Abstract
The use of sewage or wastewater in agriculture is becoming increasingly common as a result of global water scarcity. Intestinal nematode infections have been identified as the main health risk associated with this practise. To protect consumer and farmer health the WHO has set an intestinal nematode water quality standard. However because of a lack of well designed studies the validity of this guideline is questioned. This paper presents the findings of a study on the risk of intestinal nematode infections in farming families occupationally exposed to untreated and partially treated wastewater in Hyderabad, India.
The study found an increased risk of hookworm (OR: 3.5, 95% CI 2.2-5.5), Ascaris lumbricoides (OR: 5.3, 95% CI: 2.0-14) and Trichuris trichiura (OR: 5.6, 95% CI: 1.8-18) infection when untreated wastewater (150 intestinal nematode ova/L) was used for crop production. The use of partially treated wastewater (28 intestinal nematode ova/L) was only associated with an increased risk (OR: 3.2, 95% CI: 1.2-8.6) of Ascaris lumbricoides infection. The findings of the study suggest that the current WHO intestinal nematode guideline of 1 ova/L is sufficient to protect farmer health.
INTRODUCTION
In the next 25 years the world population is expected to grow by almost two billion people, a growth which will predominantly take place in urban centres in developing countries (1). As a result of this unprecedented growth fresh water will become increasingly scarce and is expected to be the primary constraint for increased food production (2). The use of (treated) wastewater for crop irrigation has been suggested as one of the possible ways out of a looming water crisis (3). The construction, operation and maintenance costs of wastewater treatment plants are high and, the reality is that in many developing countries wastewater is used without any form of treatment (4).
The use of wastewater poses a number of health risks (5). Predominant among these is the risk of intestinal helminth infection (3). A review by the World Health Organization (WHO) identified several epidemiological limitations in past studies on wastewater use in agriculture, which means that credible evidence to guide policy is lacking (6). These limitations include one or more of the following: no inclusion of a control group of unexposed farmers; no control for confounding variables; absence of test results and helminth ova concentrations in the water used for irrigation were not assessed. Moreover, few studies quantified intensity of infection, a key determinant of transmission intensity and morbidity risk (7). Finally, most studies focused almost exclusively on A. lumbricoides and/or T. trichiura, whereas hookworm often exhibits the strongest association with (irrigated) agriculture (8).
This paper presents the findings of a cross-sectional study conducted in three wastewater quality zones in and downstream of the city of Hyderabad, India. The primary objective was to investigate the risk of hookworm infection as a result of occupational exposure to wastewater with varying hookworm concentrations.
METHODS
Study area
The city of Hyderabad (78.47′E, 17.45′N) is located on the Deccan plateau in southern India and has an estimated population of 6.8 million (9). The city’s wastewater is disposed of untreated into the Musi-River from where it is used for irrigation. In the city an estimated 250 households use wastewater from the river to irrigate approximately 500 hectares of land (10). Downstream of Hyderabad, with the help of weirs, water from the Musi-River is used to irrigate 3,100 hectares of agricultural land.
Water quality
A water quality survey was undertaken on the Musi-River, which showed typical properties of domestic sewage with high E. coli and helminth concentrations (11). Water quality improves dramatically with increasing distance from Hyderabad. This led to the formulation of the hypothesis that with increased distance from the city lower hookworm prevalence could be expected in farmers occupationally exposed to Musi-River water.
Study population
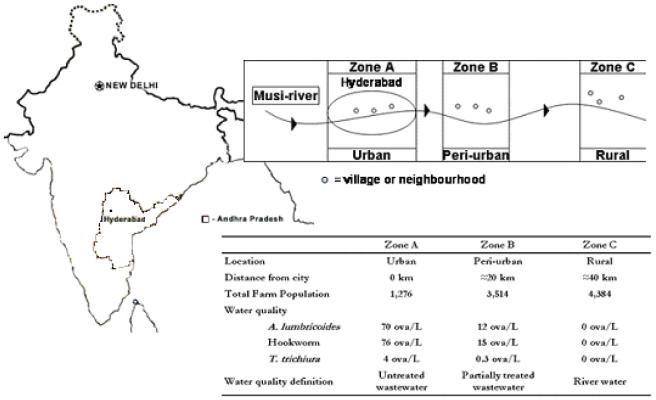
To test the hypothesis a yearlong water quality survey was setup (11) and with the help of this survey three water quality zones were identified along the river (Figure 1). Zone-A was situated in the centre of Hyderabad. Water quality was here classified as untreated wastewater with a mean hookworm ova concentration of 76 ova/L. Zone-B was situated in the peri-urban zone of Hyderabad. Farmers used water that was diverted from the first weir on the Musi-River and with a mean hookworm ova concentration of 15 ova/L, water was defined as partially treated wastewater. Zone-C is a rural zone where farmers used irrigation water free of hookworm ova, water quality was defined as river water. Each zone was separated from the downstream zone by a buffer zone in which no villages were selected. In each zone three villages, or for Zone-A neighbourhoods, were randomly selected from a list of villages/neighbourhoods within that zone. A population census was completed in each village/neighbourhood during the period December 2003 to April 2004.
Figure 1.
Schematic overview of the location and characteristics of the three study zones, villages and neighbourhoods in and downstream of Hyderabad.
Sample size for each of the three exposure groups was calculated for hookworm prevalence following cluster simulations for a study power of at least 80 percent, a 95 percent confidence interval (CI) and a compliance of 80 percent. An equal number of households per cluster (i.e. village or neighbourhoods) were selected to maximise the power of between-cluster comparison. This resulted in a required sample of 580 (adult) individuals. Based on the census it was estimated that approximately 30 households per cluster were required to meet the calculated sample size.
Households, defined here as individuals sharing food from one kitchen, were randomly selected from the census list. To ensure that the selected household was indeed involved in agriculture and exposed to the water quality in the selected zone, a brief check list was used. The aim of this check-list was to exclude farmers working as casual labourers in other water quality zones and to exclude land-owners who had agricultural labourers carrying out all agricultural activities for them. All members of the household above the age of 2 were assigned an identification number.
Data Collection
Baseline data regarding age, gender and education of the selected individuals were collected by means of a pre-tested questionnaire. A household questionnaire was designed to collect socio-economic (type of house construction, ownership of key household items), hygiene (type of water supply and household water use) and sanitation (presence or absence of a latrine) and agricultural variables (crops cultivated, type and number of cattle, landownership). On the day of stool sample collection an individual questionnaire was administered which dealt with personal hygiene (hand washing before eating, and after defection) and sanitation (preferred place of defecation), agricultural activities involved in, and the use of footwear.
Fresh stool samples were collected in the period August 2004 to May 2005 and analysed using the formalin-ether concentration technique (12). Stool sample containers were distributed in the morning and collected within 24 hours after distribution. Approximately 1 gram of stool was fixed in a pre-weighted 10 ml tube containing 6 ml of formalin (30% formaldehyde). The tube containing the stool sample was weighed. The sample was then transferred to a Parasep® (DiaSys Europe Ltd, Wokingham, UK) faecal concentrator tube, after which 2 ml of ethyl-acetate was added. Sediment was analysed for all nematode ova. A sample was declared negative if five slides yielded no ova. All sediment in the tube was analysed in case a slide was found to be positive. The intensity of infection was expressed as eggs per gram of stool (epg).
Data analysis
The association between water quality and intestinal nematode infection was explored using a pre-formulated plan of analysis. Household characteristics per zone were compared using ANOVA analysis for continuous and Chi-squared test categorical variables. Two main outcome variables were identified: prevalence of hookworm and heavy hookworm infection and two secondary outcome variables: A. lumbricoides and T. trichiura infection. The WHO has suggested a threshold for what is considered a heavy infection (13), though in reality this might vary with nutritional status, age and the species of hookworm (14). Therefore, the upper 95 percentile of egg counts in the population was chosen as a cut-off point: here defined as >160 epg.
Univariate logistic regression analysis was carried out for all four outcome variables to identify other risk factors and confounders. Variables which showed an association of P<0.20 in univariate analysis were included into a multivariate logistic regression model. Variables which in the logistic regression model showed non-significant association (P>0.05) were in a backward stepwise mode removed from the model. Risk was reported as odds ratio (OR) with corresponding 95% confidence interval (CI). The robustness of the model was tested using the Likelihood Ratio Test (LRT). Because helminth infections tend to cluster within households (15-17), household was included into the multivariate logistic regression model as a random effect and standard errors were calculated using sandwich estimation. All statistical analysis was conducted in STATA 7.0 (STATA-Corporation, College Station, USA).
Ethical considerations
Ethical clearance for this study was obtained in November 2003 from the ethics committees of the LSHTM and in June 2004 from Osmania Medical College, India. Written informed consent was given by all households during household selection. All individuals with a positive stool sample were provided with a single dose of Albendazole (Zentel, GSK) and at the end of the study a general health camp was organized for all participating households.
RESULTS
A total of 1,078 people in 251 households provided informed consent. In Zone-A insufficient eligible households were identified and therefore an average of 23 households per cluster were selected instead of the 30 households stated in the study protocol. The reasons for this lower number of selected households were two-fold: (i) only a limited number of households which were exclusively involved in agriculture were identified and (ii) reluctance to participate in the study, because of a fear that a negative outcome of the study would result in wastewater use being banned and thus a loss of subsistence. This sampling bias could potentially have affected the association between (waste)water quality and hookworm infection. However, as the results show, hookworm prevalence in farmers using untreated wastewater was very high and the lower number of households participating in the study might have affected the strength, but not the direction of the association. Compliance of those in households that had agreed to participate in the study was 93% (1007 individuals).
Population characteristics
Significant differences in socio-economic, water and sanitation characteristics were evident between the three different exposure groups (Table 1). Farming families in Zone-A had significantly lower socio-economic status, reflected in lower caste, literacy rates, house and land ownership levels relative to farmers in the other two zones. However, sanitation and hygiene, expressed by the ownership of a latrine, access to water within the household and the use of footwear was better in Zone-A as compared to the other two zones. Rice was the most commonly grown crop in Zone-C, while fodder grass and, to a lesser extent, leafy vegetables were grown in Zone-A. Zone-B was a transition zone where both rice and fodder grass was cultivated. Children had no clearly defined activities in agriculture, though they did accompany their parents to the fields and were observed playing in the agricultural fields around the village.
Table 1.
General, socio-economic, water and sanitation characteristics of the three exposure groups (values in parantheses are one standard deviation)
| Zone A | Zone B | Zone C | Test Statistic | P value | |
|---|---|---|---|---|---|
| n = 240 | n = 354 | n = 413 | |||
| Age | |||||
| Adult | 64.6% | 61.3% | 64.6% | χ2=1.1 | 0.58 |
| Child | 35.4% | 38.7% | 35.4% | ||
| Period worked in irrigated agriculture (years) | 11.4 (12.4) | 10.3 (12.1) | 12.1 (13.7) | F = 1.9 | 0.16 |
| Gender | |||||
| Female | 63.8% | 46.6% | 48.7% | χ2=19 | <0.001 |
| Male | 36.2% | 53.3% | 51.3% | ||
| Education | |||||
| Illiterate | 59.0% | 49.7% | 50.6% | χ2=7.7 | 0.10 |
| < 6 years | 14.2% | 16.9% | 19.9% | ||
| ≥ 6 years | 26.8% | 33.3% | 29.5% | ||
| Caste | |||||
| Economic weak | 68.3% | 52.8% | 33.9% | χ2=163 | <0.001 |
| Economic strong | 31.7% | 47.2% | 66.1% | ||
| Land ownership (acres) | 2.6 (1.7) | 1.4 (1.1) | 1.9 (1.8) | F = 4.7 | <0.001 |
| Presence of latrine | |||||
| No | 13.8% | 42.1% | 50.4% | χ2=88 | <0.001 |
| Yes | 86.3% | 57.9% | 49.6% | ||
| Preferred defecation | |||||
| Open defecation | 22.9% | 47.9% | 44.6% | χ2=42 | <0.001 |
| Latrine | 77.1% | 52.1% | 55.4% | ||
| Use of footwear | |||||
| Never | 27.8% | 62.3% | 40.5% | χ2=93 | <0.001 |
| Sometimes | 43.3% | 34.6% | 48.0% | ||
| Always | 29.9% | 3.1% | 11.5% | ||
| Type of water supply | |||||
| Outside the HH* | 55.0% | 67.8% | 100% | χ2=149 | <0.001 |
| HH* connection | 45.0% | 32.2% | 0% | ||
| Soap used last time when hands were washed | |||||
| No | 99.6% | 93.1% | 98.3% | χ2=24 | <0.001 |
| Yes | 0.4% | 7.0% | 1.8% |
HH= Household
Prevalence and intensity of intestinal nematode infection
Overall, 31.2 percent of individuals were infected by at least one intestinal nematode infection; of these, hookworm was found to be the most prevalent (29.8 percent) followed by A. lumbricoides and T. trichiura (Table 2). The mean intensity of infection ranged from 35 epg for hookworm to 1.7 epg for T. trichiura. The highest intensity of infection in an individual was found for hookworm (1,789 epg), followed by A. lumbricoides (1,333 epg) and T. trichiura (336 epg). A significant difference in hookworm, A. lumbricoides and T. trichiura prevalence was found between the three zones (Table 3). Farming families in zone-A had a significantly higher prevalence of hookworm, A. lumbricoides, and T. trichiura infection. In addition they also exhibited a significantly higher intensity of infection for all three infections relative to farming families in Zone-B and C. When compared to farming families in Zone-C, farming families in Zone-B had a significantly higher prevalence of A. lumbricoides infection, though no significant difference in the prevalence of hookworm or T. trichiura was found.
Table 2.
Intestinal nematode prevalence and intensity of infection in the general population and in the three exposure groups
| Total population (n = 1,007) | Zone A (n = 240) | Zone B (n = 354) | Zone C (n = 413) | P | |
|---|---|---|---|---|---|
| Prevalence (%) | |||||
| A. lumbricoides | 5.6 | 10.0 | 6.5 | 2.2 | <0.001 |
| Hookworm | 29.8 | 55.4 | 18.9 | 24.2 | <0.001 |
| Hookworm (epg>160) | 5.2 | 12.9 | 2.5 | 2.9 | <0.001 |
| T. trichiura | 3.1 | 8.3 | 1.3 | 1.6 | <0.001 |
| Intensity of infection (epg) * | |||||
| A. lumbricoides | 11 (80) | 30 (148) | 6.6 (39) | 0.3 (2.5) | <0.001 |
| Hookworm | 35 (129) | 78 (198) | 15 (86) | 19 (80) | <0.001 |
| T. trichiura | 1.7 (17) | 6.1 (32) | 0.1 (1.2) | 0.1 (1.5) | <0.001 |
Mean, values in parentheses are one standard deviation
Table 3.
Effect of exposure to untreated wastewater and partially treated wastewater on A. lumbricoides, hookworm, heavy hookworm and T. trichiura infection
| Water Quality | ||||
|---|---|---|---|---|
| OR | 95% CI | P | ||
| A. lumbricoides a | ||||
| River water | 0 ova/L | 1.0 | ||
| Partially treated wastewater | 12 ova/L | 3.2 | 1.2 - 8.6 | 0.02 |
| Untreated wastewater | 70 ova/L | 5.3 | 2.0 - 14 | 0.001 |
| Hookworm b | ||||
| River water | 0 ova/L | 1.0 | ||
| Partially treated wastewater | 15 ova/L | 0.7 | 0.4 - 1.1 | 0.11 |
| Untreated wastewater | 76 ova/L | 3.5 | 2.2 - 5.5 | <0.001 |
| Hookworm (epg>160) c | ||||
| River water | 0 ova/L | 1.0 | ||
| Partially treated wastewater | 15 ova/L | 0.8 | 0.3 - 2.2 | 0.65 |
| Untreated wastewater | 76 ova/L | 3.9 | 1.5 - 9.9 | 0.004 |
| T. trichiura d | ||||
| River water | 0 ova/L | 1.0 | ||
| Partially treated wastewater | 0.3 ova/L | 0.6 | 0.2 - 2.5 | 0.53 |
| Untreated wastewater | 4 ova/L | 5.6 | 1.8 - 18 | 0.003 |
Controlled for age, sex, education and household clustering
Controlled for age, education, caste, latrine presence and household clustering
Controlled for age, sex, education, type of water supply, agricultural activities involved in and household clustering
Controlled for sex, education and household clustering
Wastewater quality and intestinal nematode infection
Among all individuals, the use of untreated wastewater, when controlled for confounding variables, was associated with an almost four-fold increased risk of hookworm and heavy hookworm infection (Table 3). This was in contrast to the use of partially treated wastewater, which showed no significant association with hookworm infection when controlled for confounding variables. The use of untreated wastewater further showed an over five-fold increased risk of A. lumbricoides and T. trichiura infection. The use of partially treated wastewater was associated with an over three-fold increased risk of A. lumbricoides infection, while no significant association was found for T. trichiura infection when controlled for confounding variables.
DISCUSSION
This study found a significantly increased risk of A. lumbricoides, hookworm and T. trichiura infection in farming communities irrigating with wastewater. The highest risks were found to be associated with the use of untreated wastewater, while the use of partially treated wastewater was associated only with an increased risk of A. lumbricoides infection. The use of untreated wastewater was also associated with a higher intensity of infection, especially for hookworm infection.
With the exception of one study conducted in Vietnam (18), all past studies on wastewater irrigation have found an increased risk of at least one intestinal nematode infection in farming families occupationally exposed to the wastewater (3). Studies in Mexico and Morocco have associated wastewater irrigation with an increased risk of A. lumbricoides and/or T. trichiura infection (19-21), while studies on the Indian Subcontinent associated wastewater irrigation with an increased risk of hookworm, and to a lesser degree A. lumbricoides infection (22-25). The current study corroborates findings of all past studies (3) and is the first that found an increased risk for three intestinal nematode infections.
Although extended exposure to wastewater with high ova concentrations can be expected to result in a higher intensity of infections, only one study reported this; Shuval et al. (5) presented the findings of a study conducted in India in 1973, which reported a significantly higher number of medium and heavy hookworm infections in farmers using sewage as compared to regular farmers. One of the study sites in the 1973 survey corresponded with the urban sites chosen for this survey. The 1973 survey reports a similar hookworm prevalence (59 percent vs 55 percent), though a much higher A. lumbricoides prevalence (29 percent vs 10 percent). However, because no information was provided on water quality and/or hygiene and sanitation facilities it is impossible to determine whether this lower prevalence came as a result of better water quality or because of improvements in hygiene and sanitary conditions.
The results of this survey can help evaluate the current WHO guidelines on the use of wastewater in agriculture (3). To protect both farmer and consumer health the WHO in 1989 recommended a maximum nematode concentration of <1 ova/L for the unrestricted use of wastewater in agriculture (26). The validity of this water quality standard has previously been questioned and it has been suggested that the water quality standard was too strict and could be increased to 10 ova/L (27). However few studies have quantified the concentration of ova in wastewater used for irrigation, often just referring to irrigation water as either: untreated, partially treated, treated or clean (21, 22, 25, 28). Other studies which did quantify ova concentration failed to differentiate between the different helminth species (24) or ova concentrations in wastewater were too high (> 700 ova/L) to provide additional evidence to the discussion (23) .
As a result there is only a single study which has investigated the association between ova concentration in wastewater and the risk of nematode infections. Research in Mexico showed no increased risk of infection in adult farmers who worked with wastewater with ova concentrations <1 ova/L of wastewater, compared to farmers involved in rain-fed agriculture, though the same study did show an increased risk of infection in their children (19). Children often accompany their parents to the fields or play in the fields surrounding the villages and the Mexican findings resulted in a footnote to the recently updated WHO wastewater guidelines which now recommend an intestinal nematode guideline of <0.1 ova/L when children under the age of 15 are exposed (3).
The WHO has set a single nematode water quality standard. However as Table 3 shows, A. lumbricoides and T. trichiura in this study showed a significant association with the use of wastewater at concentrations ranging from 3 to 12 ova/L, while hookworm at similar ova concentrations in wastewater showed no increased risk of infection. This would suggest that the WHO guidelines should differentiate between hookworm on the one hand and A. lumbricoides and T. trichiura on the other hand. This would mean that a more lenient water quality standard can be set when hookworm is the only infection associated with wastewater irrigation, for example in Faisalabad, Pakistan (23). The findings would further suggest that the current WHO guideline value of ≤ 1 ova/L (3, 26) is of the correct order of magnitude. The fact that no increased risk of T. trichiura was detected when wastewater with a concentration of 0.3 ova/L was used would suggest that the proposed nematode guideline of ≤ 0.1 ova/L when children are exposed to wastewater (3, 19) is too strict.
In this study, examination of the associations between the use of wastewater of a particular quality and intestinal nematode infections was hampered by several epidemiological difficulties, many of which are inherent to the practise of wastewater in agriculture. The first problem is the identification of a suitable control group of ‘clean’ water farmers. The high cost of municipal water combined with the high water demand by agricultural crops, means that an urban farmer is almost always synonymous with a farmer that uses sewage and that ‘clean’ water farmers can not be identified within the urban setting. By selecting rural farmers an urban-rural bias is introduced. Both A. lumbricoides and T. trichiura infection are associated with crowded environments and tend to be higher in urban as compared to rural populations (29). In addition wastewater farmers are often the urban poor without well established land or water rights. This was clearly shown in this study where urban farmers were of lower socio-economic status than rural farmers. This could possibly mean that the association between A. lumbricoides and T. trichiura and wastewater irrigation is not as strong as is suggested in this survey.
A further difficulty is caused by the characteristics of wastewater and its geographical isolated use. The one to one comparison between geographically distinct areas (untreated wastewater vs. river water and partially treated wastewater vs. river water) which was adopted in this study, and all other ‘wastewater’ studies is in principle statistically flawed (30). However the inclusion of additional random clusters is complicated by the fact that wastewater quality, and especially pathogen concentrations, will vary widely from neighbourhood to neighbourhood, and as a result wastewater quality used at different sites is not uniform. Moreover, wastewater irrigation can only occur where sewage is disposed of, this means that it is geographically constrained to a very limited number of sites and in the case of small cites often only one. In Hyderabad, relatively uniform water quality was found because all wastewater ended up in the Musi-River where it mixed and from where it was used for agricultural purposes. This allowed for the selection of three independent clusters (villages/neighbourhoods) per water quality zone to address the one-to-one comparison problem, though from a statistical point of view this is only a marginal improvement.
The WHO water quality standards are based on the best available epidemiological evidence and on the principle that there should be no additional cases of disease as a result of wastewater use in agriculture. This approach has made the WHO guidelines vulnerable to criticism, especially from supporters of the United States Environmental Protection Agency guidelines; which advocate a much stricter water quality standard based on the principle that there should be no additional risk as a result of wastewater use in agriculture (31). This discussion seems trivial but for developing countries a water quality standard which is too strict, will either mean needless investments in wastewater treatment technology or a situation, like in Hyderabad, where municipalities ignore the use of wastewater, pretending that it does not take place (4). To help strengthen the WHO guidelines more research is needed. A potential improvement on the chosen study design, which would strengthen the association between exposure to wastewater of particular quality and nematode infection would be a re-infection study, whereby exposure to wastewater and wastewater quality will be monitored on regular intervals.
Based on WHO standards (3) wastewater used in Hyderabad to irrigate was unfit for crop production. The most obvious way to mitigate health risks would be wastewater treatment. However, this would require large investments in wastewater treatment technology and an even larger investment in the sewerage system. Currently all sewerage pipes drain naturally into the Musi-River, and a complete reconstruction of the current infrastructure in Hyderabad is prohibitively expensive. Thus, the only option would be to ban wastewater use, and consequently the removal of farmers that use wastewater from their land. In addition to the questionable legality of such a move, it also highly undesirable, as an already vulnerable group would be deprived of their sole subsistence (32). As no crops are grown that are consumed uncooked, the use of wastewater does not pose a risk to the general public, while fodder grass production is key to the dairy industry in Hyderabad (32) thus making an important contribution to overall population health in Hyderabad.
Thanks to wide availability of cheap, single dose anthelmintic drugs nematode infections pose a relatively easy containable risk (13). Farmers using wastewater in Hyderabad have fought several court cases over the last decades to retain access to wastewater and are thus well organized and easily mobilized. This would make twice yearly anthelmintic treatment programmes in the wastewater farming communities an effective way to minimize the impact of wastewater irrigation in Hyderabad.
To help formulate national guidelines the WHO recommends epidemiological studies with a water quality component, similar to the one presented here (33). This study provided further evidence that the use of wastewater was associated with an increased risk of nematode infections. Although this study is unable to provide conclusive evidence about the validity of the current WHO wastewater nematode guideline, the findings do suggest that for A. lumbricoides and T. trichiura infection the current WHO nematode guideline of ≤ 1 ova/L is appropriate but that the nematode guideline of ≤ 0.1 ova/L when children are exposed is too strict. No increased risk of hookworm infection was detected when wastewater with a mean concentration of 15 ova/L was used, which would suggest that, at least for the risk of hookworm infection, the WHO nematode guideline is too strict and that a more lenient guideline can be set if hookworm species are the predominant ova in wastewater. However more studies are needed to confirm this.
ACKNOWLEDGEMENT
This paper is dedicated to Felix P. Amerasinghe, who provided invaluable assistance to the study but untimely passed away in June 2005. We thank Urmila Mata, Rama Devi and Liaquat Ullah, who conducted the interviews, collected stool samples and organized the health camps. Simon Cousens, Chris Scott, Wim van der Hoek, Flemming Konradsen, Laura Rodrigues, Mimi Jenkins, Sandy Cairncross and Anne Peasey, were part of the PhD panel of Jeroen H. J. Ensink and in this position contributed significantly to the design of the study. The Wastewater research in Hyderabad was supported from core money from the International Water Management Institute. Simon Brooker is supported by a Wellcome Research Career Development Fellowship (081673).
REFERENCES
- 1.UNPD 2003. [Accessed July 2005]. http://esa.un.org/unup/
- 2.Seckler D, Amarasinghe U, Molden D, de Silva R, Barker R. World water demand and supply, 1990 to 2025: scenarios and issues. Colombo: International Water Management Institute; 1998. [Google Scholar]
- 3.WHO . wastewater use in agriculture. Vol. 2. Geneva: World Health Organization; 2006. Guidelines for the safe use of wastewater, excreta and greywater in agriculture. [Google Scholar]
- 4.Scott CA, Faruqui NI, Raschid-Sally L. Wastewater use in irrigated agriculture: management challenges in developing countries. In: Scott CA, Faruqui NI, Raschid-Sally L, editors. Wastewater use in irrigated agriculture: confronting the livelihood and environmental realities. Wallingford: Cabi Publishing; 2004. pp. 1–10. [Google Scholar]
- 5.Shuval HI, Adin A, Fattal B, Rawitz E, Yekutiel P. Wastewater irrigation in developing countries: Health effects and technical solutions. Washington DC: The World Bank; 1986. [Google Scholar]
- 6.Blumenthal U, Peasey A. Critical review of epidemiological evidence of the health effects of wastewater and excreta use in agriculture. Geneva: WHO; 2002. [Google Scholar]
- 7.Anderson RMC, May RM. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford University Press; 1991. [Google Scholar]
- 8.Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197–288. doi: 10.1016/S0065-308X(04)58004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UN . World urbanization prospects, the 2001 revision. New York: UN; 2002. [Google Scholar]
- 10.Buechler S, Devi G, Raschid-Sally L. Livelihoods and wastewater irrigated agriculture: Musi river in Hyderabad city, Andhra Pradesh, India. Urban Agriculture. 2002:14–17. [Google Scholar]
- 11.Ensink JHJ. PhD thesis. London: University of London; 2006. Wastewater quality and the risk of hookworm infection in Pakistani and Indian sewage farmers. [Google Scholar]
- 12.Ridley DS, Hawgood BC. The value of formal-ether concentration of faecal cysts and ova. J Clin Pathol. 1956:74–76. doi: 10.1136/jcp.9.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . Prevention and control of intestinal parasitic infections. Geneva: WHO; 1987. [Google Scholar]
- 14.Brooker S, Peshu N, Warn PA, Mosobo M, Guyatt HL, Marsh K, Snow RW. The epidemiology of hookworm infection and its contribution to anaemia among pre-school children on the Kenyan coast. Trans R Soc Trop Med Hyg. 1999;93:240–246. doi: 10.1016/s0035-9203(99)90007-x. [DOI] [PubMed] [Google Scholar]
- 15.Olsen A, Samuelsen H, Onyango-Ouma W. A study of risk factors for intestinal helminth infections using epidemiological and anthropological approaches. J Biosoc Sci. 2001;33:569–84. doi: 10.1017/s0021932001005697. [DOI] [PubMed] [Google Scholar]
- 16.Behnke JM, De Clercq D, Sacko M, Gilbert FS, Ouattara DB, Vercruysse J. The epidemiology of human hookworm infections in the southern region of Mali. Trop Med Int Health. 2000;5:343–354. doi: 10.1046/j.1365-3156.2000.00553.x. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro AE, Tukahebwa EM, Kasten J, Clarke SE, Magnussen P, Olsen A, Kabatereine NB, Ndyomugyenyi R, Brooker S. Epidemiology of helminth infections and their relationship to clinical malaria in southwest Uganda. Trans R Soc Trop Med Hyg. 2005;99:18–24. doi: 10.1016/j.trstmh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Trang do T, van der Hoek W, Cam PD, Vinh KT, Hoa NV, Dalsgaard A. Low risk for helminth infection in wastewater-fed rice cultivation in Vietnam. J Water Healt. 2006;4:321–331. doi: 10.2166/wh.2006.013. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal UJ, Cifuentes E, Bennett S, Quigley M, Ruiz-Palacios G. The risk of enteric infections associated with wastewater reuse: the effect of season and degree of storage of wastewater. Trans R Soc Trop Med Hyg. 2001;95:131–137. doi: 10.1016/s0035-9203(01)90136-1. [DOI] [PubMed] [Google Scholar]
- 20.Cifuentes E. The epidemiology of enteric infections in agricultural communities exposed to wastewater irrigation: perspectives for risk control. Int J Environ Health Res. 1998;8:203–213. [Google Scholar]
- 21.Habbari K, Tifnouti A, Bitton G, Mandil A. Geohelminthic infections associated with raw wastewater reuse for agricultural purposes in Beni-Mellal, Morocco. Parasitol Int. 2000;48:249–254. doi: 10.1016/s1383-5769(99)00026-4. [DOI] [PubMed] [Google Scholar]
- 22.Krishnamoorthi KP, Abdulappa MK, Anwikar AK. Intestinal parasitic infections associated with sewage farm workers with special reference to helminths and protozoa; Proceedings of symposium on environmental pollution; Nagpur: Central Public Health Engineering Research Institute. 1973. [Google Scholar]
- 23.Ensink JHJ, van der Hoek W, Mukhtar M, Tahir Z, Amerasinghe FP. High risk of hookworm infection among wastewater farmers in Pakistan. Trans R Soc Trop Med Hyg. 2005;99:809–818. doi: 10.1016/j.trstmh.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Feenstra S, Hussain R, van der Hoek W. Health risks of irrigation with untreated urban wastewater in the southern Punjab, Pakistan. Lahore: International Water Management Institute; 2000. [Google Scholar]
- 25.Srivastava VK, Pandey GK. Parasitic infections in sewage farm workers. Indian J Parasitol. 1986;10:193–194. [Google Scholar]
- 26.WHO . Health guidelines for the use of wastewater in agriculture and aquaculture. Geneva: WHO; 1989. [PubMed] [Google Scholar]
- 27.Ayres RM, Lee DL, Mara DD, Silva SA. The accumulation, distribution and viability of human parasitic nematode eggs in the sludge of a primary facultative waste stabilization pond. Trans R Soc Trop Med Hyg. 1993;87:256–258. doi: 10.1016/0035-9203(93)90115-7. [DOI] [PubMed] [Google Scholar]
- 28.Bouhoum K, Schwartzbrod J. Epidemiological study of intestinal helminthiasis in a Marrakech raw sewage spreading zone. Zentralbl Hyg Umweltmed. 1998;200:553–561. [PubMed] [Google Scholar]
- 29.O’Lorcain P, Holland CV. The public health importance of Ascaris Lumbricoides. Parasitology. 2000;121:S51–S57. doi: 10.1017/s0031182000006442. [DOI] [PubMed] [Google Scholar]
- 30.Blum D, Feachem RG. Measuring the impact of water supply and sanitation investments on diarrhoeal diseases: Problems of methodology. Int J Epidemiol. 1983;12:357–365. doi: 10.1093/ije/12.3.357. [DOI] [PubMed] [Google Scholar]
- 31.USEPA . Guidelines for water reuse. Washington DC: United States Environmental Protection Agency; 2004. [Google Scholar]
- 32.Buechler S, Devi G. Household food security and wastewater dependent livelihood activities along the Musi-river in Andhra Pradesh, India. Geneva: WHO; 2002. [Google Scholar]
- 33.Blumenthal UJ, Mara DD, Peasey A, Ruiz-Pallacios G, Stott R. Guidelines for the microbiological quality of treated wastewater used in agriculture: recommendations for revising WHO guidelines. Bull World Health Organ. 2000;78:1104–1016. [PMC free article] [PubMed] [Google Scholar]