Abstract
Vitamin D, the sunshine vitamin, is now recognized not only for its importance of bone health in children and adults, but also for other health benefits including reducing risk of chronic diseases including autoimmune diseases, common cancer and cardiovascular disease. Vitamin D made in the skin or ingested in the diet is biologically inert and requires two successive hydroxylations first in the liver on carbon 25 to form 25-hydroxyvitamin D [25(OH)D], and then in the kidney for a hydroxylation on carbon 1 to form the biologically active form of vitamin D, 1,25-dihydroxyvitamin D [1,25(OH)2D]. With the identification of 25(OH)D and 1,25(OH)2D, methods were developed to measure these metabolites in the circulation. Serum 25(OH)D is the barometer for vitamin D status. Serum 1,25(OH)2D provides no information about vitamin D status and is often normal or even elevated due to secondary hyperparathyroidism associated with vitamin D deficiency. Most experts agree that 25(OH)D of < 20 ng/ml is considered to be vitamin D deficiency whereas a 25(OH)D of 21-29 ng/ml is considered to be insufficient. The goal should be to maintain both children and adults at a level > 30 ng/ml to take full advantage of all the health benefits that vitamin D provides.
Historical Perspective
The association of sunlight and vitamin D for bone health began with the industrialization of northern Europe. The lack of adequate sun exposure resulted in an epidemic of children with severe growth retardation and bony deformities that was commonly known as rickets.1 In 1919, Huldschinsky et al2 reported that exposure to ultraviolet radiation cured rickets. This was followed by Hess and Unger in 19213 who observed that exposure to sunlight cured rickets.
In the 1930’s, it was appreciated that ultraviolet irradiation of yeast extract was effective in producing an antirachitic substance known as vitamin D. This vitamin D was structurally identified and called vitamin D2. Vitamin D3 was identified by the irradiation of 7-dehydocholesterol. Because vitamin D2 was inexpensive to produce, vitamin D2 was used widely for the fortification of foods including milk and bread in the United States and Europe. When 7-dehydrocholesterol was easily extracted from lanolin from sheep’s wool, vitamin D3 was inexpensively made and was used in food fortification and for supplements.
In the early 1950’s, there was an outbreak of hypercalcemia thought due to the over fortification of milk with vitamin D, and as a result, most European countries forbid the fortification of milk and other dairy products with vitamin D. In the United States, milk and orange juice are fortified with vitamin D3 whereas a majority of multivitamin supplements and pharmaceutical preparations contain vitamin D2.1, 4
The appreciation that vitamin D (D represents either D2 or D3) required a hepatic hydroxylation on carbon 25 to produce 25-hydroxyvitamin D [25(OH)D] (Fig 1) led to the development of a binding protein assay using the vitamin D binding protein (DBP) to measure circulating levels of 25(OH)D in the circulation.5-7 The identification of 1,25-hydroxyvitamin D as being the biologically active form of vitamin D led to the development of a binding protein assay using the vitamin D receptor as the binder to measure circulating levels of 1,25(OH)2D.8-10
Figure 1.
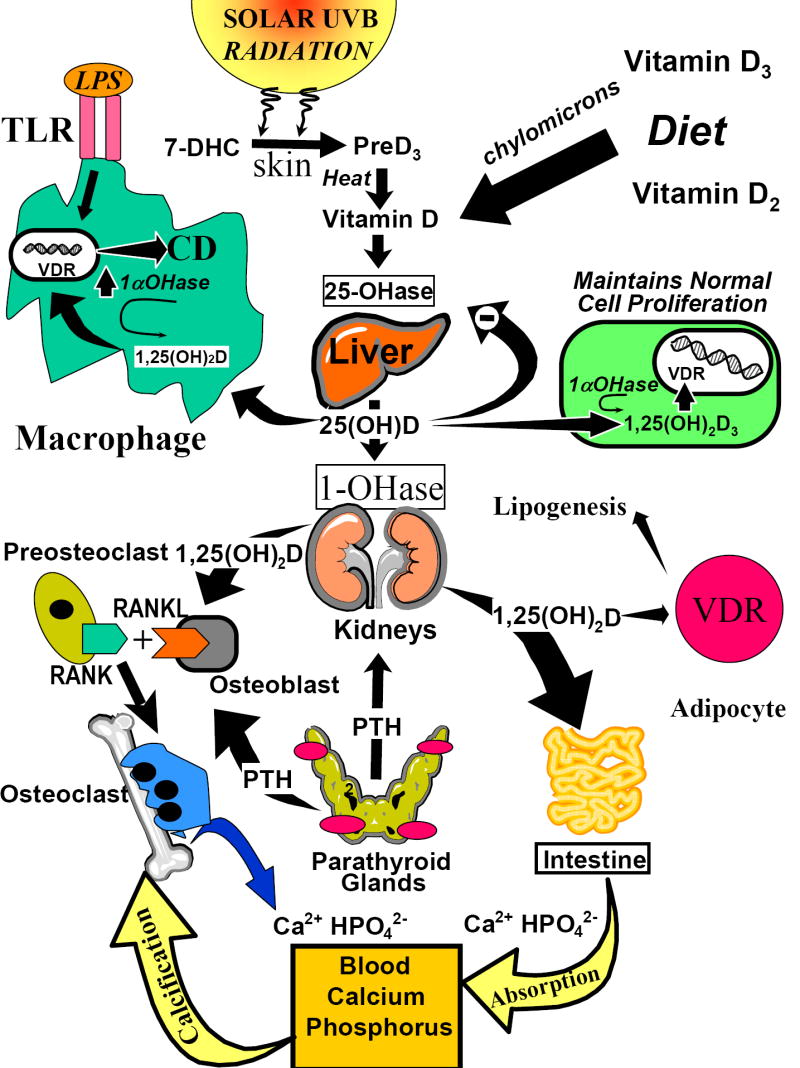
The metabolism and biologic function of vitamin D. During exposure to sunlight, 7-dihydrocholesterol (7-DHC) is photolyzed to previtamin D3 (preD3). Body heat converts preD3 to vitamin D3. Vitamin D2 and vitamin D3 in the diet and vitamin D made in the skin enters the circulation and either is stored in the body’s fat adipocytes or enters the liver and is converted to 25-hydroxyvitamin D [25(OH)D]. For regulation of calcium metabolism, 25(OH)D is converted in the kidneys to 1,25-dihydroxyvitamin D [1,25(OH)2D]. 1,25(OH)2D interacts with its vitamin D receptor (VDR) in the small intestine and on osteoblasts to regulate calcium and phosphorus metabolism. 25(OH)D is metabolized in various tissues and cells for regulating cellular proliferation and differentiation as well as inducing cathelicidin D(CD) in macrophages. The induction of 1,25(OH)2D in the macrophage is controlled by the 2/1 toll-like receptors (TLR) and its interaction with lipopolysaccharide (LPS). In addition, circulating concentrations of 1,25(OH)2D may help increase insulin production and decrease renin production and alter adipocyte lipogenesis. With permission; copyright Michael F. Holick.
25(OH)D Assays
The first assays for 25(OH)D used the competitive protein binding format with the vitamin D binding protein (DBP) as the binder. The advantage of this assay was that DBP recognized 25(OH)D2 equally as well as 25(OH)D3. The major limitation of this assay was that the assay measured 25(OH)D in a serum sample that contained other vitamin D metabolites including 24,25-dihydroxyvitamin D [24,25(OH)2D], 25,26-dihydroxyvitamin D and the 25,26-dihydroxyvitamin D -26, 23-lactone.11 However, typically these more polar metabolites of 25(OH)D circulate at less than 10-15% of the total concentration of 25(OH)D and were usually insignificant.
In 1985, a radioimmunoassay (RIA) was developed for 25(OH)D.11, 12 This assay (Diasorin@) recognized 25(OH)D2 equally as well as 25(OH)D3.11, 12 However, like the DBP competitive protein binding assay, the RIA for 25(OH)D also recognized 24,25(OH)2D and other polar metabolites to the same extent. Thus, both the DBP and the RIA assays typically overestimated 25(OH)D levels by approximately 10-20%. However, since the coefficient of variations (CV) for the intra and interassay variations were 8-15%, this was within the CV for the assay. More recently IDS developed an RIA which has a 100% specificity for 25(OH)D3 and only 75% specificity for 25(OH)D2.
To remove interfering vitamin D metabolites, simple preparative chromatography was developed to separate 25(OH)D from more polar metabolites that interfered with the assay. In the mid-1970’s, high performance liquid chromatography (HPLC) was applied to the 25(OH)D assay.11, 13 This assay included a lipid extraction of the serum followed by preparative chromatography and the 25(OH)D fraction was applied to HPLC and the UV absorption of 25(OH)D was used to measure its concentration. HPLC was considered to be the gold standard but was a very cumbersome assay, and, thus, was not routinely used by reference laboratories for clinical samples.
The advances in liquid chromatography tandem mass spectroscopy (LC-MS) was applied for the direct measurement of 25(OH)D in human serum. This assay quantitatively measured both 25(OH)D2 and 25(OH)D3.14, 15
1,25-Dihydroxyvitamin D Assays
Once 1,25(OH)2D was identified, an assay using the chicken intestinal vitamin D receptor was developed as a competitive protein binding assay to measure circulating levels of 1, 25(OH)D.8 It was observed that the bovine thymus was an excellent source for the VDR and competitive binding protein assay for 1, 25(OH)2D was developed using bovine VDR as the binding protein.9, 11
Radioimmunoassays were later developed to measure 1,25(OH)D. The Diasorin assay was reported to measure 1,25(OH)D3 equally as well as 1,25(OH)D2. However, the IDS assay recognized 1,25(OH)2D3 more effectively than 1,25(OH)2D2.
Determination of Vitamin D Status
25(OH)D is the only vitamin D metabolite that is used to determine whether a patient is vitamin D deficient, sufficient or intoxicated.1, 4, 16, 17 25(OH)D is the major circulating form of vitamin D that has a half life of approximately 2-3 weeks. 25(OH)D is a summation of both vitamin D intake and vitamin D that is produced from sun exposure.1, 4
Although 1,25(OH)D3 is the biologically active form of vitamin D, and, thus, would be thought to be the ideal measure for vitamin D status, it is not. There are several reasons for this. The circulating half life of circulating 1,25(OH)D is only 4-6 hours. Circulating levels of 1,25(OH)D are a thousand fold less than 25(OH)D. As a patient becomes vitamin D deficient, there is a decrease in intestinal calcium absorption which lowers ionized calcium transiently. This signal is recognized by the calcium sensor in the parathyroid glands to increase the production and secretion of parathyroid hormone (PTH).18 PTH regulates calcium metabolism by increasing tubular reabsorption of calcium in the kidney, increasing mobilization of calcium from the skeleton and by increasing the renal production of 1,25(OH)D.1, 4, 16 Thus, as a patient becomes vitamin D insufficient and deficient, the increase in PTH levels result in normal or elevated levels of 1,25(OH)D. This makes the 1,25(OH)2D assay useless as a measure of vitamin D status.
1,25(OH)D assay, however, has been effectively used to help in the diagnosis of several inherited and acquired disorders in calcium metabolism as they relate to alteration in the renal or extra renal production of 1,25(OH)D.4, 16, 19
Definition of Vitamin D Insufficiency and Deficiency
There is no absolute consensus as to what a normal range for 25(OH)D should be. Part of the difficulty is how a normal range is determined, i.e., typically it is done by obtaining blood from several hundred volunteers and deeming them to be normal and to perform the measurement of the analyte and do a distribution with a mean ± 2SD as the normal range. However, since it is now recognized that 30-50% of both the European and US population are vitamin D insufficient or deficient, the previously reported normal ranges of 10-55 ng/ml are totally inadequate.1, 4, 16, 20-23 Chapuy et al24 reported that a dot plot of serum 25(OH)D levels as a function of PTH levels provided an insight as to what the serum 25(OH)D levels should be to be considered sufficient. They observed that the PTH levels began to plateau at their nadir when 25(OH)D levels were between 30-40 ng/ml. A similar observation was made by Thomas et al and Holick et al.14 (Fig 2)
FIG. 2.
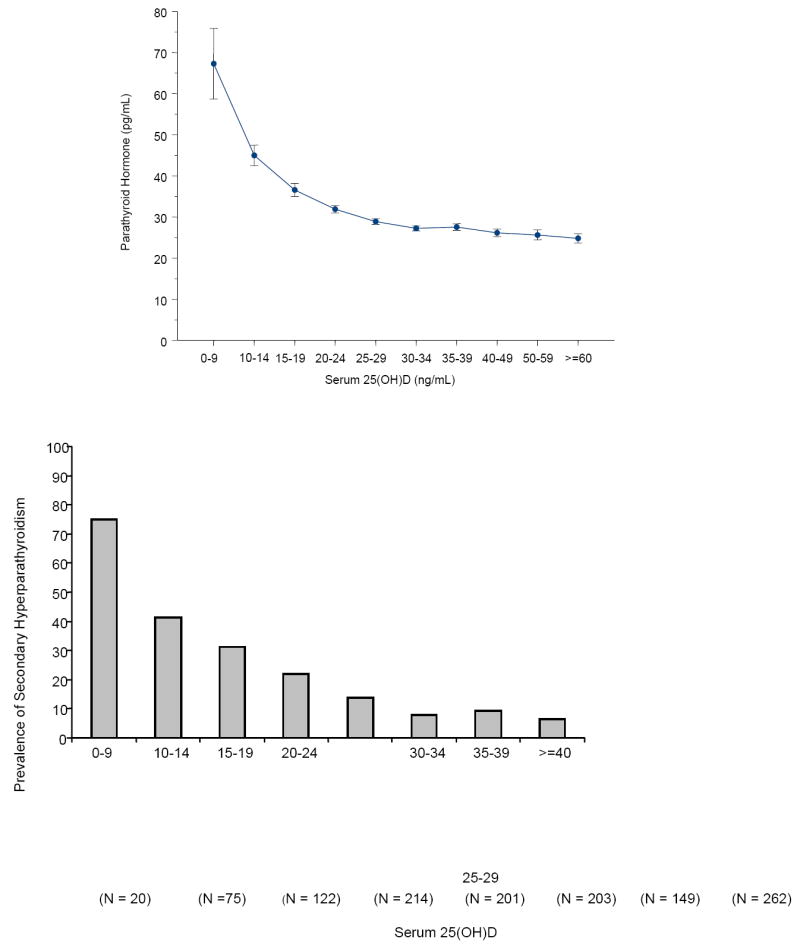
A, Mean (±SE) serum PTH (picograms per milliliter) by serum 25(OH)D subgroups. Subject PTH concentrations (picograms per milliliter) relative to serum 25(OH)D concentrations sorted by subgroups delineated by predefined cutoffs for analyses of 25(OH)D inadequacy. Serum PTH values began to increase with 25(OH)D concentrations less than 29.8 ng/ml. B, Percent of subjects with secondary hyperparathyroidism by 25(OH)D level. The percent of subjects with secondary hyperparathyroidism (PTH > 40 pg/ml) sorted by subgroups with serum 25(OH)D concentrations delineated by predefined cutoffs for analyses of 25(OH)D inadequacy. Reproduced with permission.14
Malabanan et al26 did provocative testing by giving healthy adults who had a 25(OH)D of between 11 and 25 ng/ml, 50,000 IU of vitamin D once a week for 8 weeks. At the end of 8 weeks, it was observed that 25(OH)D levels increased on average by more than 100%. An analysis of the change in PTH levels for each of the subjects revealed that on average, the mean decrease on PTH levels declined by 55% in subjects who had 25(OH)D between 11-15 ng/ml and declined by 35% for those with 25(OH)D levels of between 16-19 ng/ml. Those subjects who had 25(OH)D > 20 ng/ml had no significant change in their PTH level. (Fig 3) Thus, based on the provocative testing, it was suggested that vitamin D deficiency should be defined as 25(OH)D above 20 ng/ml. Heaney et al 27 measured the efficiency of intestinal calcium absorption in women who had on average a 25(OH)D of 20 ng/ml and then in the same women who received 25(OH)D3 to raise their blood level on average to 32 ng/ml. They reported a 45-65% increase in the efficiency of intestinal calcium transport when women were able to achieve a 25(OH)D of > 32 ng/ml.
Figure 3.
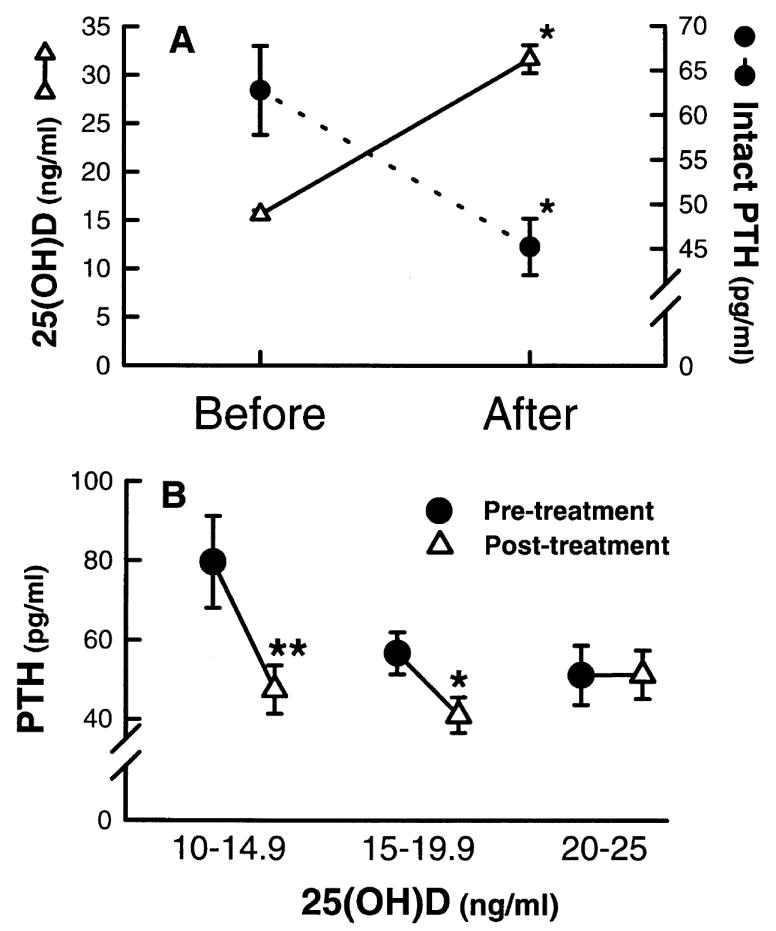
(A) Serum levels of 25(OH)D (-Δ-) and PTH (-o-) before and after therapy with 50,000 IU of vitamin D2 and calcium supplementation once a week for 8 weeks. (B) Serum levels of PTH levels in patients who had serum 25(OH)D levels of between 10 and 25 ng/ml and who were stratified in increments of 5 ng/ml before and after receiving 50,000 IU of vitamin D2 and calcium supplementation for 8 weeks. Reproduced with permission.26
With all of this information collectively, most experts now agree that vitamin D deficiency should be defined as a 25(OH)D of < 20 ng/ml. Vitamin D insufficiency is now recognized as a 25(OH)D of 21-29 ng/ml. The preferred level for 25(OH)D is now recommended by many experts to be > 30 ng/ml.1, 4, 28
The upper limit of normal has also been questioned.4, 29, 30 The upper limit being 55 ng/ml seemed to be inadequate especially since lifeguards who are exposed to a lot of sunlight typically have reported levels of 100-125 ng/ml.4, 16, 29 There has never been a reported case of vitamin D intoxication from sun exposure, and lifeguards have not been reported to be vitamin D intoxicated. Based on the literature, it appears that vitamin D intoxication does not occur until blood levels are above 150-200 ng/ml.31, 32 Vitamin D intoxication is defined as a 25(OH)D > 150 ng/ml that is associated with hypercalcemia, hypercalciuria and often hyperphosphatemia.
Based on all of this information, many of the reference laboratories are now using a normative range for 25(OH)D to be 20-100ng/ml. However, several reference laboratories are also recognizing the recommendation by some experts that a preferred level of > 30 ng/ml is most desirable.
At least two of the reference laboratories are now using LC-MS routinely to measure 25(OH)D. Since they are able to quantitatively measure 25(OH)D2 and 25(OH)D3, they report them out as individual levels. They also report the total 25(OH)D which is summation of 25(OH)D2 and 25(OH)D3. Physicians only need to be aware of the total 25(OH)D level.
Conclusion
The only way to determine whether a person is vitamin D deficient or sufficient is to measure their circulating level of 25(OH)D. There are a variety of assays used to measure 25(OH)D. The radioimmunoassays and competitive protein binding assays for 25(OH)D are useful in detecting vitamin D deficiency and sufficiency. However, these assays are fraught with technical difficulties especially if they are not run routinely.33 (Fig 4) Several reference laboratories have now switched to LC-MS which measures both 25(OH)D2 and 25(OH)D3 quantitatively. The total 25(OH)D, i.e., 25(OH)D2 plus 25(OH)D3 is what physicians need to be aware of for their patients. A level > 30 ng/ml is now considered to be the preferred healthful level that all children and adults should maintain throughout the year.
FIG. 4.
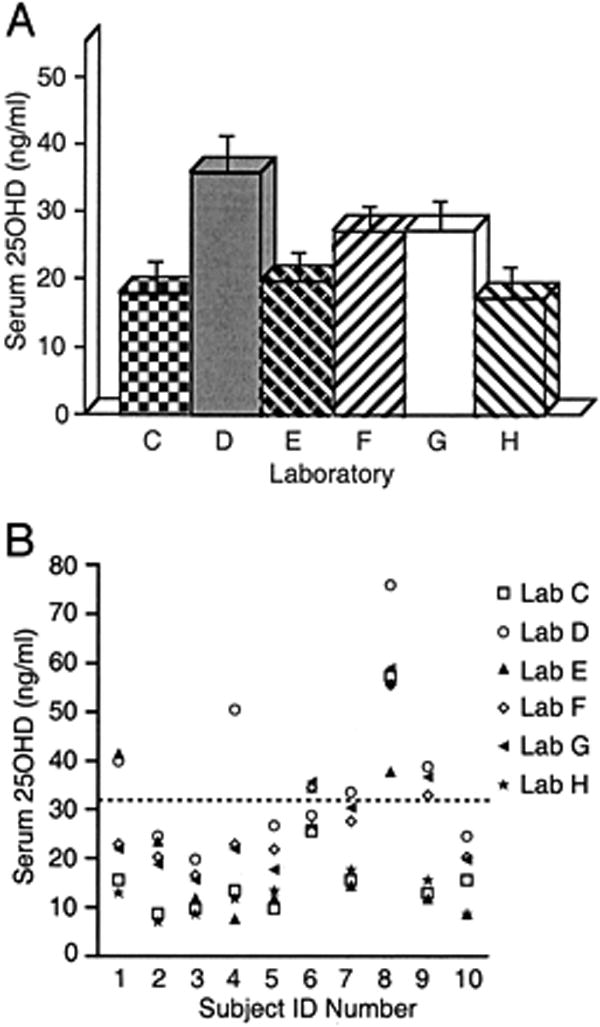
Laboratory personnel basal serum 25(OH)D concentration. A, In these 10 individuals, the mean serum 25(OH)D varied widely [from 17.1 (4.6) to 35.6 (5.2) ng/ml; P < 0.005] between laboratories (error bars represent SEM). B, Similarly, marked within-individual variation was observed in 25(OH)D measurement in different laboratories. Whether an individual has hypovitaminosis D (arbitrary threshold, 32 ng/ml shown as dashed line) depends on which laboratory was used. Note that laboratory H used HPLC and was considered to be the “gold standard” against which others were compared. Reproduced with permission.33
Acknowledgments
Sources of Support: This work was supported in part by NIH grants M01RR00533 and AR36963 and the UV Foundation.
Abbreviations
- 25(OH)D
25-Hydroxyvitamin D
- 1,25(OH)2D
1,25 Dihydroxyvitamin D
- PTH
Parathyroid hormone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 116 doi: 10.1172/JCI29449. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huldschinsky K. Heilung von Rachitis durch Kunstliche Hohensonne. Deutsche Med Wochenschr. 1919;45:712–713. [Google Scholar]
- 3.Hess AF, Unger LJ. The cure of infantile rickets by sunlight. JAMA. 1921;77:39–41. [Google Scholar]
- 4.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 5.Belsey R, Clark MB, Bernat M, Glowacki J, Holick MF, DeLuca HF, et al. The physiologic significance of plasma transport of vitamin D and metabolites. Am J Med. 1974;57:50–56. doi: 10.1016/0002-9343(74)90767-0. [DOI] [PubMed] [Google Scholar]
- 6.Haddad JG, Chuy KJ. Competitive protein binding radioassay for 25-hydroxycholecalciferol. J Clin Endocrinol Metab. 1971;33:992–995. doi: 10.1210/jcem-33-6-992. [DOI] [PubMed] [Google Scholar]
- 7.Chen TC, Turner AK, Holick MF. Methods for the determination of the circulating concentration of 25-hydroxyvitamin D. J Nutr Biochem. 1990;1:315–319. doi: 10.1016/0955-2863(90)90067-u. [DOI] [PubMed] [Google Scholar]
- 8.Brumbaugh PF, Haussler DH, Bursac KM, Haussler MR. Filter assay for 1,25dihydroxyvitamin D3. Utilization of the hormone’s target tissue chromatin receptor. Biochemistry. 1974;13:4091–4097. doi: 10.1021/bi00717a005. [DOI] [PubMed] [Google Scholar]
- 9.Chen TC, Turner AK, Holick MF. A method for the determination of the circulating concentration of 1,25-dihydroxyvitamin D. J Nutr Biochem. 1990;1:320–327. doi: 10.1016/0955-2863(90)90068-v. [DOI] [PubMed] [Google Scholar]
- 10.Chesney RW, Hamstra AJ, DeLuca HF, Horowitz S, Gilbert EF, Hong R, et al. Elevated serum 1,25-dihydroxyvitamin D concentrations in the hypercalcemia of sarcoidosis: Correction by glucocorticoid therapy. J Pediatr. 1981;98:919–922. doi: 10.1016/s0022-3476(81)80588-4. [DOI] [PubMed] [Google Scholar]
- 11.Horst RL, Hollis BW. Vitamin D assays and their clinical utility. In: Holick MF, editor. Physiology, Molecular Biology, and Clinical Applications. Totowa, NJ: Humana Press Inc; 1999. pp. 239–271. [Google Scholar]
- 12.Hollis B. The determination of circulating 25-hydroxyvitamin D: no easy task. J Clin Endocrinol Metab. 2004;89:3149–3151. doi: 10.1210/jc.2004-0682. [DOI] [PubMed] [Google Scholar]
- 13.Jones G. Assay of vitamin D2 and D3 in human plasma by high performance liquid chromatography. Clin Chem. 1978;24:287–298. [PubMed] [Google Scholar]
- 14.Holick MF. Variations in 25-hydroxyvitamin D assay results. J Clin Endocrinol Metab (Letter to the Editor) 2005;90(5):210. [Google Scholar]
- 15.Guo T, Taylor RL, Singh RJ, Soldin SJ. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta. 2006;372(12):76–82. doi: 10.1016/j.cca.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 16.Bouillon R. Vitamin D: From photosynthesis, metabolism, and action to clinical applications. In: DeGroot LJ, Jameson JL, editors. Endocrinology. Philadelphia: WB Saunders; 2001. pp. 1009–1028. [Google Scholar]
- 17.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int (Editorial) 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 18.Brown EM, Gamba G, Riccardl D, Lombardi M, Butters R, Klfor O, et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF, Garabedian M. Vitamin D: photobiology, metabolism, mechanism of action, and clinical applications. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Sixth Edition. Chapter 17. American Society for Bone and Mineral Research; Washington, DC: 2006. pp. 129–137. [Google Scholar]
- 20.Chapuy MC, Chapuy P, Thomas JL, Hazard MC, Meunier PJ. Biochemical effects of calcium and vitamin D supplementation in elderly, institutionalized, vitamin D-deficient patients. Rev Rhum. 1996;63:135–140. [PubMed] [Google Scholar]
- 21.Bakhtiyarova S, Lesnyak O, Kyznesova N, Blankenstein MA, Lips P, et al. Vitamin D status among patients with hip fracture and elderly control subjects in Yekaterinburg, Russia. Osteoporos Int. 2006;17:441–46. doi: 10.1007/s00198-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 22.Larsen ER, Mosekilde L, Foldspang A. Vitamin D and calcium supplementation prevents osteoporotic fractures in elderly community dwelling residents: a pragmatic population-based 3-year intervention study. J Bone Miner Res. 2004;19:370–378. doi: 10.1359/JBMR.0301240. [DOI] [PubMed] [Google Scholar]
- 23.McKenna MJ, Freany R. Secondary hyperparathyroidism in the elderly: means to defining hypovitaminosis D. Ospeoporos Int. 1998;8(suppl):S3–S6. doi: 10.1007/pl00022725. [DOI] [PubMed] [Google Scholar]
- 24.Chapuy MC, Preziosi P, Maaner M, Arnaud S, Galan P, Hercberg S, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteopor Int. 1997;7:439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 25.Thomas KK, Lloyd-Jones DH, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 26.Malabanan AO, Turner AK, Holick MF. Severe generalized bone pain and osteoporosis in a premenopausal black female: effect of vitamin D replacement. J Clin Densitometr. 1998;1:201–204. [Google Scholar]
- 27.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 28.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 29.Vieth R. The Pharmacology of Vitamin D, including fortification strategies. In: Feldman D, Pike JW, Glorieux FH, editors. Vitamin D. 2. Chapter 61. Elsevier Acad Press; 2005. [Google Scholar]
- 30.Vieth R. Why the optimal requirement for vitamin D3 is probably much higher than what is officially recommended for adults. J Steroid Biochem Mol Biol. 2004;89-90:575–579. doi: 10.1016/j.jsbmb.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 31.Koutkia P, Chen TC, Holick MF. Vitamin D Intoxication Associated with an Over-the-Counter Supplement. N Engl J Med. 2001;345(1):66–67. doi: 10.1056/NEJM200107053450115. [DOI] [PubMed] [Google Scholar]
- 32.Adams JS, Lee G. Gains in bone mineral denisty with resolution of vitamin D intoxication. Annals Int Med. 1997;127:203–206. doi: 10.7326/0003-4819-127-3-199708010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen K, et al. Assay variation confounds the diagnosis of hypovitaminosis D: A call for standardization. J Clin Endocrinol Metab. 2004;89:3152–3157. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]


