Senescence can be defined demographically as an age-dependent increase in mortality risk, or functionally as a decline in performance. The relationship between the two phenomena is central for understanding the biological aging process and the implications of human lifespan extension [1]. Generally, demographic and functional senescence are believed to be tightly linked [1], because aging involves a performance decline in multiple body functions, leading to increased mortality. The limited existing data support a direct connection between old age, increased mortality rate and decreased behavioral or physiological performance in organisms ranging from flies [2] to humans [3]. A recent study [4], however, suggests that the linkage may be less universal than previously postulated. To investigate this linkage directly in the non-traditional aging model Apis mellifera [5], old honey bee workers were studied with respect to survival and performance. A test battery of behavioral assays showed a significant increase in experimental mortality rate with chronological age, but no evidence for an age-dependent performance decline in locomotion, learning or responsiveness to light or sucrose. The explanation for this decoupling of intrinsic mortality and functional decline may lie in the social evolution of honey bees [6].
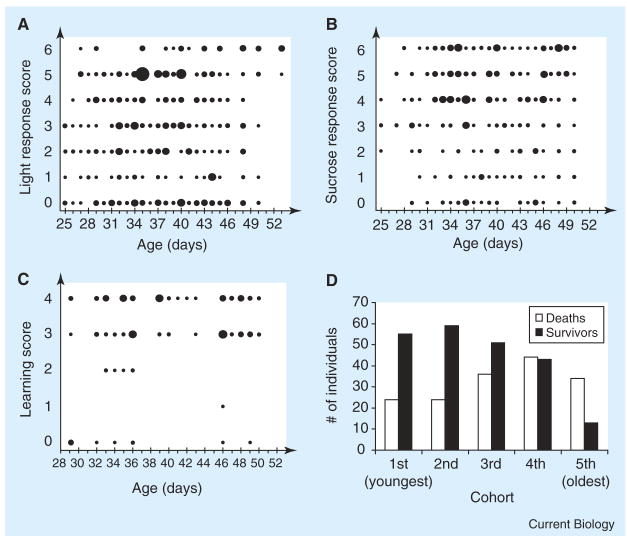
Multiple cohorts of bees between 26 and 52 days old were simultaneously sampled and studied for intrinsic mortality and performance in four diverse behavioral modalities that are central for the workers’ foraging function (detailed experimental procedures are in the Supplemental data available on-line with this issue). Light-response scores increased slightly but significantly with age (B = 0.03, R2 = 0.01, F(1,399) = 4.6, p = 0.03; Figure 1A), indicating a weak gain of function instead of the expected loss of functionality during aging. Performance during the light response assay was not different (F(1,344) = 0.84, p = 0.36) between bees dying or surviving during the remainder of the experiment, suggesting that our results cannot be explained by demographic selection. Sucrose responsiveness was unaffected by age (B = 0.001, R2 = 0.00, F(1,293) = 0.002, p = 0.96; Figure 1B); however, light responsiveness and sucrose responsiveness were correlated in bees (R = 0.22, n = 166, p = 0.004), consistent with previous results [7].
Figure 1. Honey bee worker aging.
Bubble charts of the behavioral performance of honey bee workers of varying old ages show no decline in behavioral performance, despite a wide age range tested (the size of the bubbles indicates sample size: n = 1–12). (A) light responsiveness, (B) sucrose responsiveness, (C) associative learning. In contrast, mortality rate during the behavioral experiments increased strongly with age (D).
Responsiveness to sucrose and light is crucial for the workers’ functioning as foragers and has been linked to a fundamental social behavioral syndrome [8]. Associative learning performance was correlated with the bees’ sucrose responsiveness (R = 0.22, n = 114, p = 0.016), similar to previous studies [9]. Acquisition scores, however, were not influenced by age (B = 0.03, R2 = 0.03, F(1,113) = 3.7, p = 0.06; Figure 1C) and statistical accounting for the bees’ perception of the sucrose reward did not change this conclusion (multiple regression: βsucrose = 0.19, p = 0.055; βage = 0.14, p = 0.146).
Presumably, the old workers in this study were invariably foragers with very few exceptions. However, to eliminate the age-dependent division of labor of honey bee workers as a potential explanation of our results, the behavioral analyses were restricted to the older 50% of experimental bees. No relationship between age and any behavioral performance was found, confirming that the age-dependent behavioral roles did not affect the outcome of our study. Independently, the walking speed of foragers when exiting the hive proved also to be unrelated to age (B = 0.01, R2 = 0.00, F(1,74) = 0.2, p = 0.66).
In contrast, the mortality of the bees during the behavioral experiments increased significantly with chronological age (logistic regression: B = −0.08, Wald = 19.0, p < 0.001) and with relative age from the youngest to the oldest cohort (χ2 = 30.5, df = 4, p < 0.001; Figure 1D). In addition, the residual lifespan of bees that were directly transferred from the hive into laboratory cages declined significantly with chronological age (Cox regression: Bage = −0.12, Wald = 6.8, p = 0.009, mortality hazard ratio: 1.04), with no significant cage effect (χ2 = 9.6, df = 5, p = 0.089). In sum, our data contain multiple lines of evidence for demographic senescence without functional senescence.
The two key findings of this study are that honey bee workers show no functional decline in central cognitive and locomotor functions with advanced chronological age and that their demographic senescence is age-dependent and thus decoupled from the measured aspects of functional senescence. Demographic selection cannot explain these combined results. The lack of a decline in behavioral performance with chronological age is corroborated by recent cellular studies of honey bee workers [10], which suggest that an individual’s social role, but not its chronological age, influences the accumulation of molecular damage in the brain. Social evolution and division of labor may have decoupled functional decline from intrinsic mortality rate in honey bee workers to optimize colony efficiency [6,11]. Down-regulation and gradual depletion of internal resources or costly physiological systems, such as the immune system, could lead to increasing intrinsic worker mortality while not affecting their behavioral performance and preserving resources at the colony level. The old workers considered in this study were most likely all foragers that experience a high extrinsic mortality but at the same time contribute critically to colony function, irrespective of their chronological age [5,6].
Honey bee workers are highly derived organisms that do not reproduce under normal circumstances and live much shorter than their reproducing, long-lived queens [5,12]. Advanced social evolution has resulted in an exceptional case of life history regulation and aging plasticity between castes because their social roles correlate with extrinsic mortality and reproductive value. Thus, our findings may represent an exception, and indeed they contrast with available data from other insects [2,13]. However, they generally suggest that mortality and functional decline can be decoupled [6] and emphasize the intriguing possibility of studying the biological processes that specifically extend the functional lifespan of organisms [1].
Acknowledgments
Preston Gardner, Kristen Ward, and other members of the UNCG Social Insect Group provided practical assistance. Kim Fondrk, Robert Page, Alice Haddy and Jennifer Tsuruda assisted with equipment and technical advice for the light response assay. We thank Robert Page for comments on an earlier version of the manuscript, and Kristen Ward for proof reading. This work was funded by NIA (PO1 AG22500) and the American Federation of Aging Research.
Footnotes
Supplemental data
Supplemental data are available at http://www.current-biology.com/cgi/content/full/17/8/R274/DC1
The editors of Current Biology welcome correspondence on any article in the journal, but reserve the right to reduce the length of any letter to be published. All Correspondence containing data or scientific argument will be refereed. Queries about articles for consideration in this format should be sent by e-mail to cbiol@current-biology.com
References
- 1.Williams GC. The Tithonus error in modern gerontology. Q Rev Biol. 1999;74:405–415. doi: 10.1086/394111. [DOI] [PubMed] [Google Scholar]
- 2.Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res Rev. 2005;4:372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nature Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 4.Burger JM, Promislow DEL. Are functional and demographic senescence genetically independent? Exp Gerontol. 2006;41:1108–1116. doi: 10.1016/j.exger.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Rueppell O, Amdam GV, Page RE, Jr, Carey JR. From genes to society: Social insects as models for research on aging. Sci Aging Knowl Environ. 2004:pe5. doi: 10.1126/sageke.2004.5.pe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amdam GV, Page RE. Intergenerational transfers may have decoupled physiological and chronological age in a eusocial insect. Ageing Res Rev. 2005;4:398–408. doi: 10.1016/j.arr.2005.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erber J, Hoormann J, Scheiner R. Phototactic behaviour correlates with gustatory responsiveness in honey bees (Apis mellifera L.) Behav Brain Res. 2006;174:174–180. doi: 10.1016/j.bbr.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Page RE, Amdam GV. The making of a social insect: developmental architectures of social design. Bioessays. 2007;29:334–343. doi: 10.1002/bies.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheiner R, Page RE, Erber J. Sucrose responsiveness and behavioral plasticity in honey bees (Apis mellifera) Apidologie. 2004;35:133–142. [Google Scholar]
- 10.Seehuus SC, Amdam GV. Cellular senescence in honey bee brain is largely independent of chronological age. Exp Gerontol. 2006;41:1117–1125. doi: 10.1016/j.exger.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tofilski A. Influence of age polyethism on longevity of workers in social insects. Behav Ecol Sociobiol. 2002;51:234–237. [Google Scholar]
- 12.Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- 13.Ridgel AL, Ritzmann RE. Insights into age-related locomotor declines from studies of insects. Ageing Res Rev. 2005;4:23–39. doi: 10.1016/j.arr.2004.08.002. [DOI] [PubMed] [Google Scholar]