confirmed by incubating the unstained slides with phosphatase before the application of primary antibodies. Bar = 50 μm.
Effect of Fixatives and Storage Condition on Stability of Tissue Phosphoproteins
Choice of fixative and storage condition and time of precut unstained tissue section all play an important role in preservation of antigens in paraffin-embedded tumor tissues (Jacobs et al. 1996; Atkins et al. 2004; DiVito et al. 2004; Fergenbaum et al. 2004). We performed a time course study to determine whether different fixatives have any effect on the stability of tissue phosphoproteins at selected storage conditions and time of tissue section. Geo xenograft tumor tissues that were fixed in 10% formalin, 4% PFA, and STF were used for this study. IHC staining with anti-pMET antibody was carried out on freshly cut tissue sections and repeated on sections that had been stored at both room temperature and 4C in the dark for 30 and 90 days. STF-fixed tissues consistently showed stronger staining of pMET compared with 10% formalin– and 4% PFA–fixed tissues. There was no difference in staining intensity of pMET at room temperature vs 4C, regardless of the fixative. Moreover, there were no deleterious effects for a storage period up to at least 90 days (Table 4). These results suggest that preservation of phosphoproteins in paraffin-embedded tissue sections was relatively stable for IHC when the samples were fixed quickly after removal.
Table 4.
Effect of storage conditions on preservation of phosphoproteins in unstained slides of Geo xenograft tumor
| Day 30 |
Day 90 |
||||
|---|---|---|---|---|---|
| Fixative | Day 0 | Room temperature | 4C | Room temperature | 4C |
| STF | +++ | +++ | +++ | +++ | +++ |
| 10% Formalin | + | + | + | + | + |
| 4% PFA | + | + | + | + | + |
STF, Streck’s tissue fixative; PFA, paraformaldehyde.
Discussion
Preservation of phosphoproteins in tissues by conventional fixatives, such as 10% formalin, has proven to be challenging, especially for clinical postoperative tissue samples (Baker et al. 2005; Jones et al. 2008). It has been shown that the rapid process of protein dephosphorylation occurs even when tissues are separated from their blood supply before removal from the body as part of a routine surgical procedure. It has also been reported that levels of detectable phosphoproteins in tissue biopsies were significantly higher than in surgical tissue samples (Baker et al. 2005), presumably because the time to fixation was generally shorter for biopsies than for surgical tissue samples. In our study, we observed that overall staining intensity was strikingly higher in xenograft tumors than in the human postoperative surgical tumor tissues. After the mice were sacrificed, the xenograft tumor tissues were quickly removed and immediately placed in fixative. This process incorporates a lot of similarities to tissue biopsies collected in clinical practice. Therefore, our data are consistent with the previous observation that the time to fixation is one of the most critical factors in preserving phosphoproteins in tissues (Baker et al. 2005; Jones et al. 2008).
In a typical clinical setting, however, it may not be practical to collect and fix the surgical tissue samples in a timely fashion. Therefore, alternative fixatives that can penetrate into tissues more quickly than 10% formalin were examined to address this critical issue. STF is a non–cross-linking fixative that has been suggested in a number of previous studies as a better choice for IHC detection of total proteins in tissues compared with 10% formalin and 4% PFA (Prento and Lyon 1997; Zhang et al. 1998,1999a,b). In this study, we showed that STF-fixed tissues had significantly higher levels of phosphoprotein staining compared with 10% formalin and 4% PFA in both xenograft and clinical surgical tumor tissues. In our xenograft tissue analyses, we found that nearly all STF-fixed tumor tissues tested had overwhelmingly higher staining intensity of the antiphosphoprotein antibodies than 10% formalin– and 4% PFA–fixed tissues. STF seemed to be able to quickly penetrate into the tumor tissue and effectively preserve the labile phosphoproteins before dephosphorylation. This fixative may potentially be an excellent alternative choice of fixative for clinical tissue sample (biopsy and surgical tissue samples) fixation because of its superiority of preserving phosphoproteins and total proteins (Zhang et al. 1998,1999a,b).
In this study, it was frequently observed that the staining intensity of phosphoproteins near the tissue surface was generally higher than toward the center of the tissue, regardless of fixative, in both xenograft and human tumor tissues. The specificity of these heterogeneous staining patterns was confirmed by morphological characteristics of staining patterns of different antibodies, such as membranous staining for anti-pMet, pIGF-1R/IR, pEGFR, pHER2, pHER3, and pHER4 and nuclear and cytoplasmic staining for anti-pAkt, pMAPK, and pp53 antibodies. In addition, staining specificity was confirmed by phosphatase digestions of tissues before application of primary antiphosphoprotein antibodies (Figure 3). The heterogeneous staining pattern from perimeter to center once again indicates the instability of the phosphoproteins. It is likely that fixatives begin to work on penetration of the outermost tumor tissue area, and penetration of fixative into deeper areas of the tissue takes more time, whereas dephosphorylation is rapidly ongoing. This may be a possible reason for no or weaker staining of phosphoproteins in the center of the tissue. Therefore, a combination of a better fixative and quick time to fixation is critical for successful preservation of phosphoproteins in clinical tissue samples.
Acknowledgments
We thank Jon Stek of the Department of Medical Communication at Merck & Co., for his careful language editing of the manuscript.
References
- Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, LoRusso P, et al. (2002) Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 20:110–124 [DOI] [PubMed] [Google Scholar]
- Atkins D, Reiffen K-A, Tegtmeier CL, Winther H, Bonato MS, Storkel S (2004) Immunohistochemical detection of EGFR in paraffin-embedded tumor tissues: variation in staining intensity due to choice of fixative and storage time of tissue sections. J Histochem Cytochem 52:893–901 [DOI] [PubMed] [Google Scholar]
- Baker AF, Dragovich T, Ihle NT, Williams R, Fenoglio-Preiser C, Powis G (2005) Stability of phosphoprotein as a biological marker of tumor signaling. Clin Cancer Res 11:4338–4340 [DOI] [PubMed] [Google Scholar]
- Beckstead JH (1994) A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem 42:1127–1134 [DOI] [PubMed] [Google Scholar]
- Blow N (2007) Technology feature: tissue preparation. Nature 448:959–962 [DOI] [PubMed] [Google Scholar]
- Chan ASY, Cheung KN, Chiu KY (1994) KP1 immunohistochemical demonstration of macrophages in colorectal tissues: effects of different fixatives and different fixation times. J Histotechnol 17:329–332 [Google Scholar]
- DiVito KA, Charette LA, Rimm DL, Camp RL (2004) Long-term preservation of antigenicity on tissue microarrays. Lab Invest 84:1071–1078 [DOI] [PubMed] [Google Scholar]
- Esteva FJ, Sahin AA, Smith TL, Yang Y, Pusztai L, Nahta R, Buchholz TA, et al. (2003) Prognostic significance of phosphorylated p38 mitogen-activated protein kinase and HER-2 expression in lymph node-positive breast carcinoma. Cancer 100:499–506 [DOI] [PubMed] [Google Scholar]
- Felip E, Rojo F, Reck M, Heller A, Klughammer B, Sala G, Cedres S, et al. (2008) A phase II pharmacodynamic study of Erlotinib in patients with advanced non-small cell lung cancer previously treated with platinum-based chemotherapy. Clin Cancer Res 14:3867–3874 [DOI] [PubMed] [Google Scholar]
- Fergenbaum JH, Garcia-Closas M, Hewitt SM, Lissowska J, Sakoda LC, Sherman ME (2004) Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Biomarkers Prev 13:667–672 [PubMed] [Google Scholar]
- Guix M, Granja Nd M, Meszoely I, Adkins TB, Wieman BM, Frierson KE, Sanchez V, et al. (2008) Short preoperative treatment with Erlotinib inhibits tumor cell proliferation in hormone receptor-positive breast cancers. J Clin Oncol 26:897–906 [DOI] [PubMed] [Google Scholar]
- Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ (1996) Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst 88:1054–1059 [DOI] [PubMed] [Google Scholar]
- Jones RJ, Boyce T, Fennell M, Jacobs V, Pinto F, Duffield E, Clack G, et al. (2008) The impact of delay in cryo-fixation on biomarkers of Src tyrosine kinase activity in human breast and bladder cancers. Cancer Chemother Pharmacol 61:23–32 [DOI] [PubMed] [Google Scholar]
- Lim J, Kin J-H, Paeng J-Y, Kim M-J, Hong S-D, Lee J-I, Hong SP (2005) Prognostic value of activated Akt expression in oral squamous cell carcinoma. J Clin Pathol 58:1199–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma PC, Jagadeeswaran R, Jagadeesh S, Tretiakova MS, Nallasura V, Fox EA, Hansen M, et al. (2005) Functional expression and mutations of c-Met and its therapeutic inhibition with SU11274 and small interfering RNA in non-small cell lung cancer. Cancer Res 65:1479–1488 [DOI] [PubMed] [Google Scholar]
- Massion PP, Taflan PM, Shyr Y, Rahman SMJ, Yildiz P, Shakthour B, Edgerton ME, et al. (2004) Early involvement of the phosphatidylinositol 3-kinase/Akt pathway in lung cancer progression. Am J Respir Crit Care Med 170:1088–1094 [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Sakamoto M, Yamasaki S, Todo S, Hirohashi S (2004) Akt phosphorylation is a risk factor for early disease recurrence and poor prognosis in hepatocellular carcinoma. Cancer 103:307–312 [DOI] [PubMed] [Google Scholar]
- Prento P, Lyon H (1997) Commercial formalin substitutes for histopathology. Biotech Histochem 72:273–282 [DOI] [PubMed] [Google Scholar]
- Rojo F, Tabernero J, Albanell J, Van Cutsem E, Ohtsu A, Doi T, Koizumi W, et al. (2006) Pharmacodynamic studies of Gefitinib in tumor biopsy specimens from patients with advanced gastric carcinoma. J Clin Oncol 24:4309–4316 [DOI] [PubMed] [Google Scholar]
- Serrels A, Macpherson IR, Evans TR, Lee FY, Clark EA, Sansom OJ, Ashton GH, et al. (2006) Identification of potential biomarkers for measuring inhibition of Src kinase activity in colon cancer cells following treatment with Dasatinib. Mol Cancer Ther 5:3014–3022 [DOI] [PubMed] [Google Scholar]
- Taillade L, Penault-Llorca F, Boulet T, Fouret P, Michiels S, Taranchon E, Mountzios G, et al. (2007) Immunohistochemical expression of biomarkers: a comparative study between diagnostic bronchial biopsies and surgical specimens of non-small-cell lung cancer. Ann Oncol 18:1043–1050 [DOI] [PubMed] [Google Scholar]
- Warmington AR, Wilkinson JM, Riley CB (2000) Evaluation of ethanol-based fixatives as a substitute for formalin in diagnostic clinical laboratories. J Histotechnol 23:299–308 [Google Scholar]
- Weinberg RA (2007) The Biology of Cancer. New York, Garland Science
- Williams JH, Mepham BL, Wright DH (1997) Tissue preparation for immunocytochemistry. J Clin Pathol 50:422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX (2001) Untangling the ErbB signaling network. Natl Rev 2:127–137 [DOI] [PubMed] [Google Scholar]
- Zhang Z-Q, Notermans DW, Sedgewick G, Cavert W, Wietgrefe S, Zupanic M, Gebhard K, et al. (1998) Kinetics of CD4 T cell repopulation of lymphoid tissues after treatment of HIV-1 infection. Proc Natl Acad Sci USA 95:1154–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-Q, Schuler T, Cavert W, Notermans DW, Gebhard K, Henry K, Havlir DV, et al. (1999a) Reversibility of the pathological changes in the follicular dendritic cell network with treatment of HIV-1 infection. Proc Natl Acad Sci USA 96:5169–5172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-Q, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, et al. (1999b) Sexual transmission and propagation of SIV and HIV in resting and activated CD4 T Cells. Science 286:1353–1357 [DOI] [PubMed] [Google Scholar]
- Zhuang L, Lee CS, Scolyer RA, McCarthy SW, Palmer AA, Zhang XD, Thompson JF, et al. (2005) Activation of the extracellular signal regulated kinase (ERK) pathway in human melanoma. J Clin Pathol 58:1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]