Abstract
The small molecule salubrinal has antiviral activity against herpes simplex virus-1 (HSV-1) and inhibits dephosphorylation of eIF2α mediated by the HSV-1 protein ICP34.5. We investigated whether salubrinal's activities in infected cells depend on ICP34.5. An ICP34.5 deletion mutant was as sensitive as wild type HSV-1 to salubrinal inhibition of plaque formation in Vero cells. However, salubrinal induced formation of syncytia in infected Vero cells, which was enhanced by ICP34.5 mutations. Expression of HSV-1 US11 with immediate early kinetics, which is known to suppress the effects of ICP34.5 mutations, resulted in slight resistance to salubrinal in murine embryonic fibroblasts, and substantial resistance in those cells when ICP34.5 was additionally mutated. ICP34.5 mutations, but not immediate early expression of US11, prevented salubrinal's ability to increase phosphorylation of eIF2α during HSV-1 infection of Vero cells. Taken together, our data indicate that salubrinal has both ICP34.5-dependent and - independent activities in HSV-1 infected cells.
Introduction
Virus infection elicits an innate antiviral response in which the host cell attempts to slow or stop viral replication (Schneider and Mohr, 2003). Like many viruses, HSV-1 encodes multiple gene products to counter these innate cellular defense mechanisms, allowing the virus to overcome the antiviral response of the infected cell (Schneider and Mohr, 2003). One major pathway employed by cells to inhibit viral replication is to phosphorylate the translation initiation factor eIF2α, which results in the global shutoff of translation and thus halts viral replication (Hinnebusch, 2000). HSV-1 encodes at least two gene products, ICP34.5 and US11, that act to counter this shutoff of global protein synthesis.
The viral gene product ICP34.5 mediates the dephosphorylation of eIF2α by binding protein phosphatase 1α (PP1α) and directing its activity to eIF2α (Chou and Roizman, 1992; He, Gross, and Roizman, 1997; He, Gross, and Roizman, 1998). eIF2α dephosphorylation allows viral genes to be translated although eIF2α kinases, such as PKR, remain active. ICP34.5 is homologous to the cellular protein GADD34, which can also bind PP1α and direct the dephosphorylation of eIF2 α (Fornace et al., 1989; Lord, Hoffman-Liebermann, and Liebermann, 1990; McGeoch and Barnett, 1991).
Additionally, US11, a virally encoded RNA binding protein, can bind the eIF2α kinase PKR, and inhibit its activity (Cassady and Gross, 2002; Cassady, Gross, and Roizman, 1998; Mulvey et al., 1999; Poppers et al., 2000). US11 is necessary to achieve maximal rates of protein synthesis at late times post infection, likely due to the ability of US11 to inhibit the high levels of activated PKR present at these times (Mulvey et al., 2003). US11 is normally expressed as a late gene product, but when it is ectopically expressed as an immediate early gene product, US11 has been shown to be able to compensate for ICP34.5 mutations in cells where ICP34.5 is required for viral replication and protein synthesis (He et al., 1997; Mohr and Gluzman, 1996).
The small molecule salubrinal (sal) was identified in a high throughput assay for its ability to protect cells from endoplasmic reticulum (ER) stress induced apoptosis (Boyce et al., 2005). Treatment of cells with sal increases the level of phosphorylated eIF2α, and this increase in phosphorylation was attributed to an inhibition of cellular GADD34-mediated dephosphorylation of eIF2α, rather than an increase in eIF2α kinase activity, making sal the first inhibitor of a particular dephosphorylation reaction (Boyce et al., 2005). The sal-dependent increase in eIF2α phosphorylation accounts for the resistance to ER stress induced apoptosis, presumably because the shutoff of translation and the gene expression program that is activated following eIF2α phosphorylation allow the cell to respond to and recover from the stress (Boyce and Yuan, 2006).
Given the homology between GADD34 and ICP34.5, the ability of sal to inhibit ICP34.5-mediated dephosphorylation of eIF2α and to inhibit HSV-1 replication was investigated (Boyce et al., 2005). Lysates from cells transfected with plasmids expressing ICP34.5 and treated with sal were inhibited for ICP34.5-mediated dephosphorylation of eIF2α relative to mock treated transfected cells (Boyce et al., 2005). Additionally, sal inhibited HSV-1 replication in Vero cells and murine embryonic fibroblasts with an IC50 of ~3µM (Boyce et al., 2005). Furthermore, sal had dramatically reduced antiviral activity in MEFs that contain a non-phosphorylatable eIF2α, indicating that phosphorylation of eIF2α is important for the full antiviral activity of sal (Boyce et al., 2005). However, it is not known whether ICP34.5 is required for the antiviral activity of sal or for the effects of sal on eIF2α during HSV-1 infection. In this report, we take a genetic approach to investigate these questions.
Results and Discussion
Antiviral activity of salubrinal is independent of ICP34.5 in Vero cells
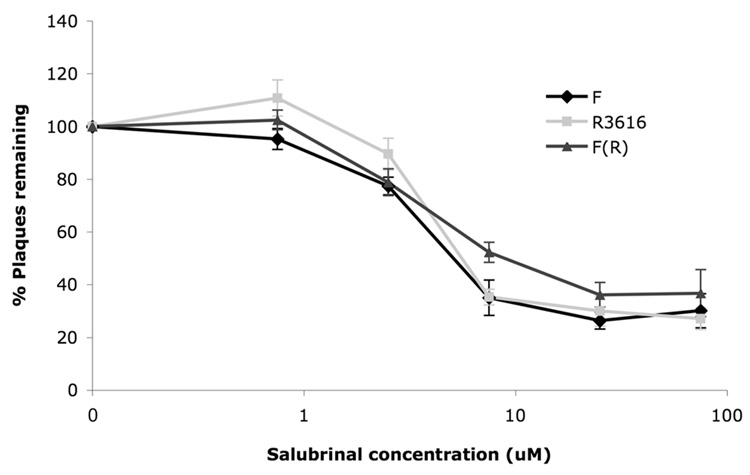
Sal has previously been shown to have anti-HSV-1 activity that is dependent on phosphorylatable eIF2α and extracts from sal treated cells expressing ICP34.5 were inhibited for the dephosphorylation of eIF2α (Boyce et al., 2005). To investigate whether expression of ICP34.5 is necessary for the antiviral activity of sal, we performed plaque reduction assays in Vero cells with HSV-1 wild type strain F; the ICP34.5 mutant R3616, which contains an approximately 1kb deletion in the gene encoding ICP34.5; or a rescued virus F(R), in which the 1kb deletion was restored with wild type strain F sequence (viruses were kindly provided by J. Chou and B. Roizman (Chou et al., 1990)). As observed before (Boyce et al., 2005), sal exhibited antiviral activity, but did not result in complete elimination of plaque formation by HSV-1 in Vero cells, even at high doses (Fig. 1). Sal treatment reproducibly inhibited plaque formation by all three viruses similarly (Fig. 1), indicating no dependence on ICP34.5 for the antiviral activity of sal in Vero cells. Possible explanations for this lack of ICP34.5 dependence are discussed towards the end of this section.
FIG. 1. Antiviral activity of sal in plaque reduction assays.
Vero cells were treated with either DMSO-containing vehicle or the indicated concentration of sal for 24 hours prior to infection with 150 pfu of HSV-1 strain F (black lines with diamonds), R3616 (light gray lines with squares), or F(R) (dark gray lines with triangles). Plaques were allowed to form for 2–3 days in the presence of the indicated concentration of sal and then were counted. Data are presented as percent of plaques remaining, relative to treatment with vehicle alone, at each sal concentration. Error bars represent standard errors of the mean (n=7 for strain F and R3616 and n=5 for F(R)). There was no significant difference (p values ranging between 0.072-0.97 by Student's t test) between F and R3616 at the various sal concentrations.
Salubrinal treatment results in a syncytial phenotype during HSV-1 infection
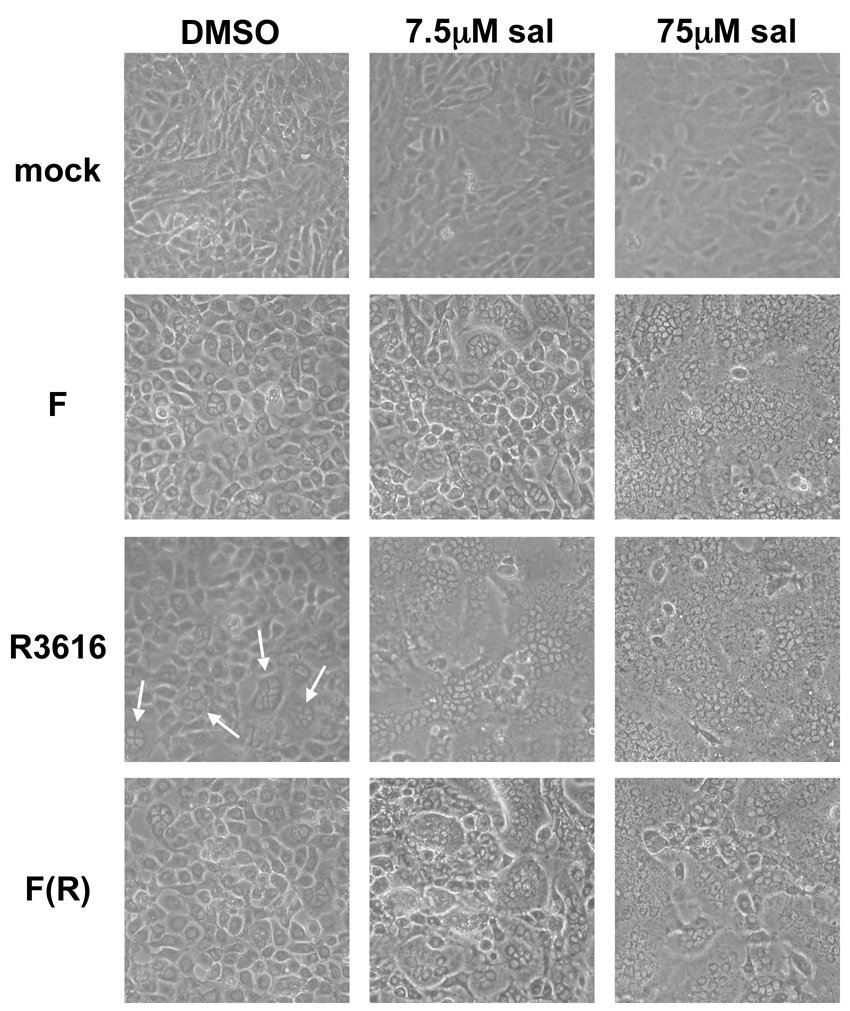
In the course of these studies, we observed that sal treatment induced syncytial cytopathic effect (CPE) in HSV-1 infected Vero cells. Syncytia were not observed in sal treated Vero cells that were mock infected (Fig. 2, top row). Following infection with strain F at a multiplicity of infection (MOI) of 3, syncytial CPE was observed upon treatment with 7.5µM sal, and nearly encompassed the entire infected monolayer at 75µM sal (Fig. 2, second row from top). However, syncytia were not evident in strain F infected cells that were not treated with sal. Interestingly, we observed sporadic small syncytia in untreated R3616-infected Vero cells (example in Fig. 2, third row from top, left panel, small syncytia indicated by arrows). Treatment with sal greatly enhanced the formation of syncytia in R3616-infected Vero cells so that they completely covered the infected monolayer following treatment with both 7.5µM and 75µM sal (Fig. 2, third row from top, middle and right panels). The effects of sal on syncytia formation in Vero cells infected with F(R), the virus with the ICP34.5 mutation restored, were similar to those observed in cells infected with strain F (Fig. 2, compare second row from top with bottom row). Thus, the more potent induction of syncytial CPE by sal in R3616-infected Vero cells was due to the ICP34.5 mutation. A similar enhancement of syncytial CPE induced by sal was observed with other ICP34.5 mutants (data not shown). Our results suggest that sal inhibits an activity that normally prevents the formation of syncytia in HSV-1 infected cells, and this prevention of syncytia formation is partially dependent on ICP34.5.
FIG. 2. Sal induces syncytial HSV-1 infection.
Vero cells were treated with either DMSO (left column) or the indicated concentration of sal (three right columns) for 24 hours prior to either mock infection (top row) or infection at a multiplicity of 3 with the indicated virus (bottom three rows). Infection was allowed to proceed for 18 hours in the presence of DMSO or the indicated concentration of sal and the cellular morphology was visualized by phase contrast light microscopy. Regions of small syncytia formation are indicated by arrows. Images were obtained by photographing with a Nikon Coolpix 4500 digital camera.
ICP34.5 has previously been linked to the syncytial phenotype; a mutation that results in syncytia formation was mapped to the region of the HSV-1 genome containing the ICP34.5 polyadenylation signal (Romanelli et al., 1991). Additionally, although mediating the dephosphorylation of eIF2α is the best characterized activity of ICP34.5 during HSV-1 infection, ICP34.5 has also been implicated as playing a role in viral egress (Brown et al., 1994; Jing et al., 2004; Jing and He, 2005). Thus, ICP34.5 itself may inhibit syncytia formation, either by permitting the synthesis of certain late viral proteins or by promoting viral egress. Therefore, sal may induce syncytia formation in part by direct or indirect inhibition of ICP34.5. Nevertheless, as sal-induced syncytia formation is enhanced by the absence of ICP34.5, sal must also act via some other target to cause this phenotype.
Effect of ectopic expression of IE US11 on the antiviral activity of salubrinal
Although ICP34.5 was not necessary for the antiviral activity of sal in Vero cells, these cells are permissive for ICP34.5 mutant virus replication, leaving the possibility that ICP34.5 may be required for the antiviral activity of sal in cells that are non-permissive for the replication of ICP34.5 mutants, such as MEFs. We could not directly test whether the antiviral activity of sal in MEFs depends on ICP34.5 because ICP34.5 null mutant viruses do not replicate in these cells. An alternate approach was suggested by previous studies that showed that expressing the late viral protein US11 as an immediate early gene (IE US11) can rescue the ICP34.5 mutant phenotypes of decreased viral replication and decreased viral protein synthesis in cells such as MEFs that are non-permissive for ICP34.5 mutant replication (He et al., 1997; Mohr and Gluzman, 1996). IE US11 viruses are able to inhibit the phosphorylation of eIF2α by interacting with and antagonizing the activity of PKR. We hypothesized that if inhibiting ICP34.5-mediated dephosphorylation was responsible for the antiviral activity of sal, then IE US11 viruses might be resistant to sal in MEFs because dephosphorylation of eIF2α is not expected to be required for replication of these mutant viruses.
To address this hypothesis we utilized two different IE US11 viruses, each in a different wild type background. We constructed a recombinant IE US11 mutant virus in the HSV-1 wild type strain KOS background using a strategy similar to one previously described (Mulvey et al., 1999). To generate this mutant, which we named NM1, we inserted the immediate early ICP27 promoter (Spector, Purves, and Roizman, 1990) upstream of the US11 open reading frame. This IE US11 expression cassette was then inserted into the thymidine kinase (tk) locus of HSV-1 strain KOS by homologous recombination, and the mutant virus was selected for loss of TK using acyclovir. The NM1 mutant virus contains a wild type US11 locus in addition to the locus encoding the ectopically expressed IE US11.
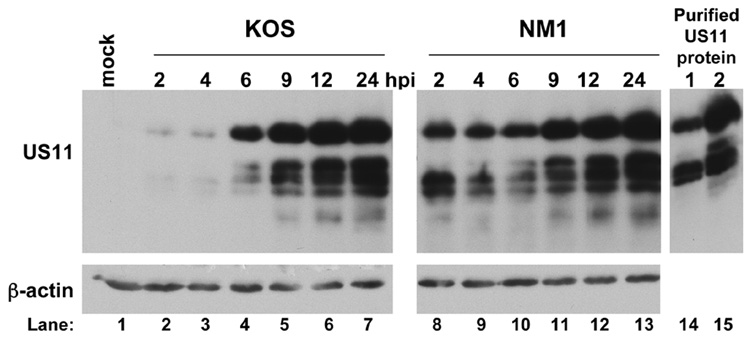
We confirmed the IE US11 expression phenotype of NM1 by western blot analysis (Fig. 3). Vero cells were infected with either KOS or NM1 for various times and lysates were prepared at the times indicated to assess US11 expression. In KOS infected cells, a protein both reacting with anti-US11 antisera and co-migrating with purified recombinant US11 (generously provided by Jennifer Baltz; Fig. 3, right panel) was readily detected starting at 6 hours post infection (Fig. 3, top left panel). In contrast, in NM1 infected cells, this protein was abundant as early as 2 hours post infection (Fig. 3, top left panel), confirming the IE expression of US11 in this mutant. Equivalent loading of each sample was demonstrated by probing for β-actin (Fig. 3, bottom left panel).
FIG. 3. Western blot analysis of US11 expression.
MEF cells were either mock infected (lane 1), infected with KOS at an MOI of 10 (lanes 2–7) or infected with NM1 at an MOI of 10 (lanes 8–13) and harvested at 2, 4, 6, 9, 12, or 24 hours post infection, as indicated above the top panel. Proteins in the cell lysates were resolved by SDS-polyacrylamide gel electrophoresis and transferred to a PVDF membrane. The membrane was cut and the bottom half was probed using an antibody against US11 while the membrane from the top half was probed with an antibody against β-actin to confirm equal loading among the samples. Purified recombinant US11 was also tested as a positive control for the US11 antibody and for migration of the immunoreactive species (lanes 14 and 15).
Additionally, we utilized a previously characterized IE US11 virus, Volney (also known as 34.5RΔSUP; (Mohr et al., 2001)), which is derived from HSV-1 wild type strain Patton and was generously provided by Ian Mohr. Volney was derived from SUP1, which was selected for its ability to replicate in otherwise non-permissive cells despite not expressing ICP34.5. SUP1 in turn was derived from the ICP34.5 mutant 5e (also known as SPBg5e), in which both copies of ICP34.5 were deleted and replaced with β-glucuronidase (Mohr and Gluzman, 1996). The ICP34.5 mutations of SUP1 were repaired, which yielded Volney (Mohr et al., 2001). Volney therefore contains wild type ICP34.5 loci but contains a deletion upstream of the US11 ORF that places the expression of the endogenous US11 gene under the control of the adjacent immediate early α47 promoter.
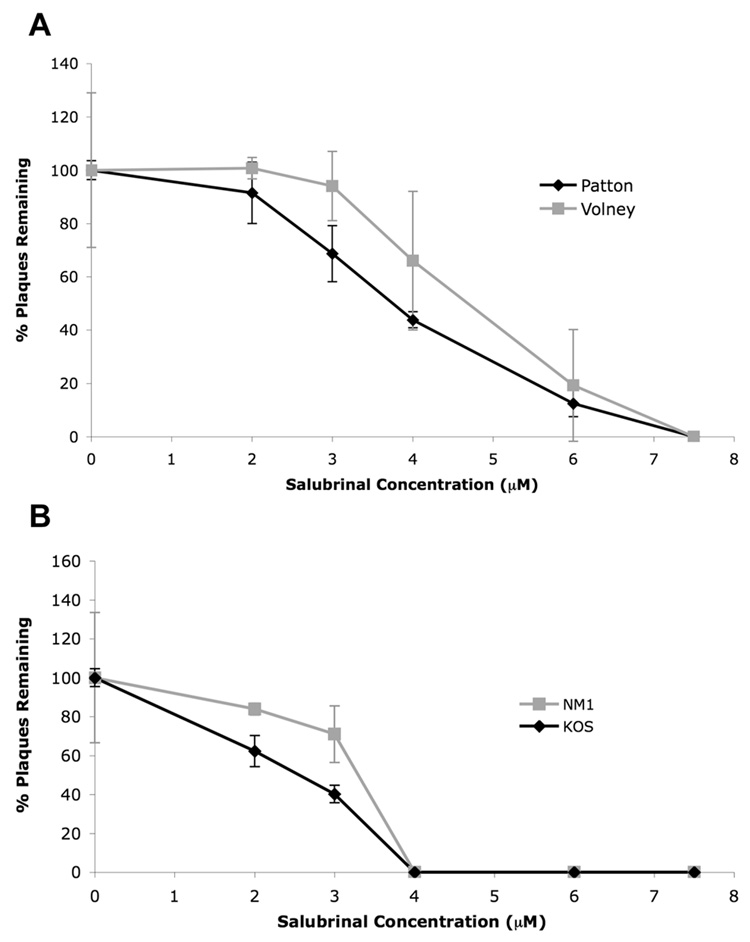
Both sets of IE US11 viruses were slightly resistant to sal in plaque reduction assays in MEFs relative to their respective wild type parental virus (Fig. 4A, B). This slight resistance was highly reproducible. The difference between the sensitivity of Patton and Volney to sal was statistically significant at both 3µM (p=0.0415) and 4µM sal (p=0.0007) by the Student's t test. The difference between the sensitivity of KOS and NM1 to sal was also significant at both 2µM (p=0.0133) and 3µM sal (p=0.0277). Thus, ectopic immediate early expression of US11 can confer slight resistance to sal in MEF cells.
FIG. 4. Slight resistance of the IE US11 viruses to sal in MEFs.
MEF cells were pretreated with DMSO or varying concentrations of sal, as indicated, for 24 hours prior to infection with 150 pfu of (A) Patton (WT, black line with diamonds) and Volney (IE US11 virus, gray line with squares) or (B) KOS (WT, black line with diamonds) and NM1 (IE US11 virus, gray line with squares). After 3 days in the presence of sal, viral plaques were quantified and results show the percent of plaques remaining, relative to treatment with DMSO alone, at each sal concentration. Mean values from three separate experiments are shown (error bars represent standard errors of the mean).
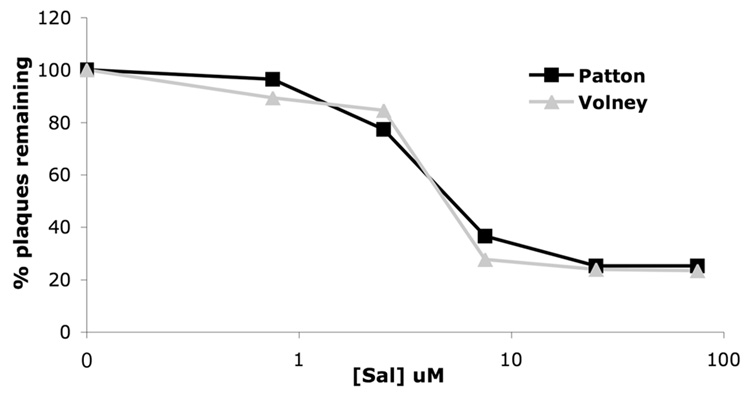
In Vero cells, the IE US11 virus Volney was not measurably resistant to sal in relative to wild type HSV-1 (Fig. 5), indicating no effect of ectopic US11 expression on sal activity in these cells, and thus suggesting that there is cell type specificity to the activity of sal. Similar results were seen when we compared the sensitivity of KOS and NM1 to sal in Vero cells (data not shown). Additionally, we observed that sal is more efficacious in MEF cells than in Vero cells (Fig. 4 and Fig. 5), further suggesting that sal activity has cell type specificity.
FIG. 5. Lack of resistance of the IE US11 virus to sal in Vero cells.
Vero cells were treated with the indicated concentrations of sal for 24 hours prior to infection with 150 pfu of Patton (black line with squares), or Volney (gray line with triangles). Plaques were allowed to form for 2–3 days in the presence of the indicated concentration of sal and were then counted. Data are presented as the percentage of plaques remaining, relative to the DMSO treatment, at each sal concentration.
The observation that IE expression of US11 conferred at most slight resistance to sal in the presence of ICP34.5 was somewhat surprising. Since IE US11 is known to antagonize the activity of the eIF2α kinase PKR (Cassady and Gross, 2002; Cassady, Gross, and Roizman, 1998; Mulvey et al., 1999; Poppers et al., 2000), our observation of minimal resistance to sal suggests that, in addition to PKR, there may be additional eIF2α kinases, such as PERK or GCN2, activated during HSV-1 infection of MEFs that are not inhibited by US11. The eIF2α kinase PERK has been reported to be activated during HSV-1 infection (Cheng, Feng, and He, 2005). PERK is not known to be inhibited by US11, and its activation could be responsible for the susceptibility to sal of the IE US11 viruses. However, more recent work has suggested that HSV-1 infection does not activate PERK (Mulvey, Arias, and Mohr, 2007).
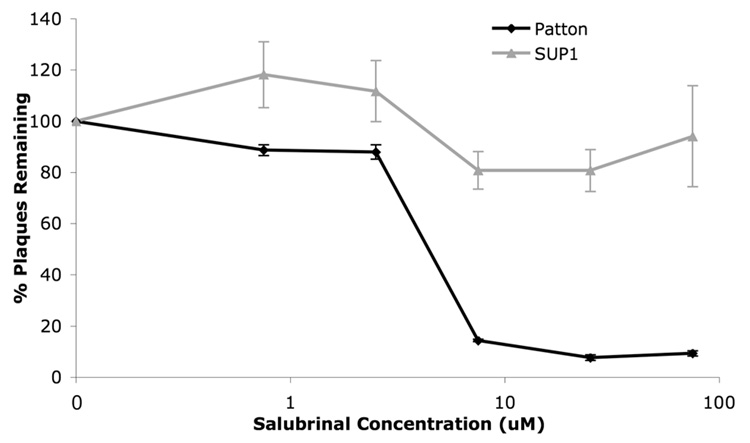
Resistance of an IEUS11-ICP34.5 double mutant to salubrinal in MEFs
Because IE US11 expression can permit HSV replication in the absence of ICP34.5 in otherwise non-permissive cells, we investigated the susceptibility to sal of the IE US11-ICP34.5 double mutant SUP1 (Mohr and Gluzman, 1996) in MEFs. Plaque formation by SUP1 was substantially resistant to inhibition by sal relative to its wild type parent, Patton (Fig. 6) and to the single IE US11 mutant, Volney, in which the ICP34.5 mutation in SUP1 was rescued (Fig. 4 and data not shown). SUP1 did not exhibit resistance to sal in Vero cells (data not shown). Thus, in MEFs, it appears that sal antiviral activity depends on ICP34.5. The simplest explanation for this result is that ICP34.5 is a direct target for Sal. This interpretation is consistent with our previous report of inhibition of eIF2α dephosphorylation in lysates of sal-treated ICP34.5-transfected cells (Boyce et al., 2005). However, less direct interpretations cannot be excluded.
FIG. 6. Resistance of SUP1 to sal in MEFs.
MEF cells were pretreated with DMSO or varying concentrations of sal, as indicated, for 24 hours prior to infection with 150 pfu of Patton (WT, black line with diamonds) and SUP1 (IE US11, Δ34.5 virus, gray line with triangles). After 3 days in the presence of sal, viral plaques were quantified and results show the percent of plaques remaining, relative to DMSO treatment alone, at each sal concentration. Data shown are from a single experiment run in triplicate. Error bars represent standard errors of the mean.
Salubrinal-mediated increases in eIF2α phosphorylation during HSV-1 infection depend on ICP34.5
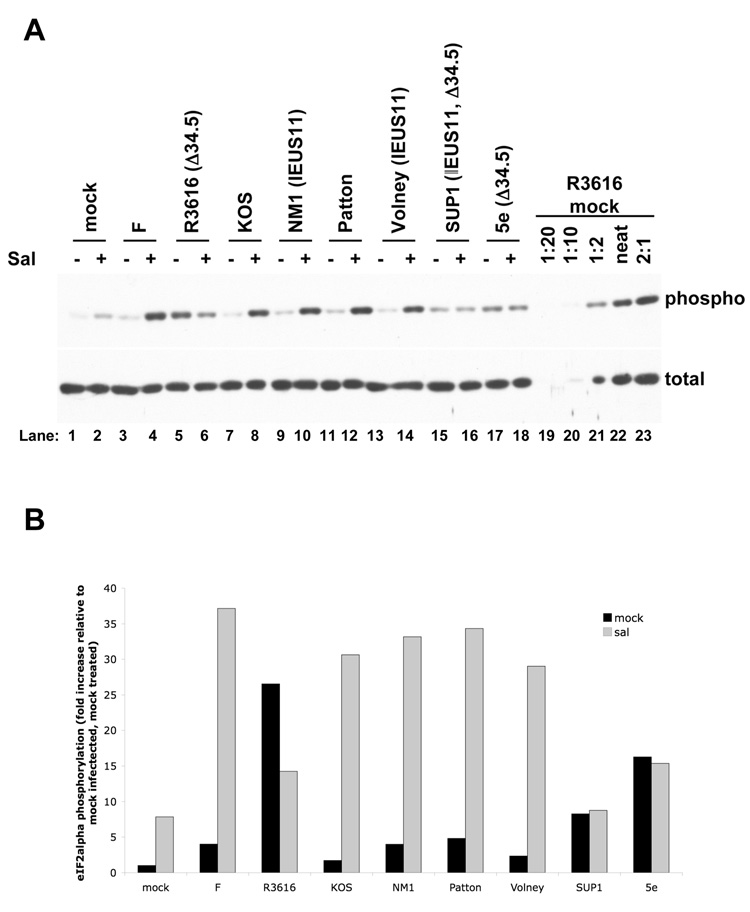
Sal has previously been shown to increase eIF2α phosphorylation during infection (Boyce et al., 2005). Therefore, we investigated whether ICP34.5 was necessary for the sal-dependent increase in eIF2α phosphorylation during HSV-1 infection. We performed western blot analyses of phosphorylated and total eIF2α on lysates from Vero cells that were treated with sal or mock treated with DMSO, and subsequently either mock infected or infected with various wild type, ICP34.5 mutant, and IE US11 viruses. The western blots were quantified using dilution series to generate standard curves (Fig. 7A, lanes 19–23), which were used to determine the relative levels of total eIF2α (which varied relatively little among samples) and phosphorylated eIF2α in each sample. Results from this quantification indicated that sal treatment increased levels of phophorylated eIF2α by approximately 8-fold in mock infected Vero cells (Fig. 7B). Sal treatment resulted in an additional increase in eIF2α phosphorylation in Vero cells infected with any of three wild type strains of HSV-1 (F, KOS, and Patton) relative to mock treatment (Fig. 7A, compare lanes 3 and 4, 7 and 8, and 11 and 12). The increase in phosphorylation relative to mock treated, mock infected Vero cells was greater than 30-fold in sal treated cells infected with any of the three wild type viruses (Fig. 7B).
FIG. 7. Effects of salubrinal on eIF2α phosphorylation.
(A) Western blot analysis of eIF2α phosphorylation. Vero cells were treated with either 75µM sal (+, lanes 2, 4, 6, 8, 10, 12, 14, 16, and 18) or DMSO-containing vehicle (−, lanes 1, 3, 5, 7, 9, 11, 13, 15, and 17) for 24 hours prior to either mock infection (lanes 1 and 2) or infection with HSV-1 strain F (lanes 3 and 4), the ICP34.5 mutant R3616 (lanes 5 and 6), KOS (lanes 7 and 8), the IE US11 mutant NM1 (lanes 9 and 10), Patton (lanes 11 and 12), the IE US11 mutant Volney (lanes 13 and 14), the IE US11, ICP34.5 double mutant SUP1 (lanes 15 and 16), or the ICP34.5 mutant 5e (lanes 17 and 18) at an MOI of 3 in the presence of DMSO or sal. At 14 hours post infection, lysates were prepared and resolved by SDS-PAGE. Samples were resolved on duplicate gels and were immunoblotted for either total eIF2α (bottom panel) or serine-51 phosphorylated eIF2α (top panel). (B) Quantification of the western blot data in (A). The dilution series at the right end of each panel (lanes 19−23) were used to generate a standard curve by determining the intensity of each band on a scanned film using Quantity One software (BioRad). The standard curve was then used to determine the relative concentration of phosphorylated or total eIF2α in each sample. The level of phosphorylated eIF2α was normalized to the level of total eIF2α in each sample. The results are presented as the fold increase in phosphorylation relative to the normalized level of phosphorylated eIF2α in the mock treated, mock infected sample.
The amount of phosphorylated eIF2α was high in mock treated R3616 infected Vero cells (Fig. 7, top panel, lane 5), as expected due to the absence of ICP34.5 mediated dephosphorylation, but the amount of phosphorylated eIF2α was not further increased upon sal treatment (Fig. 7, top panel, lane 6). Moreover, there was more phosphorylated eIF2α in Vero cells infected with wild type virus that were treated with sal than in either untreated or sal-treated Vero cells infected with R3616. Thus, the level of eIF2α phosphorylation is not maximal in mock treated Vero cells infected with the ICP34.5 mutant R3616, so phosphorylation could potentially be increased further. Phosphorylation of eIF2α in mock treated and sal treated Vero cells infected with the rescuant virus, F(R), were similar to those seen in wild type strain F-infected cells (data not shown), indicating that the lack of the sal-dependent increase in eIF2α phosphorylation was due to the ICP34.5 mutation. Similarly, sal did not increase eIF2α phosphorylation following infection with the Patton ICP34.5 mutants 5e and SUP1 (Fig. 7A, lanes 17 and 18, and 7B), and there was less induction in SUP1-infected Vero cells than in cells infected with the ICP34.5 rescued virus Volney (compare lanes 16 and 14).
These data suggest that ICP34.5 is necessary for the increase in eIF2α phosphorylation induced by sal during HSV-1 infection. Taken together with the previous result that sal inhibits eIF2α dephosphorylation in lysates of ICP34.5-transfected cells (Boyce et al., 2005), this result further suggests that ICP34.5 is a direct target for sal. ICP34.5 is a component of a high molecular weight phosphatase complex (He, Gross, and Roizman, 1998), and thus sal could potentially either inhibit ICP34.5 directly, inhibit another protein in the complex, or disrupt interactions between subunits of the ICP34.5 containing complex.
Unexpectedly, although the level of eIF2α phosphorylation was relatively high in mock treated R3616 infected Vero cells, these levels of phosphorylation were reproducibly reduced by sal treatment (Figs. 7A and 7B). What causes this surprising reduction? A general explanation is that sal in combination with the absence of ICP34.5 elicits a response that results in the reduction. One specific possibility is that PKR or another eIF2α kinase becomes less activated. Examination of data in previous reports (Cassady et al., 2002; Chou et al., 1995; He et al., 1997) suggests that PKR activation is lower in certain cells infected with R3616 relative to cells infected with wild type HSV-1. Additionally, although a more recent report did not examine the effects of wild-type virus, it suggested that the ICP34.5 mutant virus R3616 does not activate PKR in Vero cells (Smith et al., 2006). Such lower levels of PKR activation in R3616 infected cells relative to wild type HSV-1 infected cells, along with inhibition of viral replication, might account for the decrease in eIF2α phosphorylation upon treatment with sal in R3616 infected Vero cells.
IE US11 expression and the salubrinal-dependent increase in eIF2α phosphorylation during HSV-1 infection
We also investigated the effects of IE US11 expression on phosphorylation of eIF2α with or without treatment with sal. The phosphorylation of eIF2α in mock- and sal-treated NM1- and Volney-infected Vero cells was comparable to that in Vero cells infected with their wild type parental viruses, KOS and Patton respectively (Fig. 7A, lanes 9 and 10, 13 and 14, and 7B). These data suggest that immediate early expression of US11 alone does not result in a decrease in eIF2α phosphorylation. It may be that IE expression of US11 only transiently reduces eIF2α phosphorylation at early times post-infection, before it is readily detectable by western blot. Additionally, eIF2α kinases other than PKR that are not known to be inhibited by US11, such as PERK or GCN2, may be activated during HSV-1 infection and account for the relatively high levels of eIF2α phosphorylation in sal-treated Vero cells infected with the IE US11 viruses NM1 and Volney.
However, there was less phosphorylation either in the presence or absence of sal in Vero cells infected with SUP1, which exhibits IE US11 expression, relative to cells infected with 5e, which does not exhibit IE US11, but contains the same ICP34.5 mutation as SUP1 (Fig. 7A, lanes 15–18, and 7B). Thus, in the absence of ICP34.5, IE US11 expression appeared to decrease eIF2α phosphorylation, as expected. Perhaps during infection with ICP34.5 mutant viruses, PKR is the predominant eIF2α kinase activated (albeit to a lower level than wild type virus infection), allowing IE US11 to inhibit the activity of PKR and preclude the phosphorylation of eIF2α.
Relationships between antiviral activity of salubrinal and eIF2α phosphorylation
The lack of effect of IE US11 expression on eIF2α phosphorylation in Vero cells in the presence of sal (Fig. 7) is consistent with the IE US11 viruses not exhibiting resistance to Sal in Vero cells (Fig. 5), and their rather slight resistance to sal in MEFs (Fig. 4). In contrast, the dependence on ICP34.5 for induction of eIF2α phosphorylation in Vero cells by sal (Fig. 7) is consistent with the resistance conferred by the ICP34.5 mutant SUP1 in MEFs (Fig. 6). It does not, however, explain, the sensitivity of the ICP34.5 mutant R3616 to salubrinal in Vero cells. One possible way to explain that result is that the eIF2α phosphorylation observed in sal treated R3616-infected Vero cells is sufficient for full antiviral activity. However, that would not explain the ability of R3616 to replicate in Vero cells, given that this virus induces even more eIF2α phosphorylation in the absence of sal. Alternatively, one may invoke an activity of sal that inhibits HSV-1 replication independent of effects on eIF2α phosphorylation. It is possible that although sal does not globally inhibit dephosphorylation (Boyce et al., 2005), it may selectively inhibit dephosphorylation events other than eIF2α, and inhibition of these additional dephosphorylations may contribute to the activity of sal and thus result in its ICP34.5-independent antiviral activity. Alternatively, there may be off-target effects of sal that come into play, and the extent of these non-specific effects may differ between different cell types, and may be more pronounced during infection with ICP34.5 mutant viruses. In this regard, we note that sal slightly but measurably inhibited HSV-1 replication in MEFs containing a non-phosphorylatable eIF2α (Boyce et al., 2005). Although we consistently observed a decrease in eIF2α phosphorylation in R3616 infected Vero cells treated with sal at 14 hours post infection, relative to mock treated Vero cells infected with R3616, eIF2α is also phosphorylated prior to infection due to pre-treatment with sal due to inhibition of GADD34. This phosphorylation may inhibit expression of viral genes to a degree and thus result in the observed decrease in plaque formation. Further investigation is needed to address these possibilities.
This report provides evidence for ICP34.5 as a target for the antiviral activity of sal in at least some cells and also identifies sal as a molecular tool that may be useful for studying the activity of ICP34.5 during HSV-1 infection. This report also demonstrates that sal has ICP34.5-independent activity and cell type specificity, and thus lays the groundwork for future studies to further investigate the mechanism of sal activity.
Materials and Methods
Cells
African green monkey kidney (Vero) cells (originally from American Type Culture Collection) were maintained in Dulbecco’s minimal essential medium (DMEM) supplemented with 5% newborn calf serum (NCS), 1% penicillin and streptomycin (P/S), and 1% amphotericin B (amphB). Murine embryo fibroblasts (MEFs, generously provided by Randal Kaufman) were maintained in DMEM supplemented with 10% fetal calf serum, 1% P/S, and 1% amphB.
Plasmids
pKS+ICP27, which contains the ICP27 gene in pBluescript KS+ (Stratagene), was constructed and generously provided by Martha Kramer in our laboratory. pBS-ICP27prom, which contains the ICP27 promoter, was constructed by digesting pKS+ICP27 with HinfI (all enzymes from NEB), treating with the Klenow fragment of E. coli DNA polymerase I (Klenow), digesting with BamHI and ligating the ~326 bp DNA fragment, containing the ICP27 promoter region, into the SmaI and BamHI sites of pBluescript II SK+ (Stratagene). pMAL-pp-US11, which contains the US11 gene, has been previously described (Bryant et al., 2005). pBS-α US11, which contains US11 downstream of the ICP27 promoter, was constructed by ligating the EcoRI-HindIII DNA fragment containing the US11 gene from pMAL-pp-US11 into the EcoRI and HindIII sites of pBS-ICP27prom. pAG7.TK.1, which contains the HSV-1 tk gene in pBluescript II SK+ (Stratagene), was constructed and generously provided by Anthony Griffiths in our laboratory. pAG7.TK. αUS11, which contains US11 under the control of the ICP27 promoter inserted into the tk gene, was constructed by ligating the Klenow treated HincII-XbaI DNA fragment from pBS-αUS11 into the Klenow treated PstI site in pAG7.TK.1. The sequence of the IE US11 cassette in pAG7.TK.αUS11 was confirmed to be correct by DNA sequencing.
Viruses
Wild type HSV-1 strains KOS, F, and Patton, the ICP34.5 null mutants R3616 (Chou et al., 1990) and 5e (Mohr and Gluzman, 1996), the R3616 rescuant F(R) (Chou et al., 1990), the IE US11 mutants SUP1 (Mohr and Gluzman, 1996) and Volney (Mohr et al., 2001) and NM1 (described below) were propagated and titrated on Vero cells. Strain F, R3616, and F(R) were generously provided by Joany Chou and Bernard Roizman and strain Patton, 5e, Volney, and SUP1 were generously provided by Ian Mohr.
Plaque reduction assays
Sal was purchased from ChemBridge or synthesized as previously described (Long et al., 2005) and generaously provided by Kai Long and Dawei Ma. Vero cells or MEFs were treated for 24 hours with either the indicated concentration of sal in a DMSO-containing vehicle, or the equivalent volume of the DMSO-containing vehicle in normal growth medium for the cell type. Cells were subsequently infected with ~150 pfu/well of the indicated virus in the presence of the DMSO-containing vehicle or vehicle containing the indicated concentration of sal for one hour in DMEM containing 2% NCS, 1% P/S, and 1% amphB. Following the one hour viral adsorption, the inocula were removed and replaced with DMEM containing methyl cellulose, 2% NCS, 1% P/S, 1% amphB, and the indicated concentration of sal. Cells were incubated for 2–3 days to allow plaque formation. When plaques were clearly visible, infected cell monolayers were fixed with acetone and methanol (1:1, v:v), stained with crystal violet, and the number of plaques in each well were counted using a dissecting light microscope.
Construction of NM1 mutant virus
The IE US11 mutant virus NM1, which expresses US11 from the ICP27 promoter inserted into the tk locus, was generated by co-transfecting infectious KOS viral DNA and pAG7.TK.αUS11 (described above) that was linearized by digestion with SacII into Vero cells using Effectene reagents (Qiagen) according to the manufacturer’s instructions. Recombinants were selected by replicating progeny virus from this transfection in the presence of 100µM acyclovir. NM1 was plaque purified three times prior to use in experiments and the presence of the IE US11 expression cassette in the final plaque purified isolate was confirmed by PCR followed by sequencing of the PCR product.
Western blotting
Western blotting experiments were performed on samples from Vero cells grown in 60mm dishes or 6-well plates. For eIF2α western blots, cells were either treated with 75µM sal dissolved in DMSO-containing vehicle or mock treated with an equivalent volume of the vehicle for 24 hours prior to infection at a multiplicity of 3 with the indicated virus in the presence of the indicated concentration of sal, and harvested at 14 hours post infection. For US11 western blots, cells were infected at an MOI of 10 with either KOS or NM1 and harvested at the indicated time. Lysates were prepared by rinsing the monolayers with phosphate buffered saline, and then the cells were lysed in SDS-PAGE loading buffer and boiled for 5 minutes. Polypeptides were resolved by SDS-PAGE and samples were transferred to a PVDF membrane. Membranes were blocked for one hour in 5% milk in TBST prior to reacting with primary antibodies at 4°C overnight. The total eIF2α antibody was from Santa Cruz Biotech, the phospho-Ser51 antibody was from Cell Signaling Technology, the β-actin antibody was from Abcam, and the US11 antibody was generated by Promega by immunizing rabbits with purified recombinant MBP-US11 prepared by Yun-Sheng He in our laboratory. All primary antibodies were diluted 1:1000 in 5% milk in TBST. HRP-conjugated secondary antibodies (Southern Biotech) were diluted 1:5000 in 5% milk in TBST. Western blots were developed using ECL reagents (Pierce).
Acknowledgments
We would like to thank the following investigators for their kind provision of invaluable reagents: Ian Mohr for HSV-1 wild type strain Patton, the immediate early expressing US11 mutant viruses Volney and SUP1, and the ICP34.5 mutant 5e; Joany Chou and Bernard Roizman for HSV-1 wild type strain F, the ICP34.5 mutant R3616, and the R3616 rescuant F(R), Randal Kaufman for MEFs; Kai Long and Dawei Ma for sal that they synthesized; Martha Kramer for plasmid pKS+ICP27, Hongming Wang for plasmid pMAL-pp-US11; Anthony Griffiths for plasmid pAG7.TK.1; Yun-Sheng He for anti-US11 antibody; and James Hogle and Jennifer Baltz for purified recombinant US11. We would also like to thank Anthony Griffiths, David Knipe, Lee Gehrke, Steve Buratowski, and Ian Mohr for helpful advice and discussions, and anonymous reviewers for their incisive and useful comments. This work was supported by NIH ROI grants AI26077 and AI19838.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, Kaufman RJ, Ma D, Coen DM, Ron D, Yuan J. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. doi: 10.1038/sj.cdd.4401817. [DOI] [PubMed] [Google Scholar]
- Brown SM, MacLean AR, Aitken JD, Harland J. ICP34.5 influences herpes simplex virus type 1 maturation and egress from infected cells in vitro. J Gen Virol. 1994;75:3679–3686. doi: 10.1099/0022-1317-75-12-3679. [DOI] [PubMed] [Google Scholar]
- Bryant KF, Cox JC, Wang H, Hogle JM, Ellington AD, Coen DM. Binding of herpes simplex virus-1 US11 to specific RNA sequences. Nucleic Acids Res. 2005;33:6090–6100. doi: 10.1093/nar/gki919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady KA, Gross M. The herpes simplex virus type 1 US11 protein interacts with protein kinase R in infected cells and requires a 30-amino-acid sequence adjacent to a kinase substrate domain. J Virol. 2002;76:2029–2035. doi: 10.1128/jvi.76.5.2029-2035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady KA, Gross M, Gillespie GY, Roizman B. Second-site mutation outside of the US10-12 domain of Deltagamma(1)34.5 herpes simplex virus 1 recombinant blocks the shutoff of protein synthesis induced by activated protein kinase R and partially restores neurovirulence. J Virol. 2002;76:942–949. doi: 10.1128/JVI.76.3.942-949.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady KA, Gross M, Roizman B. The herpes simplex virus US11 protein effectively compensates for the gamma(1)34.5 gene if present before activation of protein kinase R by precluding its phosphorylation and that of the alpha subunit of eukaryotic translation initiation factor 2. J Virol. 1998;72:8620–8626. doi: 10.1128/jvi.72.11.8620-8626.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Feng Z, He B. Herpes simplex virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF-2alpha dephosphorylation by the gamma(1)34.5 protein. J Virol. 2005;79:1379–1388. doi: 10.1128/JVI.79.3.1379-1388.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Chen JJ, Gross M, Roizman B. Association of a M(r) 90,000 phosphoprotein with protein kinase PKR in cells exhibiting enhanced phosphorylation of translation initiation factor eIF-2 alpha and premature shutoff of protein synthesis after infection with gamma 134.5- mutants of herpes simplex virus 1. Proc Natl Acad Sci U S A. 1995;92:10516–10520. doi: 10.1073/pnas.92.23.10516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Kern ER, Whitley RJ, Roizman B. Mapping of herpes simplex virus-1 neurovirulence to gamma(1)34.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266. doi: 10.1126/science.2173860. [DOI] [PubMed] [Google Scholar]
- Chou J, Roizman B. The gamma(1)34.5 gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace AJ, Jr., Nebert DW, Hollander MC, Luethy JD, Papathanasiou M, Fargnoli J, Holbrook NJ. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989;9:4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Chou J, Brandimarti R, Mohr I, Gluzman Y, Roizman B. Suppression of the phenotype of gamma(1)34.5- herpes simplex virus 1: failure of activated RNA-dependent protein kinase to shut off protein synthesis is associated with a deletion in the domain of the alpha47 gene. J Virol. 1997;71:6049–6054. doi: 10.1128/jvi.71.8.6049-6054.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activated protein kinase. Proc Natl Acad Sci U S A. 1997;94:843–848. doi: 10.1073/pnas.94.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Gross M, Roizman B. The gamma(1)34.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J Biol Chem. 1998;273:20737–20743. doi: 10.1074/jbc.273.33.20737. [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. Mechanism and Regulation of Initiator Methionyl-tRNA Binding to Ribosomes. In: Sonenberg N, Hershey JW, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2000. pp. 185–244. [Google Scholar]
- Jing X, Cerveny M, Yang K, He B. Replication of herpes simplex virus 1 depends on the gamma 134.5 functions that facilitate virus response to interferon and egress in the different stages of productive infection. J Virol. 2004;78:7653–7666. doi: 10.1128/JVI.78.14.7653-7666.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing X, He B. Characterization of the triplet repeats in the central domain of the gamma134.5 protein of herpes simplex virus 1. J Gen Virol. 2005;86:2411–2419. doi: 10.1099/vir.0.81033-0. [DOI] [PubMed] [Google Scholar]
- Long K, Boyce M, Lin H, Yuan J, Ma D. Structure-activity relationship studies of salubrinal lead to its active biotinylated derivative. Bioorg Med Chem Lett. 2005;15:3849–3852. doi: 10.1016/j.bmcl.2005.05.120. [DOI] [PubMed] [Google Scholar]
- Lord KA, Hoffman-Liebermann B, Liebermann DA. Sequence of MyD116 cDNA: a novel myeloid differentiation primary response gene induced by IL6. Nucleic Acids Res. 1990;18:2823. doi: 10.1093/nar/18.9.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch DJ, Barnett BC. Neurovirulence factor. Nature. 1991;353:609. doi: 10.1038/353609b0. [DOI] [PubMed] [Google Scholar]
- Mohr I, Gluzman Y. A herpesvirus genetic element which affects translation in the absence of the viral GADD34 function. EMBO J. 1996;15:4759–4766. [PMC free article] [PubMed] [Google Scholar]
- Mohr I, Sternberg D, Ward S, Leib D, Mulvey M, Gluzman Y. A herpes simplex virus type 1 gamma(1)34.5 second-site suppressor mutant that exhibits enhanced growth in cultured glioblastoma cells is severely attenuated in animals. J Virol. 2001;75:5189–5196. doi: 10.1128/JVI.75.11.5189-5196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M, Arias C, Mohr I. Maintenance of endoplasmic reticulum (ER) homeostasis in herpes simplex virus type 1-infected cells through the association of a viral glycoprotein with PERK, a cellular ER stress sensor. J Virol. 2007;81:3377–3390. doi: 10.1128/JVI.02191-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M, Poppers J, Ladd A, Mohr I. A herpesvirus ribosome-associated, RNA-binding protein confers a growth advantage upon mutants deficient in a GADD34-related function. J Virol. 1999;73:3375–3385. doi: 10.1128/jvi.73.4.3375-3385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M, Poppers J, Sternberg D, Mohr I. Regulation of eIF2alpha phosphorylation by different functions that act during discrete phases in the herpes simplex virus type 1 life cycle. J Virol. 2003;77:10917–10928. doi: 10.1128/JVI.77.20.10917-10928.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppers J, Mulvey M, Khoo D, Mohr I. Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J Virol. 2000;74:11215–11221. doi: 10.1128/jvi.74.23.11215-11221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli MG, Cattozzo EM, Faggioli L, Tognon M. Fine mapping and characterization of the Syn 6 locus in the herpes simplex virus type 1 genome. J Gen Virol. 1991;72:1991–1995. doi: 10.1099/0022-1317-72-8-1991. [DOI] [PubMed] [Google Scholar]
- Schneider RJ, Mohr I. Translation initiation and viral tricks. Trends Biochem Sci. 2003;28:130–136. doi: 10.1016/S0968-0004(03)00029-X. [DOI] [PubMed] [Google Scholar]
- Smith KD, Mezhir JJ, Bickenbach K, Veerapong J, Charron J, Posner MC, Roizman B, Weichselbaum RR. Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. J Virol. 2006;80:1110–1120. doi: 10.1128/JVI.80.3.1110-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D, Purves F, Roizman B. Mutational analysis of the promoter region of the alpha 27 gene of herpes simplex virus 1 within the context of the viral genome. Proc Natl Acad Sci U S A. 1990;87:5268–5272. doi: 10.1073/pnas.87.14.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]