Abstract
Smoking causes lung cancer and chronic obstructive pulmonary disease (COPD) that impose severe health problem to humans. Both diseases are related to each other and can be induced by chronic inflammation in the lung. To identify the molecular mechanism for lung cancer formation, a CCSP-rtTA/(Teto)7Stat3C bitransgenic model was generated recently. In this model, persistent activation of the Stat3 signaling pathway induced pulmonary inflammation and adenocarcinoma formation in the lung. A group of Stat3 downstream genes were identified by Affymetrix GeneChip microarray analysis that can be used as biomarkers for lung cancer diagnosis and prognosis. To determine which human lung cancers are related to the Stat3 pathway, multiple Stat3 downstream genes were screened in human lung cancers (adenocarcinomas and squamous cell carcinomas) and lung tissue with COPD. In both cancer and COPD, the Stat3 gene was up-regulated. A panel of Stat3-up-regulated downstream genes in mice was up-regulated in human adenocarcinomas, but not in human squamous cell carcinomas. This panel of genes was also modestly up-regulated in lung tissue with COPD from patients with a history of smoking and not up-regulated in those without histories of smoking. Several Stat3-down-regulated downstream genes also showed differential expression patterns in carcinoma and COPD. These studies support a concept that Stat3 is a potent oncogenic molecule that plays a role in formation of lung adenocarcinomas in both mice and humans. The carcinogenesis of adenocarcinoma and squamous cell carcinoma is mediated by different molecular mechanisms and pathways in vivo. Stat3 and its downstream genes can serve as biomarkers for lung adenocarcinoma and COPD diagnosis and prognosis in mice and humans.
Keywords: Stat3, Adenocarcinoma, Squamous Cell Carcinoma, COPD, Biomarkers, Real-Time qRT-PCR
Introduction
Lung cancer is one of the biggest public health challenges facing the United States and many other countries. It accounts for 28% of all cancer deaths (more than colonic, mammary, prostatic and pancreatic cancers combined). Although incidence rates have stabilized, more than 173,000 new cases of lung cancer are diagnosed each year, and it continues to be the most common cause of cancer death among men and women, with more than 163,000 people succumbing each year (http://www.cancer.gov/ncicancerbulletin/NCI_Cancer_Bulletin_052306/page3). Lung cancer is difficult disease to detect in its early stages. In most cases, the tumors are detected at advanced stages and the overall 5-year survival rate is ~15 percent. Thus, it is essential to better understand the early events that initiate lung carcinogenesis and to find biomarkers for early lung cancer detection. Approximately 80% of human lung cancers are adenocarcinomas and squamous cell carcinomas. Human chronic obstructive pulmonary disease (COPD) (especially with smoking history) is a pre-cancer disease and is the fourth leading cause for human death in the United States. Importantly, both COPD and lung cancer can be induced by repeated exposure to smoking. It is well-known that smokers with COPD are elevated at risk to develop lung cancer. Both lung diseases are associated with inflammation in the lung. Nevertheless, the molecular mechanisms connecting inflammation and COPD and lung cancer are poorly understood.
Inflammation is a protective process to facilitate pathogen clearance and to repair tissue injury in the lung. The magnitudes of inflammatory responses are precisely regulated by pro- and anti-inflammatory molecules. Exuberant inflammation can cause severe consequences and lead to carcinogenesis. One commonly induced pro-inflammatory gene group during pulmonary inflammation is the interleukin 6 (IL-6) family of cytokines. Pro-inflammatory IL-6 cytokines share the common gp130 receptor subunit [1, 2]. Upon binding to their cell-surface receptors, IL-6 family members trigger Stat3 phosphorylation by Janus-activated kinases (JAKs). As we demonstrated previously, IL-6, JAKs and Stat3 are co-expressed in alveolar type II (AT II) epithelial cells in the lung [3–5]. Over-expression of dominant negative Stat3 to subvert endogenous Stat3 activity in respiratory epithelial cells causes alveolar destruction, suggesting its role in maintaining alveolar structure and function [4]. On the other hand, persistent activation of Stat3 signaling has the potential to induce lung cancer [6]. Carcinogenesis is a complex process that is influenced by both genetics and the tissue microenvironment. Based on our studies in the CCSP-rtTA/(teto)7Stat3C bitransgenic mouse model, Stat3 induces lung adenocarcinoma formation in two steps. The first step involves aberrant expression of cytokines/chemokines and abnormal inflammatory cell infiltration, which hijack the immune system to inhibit immune surveillance and promote neoplasia. The second step involves reactivation of developmental genes that stimulate epithelial cell over-growth. These observations are in agreement with previous findings that Stat3 is an oncogene [7].
Although extensive surveys of primary tumors and cell lines derived from tumors indicate that inappropriate activation of Stat3 occurs with surprisingly high frequency (50 to 90%) in a wide variety of human cancers, including lung cancers [8,9] [10–13], no Stat3 downstream gene has been used to link the Stat3 pathway to lung cancer formation. During characterization of the CCSP-rtTA/(teto)7Stat3C bitransgenic mouse model, multiple Stat3 up or down-regulated downstream genes were identified by Affymetrix GeneChip Microarray analysis, which have been confirmed by Real-Time PCR and immunohistochemistry analyses [6]. These genes can potentially serve as molecular biomarkers for cancer patient diagnosis or prognosis.
In this report, a panel of Stat3-most-up-regulated and down-regulated downstream genes in the CCSP-rtTA/(teto)7Stat3C bitransgenic mouse model was studied in human lung carcinomas and COPD tissues to assess if they can be used as biomarkers for diagnostic or prognostoc purposes. Although expression of the Stat3 gene was up-regulated in human carcinomas and tissues with COPD, its downstream genes were differentially expressed in adenocarcinomas and squamous cell carcinomas and in tissues with COPD from smokers and non-smokers and therefore can be used to distinguish them. These studies also suggest that Stat3 and its downstream genes more actively participate in the carcinogenesis of adenocarcinomas than in squamous cell carcinomas in the lungs. This is in agreement with observations made in the CCSP-rtTA/(teto)7Stat3C bitransgenic mouse model in which only adenocarcinomas were observed. In COPD, smokers seem more skewed towards the COPD-adenocarcinoma transition based on Stat3-up-regulated gene analysis.
Materials and Methods
Human Tissue
Human lung tissues were obtained from the Indiana University Simon Cancer Center Tissue Procurement Lab and the IU/Lilly Clinically-Annotated Tissue Databank. The protocol for an exempt study using these human tissues has been approved by the IUPUI/Clarian Institutional Review Committee. All samples are surgically collected from human tissues. Normal samples represent lung tissues without cancer and COPD.
Isolation of total RNAs and Real-Time PCR
Total RNA from whole lung tissues was purified using the Qiagen total RNA purification kit as recommended by the manufacturer (Qiagen, Valencia, CA). All reverse transcription reactions were set up using the TaqMan® Reverse Transcription Kit (Applied Biosystems, Foster City, CA). A total of 2 μg RNA from human whole lung tissues were used in a 100 μl reaction. The cDNA synthesis reactions were carried out using a GeneAmp® 9700 Thermocycler (Applied Biosystems) with a suggested cycling protocol of 25°C for 10 minutes, 48°C for 30 minutes, and 95°C for 5 minutes. For Real-Time PCR, a total of 2 μl of cDNA were amplified by a pair of sequence-specific DNA oligonucleotide primers for each molecule in the RT reaction in a 50 μl reaction containing SYBR® Green PCR Master Mix (Applied Biosystems). The quality of each primer pairs was checked by dissociation curves in a pre-run study. GAPDH primers were used as an endogenous control and normalizer for all cDNA samples. Reactions were analyzed using the Relative Quantification Assay of 7500 System Sequence Detection Software for the 7500 Real-Time PCR System (Applied Biosystems). The default cycling protocol for this software was 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute. Statistical differences among different human samples were measured by ANOVA. P< 0.05 was considered significant.
Human Real-Time PCR Primers
Cbln1:
Upstream: 5′-AAT CGC ACC ATG ATC ATC TAC TTC-3′
Downstream: 5′-TGC GTT CTG AAT CAA AGT TGT TC-3′
Cldn2:
Upstream: 5′- GAA ACT GAT TGG CCC TGG AA -3′
Down Stream: 5′- AAT TAG TCT GGA CAC TGT GGA GTC AT-3′
Fgl1:
Upstream: 5′- AGG GAA CTG CGC AGA AGA AG-3′
Down Stream: 5′- AGG TTT GCA GAG TGA CAC CTG TT -3′
Foxa3:
Upstream: 5′- AGG GAA CTG CGC AGA AGA AG -3′
Downstream: 5′- CCA AAC CCC ACG TCC AGT T-3′
Gjb1:
Upstream: 5′- GTG ACA GGG AGG TGT GAA TGA G-3′
Downstream: 5′- ACG CCA CTG AGC AAG GTG TAC-3′
HNF4a:
Upstream: 5′- TCT TAC GAC ACG TCC CCA TCA-3′
Downstream: 5′- GCT GAC ACC CAG GCT GTT G-3′
Krtdap:
Upstream: 5′- AAAGGACTGAGGAGCGCAACT- 3′
Downstream: 5′- CGCTAGCCCCCTCTTCCA- 3′
Lor:
Upstream: 5′- AATAGATCCCCCAGGGTACCA- 3′
Downstream: 5′- CGGTGCCCCTGGAAAAC- 3′
Rdhe2:
Upstream: 5′- AGC CAA AAG GAA GGA GTG TAT AGA GT -3′
Downstream: 5′- TGG AAA CAT CGC CGA CTT CT-3′
Stat3:
Upstream: 5′- GGCCCCTCGTCATCAAGA- 3′
Downstream: 5′- TTTGACCAGCAACCTGACTTTAGT- 3′
Tmem27:
Upstream: 5′- GGC TGC TCT TTT TTC TGG TGA CT -3′
Downstream: 5′- GCA TTT TCT GCA CCT GGT TGA -3′
Ttr:
Upstream: 5′- CTA CGG GCA CCG GTG AAT C-3′
Downstream: 5′- GCC TCG GAC AGC ATC TAG AAC T-3′
Results
Pre-screening of Human samples
Samples from 20 normal human lung tissues, 30 human pulmonary adenocarcinomas, 29 human pulmonary squamous cell carcinomas, 29 human lung tissues with COPD from non-smokers and 33 human lung tissues with COPD from smokers were obtained. The quality of all human samples was pre-screened by two standards. First, the total RNA was purified. Only samples with high yields of RNA were selected for the Real-Time PCR study. Second, the selected samples were subject to initial Real-Time PCR analyses for GAPDH mRNA expression by using sequence – specific primers. The samples with low expression levels of GAPDH mRNA were disqualified for further analysis. Based upon this screening, 7 of the normal samples, 4 of the adenocarcinomas, 3 of the squamous cell carcinomas, 9 COPD lung samples from non-smokers and 3 from smokers were eliminated from the study.
Up-regulation of the Stat3 gene
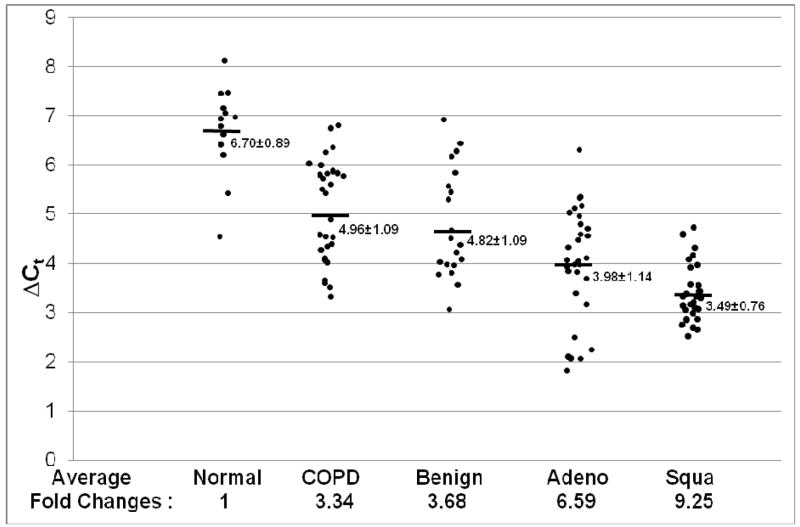
Since in the CCSP-rtTA/(teto)7Stat3C bitransgenic mouse model, Stat3C over-expression caused lung tumor formation, it is important to evaluate if up-regulation of Stat3 is associated with lung cancers and COPD in humans. The expression levels of Stat3 mRNA were examined in human adenocarcinomas and squamous cell carcinomas vs normal human samples by quantitative Real-Time PCR, Figure 1. In comparison with normal human lungs, the average of Stat3 mRNA expression levels was 6.59-fold higher in adenocarcinomas and 9.25-fold higher in squamous cell carcinomas. The average of Stat3 mRNA expression levels was 3.34-fold higher in lung tissues with COPD from non-smokers and 3.68-fold higher in lung tissues with COPD from smokers. A more detailed comparison of Stat3 mRNA expression between different tumor stages, differentiation grades of the tumor and the smoking history is presented in Table 1.
Figure 1. Stat3 mRNA expression in adenocarcinoma, squamous cell carcinoma and COPD.
Expression of Stat3 mRNA was measured by quantitative Real-Time PCR and normalized by GAPDH mRNA expression. Numbers in each column represent average ΔCt. Average fold changes were determined by 2(ΔΔCt), in which ΔΔCT= ΔCt(normal human samples) − ΔCt(cancer or COPD samples). Numbers on the bottom represent average fold changes compared to the normal human samples. Normal human samples were set up as one. Nor: normal samples. COPD: COPD samples from smokers. Benign: COPD samples from non-smokers. Adeno: adenocarcinoma. Squa: squamous cell carcinoma.
Table 1.
Stat3 mRNA expression in different tumor stages, differentiation grades and smoking history
| Diagnosis | Stat3 Expression | |
|---|---|---|
| Adenocarcinoma | Average ΔCt | Average Fold Change |
| Tumor Stage | ||
| I = 12 | 4.36±1.09 | 5.06 |
| II = 2 | 3.71±2.06 | 4.96 |
| III = 5 | 4.39±0.94 | 7.94 |
| Nuclear Grade | ||
| 1/3 = 4 | 4.40±0.56 | 4.92 |
| 2/3 = 13 | 4.28±1.12 | 5.36 |
| 3/3 = 10 | 4.11±1.40 | 6.02 |
| Smoking History | ||
| Yes = 20 | 4.43±0.89 | 4.82 |
| No = 0 | n/a | n/a |
| Not provided = 8 | 3.51±1.28 | 9.13 |
| Squamous Cell Carcinoma | ||
| Tumor Stage | ||
| I = 7 | 5.16±0.67 | 2.91 |
| II = 2 | 5.69±1.57 | 2.01 |
| III = 1 | 6.26±0.57 | 1.36 |
| Nuclear Grade | ||
| 1/3 = 0 | n/a | n/a |
| 2/3 = 10 | 4.66±1.16 | 4.11 |
| 3/3 = 8 | 5.01±0.81 | 3.23 |
| Smoking History | ||
| Yes = 8 | 4.92±1.01 | 3.43 |
| No = 0 | n/a | n/a |
| Not provided = 11 | 4.91±1.01 | 3.46 |
| COPD | 4.96±1.09 | 3.34 |
| COPD w/o Smoking | 4.82±1.09 | 3.68 |
Changes of the Stat3 up-regulated genes in human adenocarcinomas
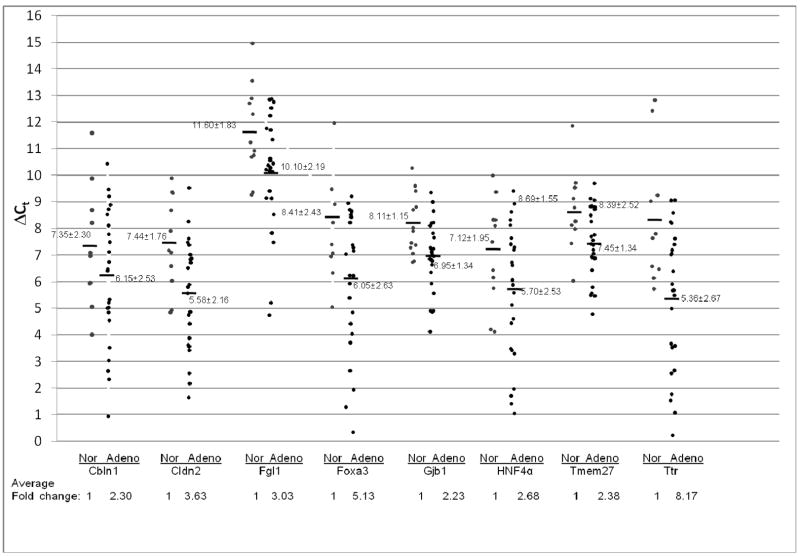
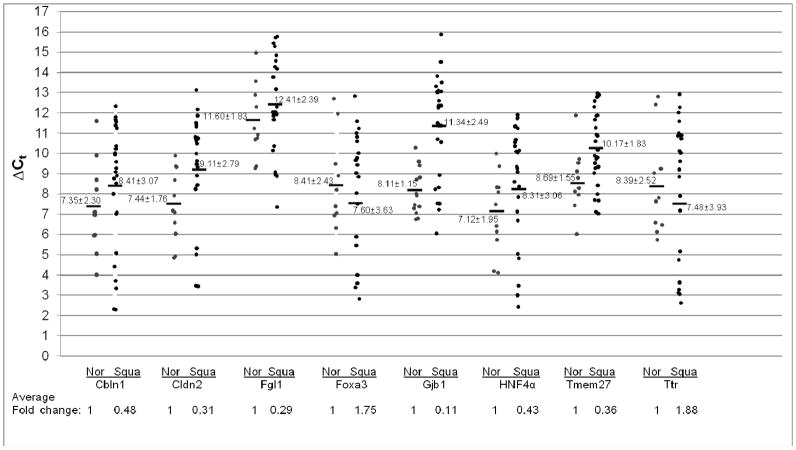
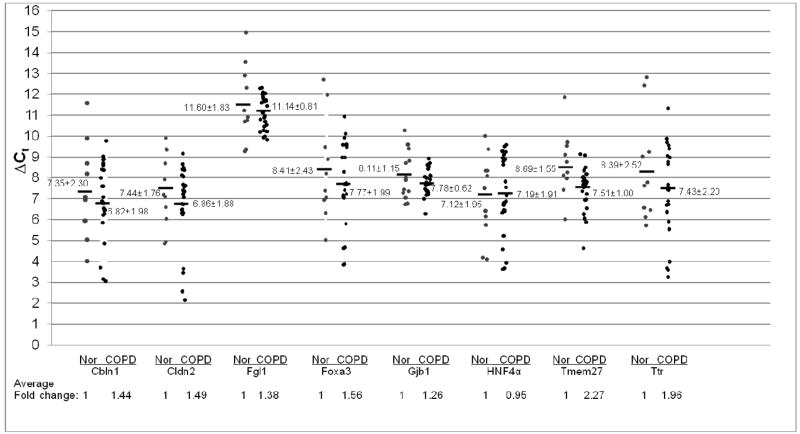
To further evaluate Stat3 signaling in pulmonary adenocarcinoma and squamous cell carcinoma, the expression levels of Stat3 downstream genes was studied. After gene search and sequence comparison, 8 Stat3 most up-regulated downstream genes in CCSP-rtTA/(teto)7Stat3C bitransgenic mice were selected for testing. These genes were Cbln1(Cerebellin 1 precursor), Cldn2 (Claudin 2), Fgl1(Fibrinogen-like protein 1 precursor), Foxa3 (forkhead box A3), Gjb1 (Gap junction beta 1 protein), HNF4α (hepatic nuclear factor 4 alpha), Tmem27 (Transmembrane protein 27) and Ttr (Transthyretin precursor). We first tested the expression levels of these Stat3-up-regulated downstream genes in adenocarcinomas by quantitative Real-Time PCR. In comparison with normal human lungs, the averages of Stat3-up-regulated gene expression levels were all significantly higher in human adenocarcinomas, ranging from 2-fold to 8-fold higher, Figure 2. In contrast to adenocarcinomas, the average expression levels of most Stat3-up-regulated genes in squamous cell carcinomas were significantly reduced (Cbln1, Cldn2, Fgl1, Gjb1, HNF4α and Tmem27). Only Foxa3 and Ttr showed modest average increases compared to normal human lungs, Figure 3.
Figure 2. Expression of Stat3-up-regulated genes in adenocarcinoma.
Expression of Stat3-up-regulated genes was measured by quantitative Real-Time PCR and normalized by GAPDH mRNA expression. Numbers in each column represent average ΔCt. Average fold changes were determined by 2(ΔΔCt), in which ΔΔCT= ΔCt(normal human samples) − ΔCt(adenocarcinoma samples). Numbers on the bottom represent average fold changes compared to the normal human samples. Normal human samples were set up as one. Nor: normal samples. Adeno: adenocarcinoma samples.
Figure 3. Expression of Stat3-up-regulated genes in squamous cell carcinoma.
Expression of Stat3 up-regulated genes was measured by quantitative Real-Time PCR and normalized by GAPDH mRNA expression. Numbers in each column represent average ΔCt. Average fold changes were determined by 2(ΔΔCt), in which ΔΔCT= ΔCt(normal human samples) − ΔCt(squamous cell carcinoma samples). Numbers on the bottom represent average fold changes compared to the normal human samples. Normal human samples were set up as one. Nor: normal samples. Squa: squamous cell carcinoma samples.
Changes of the Stat3-up-regulated genes in lung tissue with COPD
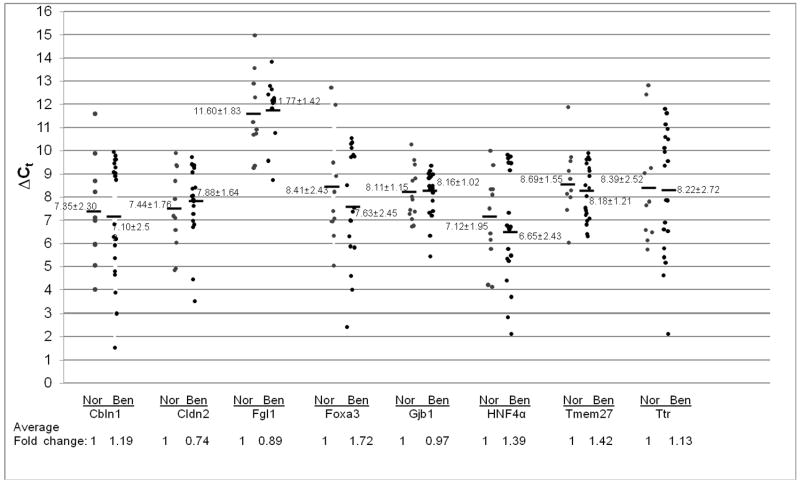
Stat3 up-regulation was observed in lung tissues with COPD from both smokers and non-smokers, as demonstrated in Figure 1. To see if the average expression levels of Stat3-up-regulated genes were altered in lung tissues with COPD, the samples were examined by quantitative Real-Time PCR. In lung tissue with COPD from non-smokers, the average expression levels of most Stat3-up-regulated genes remained unchanged (Cbln1, Cldn2, Fgl1, Gjb1 and Ttr). The average expression levels of Foxa3, HNF4a and Tmem27 genes were modestly increased compared to normal human lungs, Figure 4. In contrast, the average expression levels of Stat3-up-regulated genes were modestly increased in lung tissues with COPD from smokers (Cbln1, Cldn2, Fgl1, Foxa3, Gjb1, Tmem27 and Ttr). Only the expression of the HNF4 gene remained unchanged, Figure 5.
Figure 4. Expression of Stat3-up-regulated genes in COPD from non-smokers.
Expression of Stat3 up-regulated genes was measured by quantitative Real-Time PCR and normalized by GAPDH mRNA expression. Numbers in each column represent average ΔCt. Average fold changes were determined by 2(ΔΔCt), in which ΔΔCT= ΔCt(normal human samples) − ΔCt(benign COPD samples). Numbers on the bottom represent average fold changes compared to the normal human samples. Normal human samples were set up as one. Nor: normal samples. Benign: COPD from non-smoker samples.
Figure 5. Expression of Stat3-up-regulated genes in COPD from smokers.
Expression of Stat3 up-regulated genes was measured by quantitative Real-Time PCR and normalized by GAPDH mRNA expression. Numbers in each column represent average ΔCt. Average fold changes were determined by 2(ΔΔCt), in which ΔΔCT= ΔCt(normal human samples) − ΔCt(COPD from smokers). Numbers on the bottom represent average fold changes compared to the normal human samples. Normal human samples were set up as one. Nor: normal samples. COPD: COPD samples from smokers.
Changes of the Stat3-down-regulated genes in the lung with COPD and in adenocarcinoma and squamous cell carcinoma
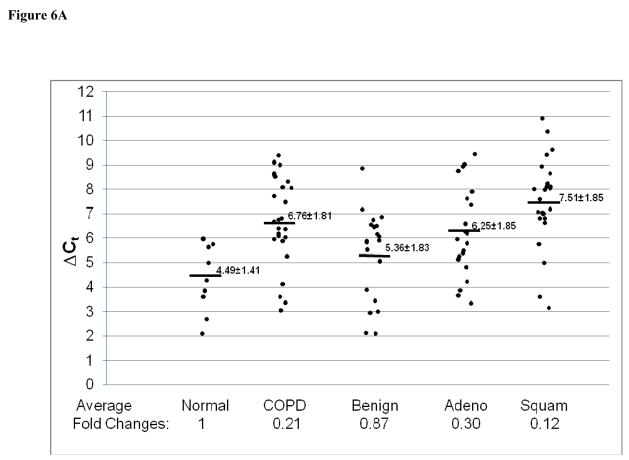
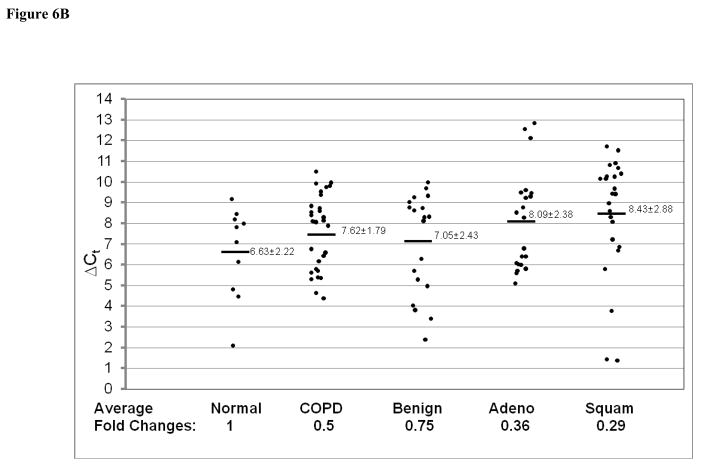
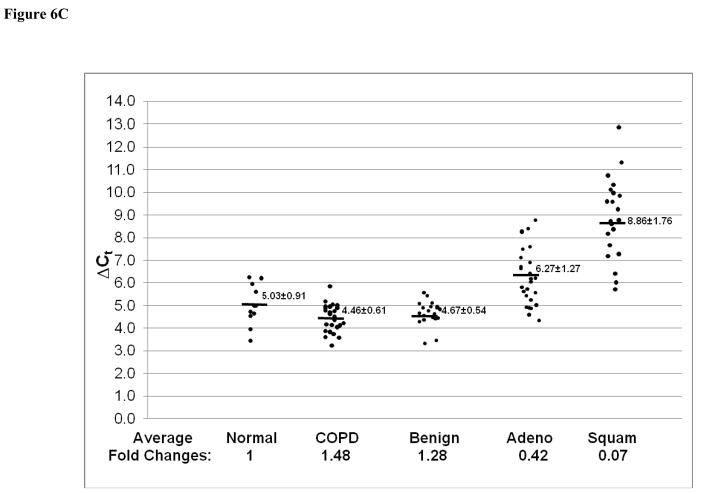
In addition to Stat3-up-regulated genes, three Stat3-down-regulated genes in CCSP-rtTA/(teto)7Stat3C bitransgenic mice were also tested in the adenocarcinomas, squamous cell carcinomas and lung tissue with COPD by quantitative Real-Time PCR. These were Krtdap (Keratinocyte differentiation associated protein), Lor (Loricrin) and Rdhe2 (Retinal short chain dehydrogenase reductase 2). The average expression levels of the Krtdap (Figure 6A) and Lor (Figure 6B) genes were significantly decreased in all samples. The average expression level of the Rdhe2 gene was decreased only in adenocarcinomas and squamous cell carcinomas (Figure 6C).
Figure 6. Expression of Stat3-down-regulated genes in adenocarcinomas, squamous cell carcinoma and COPD.
Expression of Stat3-down-regulated Krtdap (A), Lor (B) and Rdhe2 (C) genes was measured by quantitative Real-Time PCR and normalized by GAPDH mRNA expression. Numbers in each column represent average ΔCt. Average fold changes were determined by 2(ΔΔCt), in which ΔΔCT= ΔCt(normal human samples) − ΔCt(cancer or COPD samples). Numbers on the bottom represent average fold changes compared to the normal human samples. Normal human samples were set up as one. Nor: normal samples. COPD: COPD samples from smokers. Benign: Benign COPD samples from non-smokers. Adeno: adenocarcinoma. Squa: squamous cell carcinoma.
Discussion
In this study, Stat3 gene up-regulation was observed in human pulmonary adenocarcinomas and squamous cell carcinomas (Figure 1), suggesting that Stat3 plays a role in initiation and progression of these cancers. This is in agreement with our previous findings in animals. Up-regulation of Stat3 in the CCSP-rtTA/(teto)7Stat3C bitransgenic mouse model induced lung adenocarcinomas [6]. The Stat3 process of carcinogenesis is initiated by increased expression of a subset of pro-inflammatory cytokines, chemokines and their receptors. These molecules recruit inflammatory cells into the lung and change the local microenvironment in favor of tumor growth [6]. Since Stat3 signaling can be activated by upstream pro-inflammatory cytokines (e.g. IL-6 family members) and growth factors (e.g. epithelial growth factor or EGF), which are often up-regulated in various pulmonary diseases (e.g. bacterial infection, chronic inflammation), our results in humans and mice provide an insight into the mechanism whereby lung cancers are influenced and induced by other forms of diseases.
In the late stage of Stat3-induced lung adenocarcinomas, multiple Stat3 downstream genes were either up-regulated or down-regulated in the CCSP-rtTA/(teto)7Stat3C bitransgenic mouse model. To investigate whether or not these genes can be used as biomarkers in human pulmonary adenocarcinomas and squamous cell carcinoma, several Stat3-most-up-regulated or down-regulated genes were chosen for investigation. Expression of Stat3-up-regulated genes (Cbln1, Cldn2, Fgl1, Foxa3, Gjb1, HNF4α, Tmem27 and Ttr) agreed well between mouse and human adenocarcinomas (Figure 2). On the other hand, most of these genes (Cbln1, Cldn2, Fgl1, Gjb1, HNF4α and Tmem27) were significantly down-regulated in human squamous cell carcinomas (Figure 3). From among the Stat3-down-regulated genes, Krtdap, Lor and Rdhe2 were all down-regulated in both adenocarcinomas and squamous cell carcinomas (Figure 6). Therefore, some common molecular pathways under the control of Stat3 contribute to carcinogesis of both adenocarcinomas and squamous cell carcinoma in the lung. In a combined panel, these Stat3 downstream genes could serve as biomarkers for differentiating between pulmonary adenocarcinoma and squamous cell carcinoma in humans.
Lung cancer and COPD are closely related in humans, especially among smokers. The connection between the two diseases needs to be studied in order to elucidate COPD-cancer transition. Since both diseases are highly associated with inflammation, Stat3 and its downstream genes may serve as a common mechanism to connect both. Indeed, Stat3 up-regulation was observed in lung tissue with COPD from both smokers and non-smokers (Figure 1). In non-smokers, expression of most of the Stat3-up-regulated genes remained unchanged, (Figure 4). In contrast, most of these genes showed modest increases in lung tissues with COPD from smokers (Figure 5), although to less extent compared to adenocarcinomas. It appears that lung tissues with COPD from smokers is more skewed toward a COPD-adenocarcinoma transition. The degree of up-regulation of Stat3 and its downstream genes may serve as a useful parameter to predict the COPD-adenocarcinoma transition in human patients. Stat3 up-regulation may serve as an inflammatory warning sign for a pre-cancerous condition in the lung. The transition between COPD and adenocarcinomas may depend on the levels of Stat3 downstream gene expression. Among the Stat3-down-regulated genes, Krtdap and Lor genes were down-regulated, whereas Rdhe2 was modestly up-regulated in lung tissues with COPD. These parameters may also help to predict the COPD-adenocarcinoma transition.
Although Stat3 has been well studied in various forms of cancer, the functional roles of its downstream genes are poorly understood in cancers, including lung cancer. Foxa3 and HNF4 are developmental genes. Up-regulation of these genes reinforced a concept that cancer cell ontogeny is determined by gene expression that recapitulates events important in embryonic development [14]. One highly induced protein in human adenocarcinomas is Ttr. Ttr is a beta-sheet rich protein with a plasma half life of 1.9 days. It behaves as a tetramer and binds to retinol binding protein (RBP) and thyroxin in plasma. Ttr is an anti-acute phase protein, and its concentration is influenced by various conditions of inflammation and infection [15]. Cbln1 belongs to the C1q/tumor necrosis factor superfamily of secreted proteins. Its derivative hexadecapeptide cerebellin has features of a neuropeptide and has been reported to be present in the brains of many vertebrate species [16]. Cldn2 is a tetraspan transmembrane protein of tight junctions. They determine the barrier properties of cell-cell contact existing between the plasma membranes of two neighboring cells, such as occur in endothelia or epithelia [17]. Another gap junction protein Gjb1 was also up-regulated in human adenocarcinomas. Fgl1 has been implicated as a mitogen for liver cell proliferation. IL-6 induces FGL1 expression in a pattern similar to those of acute phase reactants. Following induction of acute inflammation, serum FGL1 levels are enhanced. Although FGL1 associates with the fibrin matrix, approximately 20% of the total plasma FGL1 remains free. Its presence in bound and free forms in the blood implies biological roles that extend beyond the liver regeneration [18, 19]. Tmem27 (collectrin) is a key regulator of renal amino acid uptake and involved in the process of progressive renal failure and renal organogenesis [20, 21]. Among Stat3-down-regulated genes, Krtdap and Lor are epithelial cell differentiation markers. It has been reported that tobacco smoke down-regulates Lor in epithelial cell hyperplasia and squamous metaplasia [22]. Rdhe2f is a sterol regulatory element-binding protein (SREBPs) induced membrane-bound enzyme required for the cellular synthesis and uptake of cholesterol and unsaturated fatty acids. The enzyme specifically uses NADPH to reduce a variety of short-chain aldehydes and retinaldehydes [23].
Although this group of genes has various functions in cells, in a combinatory way, they define a specific pathway for formation of adenocarcinoma and COPD in the lung. Based on our studies, even though the average expression levels of these biomarker genes are higher or lower in human adenocarcinoma and squamous cell carcinoma and COPD samples, the individual expression level of each biomarker gene varied among the conditions. For more accurate prediction of diagnosis and prognosis, it is recommended that a panel of biomarker genes is used. Biomarker is only one valuable parameter to assist diagnosis and prognosis. It has to be used in combination with other means to accurately predict lung cancer formation.
Acknowledgments
This study was supported by National Institute of Health Grants HL-061803 and HL-067862 (C. Yan and H. Du), and Indiana University Cancer Center ITRAC Award (C. Yan).
Financial Support: National Institute of Health Grants HL-061803 and HL-067862 (C. Yan and H. Du).
Footnotes
Conflict of interest
The authors have no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 2.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 3.Yan C, Naltner A, Martin M, et al. Transcriptional stimulation of the surfactant protein B gene by STAT3 in respiratory epithelial cells. J Biol Chem. 2002;277:10967–72. doi: 10.1074/jbc.M109986200. [DOI] [PubMed] [Google Scholar]
- 4.Yang L, Lian X, Cowen A, et al. Synergy between signal transducer and activator of transcription 3 and retinoic acid receptor-alpha in regulation of the surfactant protein B gene in the lung. Mol Endocrinol. 2004;18:1520–32. doi: 10.1210/me.2003-0458. [DOI] [PubMed] [Google Scholar]
- 5.Lian X, Qin Y, Hossain SA, Senior RM, Du H, Yan C, et al. Overexpression of Stat3C in Pulmonary Epithelium Protects against Hyperoxic Lung Injury. J Immunol. 2005;174:7250–6. doi: 10.4049/jimmunol.174.11.7250. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Du H, Qin Y, et al. Activation of the signal transducers and activators of the transcription 3 pathway in alveolar epithelial cells induces inflammation and adenocarcinomas in mouse lung. Cancer Res. 2007;67:8494–503. doi: 10.1158/0008-5472.CAN-07-0647. [DOI] [PubMed] [Google Scholar]
- 7.Bromberg JF, Wrzeszczynska MH, Devgan G, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 8.Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846–56. doi: 10.1172/JCI31871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kortylewski M, Jove R, Yu H. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev. 2005;24:315–27. doi: 10.1007/s10555-005-1580-1. [DOI] [PubMed] [Google Scholar]
- 10.Haura EB, Zheng Z, Song L, et al. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11:8288–94. doi: 10.1158/1078-0432.CCR-05-0827. [DOI] [PubMed] [Google Scholar]
- 11.Yeh HH, Lai WW, Chen HH, et al. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene. 2006;25:4300–9. doi: 10.1038/sj.onc.1209464. [DOI] [PubMed] [Google Scholar]
- 12.Dauer DJ, Ferraro B, Song L, et al. Stat3 regulates genes common to both wound healing and cancer. Oncogene. 2005;24:3397–408. doi: 10.1038/sj.onc.1208469. [DOI] [PubMed] [Google Scholar]
- 13.Song L, Turkson J, Karras JG, et al. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–65. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 14.Borczuk AC, Gorenstein L, Walter KL, et al. Non-small-cell lung cancer molecular signatures recapitulate lung developmental pathways. Am J Pathol. 2003;163:1949–60. doi: 10.1016/S0002-9440(10)63553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ando Y. Transthyretin-its function and pathogenesis. Rinsho Byori. 2006;54:497–502. [PubMed] [Google Scholar]
- 16.Bao D, Pang Z, Morgan JI. The structure and proteolytic processing of Cbln1 complexes. J Neurochem. 2005;95:618–29. doi: 10.1111/j.1471-4159.2005.03385.x. [DOI] [PubMed] [Google Scholar]
- 17.Krause G, Winkler L, Mueller SL, et al. Structure and function of claudins. Biochim Biophys Acta. 2008;1778(3):631–45. doi: 10.1016/j.bbamem.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z, Ukomadu C. Fibrinogen-like protein 1, a hepatocyte derived protein is an acute phase reactant. Biochem Biophys Res Commun. 2008;365:729–34. doi: 10.1016/j.bbrc.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rijken DC, Dirkx SP, Luider TM, et al. Hepatocyte-derived fibrinogen-related protein-1 is associated with the fibrin matrix of a plasma clot. Biochem Biophys Res Commun. 2006;350:191–4. doi: 10.1016/j.bbrc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Danilczyk U, Sarao R, Remy C, et al. Essential role for collectrin in renal amino acid transport. Nature. 2006;444:1088–91. doi: 10.1038/nature05475. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Wada J, Hida K, et al. Collectrin, a collecting duct-specific transmembrane glycoprotein, is a novel homolog of ACE2 and is developmentally regulated in embryonic kidneys. J Biol Chem. 2001;276:17132–9. doi: 10.1074/jbc.M006723200. [DOI] [PubMed] [Google Scholar]
- 22.Zhong CY, Zhou YM, Douglas GC, et al. MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis. 2005;26:2187–95. doi: 10.1093/carcin/bgi189. [DOI] [PubMed] [Google Scholar]
- 23.Kasus-Jacobi A, Ou J, Bashmakov YK, et al. Characterization of mouse short-chain aldehyde reductase (SCALD), an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem. 2003;278:32380–9. doi: 10.1074/jbc.M304969200. [DOI] [PubMed] [Google Scholar]