Abstract
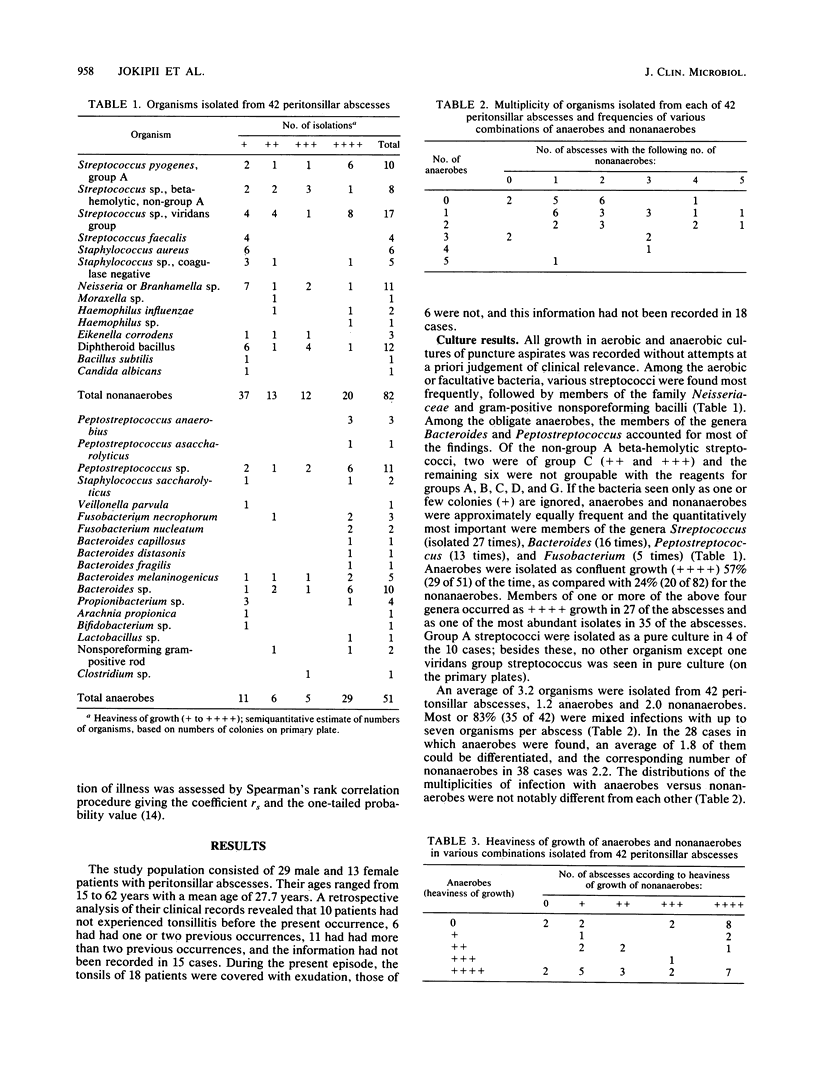
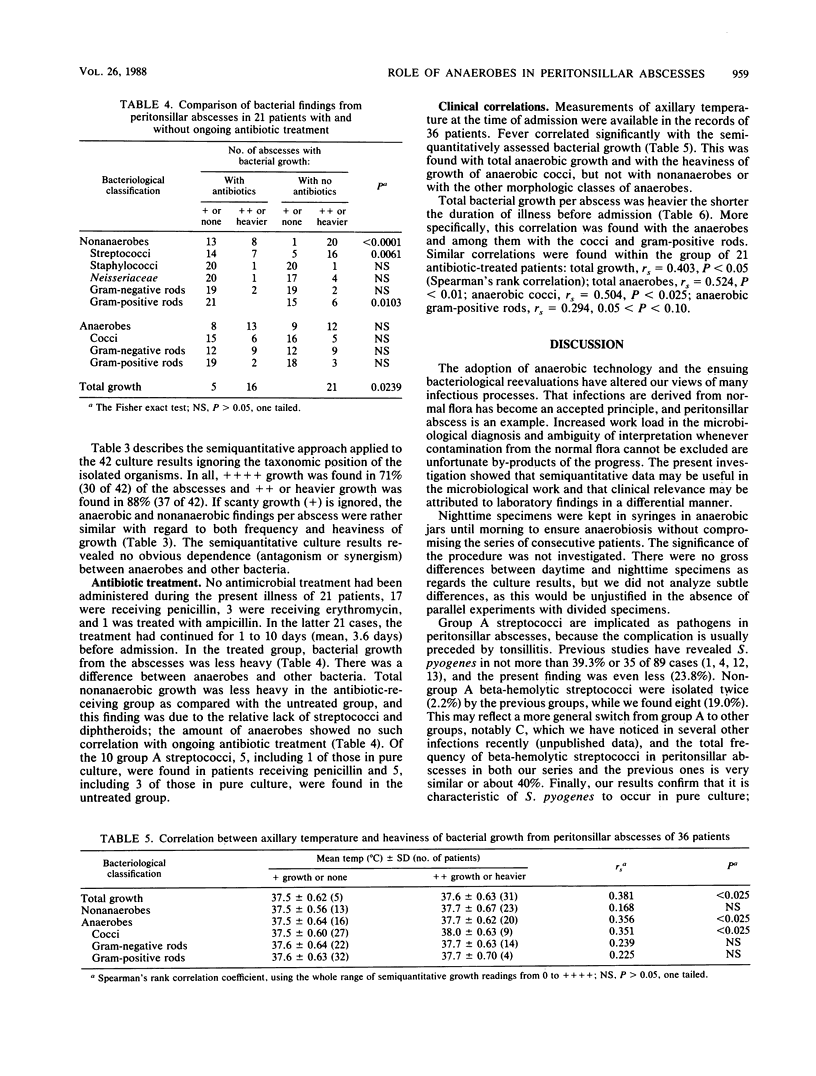
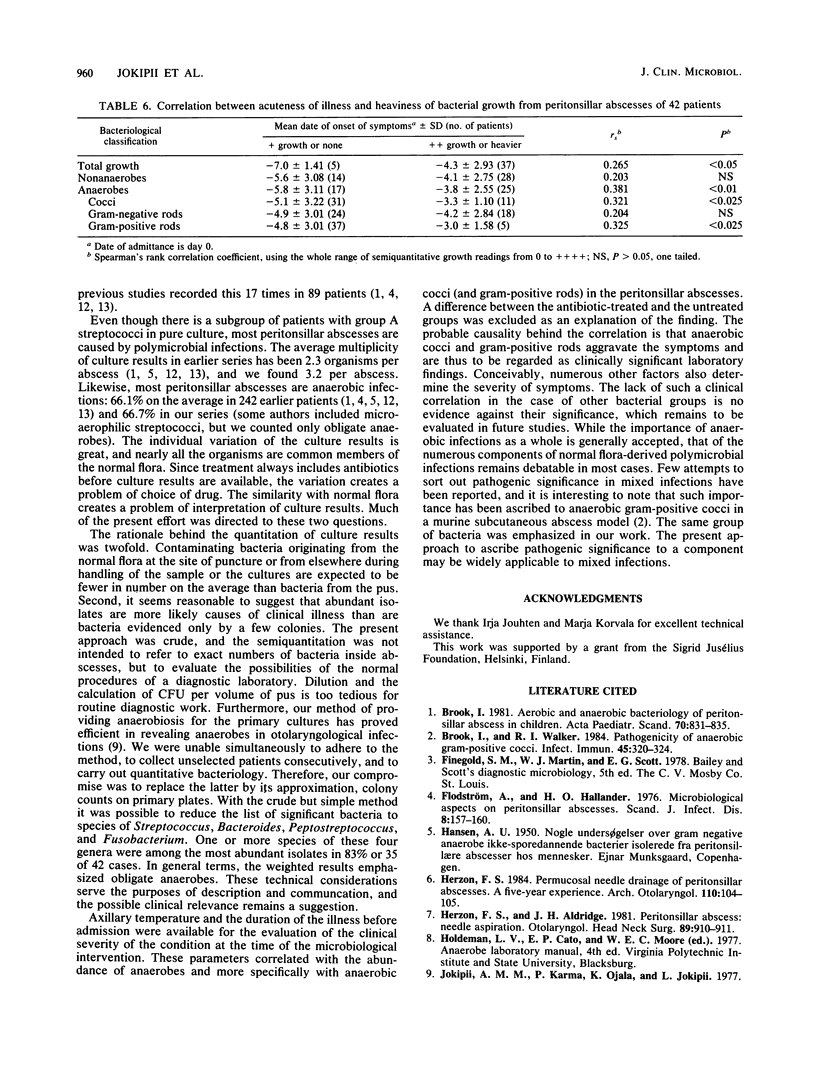
We studied the bacteria in consecutive peritonsillar abscesses using semiquantitation of the primary culture findings and correlated the results to clinical parameters. Puncture-aspirated pus from 42 abscesses yielded 133 isolates. Group A streptococci were isolated 10 times and, unlike other bacteria, were isolated 4 times in pure culture; other beta-hemolytic streptococci were found in 8 abscesses, and anaerobes were found in 28. The infections were polymicrobial, with two to seven bacteria in 83%. Anaerobes were more abundant than nonanaerobes; members of the genera Streptococcus, Bacteroides, Peptostreptococcus, and Fusobacterium were the most important quantitatively, considering both frequency and abundance. In patients with ongoing antibiotic treatment, nonanaerobes (but not anaerobes) were less abundant than in untreated patients. The abundance of obligate anaerobes (specifically cocci and gram-positive rods) correlated to the severity of illness as defined by fever and short duration before hospitalization. With other groups of bacteria, no such correlation was found. The correlation was not explained by a difference between the antibiotic-treated and the untreated patients. The results indicate the value of the semiquantitation of culture data and the frequency and pathogenic significance of obligate anaerobes in peritonsillar abscesses.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brook I. Aerobic and anaerobic bacteriology of peritonsillar abscess in children. Acta Paediatr Scand. 1981 Nov;70(6):831–835. doi: 10.1111/j.1651-2227.1981.tb06235.x. [DOI] [PubMed] [Google Scholar]
- Brook I., Walker R. I. Pathogenicity of anaerobic gram-positive cocci. Infect Immun. 1984 Aug;45(2):320–324. doi: 10.1128/iai.45.2.320-324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodström A., Hallander H. O. Microbiological aspects on peritonsillar abscesses. Scand J Infect Dis. 1976;8(3):157–160. doi: 10.3109/inf.1976.8.issue-3.06. [DOI] [PubMed] [Google Scholar]
- Herzon F. S., Aldridge J. H. Peritonsillar abscess: needle aspiration. Otolaryngol Head Neck Surg. 1981 Nov-Dec;89(6):910–911. doi: 10.1177/019459988108900606. [DOI] [PubMed] [Google Scholar]
- Herzon F. S. Permucosal needle drainage of peritonsillar abscesses. A five-year experience. Arch Otolaryngol. 1984 Feb;110(2):104–105. doi: 10.1001/archotol.1984.00800280038011. [DOI] [PubMed] [Google Scholar]
- Schechter G. L., Sly D. E., Roper A. L., Jackson R. T. Changing face of treatment of peritonsillar abscess. Laryngoscope. 1982 Jun;92(6 Pt 1):657–659. doi: 10.1002/lary.1982.92.6.657. [DOI] [PubMed] [Google Scholar]
- Sprinkle P. M., Veltri R. W., Kantor L. M. Abscesses of the head and neck. Laryngoscope. 1974 Jul;84(7):1142–1148. doi: 10.1288/00005537-197407000-00008. [DOI] [PubMed] [Google Scholar]
- Sugita R., Kawamura S., Icikawa G., Fujimaki Y., Oguri T., Deguchi K. Microorganisms isolated from peritonsillar abscess and indicated chemotherapy. Arch Otolaryngol. 1982 Oct;108(10):655–658. doi: 10.1001/archotol.1982.00790580049016. [DOI] [PubMed] [Google Scholar]


