Abstract
Immunoglobulin (Ig) molecules have thus far been found only to be produced by differentiated B lymphocytes. As we know, immune privilege in the normal adult mouse testis exists to make these privileged sites generally free of patrolling immune cells and Igs. However, in this study, the expression of Ig in normal adult mouse testis and epididymis was detected. First, by IHC, a strong pattern of Ig expression was detected in the spermatogenic cells of adult mouse testis at different development stages. Second, by Western blot analysis, different strong bands of Igs in mouse testicular spermatogenic cells and epididymal epithelial cell extract were recognized using specific antibodies against IgG. More importantly, by ISH and cell sorting–related RT-PCR, rearranged Ig γ chain and κ chain transcripts were expressed in testicular spermatogenic cells and epididymal epithelial cells. These results suggested that Ig in testis and epididymis was mainly produced by adult mouse testicular spermatogenic cells and epididymal epithelial cells. This manuscript contains online supplemental material at http://www.jhc.org. Please visit this article online to view these materials. (J Histochem Cytochem 57:339–349, 2009)
Keywords: immunoglobulin, V-(D)-J rearrangement, mouse, testis, epididymis
As we know, immunoglobulin (Ig) molecules have thus far been found only to be produced by differentiated B lymphocytes but not by other types of cells, such as spermatogenic cells and epididymal epithelial cells. Furthermore, it is well known that the testis is an immunologically privileged site in the body and that human seminal plasma possesses a generalized immunosuppressive activity. Multiple factors participate in the establishment of immunotolerance in the testis and epididymis: the blood–testis barrier (BTB) formed by Sertoli cells and the blood–epididymal barrier formed by epididymal epithelial cells; the local production of immunosuppressive molecules of Sertoli cells; and the Fas system as a regulator of immunological homeostasis in both physiological and pathological conditions (Lenzi 2001; Fijak and Meinhardt 2006). Therefore, the presence of Ig in the adult testis is generally considered abnormal and pathological. However, some studies have indicated the testis rather than the epididymis might easily become an unprivileged organ as to autoimmunity under some special conditions (Itoh et al. 2005). Furthermore, a few studies reported that IgG and IgA were present in sperm (Allen and Bourne 1978; Hjort 1996), although it had been suggested that there is an Fc receptor in sperm, which was a 16/20-kDa antibody-binding protein, suggested to be related to FcγRIII on the basis of monoclonal antibody reactivity (Kamada et al. 1991). However, Chiu and other researchers did not find FcγRIII immunoreactivity in sperm (Bronson et al. 1992; Chiu and Chamley 2002), suggesting that sperm could express Igs. Additionally, in normal adult rat testicular fluid, IgG concentrations (2.67 ± 0.98 mg/ml) were measured to be 65% of the levels measured in serum by two-site enzyme-linked immunosorbent assay; the authors postulated that the permeability of the rat testis to circulating IgG was relatively high compared with other tissues, and the testicular transendothelial transfer of IgG was unaffected by changes in testicular activity or hormone secretion (Hedger and Hettiarachchi 1994; Hedger and Meinhardt 2003). More studies of gonads and genital tracts in human embryos and fetuses showed that components of the secretory immune system (SIS) were observed in genital organs in 4- to 5-week-old embryos and were present during the whole intrauterine period, suggesting the presence of two forms of immune protection of fetal genital organs in response to antigen attack (Gurevich et al. 2003).
Currently, it has been confirmed that the rearranged Ig gene could be expressed in some epithelial tumor cells and some normal cells (Qiu et al. 2003; Babbage et al. 2006; Liu et al. 2007; Huang et al. 2008). In addition, we previously detected many Ig-positive spermatogenic cells in unstressed, injury-free, normal adult mouse testis and epididymis. As a result, we decided to evaluate whether the spermatogenic cells and epididymal epithelial cells themselves could synthesize Igs. In this study, using IHC and Western blot analysis, we further found Igs present in adult mouse testicular spermatogenic cells and epididymal epithelial cells. More importantly, by ISH and cell sorting–related RT-PCR, we found transcripts of Ig also exist in the adult mouse testicular spermatogenic cells and epididymal epithelial cells. These results strongly suggest that normal adult mouse testicular spermatogenic cells and epididymal epithelial cells could express Ig.
Materials and Methods
Animal and Tissue Preparation
Adult male BALB/c mice 6 weeks of age were obtained from Peking University Health Science Center. Under anesthesia with chloral hydrate (400 mg/kg, IP), the mice were perfused transcardially with 15 ml sterile saline solution containing 100 U/ml heparin. Less than 10 min elapsed between the time that the mice were killed and when the testis and epididymis were dissected free of fat and connective tissue. After careful removal of the blood vessels from testis and epididymis tissues, the fresh tissues were used to extract the protein and perform Western blot analysis and to prepare for the paraffin-embedded sections to perform IHC and ISH. Conditions of animal housing and all experimental procedures were conducted under institutional guidelines provided by the Institutional Animal Care and Use Committee of China.
IHC
Adult mouse testis, epididymis, and spleen were excised, sliced, fixed with 10% formalin, embedded in paraffin, and cut into 4-μm serial sections. Sections were deparaffinized in xylene and ethanol, and IHC examination was performed as described previously. Antigen retrieval was performed in 0.01 M citrate buffer (pH 6.0) twice in a microwave oven for 5 min each. The sections were incubated with 3% H2O2 at room temperature for 10 min, rinsed twice, and blocked in PBS plus 10% normal goat serum for 10 min. After excess blocking buffer was removed, affinity-purified goat anti-mouse Ig Fab fragment, IgG Fc fragment, or Ig κ light chain polyclonal antibody (Bethyl Laboratories; Montgomery, AL) and biotinylated goat anti-mouse IgG or IgM (KPL; Gaithersburg, MD) (1:200 in PBS) were used at 37C for 1 hr. After a thorough rinse, sections were incubated with horseradish peroxidase (HRP)-conjugated anti-goat IgG (KPL) or HRP-conjugated streptavidin (Vector Laboratories; Burlington, Canada) (1:200 in PBS) at 37C for 40 min. After rinsing in PBS, all sections were visualized with 0.05% DAB (Vector Laboratories; Burlingame, CA). As a negative control, sections without primary antibodies were also used.
Preparation of Testicular Single Cell Suspensions
Testicular cell suspensions were performed as described previously (Bastos et al. 2005). Cells were isolated from 6-week-old male mice using a two-step enzymatic digestion to remove interstitial cells. The albuginea was removed, and seminiferous tubules were dissociated by using enzymatic digestion with collagenase type I (Invitrogen; La Jolla, CA) at 100 U/ml for 25 min at 32C in HBSS supplemented with 20 mM HEPES (pH 7.2), 1.2 mM MgSO47H2O, 1.3 mM CaCl2 · 2 H2O, 6.6 mM sodium pyruvate, and 0.05% lactate. A filtration step with a 40-μm nylon mesh was achieved to separate, on the one hand, a fraction enriched with interstitial cells, and on the other hand, tubules retained in the filter. Tubules were collected and incubated at 32C for 25 min in the same collagenase buffer as that used for the first step. The resulting whole cell suspension was filtered through a 40-μm nylon mesh to remove cell clumps. After a wash in HBSS, the cell pellet was resuspended, and the testicular cell suspensions were prepared.
Isolation of Epididymal Epithelial Cells
Epididymal epithelial cells were isolated from the fresh epididymides of 6-week-old mice. Epididymides were dissected free of fat and connective tissue in HBSS. Whole epididymis and epididymal segments were dissected out, minced into small fragments (∼2–3 mm3), and transferred to 0.25% trypsin in HBSS (pH 7.2; 5 mg of tissue/ml of solution). After incubation at 32C for 30 min, the supernatant was discarded, and the pellet was suspended in collagenase (1 mg/ml) in salt solution (pH 7.2). After incubation at 32C for 30 min, the sample was allowed to settle for 5 min, the supernatant was discarded, and the sediment, consisting mainly of epididymal epithelial cell aggregates, was extracted. Finally, the epididymal epithelial cells were washed twice with ice-cold HBSS. Cell concentrations were estimated with trypan blue staining (90% viable cells).
Protein Extraction and Western Blot Analysis
The testicular single cell suspensions and epididymal epithelial cell suspensions were prepared as described. In addition, single spleen cells were prepared after removing erythrocytes by hypotonic lysis as a positive control. Moreover, the cell pellet was lysed in RIPA lysis buffer (150 mM NaCl, 25 mM HEPES, 2 mM NaF, 0.2% SDS, and 1% NP-40, with freshly added proteinase inhibitor cocktail) for 30 min on ice. Cell lysates were clarified by centrifugation at 4C at 16,000 × g for 15 min. Protein was separated by 12.5% SDS-PAGE and transferred onto nitrocellulose membranes (Amersham Pharmacia; Little Chalfont, UK). Membranes were blocked in Tris-buffered saline containing 0.1% (v/v) Tween-20 (TBS-T) and 5% (w/v) non-fat milk for 2 hr and were incubated overnight at 4C with the appropriate primary antibody. After being rinsed with TBS-T buffer, membranes were incubated for 1 hr in the dark with the appropriate Alexa Fluor 780-labeled secondary antibodies (LI-COR Bioscience; Lincoln, NE) prepared in TBST/5% non-fat milk. The signal was detected using the Odyssey Imaging System (LI-COR Bioscience).
ISH
Oligonucleotides were synthesized (40-nmol scale) using an ABI 392 DNA Synthesizer (Applied Biosystems; Foster City, CA) and were analyzed by acrylamide gel electrophoresis and purified, including oligo 1, GAGTCAGAGTAATGGTGAGCACATCCTTG (located in Ig γ chain constant region cDNA fragment exon1); oligo 2, GTATTTCCTGCCTCCCAGTTGCTCTTC (located in Ig γ chain constant region cDNA fragment exon2); and control oligo, CGAAGGCAGTCAGGCACCGTGTATGAACTAC, which was confirmed to have no matches with the complete mouse genome by a BLAST search in National Center for Biotechnology Information (www.ncbi.nlm.nih.gov).
The sections of mouse testis, epididymis, and spleen tissues embedded in paraffin were cut at 4 μm and mounted on poly-lysine (200 μg/ml)-coated glass slides. Deparaffinized and rehydrated sections were incubated in 0.2 M HCl for 10 min at room temperature, treated with proteinase K (10 μg/ml in 10 mM Tris, pH 8.0, and 1 mM EDTA) at 37C for 20 min, and postfixed with 4% paraformaldehyde at room temperature for 10 min. The sections were dehydrated in increasing ethanol series and air dried. Prehybridization was conducted at 42C for 1 hr. The sections were hybridized with the oligonucleotide probes at 42C for 16 hr in a humidified incubator. Unbound probes were removed by sequential washes of 2× saline-sodium citrate (SSC) containing 50% formamide at 37C for 30 min, twice in 2× SSC at 37C for 15 min, and 0.1× SSC at 37C for 15 min. DNA-RNA hybrids were immunodetected with a 1:400 dilution of anti-digoxigenin alkaline phosphatase conjugate (Boehringer Mannheim; Mannheim, Germany) at 37C for 1 hr, followed by incubation with nitroblue tetrazolium salt and 5-bromo-4-chloro-3-indolyl phosphate (Promega; Madison, WI). After mounting in a crystal mount medium, the sections were photographed on a light photomicroscope (Vanox-S; Olympus, Tokyo, Japan).
Cell Sorting
Testicular cells suspensions were performed as described previously. The isolated testicular cells were resuspended in the same incubation buffer supplemented with 1% FCS. Cell concentrations were estimated with trypan blue staining. Two million cells were diluted in 2 ml of incubation buffer and stained with bis-benzamide Hoechst 33342 (5 μg/ml; Sigma, St Louis, MO) for 1 hr at 32C. Before analysis, propidium iodide (PI, 2 μg/ml; Sigma) was added to exclude dead cells. Analysis and cell sorting were performed on a dual-laser FACStar Plus flow cytometer (Becton Dickinson; Le Pont de Claix, France) equipped with a 360-nm ultraviolet argon laser. Ten thousand primary spermatocytes were assigned to subpopulation 3 in adult mice because of their 4N DNA content.
RT-PCR
Total RNA was isolated from primary spermatocytes sorted by flow cytometry and epididymal epithelial cells using RNeasy stabilization and the total RNA isolation system according to the standard RNeasy Mini Kit (Qiagen; Hilden, Germany) protocol.
RT was carried out with the Sensiscript RT kit (Qiagen), following the standard protocol. For amplifying spermatocyte marker Pgk-2, RQ1 RNase-free DNase (Promega) was used to treat the RNA samples to eliminate any contaminating genomic DNA. RT was carried out, and 1 μl of each RT reaction was used for PCR with LA Taq Polymerase (TaKaRa Bio; Otsu, Japan). Nested PCR was performed for amplifying the epithelial cell marker cytokeratin-18 (CK18). Half-nested PCR was performed for amplifying the constant region of the Ig γ1 chain, the variable region of the Ig κ chain, and the B-cell marker CD20. PCR for Pgk-2 was also performed. GAPDH was amplified as an internal standard. All the sense and antisense primers except Pgk-2 primers used above were located in different exons so we could discriminate between cDNA and genomic DNA easily. The sequences of the primers above are listed in Table 1.
Table 1.
Sequences of primers used for PCR experiments
| Gene name | Primer sequence 5′-3′ | Product size (bp) | |
|---|---|---|---|
| Ig κvariable region | The same sense primer | GACATTCAGCTGACCCAGTCTCCA | 311 |
| External antisense primer | GTCCTGATCAGTCCAACTGTTCAG | ||
| Internal antisense primer | AGNTTGGTTCCACCRCCGAACG (N = C/T, R = T/C) | ||
| Ig γ1 constant region | External sense primer | CCTGGTCAAGGGCTATTTCC | 381 |
| Internal sense primer | TCTACACTCTGAGCAGCTCAG | ||
| The same sense primer | GGTGCATGATGGGAAGTTCAC | ||
| CD20 | The same sense primer | TTCAAACTTCCAAGCCGTATG | 219 |
| External antisense primer | GAGTTTAAGGAGCGATCTC | ||
| Internal antisense primer | GACAGCAGAACCACATTAGAT | ||
| Pgk-2 | Sense primer | AGGAGATACTGCTACTTGCTGCGCC | 300 |
| Antisense primer | GATGATGACAGAATTAAGACTTGCT | ||
| CK18 | External sense primer | TGCCGCCGATGACTTTAG | 454 |
| External antisense primer | CCTCTGCCCGAGTTTGTG | ||
| Internal sense primer | TCCGCAAGGTGGTAGATGAC | ||
| Internal antisense primer | CTCCATCTGTGCTTGTATCG | ||
| GAPDH | Sense primer | CCTTCATTGACCTCAACTAC | 591 |
| Antisense primer | GGA AGGCCATGCCAGTGAGC |
All PCR products were cloned into pGEM-T Easy Vector (Promega) and subjected to sequencing using an ABI 3100 Genetic Analyzer (Applied Biosystems); finally, these sequences were analyzed.
Results
Presence of Ig Molecules in Adult Mouse Testis and Epididymis on the Protein Level
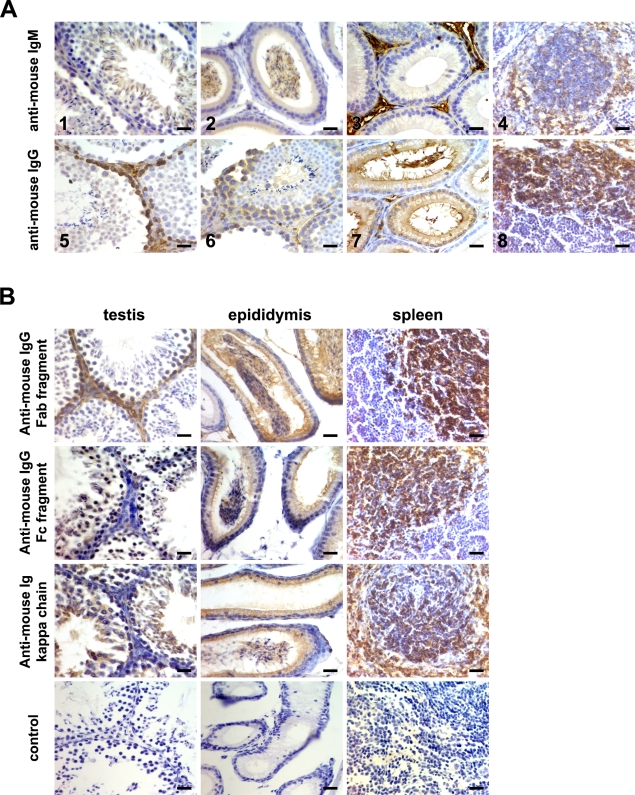
First by IHC, the sections of perfused adult mouse testis and epididymis tissues were detected with anti-mouse IgG and IgM polyclonal antibodies. IgM immunoreactivity was found in spermatids and spermatozoons of testis tissue (Figure 1A1); while IgG immunoreactivity was found in spermatogoniums and spermatocytes of testis tissue (Figures 1A5 and 1A6). Moreover, in epididymis tissue, IgM immunoreactivity existed in spermatozoons of the epididymis cavity and interstitial cells of the epididymis (Figures 1A2 and 1A3), whereas IgG immunoreactivity existed in epithelial cells of the epididymis (Figure 1A7). In addition, IgG and IgM immunoreactivity was present in plasma cells or plasmablasts in spleen tissues (Figures 1A4 and 1A8), suggesting that the polyclonal antibodies used in this experiment were specific for IgG and IgM, and no immunoreactivity was observed when the primary antibody was not used (data not shown). Moreover, it was unexpected that IgM and IgG immunoreactivity was also observed in spermatogenic cells of SCID mouse testis, whereas no IgM and IgG immunoreactivity was observed in SCID mouse spleen tissue sections (Supplemental Figure 1).
Figure 1.
Immunoglobulin (Ig) expression in adult mouse testis and epididymis was detected by IHC using goat anti-mouse IgG, IgM, and Ig different fragments antibodies. (A) The results of IgG and IgM expression. IgM immunoreactivity in spermatids and spermatozoons of testis tissue (1), spermatozoons of the epididymis cavity (2), and interstitial cells of the epididymis (3); IgG positive immunoactivity in spermatogoniums (5) and spermatocytes (6) of testis tissue and epithelial cells of the epididymis (7). Spleen sections were used as a positive control (4,8). (B) The results of Ig expression using Ig different fragment antibodies. Goat anti-mouse Ig Fab fragment, IgG Fc fragment, and Ig κ chain polyclonal antibodies were used. The sections without primary antibody were used as a negative control. Bar = 50 μm.
To further detect Ig immunoreactivity in adult mouse testis and epididymis tissue, we also selected other specific antibodies for Ig, including the anti-mouse Ig Fab fragment, the IgG Fc fragment, and κ light chain polyclonal antibodies. The perfused adult mouse testis and epididymis tissue sections were detected using these antibodies. As shown in Figure 1B, the Ig Fab fragment was detected in spermatogoniums of the testis and the IgG Fc fragment and Ig κ chain were detected in spermatocytes and spermatozoons of the testis, suggesting that there perhaps existed a different exposed epitope of Ig protein in testicular spermatogenic cells at different stages. In addition, both Ig fragments and κ chain immunoreactivity was observed in spermatozoons of the epididymis cavity and epithelial cells of the epididymis. Moreover, the different Ig fragments and κ chain immunoreactivity was present in plasma cells or plasmablasts in spleen tissues, whereas no immunoreactivity was observed when the primary antibody was not used, suggesting that the polyclonal antibodies were specific for Ig (Figure 1B).
Next we extracted the protein from the isolated testicular cells and epididymal epithelial cells, analyzed IgG in testis and epididymis tissues by Western blot analysis, and compared the results with those of isolated spleen cells. The extract treated with loading buffer without β-mercaptoethanol (β-ME) was not boiled; the observed bands were similar to IgG, at 150 kDa. In contrast, the extract treated with loading buffer with β-ME was boiled; IgG heavy chain (50 kDa) and light chain (25 kDa) were shown in mouse testis, epididymis, and spleen tissues, respectively (Figure 2).
Figure 2.
Ig expression in adult mouse testis and the epididymis by Western blot analysis. (Left) Protein treated with loading buffer without β-mercaptoethanol (β-ME). (Right) Protein treated with loading buffer with β-ME. IgG was detected in the testis, epididymis, and spleen tissues using anti-IgG whole molecular polyclonal antibody. β-actin was used as an internal control.
Presence of Ig Molecules in Adult Mouse Testis and Epididymis on the mRNA Level
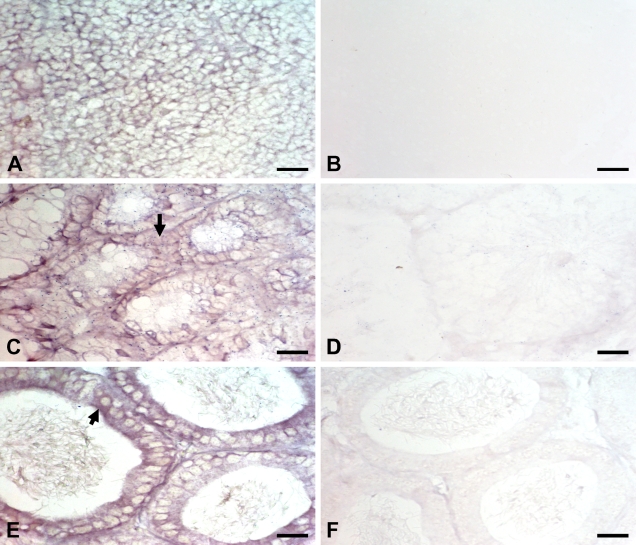
To further identify the possibility that the Ig molecule was synthesized by spermatogenic cells, two oligonucleotides of the IgG constant region were synthesized as probes by ISH. Ig gene transcripts were shown in spermatogenic cells of adult mouse testis (Figure 3C), epithelial cells of the epididymis (Figure 3E), and lymphocytes of the spleen germinal center (Figure 3A). No specific signal was shown in the absence of probes (data not shown) or when the sections were treated with the control probes (Figures 3B, 3D, and 3F).
Figure 3.
Ig mRNA expression in adult mouse testis and the epididymis was detected by ISH, showing results with digoxigenin-labeled mouse IgG-specific oligonucleotide probes. The result of oligonucleotide probes in spleen cells (A), spermatogenic cells of the testis (C), and epithelial cells of the epididymis (E); the result of nonsense oligonucleotide probes in spleen cells (B), spermatogenic cells of the testis (D), and epithelial cells of the epididymis (F). Arrows indicate positive staining on testicular spermatogenic cells (C) and epididymal epithelial cells (E). Bar = 50 μm.
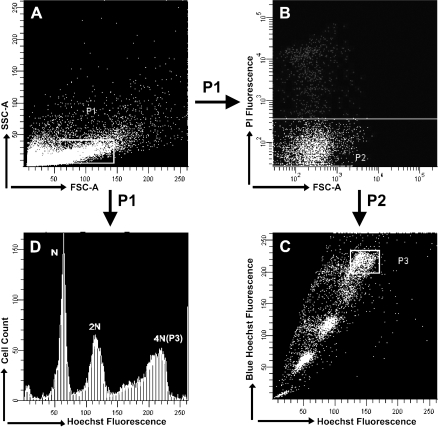
Further examination was used to detect Ig expression in adult mouse spermatogenic cells and epididymis epithelial cells, which was necessary to exclude the possibility of B-cell existence in testis tissue. The adult mouse testis tissues were made into testicular single cell suspensions and stained with Hoechst 33342 and PI. Hoechst 33342 is generally associated with PI fluorochrome to discriminate viable cells from dead cells. By flow cytometry and cell sorting, we had viable populations (negative for PI fluorescence, 90%) resolved according to PI fluorescences (Figures 4A and 4B). Primary spermatocytes were assigned to subpopulation 3 according to their 4N DNA content (Figures 4C and 4D), and nearly 10,000 cells were sorted. Additionally epididymal epithelial cells were isolated from fresh adult mouse epididymis tissues.
Figure 4.
Flow cytometric analysis of Hoechst 33342 and propidium iodide (PI) fluorescence. (A) Adult murine testicular cells. P1 indicated the sorted cells. (B) PI analysis of testicular cells to exclude dead cells. P2 indicated PI-negative cells. (C) Hoechst analysis of testicular cells according to DNA content. P3 indicated primary spermatocyte. (D) Hoechst fluorescence of cells in the mix shown in A.
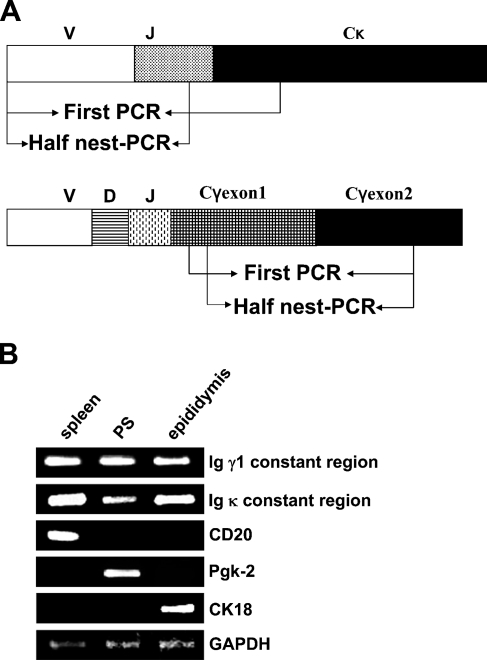
Total RNA was extracted from the sorted primary spermatocytes and isolated epididymal epithelial cells and reverse transcripted into cDNA. Using the cDNA as a template, we detected the expression of CD20 (B-cell marker) to prove that there was no contamination of B cells or plasma cells in the primary spermatocytes and epididymis epithelial cells that we had; Pgk-2 was used as a specific marker for spermatocytes, and CK18 was used as a specific marker for epididymis epithelial cells. Most importantly, we used specific primers to amplify the constant region of the Ig γ1 germline transcript and the variable region of the Ig κ chain, respectively. As shown in Figures 5A and 5B, we confirmed the transcripts of the Ig γ1 constant region and the Ig κ chain variable region present in primary spermatocytes and epididymal epithelial cells of adult mice. The identification of the PCR product was confirmed by DNA sequencing.
Figure 5.
The results of RT-PCR. (A) Representative diagrams (not to scale) of the Ig γ1 and Ig κ genes analyzed in this study. Arrows indicate positions of the primers used for amplification of the segments. (B) The transcripts of Igγ1, Igκ, CD20, Pgk-2, CK18, and GAPDH in sorted primary spermatocytes and isolated epididymal epithelial cells were analyzed by RT-PCR. PS, primary spermatocyte. Spleen was used as a positive control. GAPDH expression was amplified as an internal control.
Sequences Analysis of the Ig κ Chain Variable Region in Sorted Primary Spermatocytes and Isolated Epididymis Epithelial Cells of Adult Mice
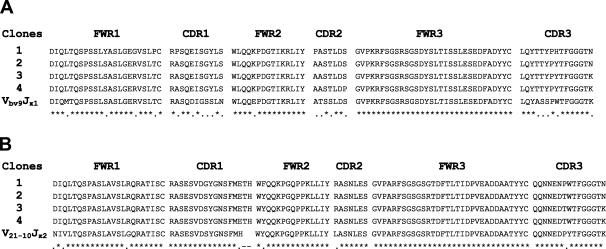
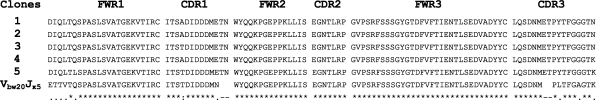
To further detect the characteristics of Igs derived from primary spermatocytes and epididymal epithelial cells of two adult BALB/c mice, we analyzed the sequences of the Ig κ chain variable region derived from different primary spermatocytes and epididymal epithelial cells. First, these sequences showed that Ig gene rearrangement (a hallmark of functional immunoglobulin genes) existed in primary spermatocytes and epididymal epithelial cells of adult mice, and these sequences did not contain a nonsense mutation. A noteworthy finding in this study was that the non–B lymphocyte–derived Ig gene repertoire exhibited distinct characteristics. Of the eight VκJκ sequences derived from primary spermatocytes, there only existed two sets of dominant VκJκ rearrangement patterns, including Vbv9Jκ1 and V21-10Jκ2. By comparison with Vbv9Jκ1 and V21-10Jκ2 germline sequences, these sequences had >5% mutation. Unexpectedly, within each set of VκJκ rearrangements, the V region sequences from different primary spermatocytes not only showed identical VκJκ rearrangement and junctions but also showed identical mutation targets over the Ig κ chain variable region (Figure 6). Most interestingly, of the five VκJκ recombinants derived from epididymal epithelial cells, there only existed one set of dominant VκJκ rearrangement patterns, which was Vbw20Jκ5. The recombinants not only showed identical VκJκ use but also showed the identical mutation targets. Despite some mutation points, there remained a high rate of homology among them (Figure 7). To exclude that the primers in this study were partial to Vbv9Jκ1, Vbw20Jκ5, or V21-10Jκ1 sequences, we used the two BALB/c mouse spleen cDNA libraries (the positive control) as the template and totally sequenced 15 VκJκ recombinants. The distinct diversity was showed in spleen cDNA (data not shown).
Figure 6.
Analysis of Ig κ chain variable region sequences in primary spermatocytes of adult mice. The PCR products from primary spermatocytes of adult mouse testis were cloned into pGEM-T Easy Vector and were sequenced. (A) Comparison with the Ig κVbv9Jκ1 germline genes. (B) Comparison with the Ig κV21-10Jκ2 germline genes.
Figure 7.
Analysis of Ig κ chain variable region sequences in epididymal epithelial cells of adult mice. The PCR products from epididymal epithelial cells of adult mice were cloned into pGEM-T Easy Vector, and five clones were sequenced. The sequences were compared with the Ig κVbw20Jκ5 germline genes.
Discussion
According to the present theory of immunology, Ig was originally thought to be rearranged and expressed uniquely in B lymphocytes and plasma cells. The accumulation of Igs in germ cells of embryos and fetuses could not only be considered a local immune response or as part of a cellular self-protection mechanism against foreign antigenic effects for these strategically important structures of the developing organism, but also emphasized that the whole immune system of fetuses and embryos had not matured and was not fully functional (Gurevich et al. 2001).
However, in this study, we used several methods to verify the expression of Ig in normal adult mouse testis and epididymis. Initial IHC studies showed IgG and IgM immunoreactivity in testicular spermatogenic cells and epididymal epithelial cells, which was observed in the cytoplasm, plasma membrane, and glandular lumen-like structures. In addition, different Ig fragments including the Fab fragment and Fc fragment and Ig κ light chain immunoreactivity were also observed in spermatogenic cells of testis and epithelial cells of the epididymis of normal adult mice, whereas no B lymphocytes or plasma cells were present in these mouse intact genital tracts. Whereas these data indicated that there was somewhat different immunoactivity among IgG and IgM, and that IgG immunoactivity existed in primary spermatocytes while IgM immunoactivity existed in secondary spermatocytes, in general the classical Ig class-switch concept showed the IgM-producing cells were the precursors of the IgG producers, so perhaps in spermatogenic cells there exist non-classical class switching. This suggests there is another class switch mechanism, which may be in operation. Souto-Carneiro et al. (2004) recently described that naive B lymphocytes could develop into IgG-secreting cells through successive cell divisions. Additionally, there is also somewhat different staining for different IgG fragments, because the different specific anti-mouse Ig fragment polyclonal antibodies recognized different exposed epitopes, suggesting that the confirmation of Ig in testicular spermatogenic cells at different stages differed.
It is worth mentioning that IgM and IgG were also expressed in SCID mouse testis and epididymis. In other words, IgM and IgG expression in spermatogenic cells and epididymal epithelial cells was unrelated to the immune status of mice or the IgG concentration in mouse serum. In particular, in SCID mice, aberrant immunoglobulin gene rearrangements resulted in a lack of mature B lymphocytes, and in our experiment, no IgM and IgG immunoactivity was observed in SCID mouse spleen tissue, serum, or milk. Therefore, SCID mice were used as the negative control for IgM and IgG; however, to our surprise, IgM and IgG immunoreactivity was also observed by IHC in some epithelia of SCID mouse mammary glands, spermatogenic cells, and epididymal epithelial cells perfused with heparinized saline, and the results showed a similar pattern and intensity of IgM and IgG immunoreactivity in the adult Balb/c mice.
More importantly, to definitively confirm expression of Ig molecules in spermatogenic cells, expression of rearranged Ig gene transcripts should be identified in these cells, because extracellular IgG could be transferred into epithelial cells through FcRn (neonatal Fc receptor) or pIgRs (polymeric immunoglobulin receptor) expressed in the reproductive ducts including the ductuli efferentes (where most IgG was reabsorbed) and the distal caput epididymidis (Gurevich et al. 2003; Hickey et al. 2004; Knee et al. 2005). By ISH and RT-PCR, we also found Ig γ1 and κ chain transcripts existed in testicular spermatogenic cells and epididymal epithelial cells. Furthermore, sequence analysis showed the sequences derived from different primary spermatocyte and epididymal epithelial cells exhibited distinct characteristics. Of the VκJκ sequences, there existed only several sets of dominant VκJκ rearrangement patterns, which showed identical VκJκ rearrangement and junctions but also showed identical mutation targets over the Ig κ chain variable region in each VκJκ pattern. Moreover, as an unexpected finding, some of these sequences were actually identical with the sequences we found previously in adult mouse brain and mammary glands (unpublished data), suggesting that these non–B-lymphocyte–derived Ig variable region sequences might be limited in non–B lymphocytes.
Furthermore, it is known that activation-induced cytidine deaminase (AID) is expressed in spermatocytes in the human testis but only infrequently in testicular germ cell tumors, because AID is essential for somatic hypermutation (SHM) and class switch recombination (CSR) of Ig genes in antigen-dependent B-cell maturation. However, we considered that SHM was not restricted to Ig gene loci and raised the possibility of a function for AID in other cell types, suggesting AID expression in spermatocytes points to a role in meiosis or continued expression of AID was not involved in the pathogenesis of germ cell tumors (Schreck et al. 2006). Moreover, Sugimura et al. (2004) reported that Ig gene heavy chain was found in embryonal carcinomas of non-seminomatous germ cell tumors of the testis, suggesting that the possibility existed that Ig gene might play important roles in immunological response to the tumor in embryonal carcinoma. In addition, we previously found that human cancers of epithelial origin, including carcinomas of the breast, colon, liver, and lung, also produced IgGs in both cytoplasmic and secreted forms. Furthermore, blockade of tumor-derived IgG by either anti-sense DNA or anti-human IgG antibody increased the incidence of programmed cell death and inhibited growth of cancer cells in vitro. More importantly, administration of anti-human IgG antibody also suppressed the growth of an IgG-secreting carcinoma line in immunodeficient nude mice. Our results supported a role of tumor-derived IgG as a growth factor for epithelial cancers (Qiu et al. 2003). From the above results, it might be that the mechanism of Ig gene rearrangement in spermatogenic cells is not the same as that in B cells. In fact, we found that Ig gene rearrangement characteristics and its regulation mechanism in the promoter region of epithelial cancer cells were not the same as those in B cells (unpublished data).
In the immune system, cytokines are regulatory proteins involved in hematopoiesis, immune cell development, inflammation, and immune responses, whereas in the reproductive system, there are a variety of cytokines, including interleukin (IL)-1β, IL-6, interferon (IFN)-γ, IL-2, transforming growth factor (TGF)-β2,macrophage inflammatory protein-1β (MIP-1β), and IL-8, in the regressing ovary, oviduct, and testis (Sundaresan et al. 2007). Several cytokines have direct effects on testicular cell functions, and a number of these are produced within the testis even in the absence of inflammation or immune activation events, suggesting cytokines in fact play an important regulatory role in the development and normal function of the testis (Hedger and Meinhardt 2003). In addition, the transcript of recombination activating gene-1 (RAG-1), which is essential for the V(D)J recombination of the Ig gene and considered to be expressed only in T or B lymphocytes, has already been discovered in zebrafish (Danio rerio) oocytes, and germline rearranged Ig genes could also be recovered from non-lymphoid tissues of zebrafish (D. rerio) oocytes to initiate recombination cleavage at the Ig gene loci. Whereas this attempted rearrangement was largely unproductive, yielding no accumulation of germline-joined Ig genes in zebrafish, the authors speculated that RAG might have been derived from a transposase, invading germ cells of ancient species, and later became a dedicated recombinase only expressed in developing lymphocytes (Zhong et al. 2007). Perhaps, in adult mouse testis, RAG initiated Ig germline rearrangement and led to accumulation of Ig genes, affecting the spermatogenesis process.
In summary, non-immunocytes including testicular spermatogenic cells and epididymal epithelial cells also expressed Ig, the sequences of which showed distinct characteristics. Although Ig specificity for spermatogenic cells needs further study, it is likely that the Ig molecule derived from testicular spermatogenic cells and epididymal epithelial cells might play an important role in the spermatogenesis process and immune responses to antigen attack.
Acknowledgments
This work was supported by the National Natural Science Foundation, China (30371609).
We thank Prof. Dalong Ma (Peking University Center for Human Disease Genomics) for comments and discussion.
References
- Allen GJ, Bourne FJ (1978) Interaction of immunoglobulin fragments with the mammalian sperm acrosome. J Exp Zool 203:271–276 [DOI] [PubMed] [Google Scholar]
- Babbage G, Ottensmeier CH, Blaydes J, Stevenson FK, Sahota SS (2006) Immunoglobulin heavy chain locus events and expression of activation-induced cytidine deaminase in epithelial breast cancer cell lines. Cancer Res 66:3996–4000 [DOI] [PubMed] [Google Scholar]
- Bastos H, Lassalle B, Chicheportiche A, Riou L, Testart J, Allemand I, Fouchet P (2005) Flow cytometric characterization of viable meiotic and postmeiotic cells by Hoechst 33342 in mouse spermatogenesis. Cytometry A 65:40–49 [DOI] [PubMed] [Google Scholar]
- Bronson RA, Fusi FM, Fleit HB (1992) Monoclonal antibodies identify Fc gamma receptors on unfertilized human oocytes but not spermatozoa. J Reprod Immunol 21:293–307 [DOI] [PubMed] [Google Scholar]
- Chiu WW, Chamley LW (2002) Use of antisperm antibodies in differential display Western blotting to identify sperm proteins important in fertility. Hum Reprod 17:984–989 [DOI] [PubMed] [Google Scholar]
- Fijak M, Meinhardt A (2006) The testis in immune privilege. Immunol Rev 213:66–81 [DOI] [PubMed] [Google Scholar]
- Gurevich A, Ben-Hur H, Moldavsky M, Szvalb S, Berman V, Zusman I (2001) Immunoprotection of gonads and genital tracts in human embryos and fetuses: immunohistochemical study. Am J Reprod Immunol 46:381–385 [DOI] [PubMed] [Google Scholar]
- Gurevich P, Zusman I, Moldavsky M, Szvalb S, Elhayany A, Halperin R, Gurevich E, et al. (2003) Secretory immune system in human intrauterine development: immunopathomorphological analysis of the role of secretory component (pIgR/SC) in immunoglobulin transport. Int J Mol Med 12:289–297 [PubMed] [Google Scholar]
- Hedger MP, Hettiarachchi S (1994) Measurement of immunoglobulin G levels in adult rat testicular interstitial fluid and serum. J Androl 15:583–590 [PubMed] [Google Scholar]
- Hedger MP, Meinhardt A (2003) Cytokines and the immune-testicular axis. J Reprod Immunol 58:1–26 [DOI] [PubMed] [Google Scholar]
- Hickey DK, Jones RC, Bao S, Blake AE, Skelding KA, Berry LJ, Beagley KW (2004) Intranasal immunization with C. muridarum major outer membrane protein (MOMP) and cholera toxin elicits local production of neutralising IgA in the prostate. Vaccine 22:4306–4315 [DOI] [PubMed] [Google Scholar]
- Hjort T (1996) Quantitative determination of IgG and IgA on sperm from infertile patients with and without antisperm antibodies. Am J Reprod Immunol 36:211–215 [DOI] [PubMed] [Google Scholar]
- Huang J, Sun X, Mao Y, Zhu X, Zhang P, Zhang L, Du J, et al. (2008) Expression of immunoglobulin gene with classical V-(D)-J rearrangement in mouse brain neurons. Int J Biochem Cell Biol 40:1604–1615 [DOI] [PubMed] [Google Scholar]
- Itoh M, Terayama H, Naito M, Ogawa Y, Tainosho S (2005) Tissue microcircumstances for leukocytic infiltration into the testis and epididymis in mice. J Reprod Immunol 67:57–67 [DOI] [PubMed] [Google Scholar]
- Kamada M, Liang Z, Koide SS (1991) Identification of IgG and Fc-binding proteins in human seminal plasma and sperm. Arch Androl 27:1–7 [DOI] [PubMed] [Google Scholar]
- Knee RA, Hickey DK, Beagley KW, Jones RC (2005) Transport of IgG across the blood-luminal barrier of the male reproductive tract of the rat and the effect of estradiol administration on reabsorption of fluid and IgG by the epididymal ducts. Biol Reprod 73:688–694 [DOI] [PubMed] [Google Scholar]
- Lenzi A (2001) Immunology and immunopathology of male genital tract. Preface. Hum Reprod Update 7:443. [PubMed] [Google Scholar]
- Liu HD, Zheng H, Li M, Hu DS, Tang M, Cao Y (2007) Upregulated expression of kappa light chain by Epstein-Barr virus encoded latent membrane protein 1 in nasopharyngeal carcinoma cells via NF-kappaB and AP-1 pathways. Cell Signal 19:419–427 [DOI] [PubMed] [Google Scholar]
- Qiu X, Zhu X, Zhang L, Mao Y, Zhang J, Hao P, Li G, et al. (2003) Human epithelial cancers secrete immunoglobulin g with unidentified specificity to promote growth and survival of tumor cells. Cancer Res 63:6488–6495 [PubMed] [Google Scholar]
- Schreck S, Buettner M, Kremmer E, Bogdan M, Herbst H, Niedobitek G (2006) Activation-induced cytidine deaminase (AID) is expressed in normal spermatogenesis but only infrequently in testicular germ cell tumours. J Pathol 210:26–31 [DOI] [PubMed] [Google Scholar]
- Souto-Carneiro MM, Longo NS, Russ DE, Sun HW, Lipsky PE (2004) Characterization of the human Ig heavy chain antigen binding complementarity determining region 3 using a newly developed software algorithm, JOINSOLVER. J Immunol 172:6790–6802 [DOI] [PubMed] [Google Scholar]
- Sugimura J, Foster RS, Cummings OW, Kort EJ, Takahashi M, Lavery TT, Furge KA, et al. (2004) Gene expression profiling of early- and late-relapse nonseminomatous germ cell tumor and primitive neuroectodermal tumor of the testis. Clin Cancer Res 10:2368–2378 [DOI] [PubMed] [Google Scholar]
- Sundaresan NR, Anish D, Sastry KV, Saxena VK, Mohan J, Ahmed KA (2007) Cytokines in reproductive remodeling of molting White Leghorn hens. J Reprod Immunol 73:39–50 [DOI] [PubMed] [Google Scholar]
- Zhong H, Li Z, Lin S, Chang Y (2007) Initiation of V(D)J recombination in zebrafish (Danio rerio) ovaries. Mol Immunol 44:1784–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]