Abstract
Thymic mesenchymal cells are known to be important for the development of the early fetal thymus into a functionally mature organ supporting T cell differentiation. We examined the expression of mesenchymal markers: pan-mesenchymal marker ER-TR7, desmin, α-smooth muscle actin (α-SMA), and α- and β-chain of platelet-derived growth factor receptor (PDGFRα, PDGFRβ) in thymi of normal adult mice. Desmin and ER-TR7 revealed specific staining in the capsule, septa, and perivascular cells. Most perivascular cells highly expressed PDGFRβ at the same levels as desmin. Low expression of PDGFRα was detected in the capsule, intralobular septa, and some perivascular cells of normal adult thymi. α-SMA, used to identify vascular smooth muscle cells, was detectable on arterioles and some large venules but not on capillaries. Thus, desmin, PDGFRα, and PDGFRβ were localized in the capsule, septa, and perivascular cells in thymus of adult mouse, although there were differences in the expression level among these markers. On the other hand, the expression of mesenchymal markers was detectable in the region of the thymic medullary epithelium of lymphotoxin β receptor–deficient mice and plt/plt mice, indicating that mesenchymal cells were abnormally localized in the region. These results suggest that disorganization of the medullary epithelium may be accompanied by aberrant distribution of mesenchyme in adult mouse thymus. (J Histochem Cytochem 57:373–382, 2009)
Keywords: thymus, mesenchymal cells, lymphotoxin, β receptor–deficient mice, Plt/plt mice, adult
T cell differentiation in thymus is dependent upon signals from thymic stromal cells. Most studies have focused on the signals provided by thymic epithelial cells, and interest in other stromal cells such as mesenchyme has lagged behind (Gray et al. 2005). However, considerable evidence for the importance of mesenchymal cells in thymic organogenesis has accumulated (Bockman and Kirby 1984). Mesenchymal cells can be seen surrounding thymic epithelial anlage by embryonic day 12 (E 12), and thereafter, they migrate into the epithelium, there to establish intrathymic networks of fibroblasts (Suniara et al. 2000). Mesenchymal cells are found to contribute to the development of epithelial cells of the early fetal thymus (Shinohara and Honjo 1996; Itoi et al. 2007). Fibroblast growth factor 7 (FGF7), FGF10, and keratinocyte growth factor produced by thymic mesenchymal cells induce proliferation of FGFR2IIIb-expressssing epithelial cells (Jenkinson et al. 2003; Alpdogan et al. 2006). Mesenchymal cells surrounding the epithelium in early fetal thymus express α-chain of platelet-derived growth factor receptor (PDGFRα) (Morrison-Graham et al. 1992; Takakura et al. 1997), and these PDGFRα-positive cells have recently been shown to play an important role in providing proliferative signals to the thymic epithelium (Jenkinson et al. 2007).
Blood vessels are composed of endothelial cells and perivascular cells. Perivascular cells include pericytes and vascular smooth muscle cells (VSMCs) (Hirschi and D'Amore 1996). Small blood vessels are composed of endothelial cells surrounded by a basal lamina and are loosely covered by a single layer of pericytes. Large veins are irregularly covered by pericytes and/or VSMCs, whereas arteries have strong, elastic vessel walls with dense layers of concentrically formed VSMCs (Cleaver and Melton 2003). VSMCs are commonly identified by α-smooth muscle actin (α-SMA). Perivascular cells are known to express mesenchymal markers, such as desmin, PDGFRα, and PDGFRβ, but the expression of these markers is highly varied among vessel types in tissues and organs (Franklin et al. 1990; Nehls and Drenckhahn 1993; Kitami et al. 1995).
Mesenchymal cells of fetal thymus are shown to originate primarily in neural crest–derived cells (Bockman and Kirby 1984; Jiang et al. 2000). A contribution of neural crest to perivascular mesenchyme was noted earlier for the developing chick thymus (Le Douarin and Jotereau 1975). Furthermore, neural crest provides pericytes and smooth muscle cells to all blood vessels of the head and neck region (Noden et al. 1995; Etchevers et al. 2001). Recent studies have demonstrated that neural crest–derived cells enter the thymus, and these cells differentiate into perivascular cells, which contribute to a postulated thymus–blood barrier (Foster et al. 2008; Müller et al. 2008). In the thymus of adult mouse, mesenchymal cells, including perivascular cells, fibroblasts, and the cells residing in capsule and septa, are detected with an antibody, ER-TR7 (van Vliet et al. 1986). However, the expression of other mesenchymal markers in adult thymus has not been studied in detail.
The lymphotoxin (LT) pathway is critical for the development and maintenance of peripheral lymphoid organs (Ware 2005). Mice with deficiencies in members of this pathway lack lymph nodes and Peyer's patches and have abnormal spleen architecture. These animals also develop autoantibodies and lymphocytic infiltrates of multiple organs, provoking speculation that the LT pathway may play a role in central tolerance induction (Chin et al. 2006). LT β receptor (LTβR) deficiency leads to abnormally differentiated thymic medullary epithelium (Boehm et al. 2003). In addition, the thymi of mice deficient in chemokine (C-C motif) receptor 7 (CCR7) or in its ligands CCL19 and CCL21-ser (plt/plt mice; Vassileva et al. 1999; Luther et al. 2000) also display altered architecture (Misslitz et al. 2004).
In this study, we examined thymic sections of normal adult mice by immunofluorescence analysis with antibodies against several mesenchymal markers to clarify the localization of mesenchyme in the thymi. Moreover, the analysis for thymi of LTβR-deficient (LTβR−/−) mice and plt/plt mice revealed that mesenechymal cells were abnormally distributed in the medullary region.
Materials and Methods
Mice Thymi
C57BL/6 and BALB/c mice were obtained from Japan SLC (Hamamatsu, Japan). Mice were sacrificed, and thymi were dissected, embedded in OCT compound, and frozen on dry ice.
Thymi of LTβR−/− mice on the B6 background (Chin et al. 2006) and plt/plt mice were gifts from Dr. Y-X. Fu (University of Chicago, Chicago, IL) and Dr. J.G. Cyster (University of California, San Francisco, CA). Mice were used at the age of 6 or 7 weeks. Animal care and experiments were reviewed and approved by the animal care committees of our institutes.
Antibodies and Reagents
The following antibodies and reagents were used: rat ER-TR7 (BMA Biomedicals; Augst, Switzerland), mouse anti-desmin (clone D33; DakoCytomatin, Glostrup, Denmark), rat anti-PDGFRα (CD140α) (clone APA5; eBioscience, San Jose, CA), rat anti-PDGFRβ (CD140β) (clone APB5; eBioscience), mouse anti-α-SMA (clone 1A4; Lab Vision, Fremont, CA), rat anti-CD31/platelet endothelial cell adhesion molecule 1 (clone MEC13. 3; eBioscience), goat anti-ephrinB2 (R and D Systems, Minneapolis, MN), rabbit anti-keratin 5 (Covance; Berkeley, CA), and rabbit anti-collagen type IV (LSL CO., LTD.; Tokyo, Japan). Alexa Fluor–labeled donkey secondary antibodies (Molecular Probes; Eugene, OR) were also used. The binding to biotinylated Ulex europaeus agglutinin-1 (UEA-1; Vector Laboratories, Burlingame, CA) was followed by allophycocyanin-conjugated streptavidin (eBioscience).
Immunofluorescence Staining and Confocal Microscopy
Cryosections were prepared, fixed in ice-cold acetone for 15 min, and air-dried. After washing with PBS, sections were blocked with 20% Block Ace (Dainippon Pharmaceutical Co.; Osaka, Japan) in PBS for 1 hr. For mouse monoclonal antibodies, the Mouse on Mouse (M.O.M.) Kit (Vector Laboratories) was used, following the manufacturer's instructions. Sections were then incubated with primary antibodies at 4C overnight, followed by washing for 5 min three times with PBS. Secondary antibodies were then applied and incubated for 1 hr. Sections were washed and mounted with fluorescent mounting medium (Vector Laboratories) using coverslips. Confocal laser-scanning microscopy analysis was performed on a Zeiss LSM 510 (Carl Zeiss; Oberkochen, Germany). Negative controls were performed by replacement of first-step antibodies with isotype-matched monoclonal antibodies or species-matched antibodies. Representative images were chosen from each experiment for figure editing.
Results
Expression of Desmin
Frozen thymus sections from normal 6-week-old BALB/c mice were stained with ER-TR7 and anti-desmin antibody, and the distribution of mesenchymal cells was analyzed by confocal microscopy. As expected from a previous study (van Vliet et al. 1986), ER-TR7 particularly marked the cells in the capsule, septa, and around blood vessels (Figure 1A). The distribution of desmin was similar to that of ER-TR7–positive reticular fibroblasts. Desmin-immunoreactive cells were situated near vasculatures including capillaries of the cortical region and postcapillary venules (PCVs) of the cortico–medullary junction (Figure 1A). Comparison with the pan-endothelial marker CD31 revealed that desmin-positive cells were localized in the wall of CD31-positive blood vessels including capillaries (Figure 1B), and desmin was restricted to perivascular cells in almost all blood vessels. When an antibody to type IV collagen was used to detect basement membranes, desmin-positive perivascular cells were seen to be associated with them (Figure 1B).
Figure 1.
Expression of desmin in the thymus of adult mouse. Immunofluorescence staining of thymic sections of adult BALB/c mice was performed to detect ER-TR7 (A) (green), CD31 (B) (green), desmin (A,B) (red), or type IV collagen (B) (blue). (A) Double staining for ER-TR7 fibroblast marker and desmin shows their colocalization along capsule and septa and around vasculature. (B) Desmin is detectable around CD31-positive blood vessels, and desmin-positive perivascular cells are associated with basement membranes. Bar = 100 μm.
Expression of α-SMA
Thymus sections of BALB/c mice were stained for VSMCs with an anti-α-SMA antibody, and the resulting α-SMA immunoreactivity was found to be restricted to some vasculature-like structures (Figure 2A). Arterial and venous endothelial cells are phenotypically distinct, and ephrinB2 is selectively expressed on arteries (Gale et al. 2001; Shin et al. 2001). To clearly discern the distribution of α-SMA in thymic vasculatures, three-color immunofluorescence staining for α-SMA, CD31, and ephrinB2 was performed (Figure 2B). α-SMA-positive VSMCs were predominately localized on ephrinB2-positive arterioles. VSMCs on arterioles were uniformly shaped, closely packed, and tightly associated with the endothelium. VSMCs were also detectable on some large ephrinB2-negative PCVs, and PCVs partially covered with VSMCs were also detectable (Figure 2B).
Figure 2.
Expression of α-smooth muscle actin (α-SMA) in the thymus of adult mouse. Immunofluorescence staining of thymic sections of adult BALB/c mice was performed to detect α-SMA (A,B) (green), CD31 (A,B) (red), or ephrinB2 (B) (blue). α-SMA reactivity is detectable on eprinB2-positive arterioles and some large ephrinB2-negative venules. α-SMA–positive vascular smooth muscle cells (VSMCs) on arterioles are uniformly shaped, closely packed, and tightly associated with the endothelium. Bar = 100 μm.
Expression of PDGFRα and PDGFRβ
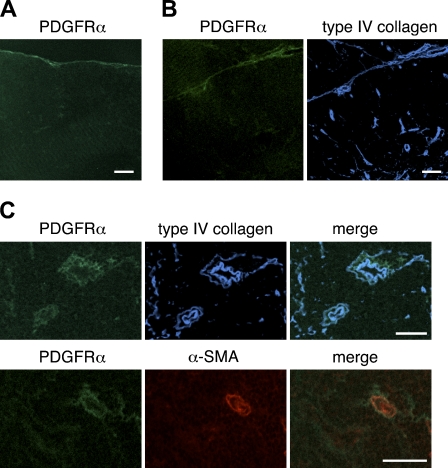
We next assessed the expression of PDGFRα and PDGFRβ in the thymi of BALB/c mice. The expression of PDGFRα was weak; nevertheless, PDGFRα-positive cells were occasionally located in the capsule (Figure 3A) and within intralobular septa (Figure 3B). Some perivascular cells expressed PDGFRα (Figure 3C). Comparison with type IV collagen reactivity indicated that PDGFRα-positive cells were frequently in close association with basement membranes around blood vessels (Figure 3C). The expression of PDGFRβ around thymic vasculatures was intense (Figure 4). Many PDGFRβ-positive cells were observed around almost all blood vessels, including capillaries in the cortical region, and desmin-positive perivascular cells highly expressed PDGFRβ (Figure 4C). PDGFRβ-positive cells were also detected in the capsule and septa (Figures 4A and 4B).
Figure 3.
Expression of the α-chain of platelet-derived growth factor receptor (PDGFRα) in the thymus of adult mouse. Immunofluorescence staining of thymic sections of adult BALB/c mice was performed to detect PDGFRα (A,B,C) (green), α-SMA (B) (red), or type IV collagen (B,C) (blue). PDGFRα reactivity is weak but detectable in the capsule (A), along intralobular septa (B), and on perivascular cells including α-SMA–positive VSMCs (C). Bar = 100 μm.
Figure 4.
Expression of PDGFRβ in the thymus of adult mouse. Immunofluorescence staining of thymic sections of adult BALB/c thymi was performed to detect PDGFRβ (A,B,C) (green), desmin (C) (red), or type IV collagen (B,C) (blue). PDGFRβ-positive cells are detectable in the capsule (A) and intralobular septa (B). PDGFRβ-positive cells are seen around almost all blood vessels, and most desmin-positive perivascular cells highly express PDGFRβ (C). Bar = 100 μm.
Localization of Medullar Epithelium and Mesenchyme in the Thymi of LTβR-deficient Mice and plt/plt Mice
We stained thymus sections from C57BL/6 wild-type, LTβR−/−, and plt/plt mice with reagents recognizing thymic medullary epithelium and analyzed them by confocal microscopy. The distribution of keratin 5 allows distinction of medullary epithelial cells, and the mature populations of thymic medullary epithelial cells bind the lectin UEA-1. In wild-type mice, UEA-1–positive epithelial cells formed a network of stellate cells that builds the thymic medulla (Figure 5). As expected, the medullary regions of LTβR−/− thymi were smaller and more sparsely populated by keratin 5–positive medullary epithelial cells than were those of wild-type thymi (Figure 5). UEA-1–positive medullary epithelial cells were detected on LTβR−/− thymic sections at a lower frequency than on wild-type sections, and were localized in small clusters, consistent with previous observations showing that the deficiency of LTβR signaling affects UEA-1–positive medullary epithelial cells (Boehm et al. 2003). Plt/plt thymi tended to have small foci of medullar epithelium, but were not disrupted to the extent of LTβR−/− thymi, and the clusters of medullary epithelial cells expressing keratin 5 and binding UEA-1 were detectable.
Figure 5.
Organization of thymic medullary epithelium in adult C57BL/6 wild-type, lymphotoxin β receptor–negative (LTβR−/−), and plt/plt mice. Immunofluorescence staining of thymic sections of adult C57BL/6 wild-type, LTβR−/−, and plt/plt mice was performed to detect keratin 5 (green) and the binding to Ulex europaeus agglutinin-1 (UEA-1) (red). Aberrant medulla formation and marked reduction in UEA-1 binding to medullary epithelial cells are seen in LTβR−/− mice. Plt/plt thymi tend to have small medullary islets, but the clusters of medullary epithelial cells expressing keratin 5 and binding to UEA-1 are present. Bar = 100 μm.
We next examined the localization of medullary epithelium and mesenchyme in wild- type, LTβR−/−, and plt/plt thymi by double staining with anti–keratin 5 and ER-TR7. Surprisingly, aberrant foci in labeling with ER-TR7 were evident in the medullary regions of both LTβR−/− and plt/plt thymi (Figure 6). Many ER-TR7–positive cells were situated in epithelial cell–free regions of the medulla as well as perivascular cells, capsule, and septa, and they were found to be frequently adjacent to the epithelial cells, but coexpression of ER-TR7 and keratin 5 was rarely detectable in LTβR−/− and plt/plt thymi. The numbers of ER-TR7–expressing cells in the medulla of LTβR−/− thymi appeared to be higher than those of plt/plt thymi. In addition, we examined the expression of desmin or PDGFRβ in wild-type, LTβR−/−, and plt/plt thymi. In wild-type mice, desmin expression was restricted in perivascular pericytes, capsule, and septa, like BALB/c thymi (data not shown). On the other hand, desmin was also detected in epithelial cell–free regions of the medulla in LTβR−/− mice (Figure 7A). Expression of PDGFRβ was found in the same area (Figure 7B). The labeling patterns of desmin and PDGFRβ were indistinguishable from those of ER-TR7 in plt/plt sections (data not shown). PDGFRα was expressed at low levels in the regions of both LTβR−/− and plt/plt thymi, whereas α-SMA was undetectable in the regions (data not shown). These mesenchymal marker–expressing cells in epithelial cell–free regions of the medulla were not associated with blood vessels, as assessed by CD31 expression (data not shown). Altogether, we conclude that mesenchymal cells are abnormally localized in epithelial cell–free regions of thymic medulla in LTβR−/− and plt/plt mice.
Figure 6.
Localization of ER-TR7 and medullary epithelium in the thymi of adult C57BL/6 wild-type, LTβR−/−, and plt/plt mice. (A,B) Immunofluorescence staining of thymic sections of adult C57BL/6 wild-type, LTβR−/−, and plt/plt mice was performed to detect ER-TR7 (green) and keratin 5 (red). Aberrant foci in labeling with ER-TR7 are evident in the thymic medullary region of both LTβR−/− and plt/plt mice. (B) High magnification. Many ER-TR7–positive cells are situated in epithelial cell–free regions of the thymic medulla and are found to be frequently adjacent to the epithelial cells, but coexpression of ER-TR7 and keratin 5 is rarely detectable in LTβR−/− thymi. Bar = 100 μm.
Figure 7.
Localization of desmin, PDGFRβ, and medullary epithelium in the thymi of adult LTβR−/− mice. Immunofluorescence staining of thymic sections of adult LTβR−/− mice was performed to detect desmin (A) (green), or PDGFRβ (B) (green), and keratin 5 (A,B) (red). Many desmin- or PDGFRβ-expressing cells are situated in epithelial cell–free regions of thymic medulla. Bar = 100 μm
Discussion
The expression of pan-mesenchymal marker ER-TR7, desmin, α-SMA, PDGFRα, and PDGFRβ was observed in the thymus of normal adult mouse by immunofluorescence staining, indicating colocalization of ER-TR7 and desmin along the capsule, intra- and interlobular septa, and around vasculature. Desmin-positive cells were present on almost all segments of thymic CD31-positive blood vessels. The distribution of pericytes or VSMCs in blood vessels varies among the tissues (Hirschi and D'Amore 1996; Bergers and Song 2005). Thymic arterioles identified by the expression of ephrinB2 were completely covered by α-SMA–positive VSMCs. Some large venules were almost covered by VSMCs, whereas some were only partially covered. α-SMA was undetectable in capillaries of adult thymus. This observation is consistent with previous studies, which demonstrated that pericytes on normal capillaries typically express desmin but not α-SMA, whereas normal venules express both molecules (Nehls and Drenckhahn 1993).
In embryos, PDGFRα is expressed on the mesenchymal layer of fetal thymus (Morrison-Graham et al. 1992; Takakura et al. 1997). These mesenchymal cells also express PDGFRβ. In the thymus of adult mouse, the distribution of PDGFRβ was similar to that of desmin, and PDGFRβ was highly expressed on perivascular cells, i.e., pericytes and α-SMA–positive VSMCs (data not shown). Recruitment and coverage of vessels with pericytes is essential for the development of vasculature, as has been shown by genetic ablation of PDGFRβ signaling (Soriano 1994; Hellstrom et al. 1999). Therefore, the presence of PDGFRβ in thymic perivascular cells also might contribute to the vascular formation and morphogenesis. Because ER-TR7 is not a cell surface marker, PDGFRα has been used as a marker for thymic mesenchymal cells instead (Jenkinson et al. 2003,2007). Although a small population of PDGFRα-positive mesenchymal cells is present in the neonatal and adult thymus (Jenkinson et al. 2007), in adult thymus, the distribution of PDGFRα was different from that of the pan-mesenchymal marker ER-TR7. The expression of PDGFRα in the thymus was weak, and PDGFRα-positive cells were occasionally located in the capsule and within the intralobular septa. Some perivascular cells expressed PDGFRα. Our observation that PDGFRα expression was low in thymic perivascular cells of adult mouse is consistent with other studies showing that neural crest–derived cells within the parenchyma of the thymus downregulate the expression of PDGFRα, whereas they maintain PDGFRβ expression (Foster et al. 2008; Müller et al. 2008). A similar downregulation of PDGFRα has also been observed during pericyte differentiation in the brain (Bondjers et al. 2006). Migration of neural crests into the thymus during embryogenesis and their differentiation into perivascular cells has been demonstrated (Foster et al. 2008; Müller et al. 2008). However, the process through which thymic mesenchymal cells differentiate into perivascular cells during thymus organogenesis remains to be clarified.
In addition, we examined the expression of mesenchymal markers in both LTβR−/− and plt/plt thymi that display the disorganization of medullary epithelium, and observed abnormal distribution of mesenchyme in these thymi. Aberrant foci of mesenchyme were found in epithelial cell–free regions of the thymic medulla. This finding is more prominent in LTβR−/− mice than in plt/plt mice. These results suggest that abnormal medullary architecture may accompany aberrant distribution of mesenchyme in adult thymus. Further studies are necessary in other mice displaying altered disruption of thymus morphology. Epithelial-mesenchymal transition (EMT) is well known as the process whereby cells undergo a switch from epithelial cells into mesenchymal phenotypes (Hay 2005). Upregulation of Snail or Slug, transcription factors for EMT, and α-SMA were undetectable in the medullary mesenchyme of LTβR−/− and plt/plt thymi by immunohistology analysis (data not shown), and so far, we do not have evidence of EMT in the thymi of these mice.
In summary, our present study indicates similar localization among pan-mesenchymal marker ER-TR7, desmin, and PDGFRβ in thymus of normal adult mouse. Expression of PDGFRα in the mesenchyme was weak, compared with the other mesenchymal markers. Almost all perivascular cells were detected with ER-TR7, desmin, and PDGFRβ, but PDGFRα was expressed in only some of perivascular cells. In several organs, such as the brain, liver, and kidney, pericytes have been shown to perform specific functions (Bergers and Song 2005), and it is therefore of interest to examine the possibility that thymic perivascular cells have specialized functions. In addition, we show abnormal distribution of thymic mesenchyme in LTβR−/− and plt/plt mice. Further studies are needed to determine the mechanism by which thymic mesenchymal cells take the place of medullary epithelial cells in LTβR−/− and plt/plt mice.
Acknowledgments
We are grateful to Dr. Y-X. Fu, Dr. M. Zhu, and Dr. J. G. Cyster for providing LTβR−/− and plt/plt mice thymi.
References
- Alpdogan O, Hubbard VM, Smith OM, Patel N, Lu S, Goldberg GL, Gray DH, et al. (2006) Keratinocyte growth factor (KGF) is required for postnatal thymic regeneration. Blood 107:2453–2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S (2005) The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 7:452–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman DE, Kirby ML (1984) Dependence of thymus development on derivatives of the neural crest. Science 223:498–500 [DOI] [PubMed] [Google Scholar]
- Boehm T, Scheu S, Pfeffer K, Bleul CC (2003) Thymic medullary epithelial cell differentiation, thymocyte emigration, and the control of autoimmunity require lympho-epithelial cross talk via LTßR. J Exp Med 198:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellström M, Lindahl P, et al. (2006) Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J 20:1703–1705 [DOI] [PubMed] [Google Scholar]
- Chin RK, Zhu M, Christiansen PA, Liu W, Ware C, Peltonen L, Zhang X, et al. (2006) Lymphotoxin pathway-directed, autoimmune regulator-independent central tolerance to arthritogenic collagen. J Immunol 177:290–297 [DOI] [PubMed] [Google Scholar]
- Cleaver O, Melton DA (2003) Endothelial signaling during development. Nat Med 9:661–668 [DOI] [PubMed] [Google Scholar]
- Etchevers HC, Vincent C, Le Douarin NM, Couly GF (2001) The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128:1059–1068 [DOI] [PubMed] [Google Scholar]
- Foster K, Sheridan J, Veiga-Fernandes H, Roderick K, Pachnis V, Adams R, Blackburn C, et al. (2008) Contribution of neural crest-derived cells in the embryonic and adult thymus. J Immunol 180:3183–3189 [DOI] [PubMed] [Google Scholar]
- Franklin WA, Christison WH, Colley M, Montag AG, Stephens JK, Hart CE (1990) In situ distribution of the beta-subunit of platelet-derived growth factor receptor in nonneoplastic tissue and in soft tissue tumors. Cancer Res 50:6344–6348 [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, McDonald DM, et al. (2001) Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev Biol 230:151–160 [DOI] [PubMed] [Google Scholar]
- Gray DH, Ueno T, Chidgey AP, Malin M, Goldberg GL, Takahama Y, Boyd RL (2005) Controlling the thymic microenvironment. Curr Opin Immunol 17:137–143 [DOI] [PubMed] [Google Scholar]
- Hay ED (2005) The mesenchymal cell, its role in the embryo, and the remarkable signaling mechanisms that create it. Dev Dyn 233:706–720 [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C (1999) Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126:3047–3055 [DOI] [PubMed] [Google Scholar]
- Hirschi KK, D'Amore PA (1996) Pericytes in the microvasculature. Cardiovasc Res 32:687–698 [PubMed] [Google Scholar]
- Itoi M, Tsukamoto N, Yoshida H, Amagai T (2007) Mesenchymal cells are required for functional development of thymic epithelial cells. Int Immunol 19:953–964 [DOI] [PubMed] [Google Scholar]
- Jenkinson WE, Jenkinson EJ, Anderson G (2003) Differential requirement for mesenchyme in the proliferation and maturation of thymic epithelial progenitors. J Exp Med 198:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson WE, Rossi SW, Parnell SM, Jenkinson EJ, Anderson G (2007) PDGFRalpha-expressing mesenchyme regulates thymus growth and the availability of intrathymic niches. Blood 109:954–960 [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM (2000) Fate of the mammalian cardiac neural crest. Development 127:1607–1609 [DOI] [PubMed] [Google Scholar]
- Kitami Y, Inui H, Uno S, Inagami T (1995) Molecular structure and transcriptional regulation of the gene for platelet-derived growth factor alpha receptor in cultured vascular smooth muscle cells. J Clin Invest 96:558–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Jotereau FV (1975) Tracing of cells of the avian thymus through embryonic life in interspecific chimeras. J Exp Med 142:17–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Tang HL, Hyman PL, Farr AG, Cyster JG (2000) Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci USA 97:12694–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misslitz A, Pabst O, Hintzen G, Ohl L, Kremmer E, Petrie HT, Förster R (2004) Thymic T cell development and progenitor localization depend on CCR7. J Exp Med 200:481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison-Graham K, Schatteman GC, Bork T, Bowen-Pope DF, Weston JA (1992) A PDGF receptor mutation in the mouse (Patch) perturbs the development of a non-neuronal subset of neural crest-derived cells. Development 115:133–142 [DOI] [PubMed] [Google Scholar]
- Müller SM, Stolt CC, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, et al. (2008) Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol 180:5344–5351 [DOI] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D (1993) The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry 99:1–12 [DOI] [PubMed] [Google Scholar]
- Noden DM, Poelmann RE, Gittenberger-de Groot AC (1995) Cell origins and tissue boundaries during outflow tract development. Trends Cardiovasc Med 5:69–75 [DOI] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, et al. (2001) Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol 230:139–150 [DOI] [PubMed] [Google Scholar]
- Shinohara T, Honjo T (1996) Epidermal growth factor can replace thymic mesenchyme in induction of embryonic thymus morphogenesis in vitro. Eur J Immunol 26:747–752 [DOI] [PubMed] [Google Scholar]
- Soriano P (1994) Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev 8:1888–1896 [DOI] [PubMed] [Google Scholar]
- Suniara RK, Jenkinson EJ, Owen JJ (2000) An essential role for thymic mesenchyme in early T cell development. J Exp Med 191:1051–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura N, Yoshida H, Ogura Y, Kataoka H, Nishikawa S, Nishikawa S (1997) PDGFRalpha expression during mouse embryogenesis: immunolocalization analyzed by whole-mount immunohistostaining using the monoclonal anti-mouse PDGFRalpha antibody APA5. J Histochem Cytochem 45:883–893 [DOI] [PubMed] [Google Scholar]
- van Vliet E, Melis M, Foidart JM, van Ewijk W (1986) Reticular fibro-blasts in peripheral lymphoid organs identified by a monoclonal antibody. J Histochem Cytochem 34:883–890 [DOI] [PubMed] [Google Scholar]
- Vassileva G, Soto H, Zlotnik A, Nakano H, Kakiuchi T, Hedrick JA, Lira SA (1999) The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J Exp Med 190:1183–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CF (2005) Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol 23:787–819 [DOI] [PubMed] [Google Scholar]