The development of new reaction methodologies requiring only a catalytic amount of promoter are fundamentally important to the advancement of organic synthesis.1 Coupled with a mode for enantioinduction, these strategies become indispensable tools for the generation of optically pure molecules in a reasonably atom-economical and environmentally conscious manner. Cycloaddition reactions constitute a special class since such multiple bond forming processes create much greater molecular complexity than single bond forming reactions.
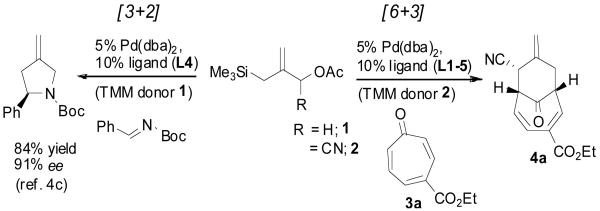
The palladium-catalyzed [3+2] cycloaddition of trimethylenemethane (Pd-TMM) to electron deficient π-systems was introduced almost thirty years ago by our laboratory and constitutes a highly efficient synthesis of substituted cyclopentanes, tetrahydrofurans, and pyrrolidines.2 Following the initial reports, direct access to bicyclo[4.3.1]decadienes via [6+3] TMM cycloaddition to cycloheptatrienones (tropones) was demonstrated to be a highly efficient process.3 Recently, a new class of chiral phosphoramidite ligands provided a major stimulus to the Pd-TMM reaction by rendering several [3+2] cycloadditions enantioselective (Scheme 1).4 Herein we report the first asymmetric Pd-TMM [6+3] cycloaddition of cyanosubstituted TMM substrate 2 with tropones to provide bicyclo[4.3.1]decadienes in high enantiomeric purity.5 Furthermore, we report their facile thermal rearrangement to yield asymmetric bicyclo[3.3.2]decadienes.
Scheme 1.
Pd-TMM [3+2] and [6+3] Cycloadditions
Our studies began with the examination of the Pd-TMM [6+3] cycloaddition of donor 24b, 6 to 4-carboethoxy-2,4,6-cyclohepatrien-1-one7 (3a; Scheme 1). Using conditions optimized for the [3+2] cycloaddition4, 8 we quickly realized high levels of conversion, with bicyclo[4.3.1]decadiene product 4a as the major constituent. The regiochemistry and relative configuration as depicted were determined by two-dimensional NMR studies and comparison with known [6+3] adducts.3
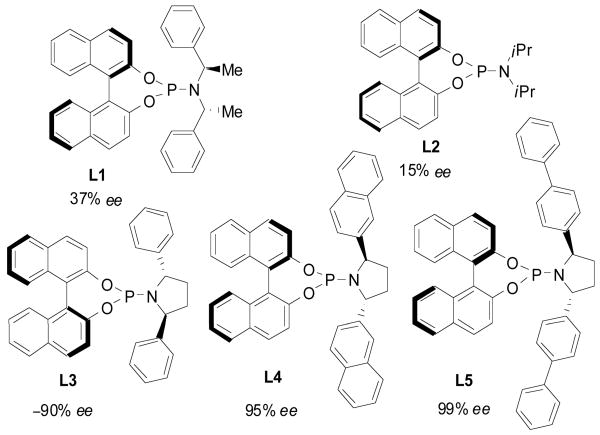
Initial efforts to render the reaction enantioselective relied on the commercially available ligand L19 (Figure 1). Unfortunately, although giving high conversion to product, the enantioselection was rather poor (37% ee). Likewise, phosphoramidite ligand L29 possessing no chirality in the amine component was largely ineffective for promoting enantioselection. In contrast to these standard phosphoramidites, the cyclic pyrrolidine phosphoramidite ligands L3-54 all gave excellent levels of enantioinduction. Various aryl substituents were examined with bis-(4-biphenyl)phosphoramidite ligand L5 attaining near perfect enantioselection in 75% isolated yield (see Table 1, entry 1). The excellent behavior of L5 is somewhat contradictory to previous observations in [3+2] cycloadditions demonstrating the efficacy of L4.4c It is noteworthy that although competing modes of cycloaddition, such as [3+2] or [4+3], could be envisioned only the [6+3] cycloaddition product was obtained. Most remarkably, only one [6+3] regioisomer was detectable and is generated as a single diastereomer.
Figure 1.
Phosphoramidite Ligand Screena
a Reactions performed at 0.1 M in toluene with 5 mol% Pd(dba)2, 10 mol% ligand, 1.0 equiv. 3a, 1.6 equiv. 2, 0-4 °C for 15 h. ee determined by chiral HPLC.
Table 1.
Scope of [6+3] Pd-TMM Cycloadditionsa
| Entry | Troponeb | Product | Yield | d.r.g | eeh |
|---|---|---|---|---|---|
| 1 |
|
|
75%e | >10:1 | 99% |
| 2 |
|
|
80%e | >10:1 | 99% |
| 3 |
|
|
77%e | >10:1 | 99% |
| 4 | R= H; 3d | R = H; 4dd | 89%f | 6:1 | 99% |
| 5 | R= Cl; 3e | R = Cl; 4e | 94%e | >10:1 | 94% |
| 6 | R= OAc; 3f | R = OAc; 4f | 90%e | >10:1 | 96% |
| 7 | R= NPhth; 3g | R = NPhth; 4g | 85%e | >10:1 | 86% |
| 8 | R= Phenyl; 3h | R = Phenyl; 4hc | 64%f | 6:1 | 93% |
| 9 |
|
|
78%f | 3:1 | 91%i |
All reactions performed at 0.2 - 0.25 M in toluene with 5 mol% Pd(dba)2, 10 mol% ligand L5, 1.6 equivalents donor 2, 0-4 °C for 15 h.
See supporting information for tropone syntheses.
Reaction at r.t.
Reaction at 45 °C.
Isolated yield of major diastereomer.
Isolated yield of both diastereomers.
Determined by NMR analysis of the crude reaction mixture.
Determined by chiral HPLC.
Of major diastereomer.
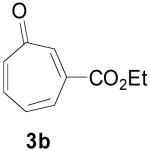
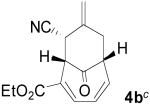
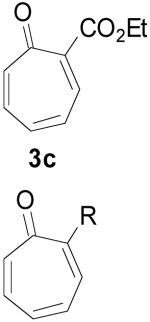
Based on these promising results, an examination of other tropone systems was undertaken (Table 1). To explore the effect of the position of the ester functionality, both the 3-carboethoxy and 2-carboethoxy tropones7 (3b, 3c) were synthesized. Gratifyingly, both tropones gave comparable reaction yields and excellent diastereo- and enantioselectivity (entries 2 and 3). In both cases, only one [6+3] regioisomer was obtained and followed what was predicted from electronic considerations.10 We also examined less electron deficient tropones, such as tropone (3d) itself. Although a higher temperature was required to obtain good conversion, the cycloaddition reaction proceeded to give the desired product 4d in good yield, diastereomeric ratio, and enantioselectivity (entry 4).
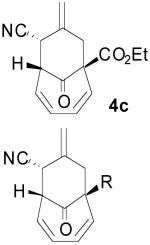
A series of 2-substituted tropones, readily available from tropolone,11 were also prepared and examined. The reaction of 2-chlorotropone (3e) proceeded very well to give the bicycle 4e in 94% yield and 94 % ee (entry 5). X-ray crystallographic analysis on the 2-chloro TMM adduct 4e unambiguously established both the absolute and relative configuration as depicted. Interestingly, 2-bromotropone failed to give any desired cycloaddition. While 2-methoxytropone also displayed no reactivity, 2-acetoxytropone (3f) delivered cycloadduct 4f, again with excellent yield and enantioinduction (entry 6). Likewise, while 2-dimethylamino tropone was unreactive, 2-phthalimido tropone (3g) was well suited to the reaction conditions, although a slightly diminished ee of 86% was observed (entry 7). These results suggest the need for an electron deficient heteroatom to enhance tropone reactivity. In addition, 2-phenyltropone (3h) provided cycloadduct 4h (entry 8) in good yield and stereoselectivity. It is interesting to note that regardless of the electronic nature of the 2-substituted tropones, exclusive regioselectivity for the products bearing the cyano group opposite to the substituent is observed. This regiochemical independence may be supportive of a concerted mechanism, an aspect of these cycloadditions that remains debatable.2, 12
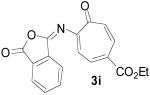
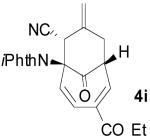
To examine the directing effects of multiple substituents, 2-amino-4-carboethoxy tropone was prepared. Not surprisingly, use of the phthalimido protecting group at C2 led to a mixture of regioisomers. However, upon changing to isophthalimido tropone 3i excellent regioselectivity was attained. Unlike previous examples, the diastereomeric ratio was lower, but high ee was still observed for the major diastereomer 4i (entry 9).
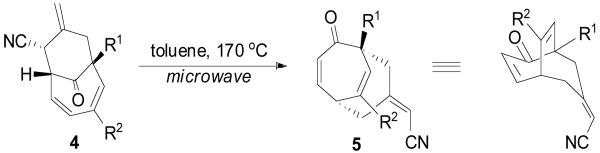
An examination of the 3-dimensional structure of the TMM adducts revealed the proximity of the exo-cyclic olefin to the endocyclic diene. Thus, it was anticipated that a [3,3] sigmatropic (Cope) rearrangement may be induced to convert the bicyclo[4.3.1]decadiene to a bicyclo[3.3.2]decadiene in a stereodefined manner. Such a process would then provide a facile, two-step asymmetric synthesis of a rather unique functionalized bicyclic motif. In the event, simply heating the TMM adducts (4a, 4d, and 4f) in toluene under microwave conditions gave good yields of the rearrangement products (5a, 5d, and 5f; Table 2).13 To verify chirality transfer during the reaction, TMM adduct 4d of 99% enantiomeric excess was converted to the [3.3.2]bicycle 5d while maintaining an ee of 98%. Presumably, rearrangement products 5a and 5f retained full stereochemistry as well.
Table 2.
Cope Rearrangements
 | |||
|---|---|---|---|
| TMM adduct | Product | Yielda | eeb |
| 4a | R1 = H, R2 = CO2Et; 5a | 75% | n.d. |
| 4d | R1, R2 =H; 5d | 72(85)% | 98% |
| 4f | R1 = OAc, R2 =H; 5f | 72(86)% | n.d. |
Isolated yield, yield in parenthesis is based on recovered starting material.
ee determined by chiral HPLC, n.d. = not determined.
In conclusion, an enantioselective palladium-catalyzed trimethylenemethane cycloaddition reaction with tropones has been developed, providing access to asymmetric substituted bicyclo[4.3.1]decadienes in a single operation. In almost all cases where cycloaddition proceeded, extremely high regio-, diastero-, and enantiocontrol was observed. The complete preference for [6+3] cycloaddition, especially in cases where [3+2] cycloaddition could be anticipated (tropones 3a, 3b and 3c) is an intriguing aspect. Additionally, the facile thermal rearrangement of the TMM adducts greatly expands the utility of this methodology by allowing access to bicyclo[3.3.2]decadienes in a straightforward manner.
Supplementary Material
Experimental procedures and spectroscopic data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
We thank the NSF and the NIH (Grant GM 33049) for their generous support of our programs. P.J.M. thanks the American Cancer Society for a postdoctoral fellowship. P. T. W. thanks AstraZeneca for a travel grant. We thank Dr. Allen Oliver at the University of California, Santa Cruz for the X-ray crystal structure and Johnson Matthey for generous gifts of palladium salts. Steven Silverman is acknowledged for donation of phosphoramidite ligands.
References
- 1.(a) Trost BM. Science. 1991;254:1471–1477. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]; (b) Trost BM. Angew Chem Int Ed Engl. 1995;34:259. [Google Scholar]
- 2.(a) Trost BM. Angew Chem Int Ed Engl. 1986;25:1. [Google Scholar]; (b) Trost BM. Pure Appl Chem. 1988;60:1615. [Google Scholar]; (c) Chan DMT. Recent Advances in Palladium-catalyzed Cycloadditions Involving Trimethylenemethane and its Analogs. In: Kobayashi S, Jorgensen KA, editors. Cycloaddition Reactions in Organic Synthesis. 1st. Wiley-VCH; Weinheim: 2002. pp. 57–83. [Google Scholar]
- 3.Trost BM, Seoane PR. J Am Chem Soc. 1987;109:615. [Google Scholar]
- 4.(a) Trost BM, Stambuli JP, Silverman SM, Schwörer U. J Am Chem Soc. 2006;128:13328. doi: 10.1021/ja0640750. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Trost BM, Cramer N, Silverman SM. J Am Chem Soc. 2007;129:12396. doi: 10.1021/ja075335w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Trost BM, Silverman SM, Stambuli JP. J Am Chem Soc. 2007;129:12398. doi: 10.1021/ja0753389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An intramolecular asymmetric [6+4] cycloaddition to a tropone (40% ee) has been reported: Rigby JH, Fleming M. Tetrahedron Lett. 2002;43:8643.
- 6.Use of donor 1 led to an intractable mixture of cycloaddition products. Work in this area is ongoing.
- 7.(a) See supporting information for tropone syntheses. Isakovic L, Ashenhurst JA, Gleason JL. Org Lett. 2001;3:4189. doi: 10.1021/ol016814h. and references therein.
- 8.Optimized reaction conditions employed 5 mol% Pd(dba)2, 10 mol% ligand, and 1.6 equivalents donor 2 in toluene at 0 °C for 15 h. Pd2(dba)3-CHCl3 complex (2.5 mol%) gave identical reactivity.
- 9.Feringa BL. Acc Chem Res. 2000;33:346. doi: 10.1021/ar990084k. [DOI] [PubMed] [Google Scholar]
- 10.For a discussion on the regioselectivity of [6+4] cycloadditions to tropones see: Garst ME, Roberts VA, Houk KN, Rondan NG. J Am Chem Soc. 1984;106:3882.
- 11.(a) Doering WvE, Knox LH. J Am Chem Soc. 1951;73:828. [Google Scholar]; (b) Doering WvE, Knox LH. J Am Chem Soc. 1952;74:5683. [Google Scholar]; (c) Doering WvE, Hiskey CF. J Am Chem Soc. 1952;74:5688. [Google Scholar]
- 12.Singleton DA, Schulmeier BE. J Am Chem Soc. 1999;121:9313. [Google Scholar]
- 13.In contrast to our flexible system, a rigidly held 1,5-diene undergoes a similar rearrangement at room temperature: Paddon-Row MN, Warrener RN. Tetrahedron Lett. 1974;15:3797.Tegmo-Larsson I, Houk KN. Tetrahedron Lett. 1978;19:941.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and spectroscopic data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.