Abstract
Functional genomic studies are dominated by transcriptomic approaches, in part reflecting the vast amount of information that can be obtained, the ability to amplify mRNA and the availability of commercially standardized functional genomic DNA microarrays and related techniques. This can be contrasted with proteomics, metabolomics and metabolic flux analysis (fluxomics), which have all been much slower in development, despite these techniques each providing a unique viewpoint of what is happening in the overall biological system. Here, we give an overview of developments in these fields 'downstream' of the transcriptome by considering the characterization of one particular, but widely used, mouse model of human disease. The mdx mouse is a model of Duchenne muscular dystrophy (DMD) and has been widely used to understand the progressive skeletal muscle wasting that accompanies DMD, and more recently the associated cardiomyopathy, as well as to unravel the roles of the other isoforms of dystrophin, such as those found in the brain. Studies using proteomics, metabolomics and fluxomics have characterized perturbations in calcium homeostasis in dystrophic skeletal muscle, provided an understanding of the role of dystrophin in skeletal muscle regeneration, and defined the changes in substrate energy metabolism in the working heart. More importantly, they all point to perturbations in proteins, metabolites and metabolic fluxes reflecting mitochondrial energetic alterations, even in the early stage of the dystrophic pathology. Philosophically, these studies also illustrate an important lesson relevant to both functional genomics and the mouse phenotyping in that the knowledge generated has advanced our understanding of cell biology and physiological organization as much as it has advanced our understanding of the disease.
Introduction
The completion of genome projects, such as those associated with the mouse [1] and humans [2], heralded the field of functional genomics, in which high-throughput approaches are used to profile a tier of organization in a cell, tissue or even organism after perturbation of a gene's function in order to deduce what the function of that gene is. By far the commonest approach used in the armory of functional genomic technology is probably the DNA microarray, which is used to profile the transcriptome that results from a gene manipulation. Although this technology has proven to be incredibly powerful in deducing the consequences of certain genetic modifications, there are situations in which such an approach may not be successful. Approaches based on transcriptomics make the assumption that altered concentrations of mRNA are reflected in the proteome, but this may not be true if the concentration of a given protein is determined by the rate of its destruction. Furthermore, although long-term changes in the function of a cell or tissue may be caused by changes in gene expression, in mammals many medium-term changes arise from protein modifications, such as phosphorylation, acetylation and ubiquitylation, although short-term changes are often caused by allosteric modifications, reflecting rapid transient changes in metabolites. This has led to tools to profile the proteome and the metabolome of a cell, tissue or organism to complement approaches using transcriptomics.
In addition to understanding gene function, functional genomic technologies have also been used to help in phenotyping organisms. One of the first applications of metabolomics was in the phenotyping of yeast (Saccharomyces cerevisiae) mutants in which genetic modifications had produced 'silent phenotypes' in terms of the growth rate, the main phenotype used to distinguish mutants [3]. Raamsdonk and colleagues [4] described an approach described as 'functional analysis by co-responses in yeast' (FANCY), which distinguishes different classes of yeast mutants by global metabolic changes [3-5]. Such a process of defining a phenotype through the global changes induced in metabolism can be used to predict the function of genes deleted or upregulated in a given system through comparative metabolomics. The definition of a metabolic phenotype, or metabotype, by large-scale analysis of metabolites using either 1H nuclear magnetic resonance (NMR) spectroscopy or mass spectrometry (MS) has found many applications in genetic engineering, toxicology and disease diagnosis in plants, animals and microbes [6-9].
Similar to metabolomics, metabolic flux analysis using isotopically labeled substrates can provide novel and unexpected insights into the metabotype of normal and diseased cells, tissues or organisms. The use of isotopes provides unique insights into the dynamics of cellular metabolism and its regulation, information that is not accessible from static measurements of mRNA or protein expression or metabolite concentration. This subdiscipline of metabolomics, which has been referred to as tracer-based metabolomics, dynamic metabolomics or fluxomics and is probably the least developed of all functional genomic technologies, builds on stable isotope methodologies that have been developed over the past two decades and involves analysis of isotopomers by MS and/or NMR (for reviews see [10-16]). In this article, we use the nomenclature followed by most biological investigators [16], in which there are two types of isotopomers: (i) positional isotopomers, which have identical global isotopic composition but differ by the position of the heavy atoms in the molecule; and (ii) mass isotopomers, which differ by the number of heavy atoms in their molecules, resulting in different molecular weights.
Here, we focus on the application of proteomics, metabolomics and the related approach of fluxomics to understand the function of dystrophin, the protein associated with muscular dystrophy, through a widely used mouse model of the disease. However, our purpose is not to focus on the disease per se, but to use this as a model for what the 'downstream' high-throughput approaches have to offer to functional genomics and high-throughput mouse phenotyping and how such approaches can contribute to our understanding of the consequences of a specific genotype and/or therapeutic strategies.
Duchenne muscular dystrophy and the mdx mouse
Duchenne muscular dystrophy (DMD) is an X-linked recessive disorder, affecting one in roughly 3,500 male births, and is characterized by progressive muscle wasting, culminating in death from respiratory or cardiac failure [17,18]. The mutated gene that causes DMD was identified in 1986 [19] and is currently the longest gene known, covering 2,400 kilobases. It is composed of at least 85 exons, which have been conserved throughout evolution, and introns make up 98% of the gene. The dystrophin gene encodes many different tissue-specific dystrophin isoforms generated by use of alternative promoters and differential splicing [20,21]. In 1987, the product of the gene was identified as dystrophin [22]. It is a rod-shaped cytoplasmic protein consisting of over 3,500 amino acids and is a central part of the dystrophin-associated protein (DAP) complex that connects the cytoskeleton of muscle fibers to the extracellular matrix.
Most DMD patient mutations introduce premature stop codons that disrupt the reading frame, resulting in very little or a complete lack of dystrophin, whereas patients with Becker muscular dystrophy (BMD), a less severe form of muscular dystrophy, have mutations that maintain dystrophin's open reading frame, resulting in reduced expression of dystrophin or generating a truncated partially functional protein [23]. Of potential broader clinical relevance, changes in the structural integrity of dystrophin have recently been documented in patients with end-stage dilated, ischemic and viral cardiomyopathies and thus may represent a common pathway for contractile dysfunction in the failing heart [24-26].
There are several human isoforms of dystrophin proteins in various parts of the body. In fact, there are three full-length isoforms of dystrophin: M-dystrophin, found in muscle and, to a small extent, glial cells in the brain; P-dystrophin, found in the Purkinje cells of the cerebellum; and C-dystrophin, found in the cerebral cortex [27]. Furthermore, there are several truncated forms of dystrophin [28]. The purpose of these isoforms, particularly those found in non-muscle tissue and the truncated forms, are not understood [27,28]. This illustrates the difficulty with moving from the genome to an understanding of the proteome.
The role of dystrophin in the cell
Despite the wealth of information now available on the structure and role of dystrophin, predominantly in skeletal muscle, its exact function is still hotly debated. Being an integral member of the link between the cytoskeleton and the extracellular matrix, dystrophin is hypothesized to have a role in stabilizing the sarcolemma (the muscle cell membrane) and maintaining muscle fiber integrity during muscle contraction [29-31]. Dystrophin may also participate in the regulation of intracellular calcium levels [32]. There is also evidence of increased activity of the calcium leak channel in dystrophic muscle [33], which in turn can lead to elevated levels of proteases in dystrophic tissue. Additional roles hypothesized for dystrophin include a role in force and signal transduction processes and in the aggregation of neurotransmitter receptors. In normal muscle, neuronal nitric oxide synthase (nNOS) associates with syntrophin in the DAP complex. However, nNOS is absent from the skeletal-muscle sarcolemma in DMD patients [34]. Interestingly, a recent study by Acharyya et al. [35] provided evidence that dystrophin itself can mediate signals relevant to muscle atrophy and hypertrophy, possibly involving Akt signaling. Finally, at least in the heart, dystrophin has also been recently proposed to be an end-target of ischemic preconditioning, a powerful cardioprotective intervention [36].
One of the most widely used models of this disease is the mdx mouse, which also has a missense mutation in the gene for dystrophin [37]. The skeletal muscle of mdx mice has a disrupted membrane skeleton with regions of the sarcolemma devoid of costameres (the structures connecting the force-generating sarcomeres with the extracellular matrix) [38], and their cytoskeletal γ-actin has been found not to be stably associated with the sarcolemma [39]. Although the mdx mouse has a pronounced hunch, lower muscle regenerative capacity and cardiomyopathic abnormalities compared with wild-type controls [40,41], the phenotype of the disorder is milder in the mouse than in human sufferers unless the mouse is subjected to physical exercise [42] or to ex vivo or in vivo increases in mechanical workload [43,44]. The mouse has only a modest decrease in life expectancy, whereas human sufferers of DMD experience severe muscle wasting culminating in death within the second decade.
This suggests two alternative and not mutually exclusive hypotheses. Firstly, other proteins may compensate the failure to express dystrophin in the mouse but not in humans. Alternatively, it may be that the mouse in a cage is not subject to the same stressor events as humans. It also highlights the difficulties that remain moving from a genomic description of a disease to an understanding of the functional phenotype in different organisms. In terms of species differences, one important difference in the development of the mdx mouse compared with human DMD sufferers is that skeletal muscle tissue in the mouse undergoes a wave of necrosis followed by recovery at 2-3 months. This recovery is absent in the development in humans. Identification of the mechanisms involved in muscle recovery may identify potential ways to treat the disorder in humans. It also shows that tools are needed to place the milder phenotype detected in the mouse in the biological context of an altered proteome and metabolome.
Proteomics and the mdx mouse
Given the central importance of dystrophin in maintaining the connection between the cytoskeleton and the extracellular matrix, a proteomic approach to understanding the disease, perhaps investigating the changes induced in the DAP complex, would be expected to be highly profitable. However, when Ge and colleagues [45] examined the changes in protein expression in the hindlimb muscles of three-month old mdx mice using a combination of two-dimensional gel electrophoresis and MS, the largest change was associated with a decrease in abundance of adenylate kinase 1, which was confirmed by biochemical assay. Doran and colleagues [46] used a similar approach based on two-dimensional fluorescence difference gel electrophoresis (DIGE) to demonstrate an increase in levels of the heat-shock protein cvHSP in the diaphragm, along with alterations in 35 out of 2,398 detected proteins, with decreased expression of the F-box protein Fbxo11, adenylate kinase, β-hemoglobin and dihydrolipoamide dehydrogenase and increased expression of cvHSP, aldehyde reductase, desmin, vimentin, chaperonin and cardiac and muscle myosin heavy chain.
So why have these studies highlighted alterations in metabolism and responses to stress, rather than alterations in the DAP complex? It is important to consider some of the limitations of proteomic approaches based on two-dimensional gels. Many low-concentration proteins can be obscured by highly expressed proteins, which dominate the two-dimensional gels. In particular, many of the enzymes involved in key metabolic pathways, such as adenylate kinase, are both readily separated on a two-dimensional gel and found in high concentrations in the cell and thus would be expected to be prominent in a global analysis of proteins that separate by two-dimensional gel electrophoresis. An alternative approach is to try a targeted analysis. To test the hypothesis that dystrophic muscle is more susceptible to cell death because of altered Ca2+ handling, Doran and colleagues [47] used the cationic carbocyanine dye 'stains-all' to specifically examine the Ca2+ binding proteins and demonstrated a reduction in luminal Ca2+-binding proteins, including calsequestrin, in skeletal muscle. The deregulation of Ca2+ homeostasis has since been confirmed by proteomics in the diaphragm, skeletal muscle and heart [48].
However, to test a hypothesis one does not need to always target the analysis, but can tailor the method for processing the data. Gulston and co-workers [49] used the multivariate regression technique prediction to latent structures by partial least squares (PLS) to regress proteomic changes by DIGE in order to investigate age-related changes in heart tissue of the mdx mouse. Intriguingly, this approach showed that the dominant proteomic changes associated with age were the same in control and mdx mice. However, at each time point the dystrophic heart tissue showed differences in mitochondrial proteins, particularly those associated with the electron transport chain and ATP synthesis.
With recent advances in MS, two-dimensional gel-based proteomics has been faced with the problem of too many proteins being detected in a dataset, with spots on the gel often containing several proteins. To address this problem, several groups involved in heart and skeletal muscle proteomics have focused on organelle proteomics or tried targeted approaches by looking at proteins that bind to a given target.
Metabolomics and the mdx mouse
Metabolic deficits or alterations have been reported in both muscle - skeletal and heart - and brain tissue [40,50-54]. Furthermore, many of these changes are age specific and will vary across the lifespan of the mouse. Although these studies often confined themselves to one tissue and a small subset of metabolites, in many cases these could be considered the front-runner studies to a metabolomics study of the mdx mouse.
One particularly elegant set of studies was performed by McIntosh and co-workers [40,41], who established that skeletal muscle taurine content correlated with the regenerative capacity of muscle tissue in three mouse models: mdx, MyoD (mutant in a protein with a key role in regulating muscle differentiation) and mdx:MyoD cross mice, all at 4 months of age. Using in situ hybridization and autoradiography, the transcription of key genes involved in muscle cell proliferation (myogenin and Myf5) were monitored and compared with the tissue content of taurine, with mdx tissue having the highest concentration of muscle taurine as well as the greatest number of muscle proliferative cells of the three mouse types.
Metabolic abnormalities have also been characterized in cerebral tissue in sufferers of DMD and the mdx mouse using chemical assays or 1H, 31P and 13C NMR spectroscopy [55-57]. This reflects the fact that DMD sufferers experience a non-progressive intellectual deficit. In both mdx mice and DMD sufferers the cerebellum contains an increased concentration of choline-containing compounds, as measured by the choline:N-acetyl aspartate ratio and the choline:creatine ratio [57]. Curiously, the choline:N-acetyl aspartate ratio correlated with improved neurological performance in DMD boys, suggesting that it may be involved in a compensatory mechanism.
Applying techniques developed for following drug toxicity using a combination of 1H NMR spectroscopy and principal components analysis [58], Griffin and co-workers [7] examined a range of tissues from the mdx mouse, including cardiac (Figure 1a), diaphragm, soleus (calf muscle), cerebral cortex and cerebellum at 6 months of age. Dystrophic muscle tissue from heart, diaphragm and soleus were similarly perturbed metabolically by a failure to express dystrophin, despite being very different types of muscle; this was characterized by an increase in taurine and lactate and a decrease in creatine in tissue extracts from dystrophic tissue. This is in keeping with the single protein isoform known to be involved in all these tissue types, and that presumably has a similar role in all these tissues. These changes could be detected even in intact tissue using high-resolution magic angle spinning 1H NMR spectroscopy, which also identified a significant increase in lipid infiltration in dystrophic tissue [59]. Similarly, extracts of cerebellum tissue from mdx mice were readily distinguished from wild-type controls by their metabolic profiles, including a characteristic increase in phosphocholine. The approach even found a weak separation in cortical tissue extracts, although this had not been detected by previous studies. The changes in profiles of dystrophic muscle, cortex and cerebellum tissue were all distinct, suggesting very different roles for the three iso-forms of dystrophin in the tissues.
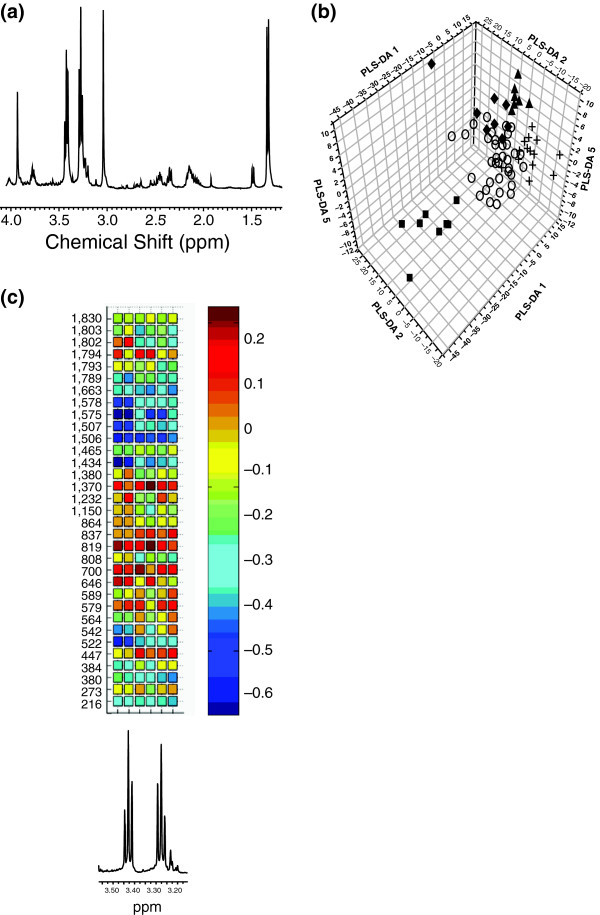
Figure 1.
Applications of metabolomics and proteomics to the mdx mouse. (a) A typical high-resolution 1H NMR spectrum from an aqueous extract of cardiac tissue from the mdx mouse. The chemical shift and splitting pattern of a given resonance (peak) enables the identification of the metabolite it belongs to, and the area under the resonance determines the concentration of that metabolite. (b) An orthogonal signal corrected partial least squares discriminate analysis plot of various mouse models of cardiac disease using solution state NMR spectroscopy. Key: circles, control + mdx; diamonds, model of cardiac hypertrophy (MLPKO); squares, model of cardiac arrhythmia (Scn-/+); triangles, model of cardiac arrhythmia (ScnΔ /+). (c) Correlation analysis between identified proteins in a proteomic study of heart tissue from mdx mice and the intracellular concentration of taurine. When detected by 1H NMR spectroscopy (bottom graph), taurine can be identified by two triple peaks at δ 3.25-3.27 and δ 3.42-3.46. The correlation heat map between spectral intensity and protein expression was used to determine which proteomic changes were associated with the increase in taurine in dystrophic muscle. The x axis is the chemical shift region containing the resonances from taurine; the y axis consists of protein spots detected in the two-dimensional gel electrophoresis. The color scale displays the correlation coefficients between the two sets of data (concentration of taurine against concentration of protein).
To investigate whether metabolic profiles could be used to predict the proteomics of a disease or therapy regime, the 1H NMR derived metabolic profiles of cardiac and diaphragm tissue from four different mouse models with mutations in the dystrophin or utrophin genes were examined [60]. Utrophin is functionally related to dystrophin, and its up-regulation in skeletal muscle has been considered as a gene therapy approach for treating DMD. During fetal development, utrophin is found over the entire surface of muscle fibers, but is replaced by dystrophin during development, and utrophin becomes localized to the neuromuscular junction in adult skeletal muscle [61-63]. The dystrophic phenotype normally observed in mdx mice is absent when muscles overexpress utrophin [64,65]. Furthermore, utrophin has a particularly favorable characteristic in dystrophic tissue; although the body can treat dystrophin as a foreign protein, mounting an immune response; increasing the innate expression of utrophin does not have this pitfall [66].
In this metabolomic study [60], the PLS technique was used to regress the metabolic profiles of tissue extracts against the relative protein content of dystrophin and utrophin in four mouse strains: (i) the mdx mouse, which does not express dystrophin; (ii) Tgfull-length/Dmdmdx, a transgenic mouse expressing full-length utrophin in skeletal muscle but not heart crossed with the mdx mouse to produce mice lacking dystrophin but with utrophin localized at the sarcolemma [67]; (iii) Tg/Dmdmdx;utrn-/-, a transgenic mouse expressing a truncated utrophin transgene crossed onto a Dmdmdx/utrn-/-double mutant background, resulting in a mouse with no dystrophin in skeletal muscle but with a truncated utrophin transgene and heart with no dystrophin or utrophin [68]; and (iv) the control mouse to (iii), Tgtruncated/Dmdmdx, which has no dystrophin but expresses utrophin. The PLS models readily classified tissue according to dystrophin content for the heart and diaphragm [60]. Examining the diaphragm, models were built that described both dystrophin and utrophin expression. Furthermore, increased utrophin expression in dystrophic diaphragm tissue produced a metabolic phenotype between that of the wild-type tissue and that of the dystrophic tissue, suggesting that increased utrophin expression counteracts some of the deficits associated with dystrophic tissue. The major metabolite responsible for classification of tissue into controls and dystrophic was taurine, in keeping with it being a potential marker of muscle regenerative capability. This suggests that metabolic profiles of muscle tissue could be useful in monitoring the efficacy of potential treatment regimes, such as gene therapy.
Cole and colleagues [69] have also examined the impact that protein expression of utrophin and dystrophin has on muscle metabolism, using 1H and 31P NMR spectroscopy to examine ATP turnover in mdx mice, utrophin and dystrophin knockout mice and controls. In particular, the hind-limb muscle in the double knockouts had reduced concentrations of ATP and creatine and increased intracellular phosphate and ADP. Indeed, ADP could distinguish all three mouse models examined.
Whereas it is clear that metabolomics and related approaches can readily distinguish dystrophic tissue from that obtained from wild-type controls for skeletal muscle, heart and brain tissues, a word of caution is necessary when considering how these results relate to human sufferers. One must remember that the mdx mouse is relatively healthy in comparison with human sufferers of DMD. Jones et al. [70] used high-resolution 1H NMR spectroscopy to investigate the metabolome of heart tissue from the mdx mouse, two mouse models of cardiac arrhythmia and one of cardiac hypertrophy (Figure 1b). Surprisingly, the dominant effect was the strain background of the mice rather than the diseases they modeled, suggesting that mouse models may have very different phenotypes depending on the strain background on which they are generated. However, multivariate statistics were capable of separating each mouse model from its control strain, showing that metabolic profiles could be generated for each disease.
So far, the steady state concentrations of metabolites in heart tissue have mainly been assessed by NMR spectroscopy. However, because of the small population differences associated with NMR spectroscopy, only a relatively small number of metabolites can be detected. To expand the metabolome, Atherton and colleagues [71,72] have used a combination of NMR spectroscopy and gas chromatography mass spectrometry (GC-MS) to detect over a hundred aqueous metabolites and fatty acids. This has expanded the coverage of the metabolome to over 250 individual species, an order of magnitude more than those measured in the mdx mouse. Han and co-workers [73] have used shotgun (direct infusion) MS to show that cardiolipin depletion in the diabetic myocardium is a potential cause of mitochondrial dysfunction. Expanding the shotgun approach to negatively charged water-soluble metabolites using matrix-assisted laser desorption ionization tandem time of flight MS (MALDI-TOF/TOF MS), the group [74] have profiled over 285 chromatographic peaks in heart tissue. These studies all indicate that metabolomic studies of the mdx mouse have only scratched the surface of the metabolome.
Fluxomics and the mdx mouse
The recent application of metabolic flux analysis to the mdx mouse heart has provided an unexpected insight into the metabotype [54,75]. Khairallah et al. [54] compared control C57BL/10 and dystrophin-deficient mdx hearts from mice at an age (10-12 weeks) during which there are no major histological or echocardiographic abnormalities or differences in metabolic gene expression or metabolite concentrations as revealed by metabolomics [49]. This was achieved using ex vivo heart perfusion in the working mode combined with a 13C labeling methodology involving mass isotopomer analysis by GC-MS. Principles and theoretical ideas relevant to the use of 13C-isotopomer analysis as applied to cardiac metabolism and methodological details on the working heart perfusion model have been described in detail [15,54,76]. This experimental paradigm enables detailed and simultaneous determinations of various hemodynamic and metabolic flux parameters relevant to energy production.
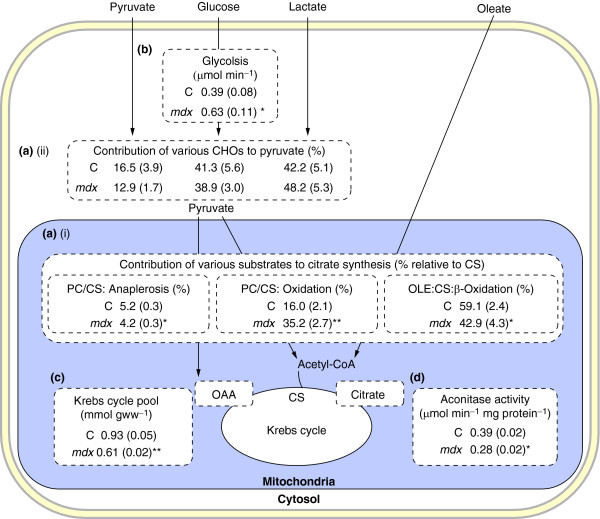
In the heart studies described by Khairallah et al. [54] four different substrates uniformly labeled with carbon 13 (U-13C) were used to probe metabolism in the mdx heart: the long-chain fatty acid (LCFA) [U-13C18]oleate and the carbohydrates (CHO) [U-13C3]lactate, [U-13C3]pyruvate and [U-13C6]glucose. Metabolic flux ratios that were assessed revealed cytosolic glycolysis, substrate selection (CHO versus LCFA) for mitochondrial acetyl-CoA formation for citrate synthesis (energy production), and pyruvate partitioning between decarboxylation (oxidation by pyruvate dehydrogenase) and carboxylation (by pyruvate carboxylase); see Figure 2. It is noteworthy that pyruvate carboxylation participates in the refueling of catalytic Krebs cycle intermediates, that is, in anaplerosis, a process that is proposed to have a crucial role in optimal cardiac energy production [77].
Figure 2.
Metabolic flux ratios assessed in working control (C) and mdx mouse heart perfused with 13C-labeled substrates. Data are given as means ± standard errors (indicated in parentheses); n = 4-8 in each group. Reactions are shown in the part of the cell in which they take place. (a) Flux ratios. (i) Flux ratios reflecting the contribution of exogenous fatty acids (oleate) and carbohydrates (CHOs: lactate, pyruvate and glucose) to acetyl-CoA formation (energy) and oxaloacetate (OAA; anaplerosis) via oleate β-oxidation (OLE), pyruvate decarboxylation (PDC) and carboxyation (PC), respectively, and expressed relative to citrate synthesis (CS). (ii) Flux ratios reflecting the contribution of individual CHOs - as indicated by the individual arrows - to pyruvate formation, expressed in percentage of total. (b) Glycolytic rate, which reflects the production of lactate and pyruvate, in μmol × min-1. (c) Tissue concentration of Krebs cycle intermediates, in μmol × g wet weight-1. (d) Tissue aconitase activity, in μmol × min-1 × mg protein-1. *p < 0.05, #p < 0.001 for mdx versus control mouse hearts. Adapted from Khairallah et al. [54].
Compared with controls, working mdx mouse hearts had an altered mitochondrial energy substrate metabolism, as reflected by a marked shift from LCFA to CHO oxidation for energy production; decreased anaplerotic pyruvate carboxylation; and enhanced glycolysis [54]. These changes were associated with exacerbated oxygen consumption and with compromised function and integrity of the sarcolemma, as reflected by lactate dehydrogenase release. Subsequent targeted metabolite and protein analyses also revealed a marked decrease in the myocardial mitochondrial Krebs cycle pool size and lower activity of the Krebs cycle enzyme aconitase, which further substantiate the presence of mitochondrial metabolic alterations in the heart of young mdx mice. Although a substrate shift from LCFA to CHO, which is a characteristic of several models of cardiomyopathies, could be beneficial for the heart (for recent reviews see [78,79]), the decreased levels of Krebs cycle intermediates and decreased aconitase activity would be expected to limit the ability of the dystrophic heart to enhance Krebs cycle flux and thus limit mitochondrial energy production under conditions of high energy demand.
A shift from LCFA to CHO oxidation in the dystrophic mdx mouse heart agrees with results from cardiac positron emission tomography studies in patients with DMD, using 18F-deoxyglucose or a radioiodinated branched fatty acid ([123I]15-(p-iodophenyl)-3-(R,S)-methylpentadecanoic acid (BMIPP)) [80,81]. However, the aforementioned substantial differences in the concentrations of Krebs cycle intermediates between control and mdx groups documented in ex vivo hearts perfused in the working mode contrasted with the small changes documented in these hearts freeze-clamped in vivo [54], which also agreed with recent metabolomic data [49]. This discrepancy between results obtained ex vivo and in vivo suggest that despite providing what are generally accepted to be physiological levels of workload, nutrients and calcium supply, the ex vivo perfusion environment unmasked major abnormalities of mdx cardiac function and energy metabolism thereby exacerbating the observed changes. Because these abnormalities are not readily detected in vivo at this stage of the disease in the mdx mouse, one would presume that homeostatic compensatory mechanisms in the intact animal obscure these changes.
Numerous candidate mechanisms have been considered to explain the documented metabolic flux alterations in perfused dystrophin-deficient hearts at such an early compensatory phase of the cardiomyopathy before heart failure. These include the activity of cell signaling pathways and changes in metabolic gene expression. Ultimately, defective NO/cGMP signaling was hypothesized, as it seemed to reconcile all data. Subsequent testing for this cGMP signaling defect using transgenic and pharmacological approaches showed improvement in contractile performance, myocardial metabolic status and sarcolemmal integrity, thus suggesting a potential clinical avenue for the treatment of the dystrophin-related cardiomyopathies [75].
In summary, results obtained using the ex vivo working mdx mouse perfused with 13C-labeled substrates together show how changes in myocardial metabolic fluxes may be a subclinical signature of defective cell signaling that occurs before overt cardiomyopathy as well as providing a basis for therapeutic strategies. The concomitant documentation of pathophysiological changes (impaired contractile function and membrane integrity) highlights the power inherent in measuring metabolic fluxes as one part of predicting the physiological phenotype of any organism. These data also suggest a crucial role for myocardial dystrophin in maintaining optimal coupling between energy metabolism and contraction. Although the described 13C-labeling paradigm focused on the measurements of carbon flow through the Krebs cycle and related energy-producing pathways in the heart, additional flux parameters could have been easily assessed by extending the number of metabolites that were analyzed by GC-MS. For example, in another study, Khairallah et al. [82] also evaluated the 13C-enrichment and concentration of LCFA in triglycerides to assess flux pertaining to lipid partitioning between mitochondrial β-oxidation (for energy) and triglyceride synthesis (for storage), a process that can be used to monitor the toxic effects of lipids, referred to as lipotoxicity.
Although the number of molecules analyzed can be increased by using a combination of MS and NMR techniques [15,83], the choice of 13C-labeled substrates can also determine the number of metabolic flux parameters that can be extrapolated in a given single experiment. In this regard, recent studies demonstrated the potential of other 13C-labeled substrates (for example [1,2-13C]glucose, [U-13C]glutamine and deuterated water) [12,83-90].
However, the use of isolated intact organs or cells can also unmask abnormalities that are not readily observed in vivo because of the existence of compensatory mechanisms in the intact organism, as was the case for the isolated mdx heart study [54,75]. Finally, there are several pitfalls to be wary of in the application of metabolic flux analyses, including corrections for natural stable isotope abundance and the presence of overlapping fragments, which affect the accuracy and precision of MS-based analyses [15,16,77,91-95]. There are also limitations associated with the assumptions on which the equations that form the metabolic models from which flux ratios are calculated [96-98].
Conclusions: towards systems biology
Technological advances are improving the number of proteins and metabolites that can be measured by combining NMR- and MS-based technologies. Recent studies clearly show the added value of dynamic metabolomics or fluxomics as applied to the discovery of metabolic pathways related to hepatic gluconeogenesis [87,88] or to the probing of drug therapy in cultured cells [84-86]. However, although metabolomics and proteomics are approaches that lend themselves to functional genomic analyses, the accurate and precise determination of metabolite fluxes is substantially more time consuming and cannot be readily adapted for functional genomic analyses. Furthermore, even with improvements in analytical technology, one problem common to proteomics, metabolomics and fluxomics is the incomplete coverage of the tiers of organization. Proteomics and metabolomics detect only a small subset of the total proteome and metabolome, and fluxomics tends to focus on discrete pathways determined in part by the substrates that are used for the analysis. One method to remedy this is to combine the analysis of different '-omes'. Although, so far, there are only few examples of such studies [49,99-102], this is likely to be a rapidly expanding field. We have illustrated the potential added value of combining information from proteomics, metabolomics and fluxomics as applied to the mdx mouse. Indeed, the combined metabolomics and proteomics (by DIGE) approach used by Gulston and coworkers [49] enabled the demonstration of a correlation between the characteristic increase in taurine found in the heart and skeletal muscle of these mice and proteins associated with oxidative phosphorylation and mitochondrial metabolism (Figure 1c). Conversely, flux studies in the working mdx mouse heart further substantiated the specific mitochondrial alterations related to energy substrate metabolism in the Krebs cycle [54], which were not apparent in histology or echocardiograms, or detected by transcriptomics or metabolomics at this age [49] and provided the basis for a successful therapeutic intervention [75]. Collectively, these data point to perturbations in proteins, metabolites and metabolic fluxes that reflect mitochondrial energetic alterations in skeletal and heart muscles at an early stage of the dystrophic pathology.
In conclusion, we have examined how recent advances in proteomics, metabolomics and fluxomics have been used to study various aspects of a pathophysiology, as applied to muscular dystrophy. These approaches have produced new insights not only into the disease process, which provides a basis for therapeutic strategies or for biomarker screening, but also into the basic biology of the cell. Such studies will take the field from a genomic description towards a fuller understanding of the systems biology of the disease.
Abbreviations
BMD: Becker muscular dystrophy; CHO: carbohydrate; DAP: dystrophin-associated protein complex; DIGE: two-dimensional fluorescence difference gel electrophoresis; DMD: Duchenne muscular dystrophy; FANCY: functional analysis by co-responses in yeast; GC-MS: gas chromatography mass spectrometry; LCFA: long-chain fatty acid; MALDI-TOF/TOF MS: matrix-assisted laser desorption ionization tandem time of flight mass spectrometry; MS: mass spectrometry; NMR: nuclear magnetic resonance spectroscopy; nNOS: neuronal nitric oxide synthase; PLS: prediction to latent structures by partial least squares.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
The authors contributed equally to the writing of this article.
Contributor Information
Julian L Griffin, Email: jlg40@cam.ac.uk.
Christine Des Rosiers, Email: christine.des.rosiers@umontreal.ca.
Acknowledgements
Cardiac work in the Griffin laboratory is supported by the Royal Society, UK (University Research Fellowship to JLG), the Wellcome Trust, the British Heart Foundation and GlaxoSmithKline. This study was supported by the Canadian Institutes of Health Research (Grant no. 9575 to CDR). The authors thank Melanie Gulston for careful proofreading of the manuscript.
References
- Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Oliver SG, Winson MK, Kell DB, Baganz F. Systematic functional analysis of the yeast genome. Trends Biotech. 1998;16:373–378. doi: 10.1016/s0167-7799(98)01214-1. [DOI] [PubMed] [Google Scholar]
- Raamsdonk LM, Teusink B, Broadhurst D, Zhang N, Hayes A, Walsh MC, Berden JA, Brindle KM, Kell DB, Rowland JJ, Westerhoff HV, van Dam K, Oliver SG. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat Biotechnol. 2001;19:45–50. doi: 10.1038/83496. [DOI] [PubMed] [Google Scholar]
- Castrillo JI, Hayes A, Mohammed S, Gaskell SJ, Oliver SG. An optimized protocol for metabolome analysis in yeast using direct infusion electrospray mass spectrometry. Phytochemistry. 2003;62:929–937. doi: 10.1016/s0031-9422(02)00713-6. [DOI] [PubMed] [Google Scholar]
- Fiehn O, Kopka J, Dormann P, Altmann T, Tretheway RN, Willmitzer L. Metabolite profiling for plant functional genomics. Nat Biotechnol. 2000;18:1157–1161. doi: 10.1038/81137. [DOI] [PubMed] [Google Scholar]
- Griffin JL, Williams HJ, Sang E, Clarke K, Rae C, Nicholson JK. Metabolic profiling of genetic disorders: a multitissue (1)H nuclear magnetic resonance spectroscopic and pattern recognition study into dystrophic tissue. Anal Biochem. 2001;293:16–21. doi: 10.1006/abio.2001.5096. [DOI] [PubMed] [Google Scholar]
- Gavaghan CL, Holmes E, Lenz E, Wilson ID, Nicholson JK. An NMR-based metabonomic approach to investigate the biochemical consequences of genetic strain differences: application to the C57BL10J and Alpk:ApfCD mouse. FEBS Lett. 2000;484:169–174. doi: 10.1016/s0014-5793(00)02147-5. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Connelly J, Lindon JC, Holmes E. Metabonomics: a platform for studying drug toxicity and gene function. Nat Rev Drug Disc. 2002;1:153–161. doi: 10.1038/nrd728. [DOI] [PubMed] [Google Scholar]
- Lee WN, Go VL. Nutrient-gene interaction: tracer-based metabolomics. J Nutr. 2005;135(12 Suppl):3027S–3032S. doi: 10.1093/jn/135.12.3027S. [DOI] [PubMed] [Google Scholar]
- Hellerstein MK. In vivo measurement of fluxes through metabolic pathways: the missing link in functional genomics and pharmaceutical research. Annu Rev Nutr. 2003;23:379–402. doi: 10.1146/annurev.nutr.23.011702.073045. [DOI] [PubMed] [Google Scholar]
- Boros LG, Brackett DJ, Harrigan GG. Metabolic biomarker and kinase drug target discovery in cancer using stable isotope-based dynamic metabolic profiling (SIDMAP). Curr Cancer Drug Targets. 2003;3:445–453. doi: 10.2174/1568009033481769. [DOI] [PubMed] [Google Scholar]
- Kelleher JK. Probing metabolic pathways with isotopic tracers: insights from mammalian metabolic physiology. Metab Eng. 2004;6:1–5. doi: 10.1016/j.ymben.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Birkemeyer C, Luedemann A, Wagner C, Erban A, Kopka J. Metabolome analysis: the potential of in vivo labeling with stable isotopes for metabolite profiling. Trends Biotechnol. 2005;23:28–33. doi: 10.1016/j.tibtech.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Des Rosiers C, Lloyd S, Comte B, Chatham JC. A critical perspective of the use of (13)C-isotopomer analysis by GCMS and NMR as applied to cardiac metabolism. Metab Eng. 2004;6:44–58. doi: 10.1016/j.ymben.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Brunengraber H, Kelleher JK, Des Rosiers C. Applications of mass isotopomer analysis to nutrition research. Annu Rev Nutr. 1997;17:559–596. doi: 10.1146/annurev.nutr.17.1.559. [DOI] [PubMed] [Google Scholar]
- Duchenne GBA. De l'Electrisation Localisee et son Application a la Therapeutique. 2. Paris: Balliere et fils; 1868. [Google Scholar]
- Emery AE. Clinical and molecular studies in Duchenne muscular dystrophy. Prog Clin Biol Res. 1989;306:15–28. [PubMed] [Google Scholar]
- Monaco A, Neve R, Colletti-Feener C. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986;323:646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Ahn AH, Kunkel LM. The structural and functional diversity of dystrophin. Nat Genet. 1993;4:283–291. doi: 10.1038/ng0493-283. [DOI] [PubMed] [Google Scholar]
- Feener CA, Koenig M, Kunkel LM. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989;338:509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E, Brown R, Kunkel L. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Monaco AP, Bertelson CJ, Liechti GS, Moser H, Kunkel LM. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988;2:90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Rodríguez M, Cai WJ, Kostin S, Lucchesi BR, Schaper J. Ischemia depletes dystrophin and inhibits protein synthesis in the canine heart: mechanisms of myocardial ischemic injury. J Mol Cell Cardiol. 2005;38:723–733. doi: 10.1016/j.yjmcc.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Badorff C, Lee GH, Lamphear BJ, Martone ME, Campbell KP, Rhoads RE, Knowlton KU. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999;5:320–326. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- Vatta M, Stetson SJ, Perez-Verdia A, Entman ML, Noon GP, Torre-Amione G, Bowles NE, Towbin JA. Molecular remodelling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359:936–941. doi: 10.1016/S0140-6736(02)08026-1. [DOI] [PubMed] [Google Scholar]
- Nudel U, Zuk D, Einat P, Zeelon E, Levy Z, Neuman S, Yaffe D. Duchenne muscular dystrophy gene product is not identical in muscle and brain. Nature. 1989;337:76–78. doi: 10.1038/337076a0. [DOI] [PubMed] [Google Scholar]
- Lederfein D, Levy Z, Augier N, Mornet D, Morris G, Fuchs O, Yaffe D, Nudel U. A 71-kilodalton protein is a major product of Duchenne muscular dystrophy gene in brain and other nonmuscle tissue. Proc Natl Acad Sci USA. 1992;89:5346–5350. doi: 10.1073/pnas.89.12.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak C, Wong S, Elson EL. Mechanical function of dystrophin in muscle cells. J Cell Biol. 1995;128:355–361. doi: 10.1083/jcb.128.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller B, Karparti G, Carpenter S. Dystrophin-deficient mdx muscle fibres are preferentially vulnerable to necrosis induced by experimental lengthening contractions. J Neurol Sci. 1990;100:9–13. doi: 10.1016/0022-510x(90)90005-8. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- Fong P, Turner PR, Denetclaw WF, Steinhardt RA. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science. 1990;250:673–676. doi: 10.1126/science.2173137. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Acharyya S, Butchbach ME, Sahenk Z, Wang H, Saji M, Carathers M, Ringel MD, Skipworth RJ, Fearon KC, Hollingsworth MA, Muscarella P, Burghes AH, Rafael-Fortney JA, Guttridge DC. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8:421–432. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Kyoi S, Otani H, Hamano A, Matsuhisa S, Akita Y, Fujiwara H, Hattori R, Imamura H, Kamihata H, Iwasaka T. Dystrophin is a possible end-target of ischemic preconditioning against cardiomyocyte oncosis during the early phase of reperfusion. Cardiovasc Res. 2006;70:354–363. doi: 10.1016/j.cardiores.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx-mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Williams MW, Bloch RJ. Extensive but coordinated reorganisation of the membrane skeleton in myofibres of dystrophic (mdx) mice. J Cell Biol. 1999;144:1259–1270. doi: 10.1083/jcb.144.6.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybakova IN, Patel JR, Ervasti JM. The dystrophin complex forms a mechanically strong link between the sarcolemma and costameric actin. J Cell Biol. 2000;150:1209–1214. doi: 10.1083/jcb.150.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh LM, Baker RE, Anderson JE. Magnetic resonance imaging of regenerating and dystrophic mouse muscle. Biochem Cell Biol. 1998;76:532–541. doi: 10.1139/bcb-76-2-3-532. [DOI] [PubMed] [Google Scholar]
- McIntosh LM, Garrett KL, Megeney L, Rudnicki MA, Anderson JE. Regeneration and myogenic cell proliferation correlate with taurine levels in dystrophin- and MyoD-deficient muscles. Anat Rec. 1998;252:311–324. doi: 10.1002/(SICI)1097-0185(199810)252:2<311::AID-AR17>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Harrod GV, Davies KE. Activation of calcineurin and stress activated protein kinase/p38-mitogen activated protein kinase in hearts of utrophin-dystrophin knockout mice. Neuromuscul Disord. 2001;11:251–259. doi: 10.1016/s0960-8966(00)00201-7. [DOI] [PubMed] [Google Scholar]
- Kamogawa Y, Biro S, Maeda M, Setoguchi M, Hirakawa T, Yoshida H, Tei C. Dystrophin-deficient myocardium is vulnerable to pressure overload in vivo. Cardiovasc Res. 2001;50:509–515. doi: 10.1016/s0008-6363(01)00205-x. [DOI] [PubMed] [Google Scholar]
- Danialou G, Comtois AS, Dudley R, Karpati G, Vincent G, Des Rosiers C, Petrof BJ. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J. 2001;15:1655–1657. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- Ge Y, Molloy MP, Chamberlain JS, Andrews PC. Proteomic analysis of mdx skeletal muscle: Great reduction of adenylate kinase 1 expression and enzymatic activity. Proteomics. 2003;3:1895–1903. doi: 10.1002/pmic.200300561. [DOI] [PubMed] [Google Scholar]
- Doran P, Martin G, Dowling P, Jockusch H, Ohlendieck K. Proteome analysis of the dystrophin-deficient MDX diaphragm reveals a drastic increase in the heat shock protein cvHSP. Proteomics. 2006;6:4610–4621. doi: 10.1002/pmic.200600082. [DOI] [PubMed] [Google Scholar]
- Doran P, Dowling P, Lohan J, McDonnell K, Poetsch S, Ohlendieck K. Subproteomics analysis of Ca+-binding proteins demonstrates decreased calsequestrin expression in dystrophic mouse skeletal muscle. Eur J Biochem. 2004;271:3943–3952. doi: 10.1111/j.1432-1033.2004.04332.x. [DOI] [PubMed] [Google Scholar]
- Doran P, Dowling P, Donoghue P, Buffini M, Ohlendieck K. Reduced expression of regucalcin in young and aged mdx diaphragm indicates abnormal cytosolic calcium handling in dystrophin-deficient muscle. Biochim Biophys Acta. 2006;1764:773–785. doi: 10.1016/j.bbapap.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Gulston MK, Rubtsov DV, Atherton HJ, Clarke K, Davies KE, Lilley KS, Griffin JL. A combined metabolomic and proteomic investigation of the effects of a failure to express dystrophin in the mouse heart. J Proteome Res. 2008;7:2069–2077. doi: 10.1021/pr800070p. [DOI] [PubMed] [Google Scholar]
- Even PC, Decrouy A, Chinet A. Defective regulation of energy metabolism in mdx-mouse skeletal muscles. Biochem J. 1994;304:649–654. doi: 10.1042/bj3040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhatarian A, Decrouy A, Chinet A, Even PC. Components of energy expenditure in the mdx mouse model of Duchenne muscular dystrophy. Pfugers Arch. 1996;431:527–532. doi: 10.1007/BF02191899. [DOI] [PubMed] [Google Scholar]
- Kemp GJ, Taylor DJ, Dunn JF, Frostick SP, Radda GK. Cellular energetics of dystrophic muscle. J Neurol Sci. 1993;116:201–206. doi: 10.1016/0022-510x(93)90326-t. [DOI] [PubMed] [Google Scholar]
- Tracey I, Scott RB, Thompson CH, Dunn JF, Barnes PR, Styles P, Kemp GJ, Rae CD, Pike M, Radda GK. Brain abnormalities in Duchenne muscular dystrophy: a 31P magnetic resonance spectroscopy and neuropsychological study. Lancet. 1995;345:1260–1264. doi: 10.1016/s0140-6736(95)90923-0. [DOI] [PubMed] [Google Scholar]
- Khairallah M, Khairallah R, Young ME, Dyck JR, Petrof BJ, Des Rosiers C. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J Mol Cell Cardiol. 2007;43:119–129. doi: 10.1016/j.yjmcc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Tracey I, Dunn JF, Radda GK. Brain metabolism is abnormal in the mdx mouse model of Duchenne muscular dystrophy. Brain. 1996;119:1039–1044. doi: 10.1093/brain/119.3.1039. [DOI] [PubMed] [Google Scholar]
- Kato T, Nishina M, Matsushita K, Hori E, Akaboshi S, Takashima S. Increased cerebral choline-compounds in Duchenne muscular dystrophy. NeuroReport. 1997;8:1435–1437. doi: 10.1097/00001756-199704140-00022. [DOI] [PubMed] [Google Scholar]
- Rae C, Scott RB, Thompson CH, Dixon RM, Dumughn I, Kemp GJ, Male A, Pike M, Styles P, Radda GK. Brain biochemistry in Duchenne muscular dystrophy: A 1H magnetic resonance and neuropsychological study. J Neurol Sci. 1998;160:148–157. doi: 10.1016/s0022-510x(98)00190-7. [DOI] [PubMed] [Google Scholar]
- Beckwith-Hall BM, Nicholson JK, Nicholls A, Foxall PJD, Lindon JC, Connor S, Abdi M, Connelly J, Holmes E. Nuclear magnetic resonance spectroscopic and principal component analysis investigations into biochemical effects of three model hepatotoxins. Chem Res Toxicol. 1998;11:260–272. doi: 10.1021/tx9700679. [DOI] [PubMed] [Google Scholar]
- Griffin JL, Williams HJ, Sang E, Nicholson JK. Abnormal lipid profile of dystrophic cardiac tissue as demonstrated by one- and two-dimensional magic-angle spinning (1)H NMR spectroscopy. Magn Reson Med. 2001;46:249–255. doi: 10.1002/mrm.1185. [DOI] [PubMed] [Google Scholar]
- Griffin JL, Sang E, Evens T, Davies K, Clarke K. Metabolic profiles of dystrophin and utrophin expression in mouse models of Duchenne muscular dystrophy. FEBS Lett. 2002;530:109–116. doi: 10.1016/s0014-5793(02)03437-3. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Beggs AH, Koenig M, Kunkel LM, Angelini C. Cross-reactive protein in Duchenne muscle. Lancet. 1989;2:1211–1212. doi: 10.1016/s0140-6736(89)91812-6. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Hoffman EP, Kunkel LM. Identification of a chromosome 6-encoded dystrophin-related protein. J Biol Chem. 1990;265:16717–16720. [PubMed] [Google Scholar]
- Khurana TS, Watkins SC, Chafey P, Chelly J, Tomé FM, Fardeau M, Kaplan JC, Kunkel LM. Immunolocalization and developmental expression of dystrophin related protein in skeletal muscle. Neuromuscul Disord. 1991;1:185–194. doi: 10.1016/0960-8966(91)90023-l. [DOI] [PubMed] [Google Scholar]
- Tinsley JM, Potter AC, Phelps SR, Fisher R, Trickett JL, Davies KE. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature. 1996;384:349–353. doi: 10.1038/384349a0. [DOI] [PubMed] [Google Scholar]
- Deconinck N, Tinsley J, De Backer F, Fisher R, Kahn D, Phelps S, Davies K, Gillis JM. Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nat Med. 1997;3:1216–1221. doi: 10.1038/nm1197-1216. [DOI] [PubMed] [Google Scholar]
- Burton EA, Davies KE. Muscular dystrophy--reason for optimism? Cell. 2002;108:5–8. doi: 10.1016/s0092-8674(01)00626-2. [DOI] [PubMed] [Google Scholar]
- Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, Davies K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med. 1998;4:1441–1444. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- Rafael JA, Tinsley JM, Deconinck AE, Davies KE. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nat Genet. 1998;19:79–82. doi: 10.1038/ng0598-79. [DOI] [PubMed] [Google Scholar]
- Cole MA, Rafael JA, Taylor DJ, Lodi R, Davies KE, Styles P. A quantitative study of bioenergetics in skeletal muscle lacking utrophin and dystrophin. Neuromuscul Disord. 2002;12:247–257. doi: 10.1016/s0960-8966(01)00278-4. [DOI] [PubMed] [Google Scholar]
- Jones GL, Sang E, Goddard C, Mortishire-Smith RJ, Sweatman BC, Haselden JN, Davies K, Grace AA, Clarke K, Griffin JL. A functional analysis of mouse models of cardiac disease through metabolic profiling. J Biol Chem. 2005;280:7530–7539. doi: 10.1074/jbc.M410200200. [DOI] [PubMed] [Google Scholar]
- Atherton HJ, Bailey NJ, Zhang W, Taylor J, Major H, Shockcor J, Clarke K, Griffin JL. A combined 1H-NMR spectroscopy- and mass spectrometry-based metabolomic study of the PPAR-alpha null mutant mouse defines profound systemic changes in metabolism linked to the metabolic syndrome. Physiol Genomics. 2006;27:178–186. doi: 10.1152/physiolgenomics.00060.2006. [DOI] [PubMed] [Google Scholar]
- Atherton HJ, Gulston MK, Bailey NJ, Cheng K-K, Zhang W, Clarke K, Griffin JL. Metabolomics of the interaction between PPAR-alpha and age in the PPAR-alpha null mouse. Mol Syst Biol. in press . [DOI] [PMC free article] [PubMed]
- Han X, Yang J, Cheng H, Yang K, Abendschein DR, Gross RW. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry. 2005;44:16684–16694. doi: 10.1021/bi051908a. [DOI] [PubMed] [Google Scholar]
- Sun G, Yang K, Zhao Z, Guan S, Han X, Gross RW. Shotgun metabolomics approach for the analysis of negatively charged water-soluble cellular metabolites from mouse heart tissue. Anal Chem. 2007;79:6629–6640. doi: 10.1021/ac070843+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, Deschepper CF, Petrof BJ, Des Rosiers C. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci USA. 2008;105:7028–7033. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairallah M, Labarthe F, Bouchard B, Danialou G, Petrof BJ, Des Rosiers C. Profiling substrate fluxes of the mouse heart using 13C-substrates. Focusing the origin and fate of pyruvate and citrate carbons. Am J Physiol Heart Circ Physiol. 2005;286:H1461–H1470. doi: 10.1152/ajpheart.00942.2003. [DOI] [PubMed] [Google Scholar]
- Des Rosiers C, Chatham JC. Myocardial phenotyping using isotopomer analysis of metabolic fluxes. Biochem Soc Trans. 2005;33:1413–1417. doi: 10.1042/BST0331413. [DOI] [PubMed] [Google Scholar]
- Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- Momose M, Iguchi N, Imamura K, Usui H, Ueda T, Miyamoto K, Inaba S. Depressed myocardial fatty acid metabolism in patients with muscular dystrophy. Neuromuscul Disord. 2001;11:464–469. doi: 10.1016/s0960-8966(01)00191-2. [DOI] [PubMed] [Google Scholar]
- Lee JS, Pfund Z, Juhász C, Behen ME, Muzik O, Chugani DC, Nigro MA, Chugani HT. Altered regional brain glucose metabolism in Duchenne muscular dystrophy: a pet study. Muscle Nerve. 2002;26:506–512. doi: 10.1002/mus.10238. [DOI] [PubMed] [Google Scholar]
- Khairallah R, Khairallah M, Gelinas R, Bouchard B, Young ME, Allen BG, Lopaschuk GD, Deschepper CF, Des Rosiers C. Cyclic GMP signaling in cardiomyocytes modulates fatty acid trafficking and prevents triglyceride accumulation. J Mol Cell Cardiol. 2008;45:230–239. doi: 10.1016/j.yjmcc.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane AN, Fan TW, Higashi RM. Isotopomer-based metabolomic analysis by NMR and mass spectrometry. Methods Cell Biol. 2008;84:541–588. doi: 10.1016/S0091-679X(07)84018-0. [DOI] [PubMed] [Google Scholar]
- Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros LG, Steinkamp MP, Fleming JC, Lee WN, Cascante M, Neufeld EJ. Defective RNA ribose synthesis in fibroblasts from patients with thiamine-responsive megaloblastic anemia (TRMA). Blood. 2003;102:3556–3561. doi: 10.1182/blood-2003-05-1537. [DOI] [PubMed] [Google Scholar]
- DeBerardenis RJ, Mancuso A, Daikhin E, Nissim H, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kombu RS, Kasumov T, Zhu SH, Cendrowski AV, David F, Anderson VE, Kelleher JK, Brunengraber H. Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle. I. Interrelation between gluconeogenesis and cataplerosis; formation of methoxamates from aminooxyacetate and ketoacids. J Biol Chem. 2008;283:21978–21987. doi: 10.1074/jbc.M803454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kasumov T, Kombu RS, Zhu SH, Cendrowski AV, David F, Anderson VE, Kelleher JK, Brunengraber H. Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle: II. Heterogeneity of metabolite labeling pattern. J Biol Chem. 2008;283:21988–21996. doi: 10.1074/jbc.M803455200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufner D, Previs SF. Measuring in vivo metabolism using heavy water. Curr Opin Clin Nutr Metab Care. 2003;6:511–517. doi: 10.1097/00075197-200309000-00004. [DOI] [PubMed] [Google Scholar]
- Voogt JN, Awada M, Murphy EJ, Hayes GM, Busch R, Hellerstein MK. Measurement of very low rates of cell proliferation by heavy water labeling of DNA and gas chromatography/pyrolysis/isotope ratio-mass spectrometric analysis. Nat Protoc. 2007;2:3058–3062. doi: 10.1038/nprot.2007.421. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Accurate assessment of amino acid mass isotopomer distribution for metabolic flux analysis. Anal Chem. 2007;79:7554–7559. doi: 10.1021/ac0708893. [DOI] [PubMed] [Google Scholar]
- Cassano AG, Wang B, Anderson DR, Previs S, Harris ME, Anderson VE. Inaccuracies in selected ion monitoring determination of isotope ratios obviated by profile acquisition: nucleotide 18O/16O measurements. Anal Biochem. 2007;367:28–39. doi: 10.1016/j.ab.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley IS, Sharp BL. Characterisation and correction of instrument bias in inductively coupled plasma. J Analyt At Spectrom. 1997;12:395–402. [Google Scholar]
- Fernandez CA, Des Rosiers C, Previs SF, David F, Brunengraber H. Correction of 13C mass isotopomer distributions for natural stable isotope abundance. J Mass Spectrom. 1996;31:255–262. doi: 10.1002/(SICI)1096-9888(199603)31:3<255::AID-JMS290>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Wolfe RR. Radioactive and Stable Isotope Tracers in Biomedicine. New York: Wiley-Liss; 1992. [Google Scholar]
- Fernandez CA, Des Rosiers C. Modeling of liver citric acid cycle and gluconeogenesis based on 13C mass isotopomer distribution analysis of intermediates. J Biol Chem. 1995;270:10037–10042. doi: 10.1074/jbc.270.17.10037. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metabol Eng. 2006;8:324–337. doi: 10.1016/j.ymben.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Bequette BJ, Sunny NE, El-Kadi SW, Owens SL. Application of stable isotopes and mass isotopomer distribution analysis to the study of intermediary metabolites of nutrients. J Animal Sci. 2006;84:E50–E59. doi: 10.2527/2006.8413_supple50x. [DOI] [PubMed] [Google Scholar]
- Simizu K. Metabolic flux analysis based on 13C-labeled experiments and integration of the information with gene and protein expression and patterns. Adv Biochem Eng Biotechnol. 2004;91:1–49. doi: 10.1007/b94204. [DOI] [PubMed] [Google Scholar]
- Laaksonen R, Katajamaa M, Päivä H, Sysi-Aho M, Saarinen L, Junni P, Lütjohann D, Smet J, Van Coster R, Seppänen-Laakso T, Lehtimäki T, Soini J, Oresic M. A systems biology strategy reveals biological pathways and plasma biomarker candidates for potentially toxic statin-induced changes in muscle. PLoS ONE. 2006;1:e97. doi: 10.1371/journal.pone.0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Krausz KW, Chen C, Idle JR, Gonzalez FJ. Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor alpha expression and activation. Mol Endocrinol. 2007;21:2136–2151. doi: 10.1210/me.2007-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara CT, Wang P, Neto EC, Stevens RD, Bain JR, Wenner BR, Ilkayeva OR, Keller MP, Blasiole DA, Kendziorski C, Yandell BS, Newgard CB, Attie AD. Genetic networks of liver metabolism revealed by integration of metabolic and transcriptional profiling. PLoS Genet. 2004;4:e1000034. doi: 10.1371/journal.pgen.1000034. [DOI] [PMC free article] [PubMed] [Google Scholar]