Abstract
The conidiation rhythm in the fungus Neurospora crassa is a model system for investigating the genetics of circadian clocks. Null mutants at the frq (frequency) locus (frq9 and frq10) make no functional frq gene products and are arrhythmic under standard conditions. The white-collar strains (wc-1 and wc-2) are insensitive to most effects of light, and are also arrhythmic. All three genes are proposed to be central components of the circadian oscillator. We have been investigating two mutants, cel (chain-elongation) and chol-1 (choline-requirer), which are defective in lipid synthesis and affect the period and temperature compensation of the rhythm. We have constructed the double mutant strains chol-1 frq9, chol-1 frq10, chol-1 wc-1, chol-1 wc-2, cel frq9, cel frq10, and cel wc-2. We find that these double mutant strains are robustly rhythmic when assayed under lipid-deficient conditions, indicating that free-running rhythmicity does not require the frq, wc-1, or wc-2 gene products. The rhythms in the double mutant strains are similar to the cel and chol-1 parents, except that they are less sensitive to light. This suggests that the frq, wc-1, and wc-2 gene products may be components of a pathway that normally supplies input to a core oscillator to transduce light signals and sustain rhythmicity. This pathway can be bypassed when lipid metabolism is altered.
The filamentous fungus Neurospora crassa is an excellent model system for investigating the mechanism of circadian rhythmicity (1, 2) because of the wealth of genetic and biochemical techniques available. Its rhythm of asexual spore-formation (conidiation) produces easily assayed bands of conidiospores in cultures growing on solid agar medium. A number of mutations are available that affect circadian rhythmicity, and molecular analyses of some of these genes have contributed to models for circadian oscillators that are currently thought to be applicable to many other organisms. This paper reports the interactions between clock-affecting mutations that are said to be “arrhythmic” with no functional circadian clock and mutations affecting lipid synthesis that also have effects on rhythmicity. Our results raise questions about the proposed roles of the well-known clock genes and point toward an important role for lipid metabolism in the mechanism of rhythmicity.
The current model for the oscillator mechanism in Neurospora is a transcription/translation feedback loop involving the rhythmic expression of the frq (frequency) gene and its protein product FRQ (2). Both short-period and long-period alleles are known at this locus. Mutations in the FRQ protein affect temperature compensation of the circadian rhythm (3) and sensitivity to light-induced phase resetting (4, 5). Both the RNA and protein products of the frq locus are rhythmically accumulated (6, 7). The level of frq mRNA is affected by light pulses and steps (8) and by temperature steps (9). The FRQ protein is found in the nucleus, and nuclear localization is necessary for its function (10). The FRQ protein is proposed to negatively regulate its own transcription (6), although transcriptional control of frq expression has not yet been demonstrated.
There are two null mutations at the frq locus: frq9, which has a single base pair deletion producing a truncated protein, and frq10, which is a deletion mutation constructed by targeted gene disruption (11). These two mutations have apparently identical phenotypes: both are arrhythmic during the first few days of growth on race tubes, but cultures often produce rhythmic banding later during growth. The period of this rhythm is sensitive to the carbon source in the growth medium and has poor temperature compensation (11, 12).
Two other genes that have recently been proposed as essential to circadian rhythmicity are the white-collar genes, wc-1 and wc-2. These were shown to be “blind” mutants insensitive to the effects of blue light (see ref. 13). Both WC proteins are likely to be DNA-binding transcription factors (14, 15) and may therefore be both blue-light photoreceptors and transcriptional regulators of blue-light-induced genes (16). The wc gene products are required for induction of many light-induced genes, and both white-collar genes are themselves induced by light (14, 15). The wc gene products also appear to be required for normal rhythmicity in Neurospora. White-collar mutants fail to produce bands of conidiation under standard culture conditions (17), and rhythmicity cannot be induced by light or temperature steps (18), indicating that arrhythmicity is not simply caused by the lack of a synchronizing stimulus. The level of expression of the frq gene is very low in wc mutants, and temperature steps fail to induce sustained oscillations of frq gene products (18). The WC proteins are proposed to be activators of frq transcription, acting as positive elements in a transcription/translation feedback loop (2).
We have been working with two mutations that affect rhythmicity and that, in contrast to frq and wc, have known metabolic deficiencies that affect growth rate. These are both lipid-deficient mutants, cel (chain-elongation) and chol-1 (choline-requiring). The cel (or fas, fatty-acid synthetase) mutant is defective in fatty acid synthesis and grows slowly in the absence of exogenous fatty acids. Unsaturated fatty acids such as linoleic acid (18:2) lengthen the period of the conidiation rhythm, but normal growth and rhythmicity can be restored by saturated fatty acids such as palmitic acid (16:0) (19). Without fatty acid supplement, or with unsaturated fatty acids, temperature compensation of the conidiation rhythm is poor, such that the period lengthens at low temperatures (20).
The chol-1 mutation is defective in the synthesis of the phospholipid phosphatidylcholine (PtdCho). On minimal medium, growth is slow, the phospholipid composition is altered (21), the period of the conidiation rhythm is lengthened (22), and temperature compensation is poor (23). Choline supplementation restores normal growth and rhythmicity.
Double mutant strains have been constructed between cel or chol-1 and the series of long- and short-period frq mutations (23, 24). In both series, the long periods of cel or chol-1 were shortened by the short-period frq mutations but were relatively unaffected by the long-period frqs, indicating an interaction, or partial epistasis, between the frq gene product and the lipid synthesis pathways affected by cel and chol-1. A double mutant strain carrying both chol-1 and the null frq mutation frq10 was found to be arrhythmic with choline supplementation but rhythmic with a long period on minimal medium (23).
This unexpected epistasis of chol-1 over the null frq mutation raised the question of whether other “arrhythmic” mutations might also be restored to rhythmicity. The experiments reported here were designed to answer these questions: Are the chol-1 and cel mutations similar in their interactions with null frq and wcs? Under what conditions can rhythmicity be reliably restored to these “arrhythmic” mutants? We have found that both cel and chol-1 mutations can restore robust rhythmicity to null frq and wc mutant strains, and that this rhythmicity is seen under conditions that are expected to alter the lipid composition of these strains. We have also found that these double mutant strains are less sensitive to light, supporting a role for the frq and wc genes in a pathway that supplies light input to a core oscillator.
Methods
Strains.
All strains analyzed carry the bd (band) mutation, which prevents inhibition of conidiation by high CO2 concentrations in closed culture vessels. Most strains (with the exception of cel bd; frq10) carry the csp-1 (conidial separation) mutation, which prevents the release of contaminating conidiospores. Multiple mutant strains were constructed by standard crossing techniques (25) by using low-nitrogen Vogel's medium. Details of strain construction and verification can be found in the supplemental Methods on the PNAS web site (www.pnas.org). Strains carrying either chol-1 or cel and either frq or wc are described as “double mutant strains,” ignoring the presence of bd and csp-1. Strains are designated in the text by using the shorthand notation in supplemental Table 1 on the PNAS web site.
Culture Conditions and Assays, chol-1 Strains.
Periods and growth rates were assayed on solid agar medium in race tubes, as previously described (22, 23), by using Vogel's minimal medium with 2% agar/0.01% arginine/0.5% maltose/choline chloride, as indicated. Race tubes were inoculated with plugs of mycelia on agar removed from cultures grown from conidial inocula on solid agar medium in small Petri plates for 2 d in constant light (LL). Race tube cultures received a further 24 h of light before the light-to-dark transition after approximately 72 h in LL since conidial inoculation. Cultures were subsequently kept in constant dark (DD) at 22°C, except where indicated. For LL and light/dark cycle (LD) conditions, cultures either were left in LL after the initial 72 h in LL after inoculation or were transferred to the first dark period of an LD cycle. Light intensity was approximately 20 μmol/m2/s. Periods and growth rates were calculated as previously described (22, 23). In supplemental Tables 2 and 4, the periods were compared by using the two-tailed Student's t test for two samples with unequal variance.
Culture Conditions and Assays, cel Strains.
Periods and growth rates were assayed on solid agar medium in 15-cm Petri plates by using Vogel's medium with 2% agar and 0.5% maltose, supplemented with fatty acids as indicated. Petri plates were inoculated with conidia from stock tube cultures and exposed to LL for approximately 16 h before transfer to DD at 22°C, except where indicated. For LD conditions, cultures were exposed to either cool-white fluorescent light alternating with complete darkness or to natural LD cycles, consisting of ambient daylight near a window. Periods and growth rates were calculated as previously described (19).
Fatty Acid Supplements.
The cel strains were grown on media supplemented with fatty acids in ethanolic solution at a final concentration of 0.004% (wt/vol) fatty acid and 0.5% (wt/vol) ethanol. This concentration of ethanol has been shown to have negligible effects on the period of cel strains (20). Fatty acid supplements used were: palmitic acid (16:0), linoleic acid (18:2), linolenic acid (18:3), and eicosatrienoic acid (20:3). (The X:Y nomenclature indicates the number of carbon atoms and the number of double bonds.)
Results
Effects of Choline Depletion on Rhythmicity in chol-1 Strains.
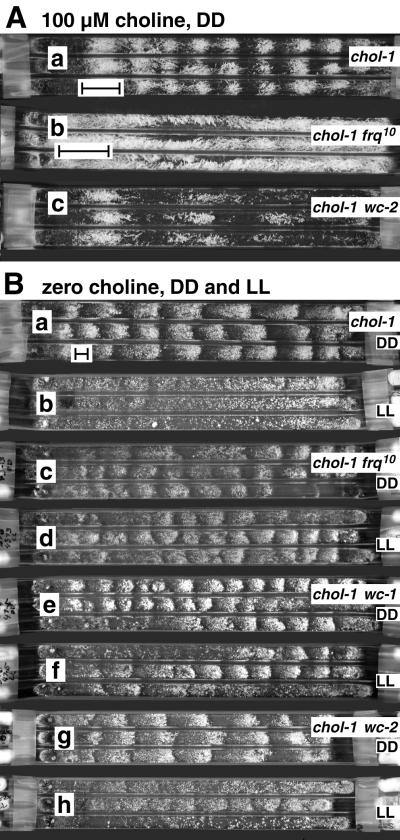
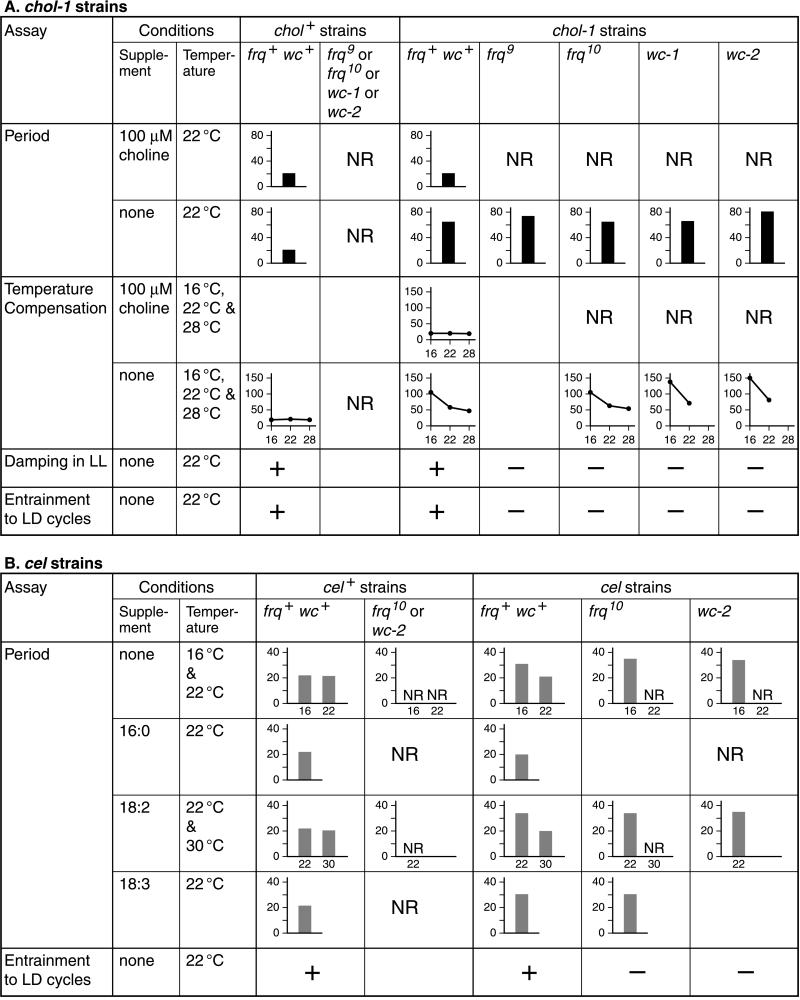
The chol-1 frq9, chol-1 frq10, chol-1 wc-1, and chol-1 wc-2 double mutants were constructed as described in the supplemental Methods and were tested for rhythmicity on minimal medium and high choline (100 μM). Rhythmicity was evaluated by eye, and cultures were judged to be rhythmic if more than three consecutive bands could be discerned. As shown in Fig. 1A, the double mutants were arrhythmic when supplemented with sufficient choline (100 μM) to restore the wild-type growth rate and were indistinguishable from their chol+ parents. On minimal medium without choline, all double mutant strains were robustly rhythmic in DD (Fig. 1 B, c, e, and g), indicating that the products of the frq, wc-1, and wc-2 genes are not required for rhythmicity under these conditions. The periods of the double mutant strains are presented in supplemental Table 2 and are summarized in Fig. 3A. (All tables can be found in the supplementary material on the PNAS web site, www.pnas.org.) The periods of all three isolates of genotype chol-1 wc-2 were significantly longer than chol-1. This may reflect the random segregation of another period-lengthening factor in this cross or may indicate a consistent effect of wc-2 on the period of chol-1. The number of isolates tested is not sufficient to distinguish between these possibilities.
Figure 1.
Conidiation rhythm in chol-1 cultures. Growth is from left to right. Bars = 24 h of growth. (A) Chol-1 strains on high choline. Cultures were grown in constant darkness on 100 μM choline. a, chol-1; b, chol-1 frq10; c, chol-1 wc-2. The chol-1 frq9 and chol-1 wc-1 strains were similar to chol-1 frq10 and chol-1 wc-2, respectively. This level of choline completely repairs the lipid synthesis defect in chol-1, and consequently chol-1 strains on high choline are indistinguishable from the corresponding chol+ strains on the same medium. (B) Effects of constant light on chol-1 strains. Cultures were grown on minimal medium without choline. a, c, e, g: Cultures were grown in constant darkness. b, d, f, h: Cultures were grown in constant light (cool-white fluorescent at approximately 20 μmol/m2/s). a, b, chol-1; c, d, chol-1 frq10; e, f, chol-1 wc-1; g, h, chol-1 wc-2.
Figure 3.
Summary of results with double mutant strains. Period bar charts represent the mean period of several isolates. Temperature compensation graphs represent the period at several different temperatures. Plus and minus symbols for damping and entrainment represent relative responses to light. NR, not rhythmic under these conditions. Empty blocks indicate no data available. Numerical data can be found in the supplemental tables. The frq+ wc+ columns include published data from refs. 20 and 23.
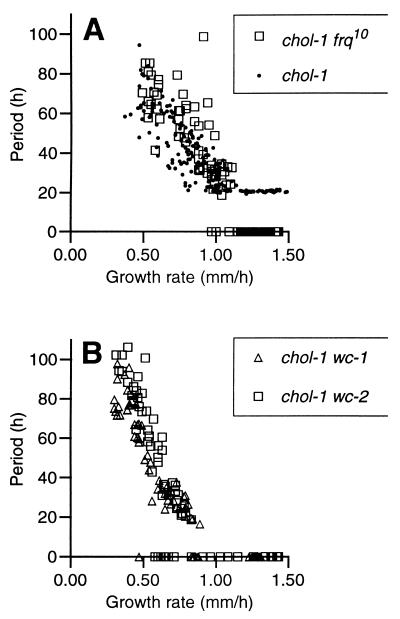
In Fig. 2, three of the double mutants (chol-1 frq10, chol-1 wc-1, and chol-1 wc-2) have been tested on a range of choline concentrations, and the periods are plotted against growth rate as a measure of the available intracellular choline. All three strains are rhythmic at choline concentrations giving growth rates less than about 1.0 mm/h and are arrhythmic at higher choline concentrations. At intermediate choline concentrations (growth rates approximately 0.8–1.0 mm/h), the periods are in the circadian range (approximately 24 h). These results may indicate that a change in lipid metabolism occurs at about 1.0 mm/h, which restores rhythmicity by bypassing or replacing the activities of the frq and wc gene products. A negative correlation between period and growth rate is seen in all three strains that is similar to the correlation seen in chol-1 (22).
Figure 2.
Relationship between period and growth rate in chol-1 double mutants. Growth rate was varied by using a range of choline concentrations in the medium, from 0 to 100 μM. Period is plotted against growth rate rather than choline concentration on the assumption that growth rate more accurately reflects available intracellular choline. [In supplemental Fig. 4 on the PNAS web site (see www.pnas.org.), the same data are replotted as period vs. choline concentration for comparison.] Each point represents one race tube culture. Points on the x axis at period = 0 represent arrhythmic cultures. (A) Data for chol-1 frq10 (squares). Data for chol-1 (dots) have been replotted from ref. 22 for comparison. (B) Data for chol-1 wc-1 (triangles) and chol-1 wc-2 (squares).
Temperature Compensation of chol-1 Strains.
To test the effects of choline depletion on temperature compensation, the chol-1 frq10 strain was assayed at five temperatures and five choline concentrations. The results can be found in supplemental Fig. 5 on the PNAS web site and are summarized in Fig. 3A. This strain is similar to chol-1 (23) in that it shows very poor temperature compensation of period in the lower temperature range (16–22°C) at low choline concentrations and good temperature compensation above 22°C. Growth rate as a function of temperature (supplemental Fig. 5B) was similar to previously reported data for chol-1 (ref. 23): growth rate is not temperature compensated on high choline, as with wild-type strains, but chol-1 and the double mutants show better compensation of growth rate on low choline and in the higher temperature range. This finding emphasizes that period and growth rate are controlled independently in these strains, and period is not a simple function of growth rate.
To test the effects of the wc mutations on temperature compensation, the chol-1 wc-1 and chol-1 wc-2 strains were assayed at 16°C and 22°C on the same choline concentrations as for chol-1 frq10. The data presented in supplemental Tables 4 and 5 and summarized in Fig. 3A demonstrate that temperature compensation is poor in this temperature range as for chol-1 and chol-1 frq10. The chol-1 wc double mutants were not rhythmic at 16°C on high choline concentrations and were not tested above 22°C.
Effects of Light on chol-1 Strains.
The effects of light on the rhythm of conidiation were assayed in the chol-1 double mutants strains. Two effects of light were assayed: the damping (suppression) of rhythmicity in cultures grown in LL, and the entrainment of the period of the rhythm by an imposed LD cycle. The results are presented in Fig. 1 and supplemental Tables 6 and 7 and are summarized in Fig. 3A. In the chol-1 strain as previously reported (22), LL damps out the rhythm (Fig. 1B, b). All four chol-1 double mutant strains were tested in LL (Fig. 1B), but none of the strains responded as strongly as chol-1 with complete damping of all replicate tubes. The chol-1 wc-2 strain appeared to respond more strongly to light than the other double mutant strains.
The chol-1 strain will entrain to LD cycles near its intrinsic period, as judged by the consistent phase angle between the band and the LD cycle, and the change in period (ref. 22 and supplemental Tables 6 and 7). In contrast, the double mutant strains did not consistently entrain to a 48-h 24:24 LD cycle, although the LD cycle period was close to the intrinsic period of the cultures in continuous darkness (supplemental Tables 6 and 7). All four strains were tested for entrainment in a 72-h 24:48 LD cycle on zero choline, and only the chol-1 strain entrained. The chol-1 frq10 strain also failed to entrain to a 24-h 12:12 LD cycle when supplemented with 5, 10, or 100 μM choline, although the chol-1 strain did entrain under these conditions. The chol-1 frq9 strain was not assayed for entrainment. These results indicate that the null frq mutation and the two wc mutations decrease the sensitivity of the rhythm to the effects of light.
Analysis of cel Double Mutants.
The cel frq10 double mutants were constructed as described in the supplemental Methods. The cel strain is rhythmic with a circadian period on minimal medium at 22°C, but all cel frq10 double mutant isolates tested were arrhythmic under these conditions (Fig. 3B and supplemental Table 3). The frq9 and frq10 strains are sometimes rhythmic after several days in culture (11, 12). Therefore a cel frq10 strain was tested on long growth tubes in addition to Petri plates to allow more days of growth, but no rhythmicity was seen up to 150 h of growth on minimal medium. The period of the cel strain can be lengthened by addition of the unsaturated fatty acids 18:2 (linoleic acid), 18:3 (linolenic acid), and 20:3 (eicosatrienoic acid) to the growth medium. Under these conditions, the cel frq10 double mutant strains showed clear banding, with long periods comparable to the cel mutant strain (Fig. 3B and supplemental Table 3). None of the fatty acids tested restored rhythmicity to cel+ frq10 strains.
The period of the cel strain can be lengthened not only by unsaturated fatty acids but also by growth at low temperature (20), and the double mutant strains also produced long periods at low temperature (Fig. 3B). On minimal medium at temperatures below 22°C, the period of the cel frq10 strain was in the range of 30–40 h, similar to the cel strain. At higher temperatures, the period-lengthening effects of unsaturated fatty acids can be reversed (20). At 30°C on 18:2-supplemented medium, the cel strain has a period of about 20 h, but cel frq10 was arrhythmic. These results indicate that the cel frq10 double mutant strains produced long-period rhythms only under conditions that lengthen the period of cel.
A cel frq9 strain was also constructed, as in the supplemental Methods. All of the cel frq9 progeny from the original cross and the backcross were arrhythmic on minimal medium but produced clear bands when supplemented with 18:2. The cel frq9 strains were not characterized further.
The cel wc-2 strains were constructed as in the supplemental Methods and the results were similar to those for cel frq10 (Fig. 3B and supplemental Table 3). The cel wc-2 strains failed to produce bands on minimal medium or when supplemented with palmitic acid (16:0), but produced long-period rhythms when supplemented with 18:2. At low temperature (16°C), the double mutants were again rhythmic, with the long periods characteristic of the cel parent (>34 h).
The cel double mutant strains were also tested for sensitivity to light, and the results are summarized in Fig. 3B. Rhythmic cel frq10 cultures on 18:2 supplementation failed to entrain to artificial 12:12 LD cycles or to natural ambient daylight cycles, although approximately half of the cel cultures grown under similar conditions were entrained. The long-period cel frq10 cultures also failed to entrain to LD cycles of 18:18, 24:24, or 36:36. Attempts to entrain the cel wc-2 strains to 12:12 LD cycles were also unsuccessful. These results indicate that the cel frq10 and cel wc-2 strains are less sensitive to light than the cel parental strain.
Discussion
Our results indicate that rhythmic conidiation in the chol-1 and cel strains does not require the frq, wc-1, or wc-2 gene products when the strains are grown in lipid-deficient conditions. However, the frq, wc-1 and wc-2 gene products are required for light sensitivity of the rhythm. We have previously shown that short-period alleles of frq significantly shorten the period of the rhythm in the lipid-deficient strains (23, 24), and the wc-2 mutation may lengthen the period in chol-1 (supplemental Table 3). The frq, wc-1, and wc-2 gene products therefore interact with the rhythms in lipid-deficient conditions, providing light input and influencing the period in constant darkness.
Rhythmicity has previously been reported in frq null and wc mutants under some conditions: in frq null mutants, cultures are initially arrhythmic but often become rhythmic after several days of growth (11, 12). Merrow et al. (27) have recently reported that a null frq mutant and wc mutants will produce rhythmic conidiation when exposed to temperature cycles but not light/dark cycles. The rhythmicity in chol-1 and cel double mutants differs from these previous reports in that it is robust and free running: it is found in nearly all cultures from the first day after inoculation and does not require an entraining temperature cycle to sustain it.
What is the relationship between the circadian rhythms seen in the wild type, the rhythmicity seen in null frq mutants after several days of growth, the rhythmicity seen in frq null and wc mutants in temperature cycles, and the long-period rhythms in chol-1 and cel? There are several possibilities, which can be polarized into two extreme options: (i) There may be many separate oscillators in Neurospora that control conidiation under different conditions: (a) a FRQ- and WC-based circadian oscillator in wild type; (b) a FRQ- and WC-independent oscillator that appears in frq null mutants after several days of growth and in temperature cycles; (c) a metabolic oscillator that overrides the circadian oscillator in lipid-deficient conditions; (d) possibly others. Each oscillator may have its own unique set of components that generate rhythmic conidiation. (ii) All forms of rhythmic conidiation may be driven by the same basic oscillator made of the same set of components. This oscillator would be capable of displaying a range of properties depending on the conditions.
There are several lines of evidence supporting option ii: (a) The frq, wc-1 and wc-2 gene products interact with the oscillator driving rhythmic conidiation in lipid-deficient conditions; (b) the rhythms seen in lipid-deficient conditions retain light-sensitivity (in frq+ wc+ strains); (c) the properties of the conidiation rhythm in cel and chol-1 change gradually as the concentration of growth supplement is varied, or as the temperature is varied, without any obvious or abrupt switch from one type of rhythmicity to another. For example, the period of chol-1 can be varied gradually from 20 h to 60 h by changing the choline concentration in the medium (Fig. 2A). (d) The frq alleles also change the properties of the oscillator in a gradient of effects: compared with the wild type, the short-period frq alleles are more sensitive to light (4, 5) and have better temperature compensation (3); the long-period alleles are less sensitive to light and have poor temperature compensation (3–5), and the null frq alleles have very poor temperature compensation (11, 12) and very poor sensitivity to light (our results). Therefore, it is possible that there is one oscillator driving rhythmic conidiation in Neurospora, and its parameters can be changed by frq and wc mutations or by lipid deficiency such that its period, temperature compensation, and light sensitivity are altered.
A mathematical model recently published by Roenneberg and Merrow (28) provides a third option. These authors propose a system of two interacting oscillators in which a “core” metabolic oscillator depends for its period and stability on rhythmic input from a pathway that is itself rhythmically modulated by output from the core oscillator. The white-collar mutants were originally isolated as blue-light “blind,” and their phenotypes place them within the light input pathway to the circadian oscillator (18). Both gene products may be photoreceptors and are required for the light induction of many other genes. The arrhythmicity of the wc mutants has been interpreted as indicating that these gene products are essential components of the circadian oscillator (18). As their blind phenotypes indicate, these genes may instead be components of blue-light input pathways. The arrhythmicity of wc mutants under standard culture conditions may therefore indicate that the activities of the light input pathway(s) are crucial to normal functioning of the circadian oscillator. The observation that rhythmicity can be restored to wc mutants in the chol-1 and cel strains suggests that the requirement for an intact light input pathway can be bypassed in these lipid-deficient mutants. Rhythmicity in frq9 and frq10 can also be restored in cel and chol-1, although the double mutants are relatively insensitive to light (our results). The frq, wc-1, and wc-2 gene products do not seem to be essential to generate rhythmicity and may therefore act primarily to provide input to a core oscillator. This type of model can explain the major experimental findings cited as support for a frq-based autoregulatory feedback loop oscillator (2, 6) and can in addition explain the persistence of rhythmicity in the absence of any frq gene product.
How might changes in lipid composition alter the properties of the circadian system and bypass the requirement for FRQ and WC? In the chol-1 mutant, choline deficiency leads to a decrease in the level of phosphatidylcholine and an increase in the levels of phosphatidylethanolamine, phosphatidylinositol, and neutral lipids (21). In cel, the fatty acid composition is altered under long-period conditions (29). Both mutations should alter membrane properties, and the phenotypes of these mutants lend weight to the proposal that membrane properties participate in regulating circadian rhythmicity (reviewed in refs. 30 and 31). Alternatively, both mutations may alter the level and/or fatty acid composition of lipids involved in intracellular signaling pathways.
Supplementary Material
Acknowledgments
Data for the chol-1 strains were collected in the laboratory of P.L.-T. in the Department of Zoology, University of Cambridge, with technical assistance from Ingrid Wesley and financial support from the Wellcome Trust (grant ref. 045355). Data for the cel strains were collected in the laboratory of S.B. in the Department of Biology, University of California, San Diego, CA.
Abbreviations
- LL
constant light
- LD
light/dark
- DD
constant dark
References
- 1.Lakin-Thomas P L, Coté G G, Brody S. Crit Rev Microbiol. 1990;17:365–416. doi: 10.3109/10408419009114762. [DOI] [PubMed] [Google Scholar]
- 2.Dunlap J C. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 3.Gardner G F, Feldman J F. Plant Physiol. 1981;68:1244–1248. doi: 10.1104/pp.68.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dharmananda S. Ph.D. thesis. Santa Cruz, CA: University of California; 1980. [Google Scholar]
- 5.Lakin-Thomas P L, Brody S, Coté G G. J Biol Rhythms. 1991;6:281–297. doi: 10.1177/074873049100600401. [DOI] [PubMed] [Google Scholar]
- 6.Aronson B D, Johnson K A, Loros J J, Dunlap J C. Science. 1994;263:1578–1584. doi: 10.1126/science.8128244. [DOI] [PubMed] [Google Scholar]
- 7.Garceau N Y, Liu Y, Loros J J, Dunlap J C. Cell. 1997;89:469–476. doi: 10.1016/s0092-8674(00)80227-5. [DOI] [PubMed] [Google Scholar]
- 8.Crosthwaite S K, Loros J J, Dunlap J C. Cell. 1995;81:1003–1012. doi: 10.1016/s0092-8674(05)80005-4. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Merrow M, Loros J J, Dunlap J C. Science. 1998;281:825–829. doi: 10.1126/science.281.5378.825. [DOI] [PubMed] [Google Scholar]
- 10.Luo C, Loros J J, Dunlap J C. EMBO J. 1998;17:1228–1235. doi: 10.1093/emboj/17.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronson B D, Johnson K A, Dunlap J C. Proc Natl Acad Sci USA. 1994;91:7683–7687. doi: 10.1073/pnas.91.16.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loros J J, Feldman J F. J Biol Rhythms. 1986;1:187–198. doi: 10.1177/074873048600100302. [DOI] [PubMed] [Google Scholar]
- 13.Ballario P, Macino G. Trends Microbiol. 1997;5:458–462. doi: 10.1016/S0966-842X(97)01144-X. [DOI] [PubMed] [Google Scholar]
- 14.Ballario P, Vittorioso P, Magrelli A, Talora C, Cabibbo A, Macino G. EMBO J. 1996;15:1650–1657. [PMC free article] [PubMed] [Google Scholar]
- 15.Linden H, Macino G. EMBO J. 1997;16:98–109. doi: 10.1093/emboj/16.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballario P, Talora C, Galli D, Linden H, Macino G. Mol Microbiol. 1998;29:719–729. doi: 10.1046/j.1365-2958.1998.00955.x. [DOI] [PubMed] [Google Scholar]
- 17.Russo V E A. J Photochem Photobiol B. 1988;2:59–65. doi: 10.1016/1011-1344(88)85037-1. [DOI] [PubMed] [Google Scholar]
- 18.Crosthwaite S K, Dunlap J C, Loros J J. Science. 1997;276:763–769. doi: 10.1126/science.276.5313.763. [DOI] [PubMed] [Google Scholar]
- 19.Brody S, Martins S A. J Bacteriol. 1979;137:912–915. doi: 10.1128/jb.137.2.912-915.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattern D L, Forman L R, Brody S. Proc Natl Acad Sci USA. 1982;79:825–829. doi: 10.1073/pnas.79.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard S C, Brody S. J Biol Chem. 1975;250:7173–7181. [PubMed] [Google Scholar]
- 22.Lakin-Thomas P L. Biol Rhythm Res. 1996;27:12–30. [Google Scholar]
- 23.Lakin-Thomas P L. J Biol Rhythms. 1998;13:268–277. doi: 10.1177/074873098129000101. [DOI] [PubMed] [Google Scholar]
- 24.Lakin-Thomas P L, Brody S. Genetics. 1985;109:49–66. doi: 10.1093/genetics/109.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis R H, deSerres F J. Methods Enzymol. 1970;17A:79–143. [Google Scholar]
- 26.Lakin-Thomas P L. J Biol Rhythms. 1992;7:227–239. doi: 10.1177/074873049200700304. [DOI] [PubMed] [Google Scholar]
- 27.Merrow M, Brunner M, Roenneberg T. Nature (London) 1999;399:584–586. doi: 10.1038/21190. [DOI] [PubMed] [Google Scholar]
- 28.Roenneberg T, Merrow M. J Biol Rhythms. 1998;13:167–179. doi: 10.1177/074873098129000011. [DOI] [PubMed] [Google Scholar]
- 29.Coté G G, Brody S. Biochim Biophys Acta. 1987;898:23–36. doi: 10.1016/0005-2736(87)90106-4. [DOI] [PubMed] [Google Scholar]
- 30.Coté G G, Lakin-Thomas P L, Brody S. In: Membranes and Circadian Rhythms. Vanden Driessche Th, Vanden Driessche Th., editors. Berlin: Springer; 1996. pp. 13–46. [Google Scholar]
- 31.Lakin-Thomas P L, Brody S, Coté G G. Chronobiol Intl. 1997;14:445–454. doi: 10.3109/07420529709001467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.