Abstract
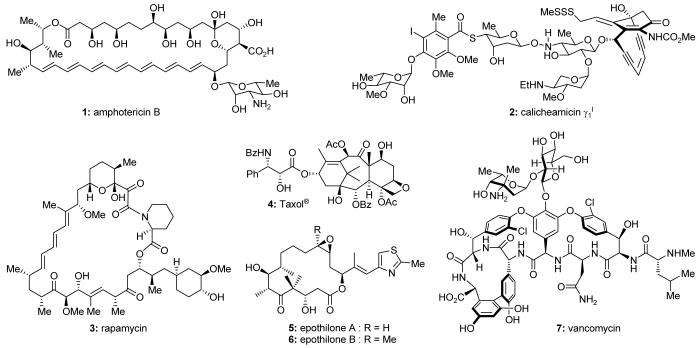
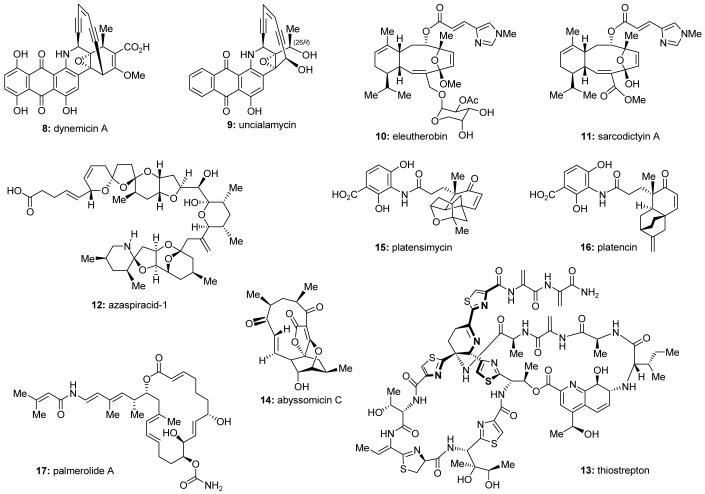
Natural products possess a broad diversity of structure and function, and they provide inspiration for chemistry, biology, and medicine. In this review article, we highlight and place in context our laboratory’s total syntheses of, and related studies on, complex secondary metabolites that were clinically important drugs, or have since been developed into useful medicines, namely amphotericin B (1, Figure 1), calicheamicin γ1I (2), rapamycin (3), Taxol® (4), the epothilones [e.g. epothilones A (5) and B (6)], and vancomycin (7). We also briefly highlight our research with other selected inspirational natural products possessing interesting biological activities [i.e. dynemicin A (8, Figure 2), uncialamycin (9), eleutherobin (10), sarcodictyin A (11), azaspiracid-1 (12), thiostrepton (13), abyssomicin C (14), platensimycin (15), platencin (16), and palmerolide A (17)].
1. Introduction
The vast array of secondary metabolites found in nature provides a veritable treasure trove for drug discovery and development.1,2 Natural products arise from a limited selection of simple building blocks and biosynthetic pathways, and yet the resulting diversity in both structure and function of these molecules far exceeds that found in synthetic compound libraries. Natural products are, therefore, a unique source of inspiration for chemists and biologists alike, and it is not surprising that they are the lead compounds for many drug discovery and development programs. Indeed, drugs developed from natural products are ubiquitous in modern medicine, particularly in the areas of anti-infectives, immunotherapy, and cancer chemotherapy.
We have had the opportunity and privilege to explore the chemistry and biology of several interesting secondary metabolites that were clinically important drugs, or have since been developed into clinically useful medicines. This review will highlight and put in a broader context our laboratory’s work on these projects, namely the total synthesis of, and related studies on, amphotericin B (1, Figure 1), calicheamicin γ1I (2), rapamycin (3), Taxol® (4), the epothilones [e.g. epothilones A (5) and B (6)], and vancomycin (7). Knowledge gleaned from these endeavors has advanced the understanding of the chemistry, biology, and medicine of these complex and structurally diverse molecules. The total synthesis of these secondary metabolites has led to the development of a range of useful synthetic strategies and technologies. Such studies have also enabled investigations into the biological function of these agents, resulting in the establishment of structure-activity relationships (SARs) within their classes and new insights into their mechanisms of action, and, in some cases, the discovery of potential drug candidates. Before concluding, we will also briefly highlight our studies with selected other bioactive natural products [i.e. dynemicin A (8, Figure 2), uncialamycin (9), eleutherobin (10), sarcodictyin A (11), azaspiracid-1 (12), thiostrepton (13), abyssomicin C (14), platensimycin (15), platencin (16), and palmerolide A (17)] that provided useful insights into their chemistry and biology as part of our endeavors to develop useful biological tools and potential drug candidates.
Figure 1.
Molecular structures of selected natural product drugs and drug leads.
Figure 2.
Molecular structures of selected bioactive natural products.
2. Amphotericin B
Isolated from a strain of Streptomyces nodosus collected in 1955 from the Orinoco delta in Tembladora, Venezuela,3 amphotericin B (1, Figure 3) is the flagship member of the polyene macrolide family of natural products.4 For nearly fifty years, amphotericin B as a deoxycholate complex has been, and continues to be, the gold standard for the treatment of life-threatening systemic fungal infections.5 Despite its significant nephrotoxicity, the broad-spectrum activity and low incidence of fungal resistance after decades of use6 has assured amphotericin B a continued and important role in modern medicine. Alternative formulations have been developed to address the observed nephrotoxicity of the deoxycholate complex, and some of these formulations are now in clinical use.5c,7
Figure 3.
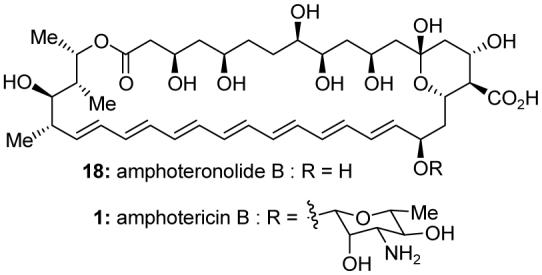
Molecular structures of amphoteronolide B (18) and amphotericin B (1).
The mechanism of action of amphotericin B is not well understood. In a widely accepted model, several molecules of amphotericin B bind ergosterol and form an ion channel in the cellular membrane, disrupting potassium gradients.8 However, amphotericin B appears to have multiple molecular functions, none of which is completely characterized.7a Recent findings by Burke and coworkers suggest either key details of the pore structure may be in error, or ion channel formation may not be essential for antifungal activity.9 The chemical and photochemical instability of amphotericin B has impeded efforts toward elucidating its SARs and its mechanism of action. Hoping to enable investigations into the biology of amphotericin B and other polyene macrolide antibiotics, we embarked in the 1980s on a total synthesis of amphoteronolide B (18, Figure 3) and amphotericin B (1).10
The highlight of our total syntheses of amphoteronolide B (18) and amphotericin B (1), completed in 1987, was a macrocyclization process featuring a ketophosphonate-aldehyde condensation (Scheme 1). This strategy had been previously developed and deployed by us11 and by others,12 but this application remains to this day one of the most, if not the most, demanding tests of this macrocyclization technique. Pleasantly, the yellow-colored ketophosphonate 19 was converted into red/orange-colored 38-membered macrocycle 20 in 70% yield upon exposure to mildly basic conditions. Macrocycle 20 was then successfully transformed into amphoteronolide B (18) and amphotericin B (1), validating our synthetic strategy. Ketophosphonate-aldehyde macrocyclizations have since been successfully applied to many other total syntheses.13
Scheme 1.
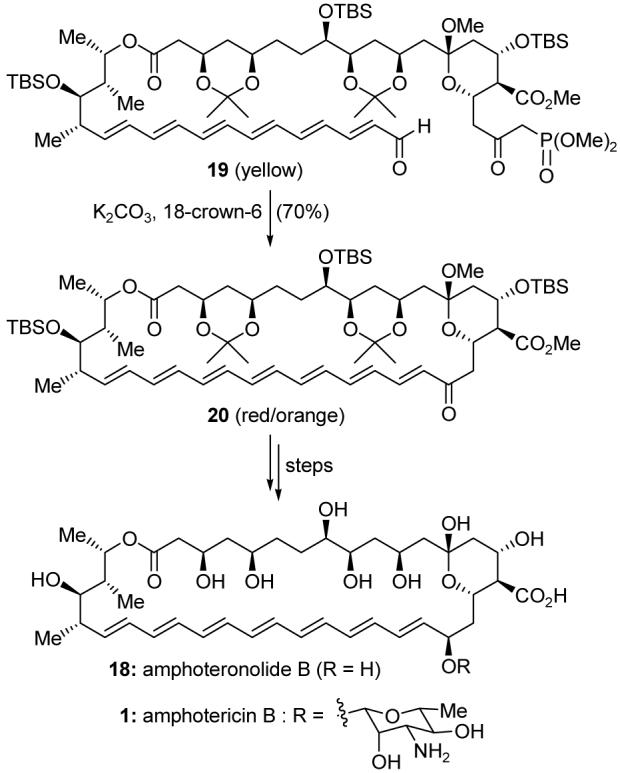
Application of the ketophosphonate-aldehyde macrocyclization to the total synthesis of amphoteronolide B (18) and amphotericin B (1) (Nicolaou et al., 1987).10
3. Calicheamicin γ1I
The calicheamicins, reported in 1987,14 are enediyne antitumor antibiotics15 isolated from Micromonospora echinospora ssp. calichensis, a bacterium residing in a rock (caliche in Greek) collected from Texas. Calicheamicin γ1I (2, Figure 4), the most prominent member of the enediyne class of natural products, possesses phenomenal cytotoxicity against tumor cells. Although calicheamicin γ1I is too toxic and indiscriminant for use as a drug, gemtuzumab ozogamicin (Mylotarg®, 21, Figure 4), a derivative of calicheamicin γ1I conjugated to a humanized anti-CD33 antibody, was developed as an anticancer agent.16 In 2000, Mylotarg® became the first antibody-drug conjugate to be approved for clinical use by the Food and Drug Administration (FDA) in the USA. It is indicated for the treatment of certain acute myeloid leukemias (AML).
Figure 4.
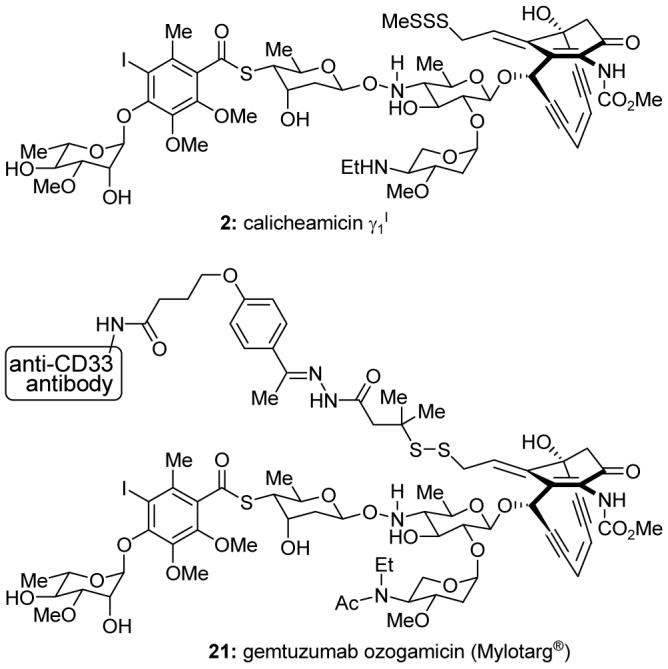
Molecular structures of calicheamicin γ1I (2) and gemtuzumab ozogamicin (Mylotarg®, 21).
The complex structure of calicheamicin γ1I (2) possesses three distinct domains: an oligosaccharide chain, a trisulfide moiety, and an enediyne core. The oligosaccharide chain recognizes and targets selected base pair sequences in the minor groove of DNA. The trisulfide moiety then serves as a molecular trigger, and upon reductive activation, the resulting thiolate performs an intramolecular Michael addition onto the proximally positioned enone moiety to unlock the enediyne warhead. Bergman cycloaromatization17 of the enediyne structural motif then generates a para-benzyne diradical,18 which abstracts hydrogen radicals from the DNA backbone. Reaction of the so-formed DNA backbone radicals with molecular oxygen results in double strand cuts, leading to cell death. By virtue of this ingenious mechanism of action, the calicheamicins and other enediynes have been hailed as masterpieces of molecular design by nature that have captured the imagination of chemists and biologists alike.15,16
Intrigued by the structure and the mechanism of action of the calicheamicins, we set out on a study of enediyne reactivity.19 Simple cyclic enediyne model systems 23 (Scheme 2) were prepared by the Ramberg-Bäcklund reaction20 of halosulfones 22, and by comparing the geometric parameters of these designed structures with their propensity toward Bergman cycloaromatization, we concluded that the cd distance (see structure 23, Scheme 2) of an enediyne was well correlated with its propensity to undergo Bergman cycloaromatization. Thus, enediynes within this series with a cd distance greater than 3.31 Å were found to be stable at room temperature, whereas those predicted to have a cd distance below 3.20 Å could not be isolated, presumably because they rapidly underwent Bergman cycloaromatization. Ten-membered ring enediyne natural products have cd distances near this critical range, and thus are prone to cycloaromatization upon relatively minor structural changes within the molecule. Separately, our collaborative studies on the interaction of the oligosaccharide portion of calicheamicin γ1I with DNA led to interesting insights relating to DNA-carbohydrate interactions.21
Scheme 2.
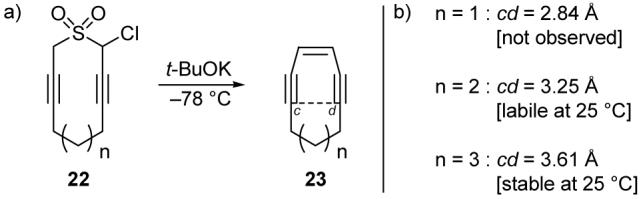
a) Application of the Ramberg-Bäcklund reaction to the preparation of simple enediyne model systems. b) Calculated cd distances (Nicolaou et al., 1988-1992).19
As one might expect from the novelty and complexity of the target molecule, our campaign for the total synthesis of calicheamicin γ1I (2),22 completed in 1992, was rich in chemical challenges and discoveries, a few of which are highlighted here. The unusual N-O glycoside linkage within the oligosaccharide domain was constructed through a Mitsunobu reaction23 with hemiacetal 24 (Scheme 3). The neighboring ester did not control the stereochemistry of the newly-formed acetal moiety, but rather, the stereochemical distribution of products was consistent with an SN2 reaction. Condensation of the N-deprotected material (25) with ketone 26 completed the installation of the N-O linkage to give oxime ether 27. This was converted into thiocarbonyl-containing intermediate 28, which underwent a stereospecific [3,3]-sigmatropic rearrangement to give thioester 29, an intermediate that was successfully elaborated into protected oligosaccharide fragment 30.
Scheme 3.
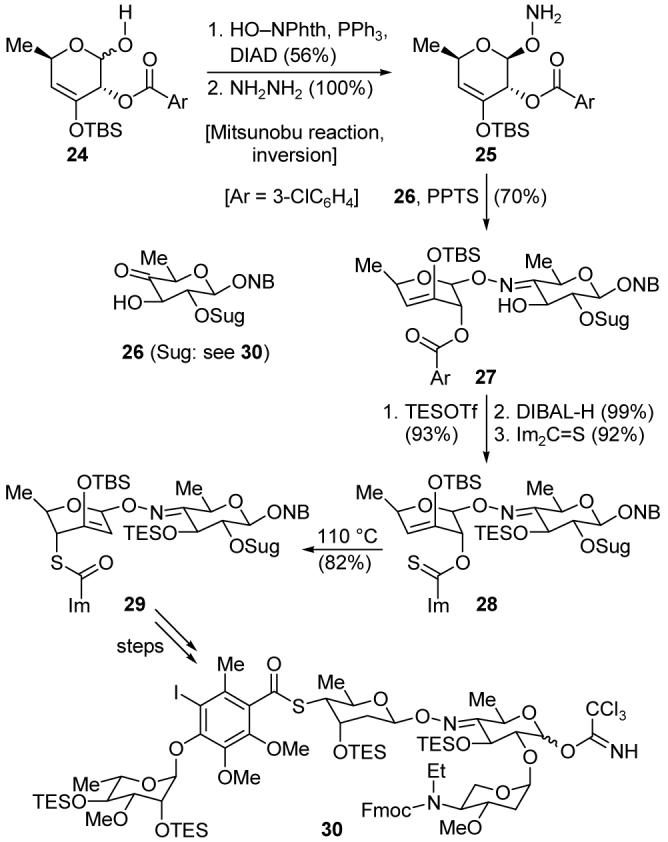
Highlights of the synthesis of the calicheamicin γ1I oligosaccharide domain (30) (Nicolaou et al., 1990).22a,d
The calicheamicinone core (32, Scheme 4) was constructed through an acetylene-aldehyde cyclization of precursor 31. The resulting propargylic hydroxyl group possessed the incorrect stereochemical arrangement, and all attempts at Mitsunobu inversion or similar reactions were unsuccessful. However, we discovered that formation of the corresponding mesylate and exposure to silica gel gave lactone 33 with clean inversion of stereochemistry. This material was elaborated to provide advanced intermediate 34, which was coupled with oligosaccharide fragment 30 and successfully transformed into calicheamicin γ1I (2). A total synthesis of calicheamicin γ1I similarly rich in discoveries was later completed by the Danishefsky camp.24
Scheme 4.
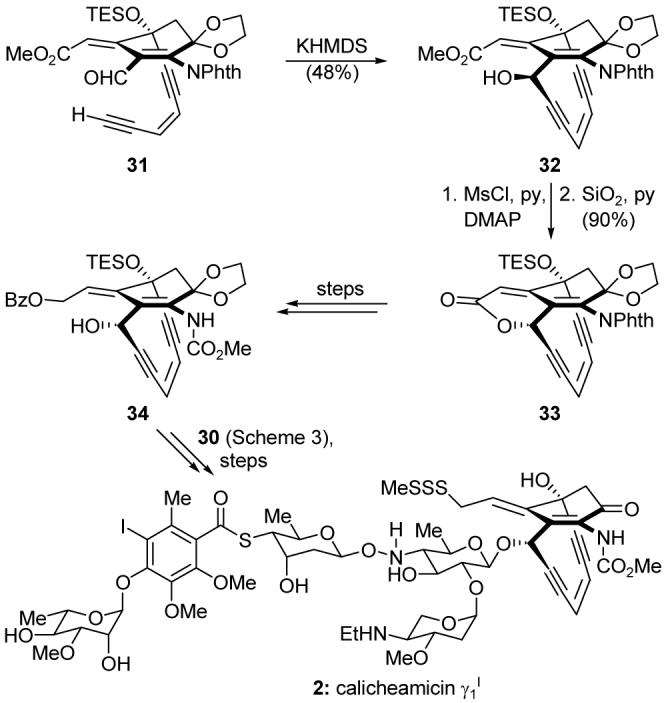
Highlights of the total synthesis of the calicheamicinone core and the total synthesis of calicheamicin γ1I (2) (Nicolaou et al., 1992).22
4. Rapamycin
Rapamycin (sirolimus, 3, Figure 5)25 was isolated in the early 1970s from Streptomyces hygroscopicus, a bacterium found on Rapa Nui (Easter Island).26 First discovered as an antifungal agent, rapamycin was soon found to be too toxic for that purpose.27 However, it was revisited because of its immunosuppressive properties, and, in 1997, rapamycin was launched as the immunosuppressant Rapamune®. A rapamycin-eluting stent was approved by the FDA in 2003. In 2007, the semisynthetic analog temsirolimus (Torisel®, 35, Figure 5) was approved by the FDA for the treatment of advanced renal cell carcinoma.
Figure 5.
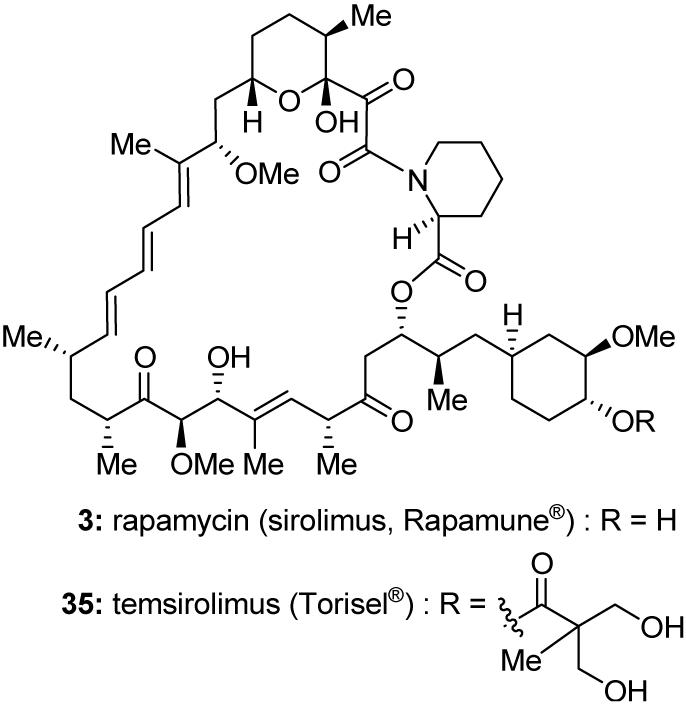
Molecular structures of rapamycin (Rapamune®, 3) and temsirolimus (Torisel®, 35).
Rapamycin interacts with FK506 binding protein (FKBP) to form a protein-drug complex.28 Despite sharing the same protein target, rapamycin’s mechanism of action is different from that of FK506. Indeed, rapamycin and FK506 are competitive antagonists to each other.29 The FKBP-rapamycin combination forms a ternary complex with the mammalian target of rapamycin (mTOR), and it is through this supramolecular assemblage that rapamycin exerts its biological properties, including immunosuppressive and cytostatic activity.25,30 Rapamycin and other TOR ligands may play a role in a wide range of disease states and health conditions that are not yet fully explored.
The first total synthesis of rapamycin was reported from our laboratory in 1993.31 Our synthetic strategy featured as final step a “double stitching” cyclization, a concept first introduced specifically for this project. Thus, as shown in Scheme 5, diiodide 36, free of protecting groups, was subjected to a double Stille coupling32 with distannylethylene 37 to furnish rapamycin (3) in 27% yield. This strategy is notable for its brevity and for affording the natural product directly and without further manipulations upon ring closure.33 Subsequent total syntheses of rapamycin include those from the laboratories of Schreiber,34 Danishefsky,35 Smith,36 and Ley.37
Scheme 5.
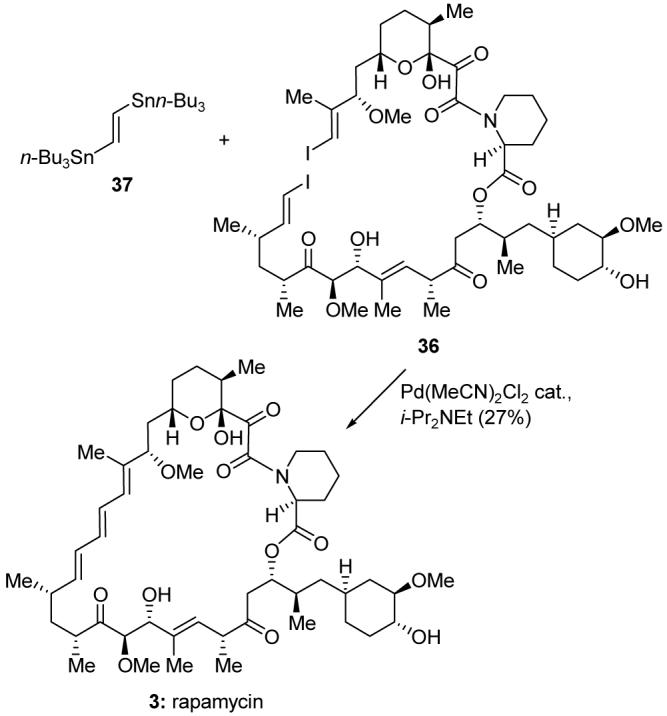
Application of the “double stitching” macrocyclization to the total synthesis of rapamycin (3) (Nicolaou et al., 1993).31
5. Taxol®
The toxic properties of yew trees have been recognized since ancient times. However, the modern story of Taxol® (paclitaxel, 4, Figure 6)38 began in 1962 with the collection of bark from the Pacific yew tree Taxus brevifolia. The molecular structure of Taxol® was disclosed in 1971,39 and its then novel mechanism of action was reported in the late 1970s.40 Taxol® was launched in 1992 for the treatment of refractory ovarian cancer, and the semisynthetic taxoid docetaxel (Taxotere®, 38, Figure 6) in 1996 for certain breast cancers. Both anticancer agents have since been approved by the FDA for other indications. In 2004, the FDA approved the use of a paclitaxel-eluting stent. Abraxane®, a paclitaxel nanoparticle formulation, was approved in 2006.
Figure 6.
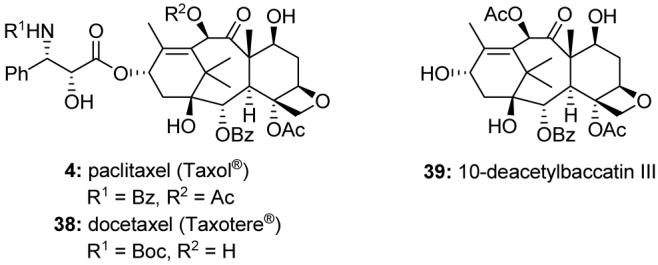
Molecular structures of paclitaxel (Taxol®, 4), docetaxel (Taxotere®, 38), and 10-deacetylbaccatin III (39).
Taxol® exerts its cytotoxic effect by interacting with tubulin.40 In contrast to other antimitotic anticancer drugs such as vinblastine, which act to inhibit microtubule formation, Taxol® acts by promoting microtubule formation and stabilizing microtubules. The disclosure of this mechanism of action ignited interest in this long-overlooked compound. In light of the importance of Taxol® in cancer chemotherapy, its SARs have been extensively studied.41
By the early 2000s, Taxol® (4) had become the best-selling anticancer drug of all time. However, there was a time when its development was in doubt because of the low availability from its natural source, the rare and slow-growing Pacific yew tree T. brevifolia. Indeed, the issues associated with Taxol® supply prompted the National Cancer Institute (NCI) to reevaluate its policies regarding the development of large-scale production methods for promising anticancer agents.42 Fortunately, 10-deacetylbaccatin III (39, Figure 6), readily available from the European yew tree Taxus baccata, was found to be a suitable precursor for industrial scale semisynthetic production of both Taxol® (4) and docetaxel (38),43 and alternative methods of production have since been developed.44 Before these discoveries, however, total synthesis was thought to be the only solution for the sustainable supply of Taxol®. The synthetic chemistry community responded, and several total syntheses have been disclosed.
Completed in 1994, our total synthesis of Taxol®45 featured two early-stage Diels-Alder cycloadditions46 for the construction of the A- and C-ring equivalents. Heating diene 40 (Scheme 6) and ketene equivalent 41 afforded, after exposure to strong base, cyclohexene 42, which was transformed into hydrazone 43. The Diels-Alder based construction of the C-ring surrogate was originally problematic due to poor regioselectivity and low yield. However, taking inspiration from the work of Narasaka,47 phenylboronic acid was used as a tether to preorganize pyrone 44 (Scheme 7) and dienophile 45 as shown in intermediate 46, enhancing the propensity of the reactants toward a Diels-Alder cycloaddition and enforcing the desired regioselectivity. The boronate tether was then cleaved by the addition of a diol, providing lactone 48 (formed by spontaneous lactone migration of Diels-Alder cycloadduct 47) in 61% yield. Lactone 48 was elaborated into C-ring fragment 49, which also possesses the functionality required for installation of the oxirane ring.
Scheme 6.
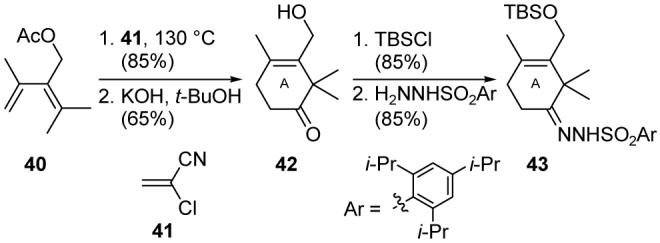
Diels-Alder based construction of the A-ring fragment of Taxol® (Nicolaou et al., 1994).45
Scheme 7.
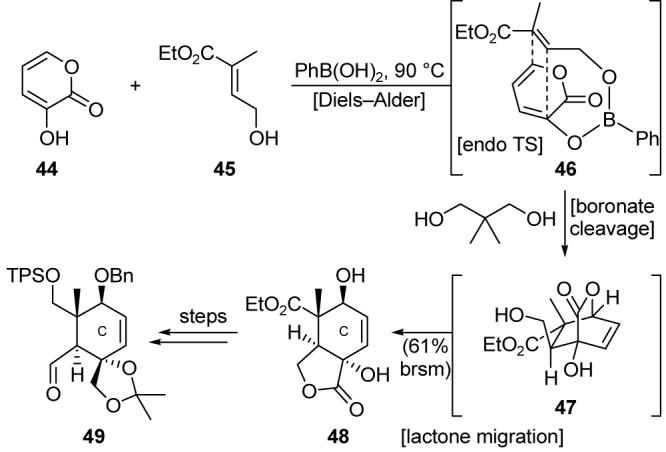
Boron-tethered Diels-Alder based construction of the C-ring fragment of Taxol® (Nicolaou et al., 1994).45
A Shapiro reaction48 converted the A-ring fragment 43 into a vinyl lithium species, which was trapped with C-ring aldehyde 49 to give intermediate 50 (Scheme 8) in 82% yield. The latter compound was converted into dialdehyde 51, setting the stage for the critical 8-membered B-ring formation. The action of TiCl3·(DME)1.5 and Zn-Cu couple effected an intramolecular McMurry coupling49 on dialdehyde 51 and forged the strained B-ring to yield ABC-ring intermediate 52. Further manipulations and side chain attachment then furnished Taxol® (4). The Holton group reported their total synthesis of Taxol®50 at about the same time. Additional total syntheses of Taxol® followed in the next few years from the laboratories of Danishefsky,51 Wender,52 Kuwajima,53 and Mukaiyama.54
Scheme 8.
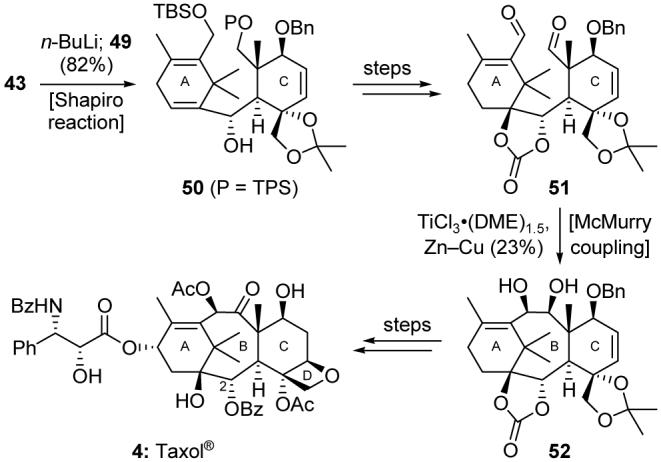
Highlights of the total synthesis of Taxol® (4) (Nicolaou et al., 1994).45
Our Taxol® campaign yielded, in addition to its coveted target, a plethora of other discoveries. A practical avenue to C2 Taxol® analogs (see numbering on Scheme 8) was developed and exploited to construct numerous such taxanes.45b,55 In addition, several water-soluble Taxol® prodrugs,56 aromatic C ring analogs,57 and fluorescent Taxol® derivatives for use as biological probes58 were designed and synthesized.
6. Epothilones
Epothilones A (5, Figure 7) and B (6, Figure 7)59 were first discovered in the 1980s as novel antifungal agents.60 They were soon recognized as potent antitumor agents, but they would not garner the attention they deserved until the compounds were rediscovered in the 1990s,61 when their mechanism of action was determined. The epothilones have the same mechanism of action as Taxol®,61,62 but with some improved properties, including the ability to overcome Taxol® resistance.61-63 By 2007, at least seven epothilones, including the naturally occurring epothilone B, had been advanced to clinical trials. In October 2007, ixabepilone (Ixempra®, 53, Figure 7), a semisynthetic analog of epothilone B, was approved by the FDA for the treatment of certain advanced breast cancers. Ixempra® is the first epothilone to reach the clinic as an approved medication for cancer patients.
Figure 7.
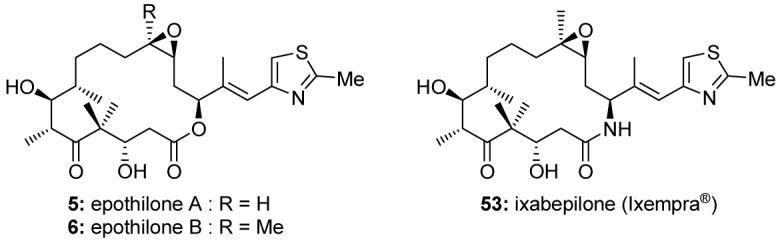
Molecular structures of epothilones A (5) and B (6) and of ixabepilone (Ixempra®, 53).
The epothilones bind to tubulin in the same protein pocket as Taxol®,61 although apparently with different key interactions.64 However, in contrast to most other antitumor agents, they show little susceptibility to phosphoglycoprotein-mediated drug efflux, giving them a distinctly different activity profile against multidrug-resistant (MDR) cancer cell lines.61-63 Over thirty total syntheses of epothilones A (5) and B (6) have been reported,39a,65 beginning with the pioneering syntheses by Danishefsky et al.,66 us,67,68 and Schinzer et al.69 The development of flexible and efficient de novo syntheses of the epothilones has enabled detailed SARs investigations on all domains of the epothilone structure.59,60,63,65
In 1997, we disclosed the total synthesis of the epothilones by two distinct strategies: macrolactonization67 and ring-closing olefin metathesis.68 The latter strategy, as applied to the total synthesis of epothilone A (5), is shown in Scheme 9. Thus, triene 54 was exposed to catalytic ruthenium metathesis initiator 5570 to provide macrocycle 56 in 46% yield (plus 39% yield of the E isomer). While this was a relatively untested strategy at the time, its success on such a multifunctional substrate helped to propel olefin metathesis71 to become a popular reaction in the total synthesis of complex molecules. Desilylation and epoxidation (promoted by dioxirane 57) then completed the total synthesis of epothilone A (5).
Scheme 9.
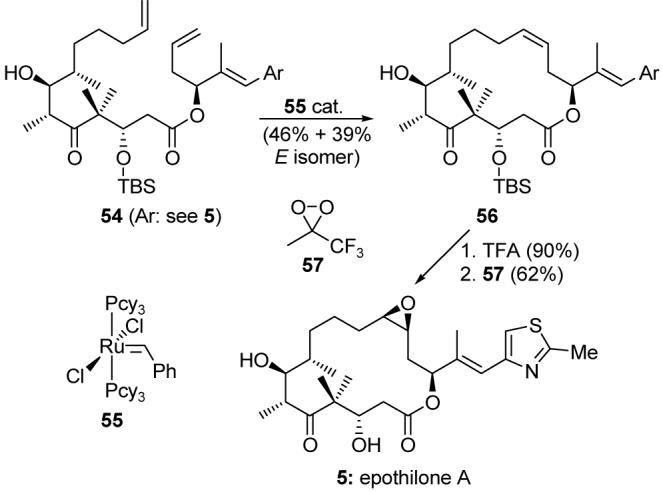
Application of the olefin metathesis macrocyclization to the total synthesis of epothilone A (5) (Nicolaou et al., 1997).68
Applying our solution and solid phase synthetic technologies, we synthesized hundreds of epothilone analogs and, through collaborative biological investigations, established useful SARs.72 For example, we demonstrated that the presence and precise position of the basic nitrogen of the side chain is crucial for potent activity, and that analogs with cyclopropane moieties with cis or trans configurations [e.g. 58 (Figure 8)] possess comparable potency to the natural epothilones.72d Based on these studies, later efforts focused on analogs of general structure 64 (Scheme 10), possessing a heteroaromatic ring system with an ortho-positioned basic nitrogen.73 These analogs were synthesized in one step by a Stille cross-coupling32 reaction of vinyl iodide 62 with heteroaromatic stannanes of general structure 63. Analogs containing pyridines, thiazoles, pyrazoles, imidazoles, triazoles, tetrazoles, and benzothiazoles were designed, synthesized, and evaluated for antitumor potency. Selected analogs (59-61)72e,73 that display higher potency than the natural epothilones against a panel of tumor cell lines (including Taxol®- and epothilone-resistant strains) are shown in Figure 8. Thiazole analog 59 entered phase I clinical trials, and pyrazole analog 61 was recently licensed by a biotechnology company for further evaluation and development.
Figure 8.
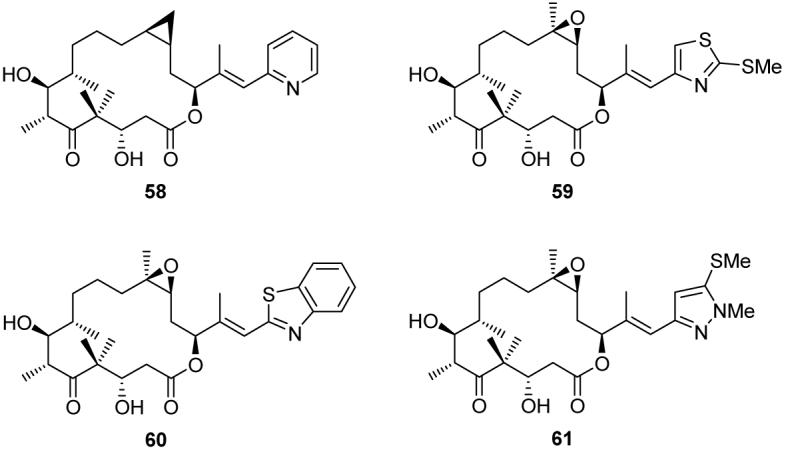
Molecular structures of selected highly potent epothilone analogs (Nicolaou et al., 1998-2006).72,73
Scheme 10.
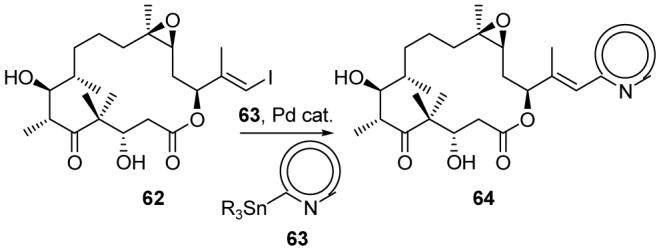
General one-step synthesis of epothilone analogs with various heteroaromatic ring systems (Nicolaou et al., 2002).73
7. Vancomycin
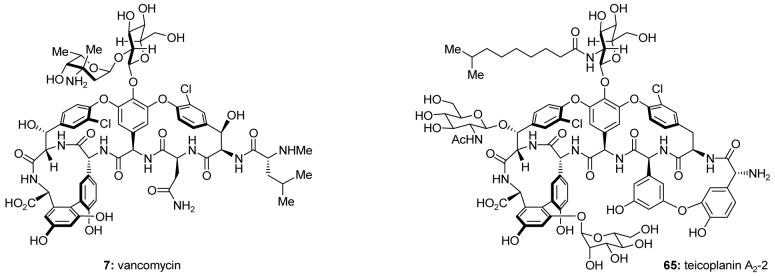
Vancomycin (7, Figure 9)74 was discovered in 1956 from a soil sample collected in the jungles of Borneo.75 Due to its unprecedented broad-spectrum activity against Gram-positive bacteria, the compound was renamed from O5865 to vancomycin (derived from “to vanquish”), and was approved as an antibiotic by the FDA in 1958. Though vancomycin and its sister antibiotic, teicoplanin [see teicoplanin A2-2 (65), Figure 9], are not the most widely used antibiotics, they nonetheless are critical medications in our arsenal against drug-resistant Gram-positive bacteria because they serve as antibiotics of last resort when other drugs fail. With the growing problem of antibiotic resistance,76 vancomycin has taken on an increasingly important role. However, no antibiotic can vanquish bacteria forever. With the discovery of vancomycin-resistant strains of methicillin-resistant Staphylococcus aureus (MRSA) in 1997,77 the urgent need to develop new antibiotics effective against such superbugs became clearly evident.
Figure 9.
Molecular structures of vancomycin (7) and teicoplanin A2-2 (65).
Vancomycin and other glycopeptide antibiotics attack Gram-positive bacteria by binding to an l-Lys-d-Ala-d-Ala fragment of the peptidoglycan structure of their cell wall.74,78 The disclosure of the molecular structure of vancomycin in the early 1980s allowed this interaction to be studied in detail, and five important hydrogen bonding interactions between vancomycin and the bacterial cell wall have been identified.78 In order to further the understanding of vancomycin’s mechanism of action and possibly develop next-generation vancomycin-based antibiotics, synthetic chemists sought to construct synthetic vancomycin and to gain further insights into its structure and activity. The Evans laboratory80 and our group81 reported total syntheses of vancomycin aglycon simultaneously in 1998. In 1999, Boger and coworkers completed their total synthesis of vancomycin aglycon,82 and our laboratory completed the total synthesis of vancomycin (7).83 Since then, the Boger82c,84 and Evans85 groups have also prepared teicoplanin aglycon by total synthesis.
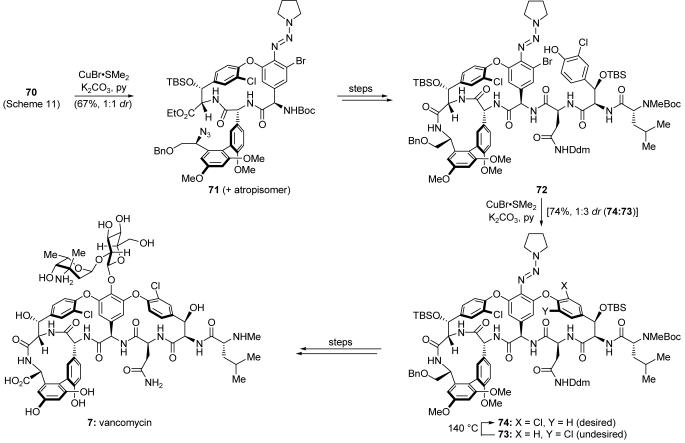
The first challenge addressed by our total synthesis of vancomycin was the atropselective construction of the axially chiral biaryl system. Diol 66 (Scheme 11) was monoprotected, and the resulting product was transformed into cyclic boronate monoester 67. Suzuki cross-coupling32 with aryl iodide 68 then furnished biaryl system 69 in 84% yield as a 2:1 mixture of atropisomers. The desired atropisomer was converted into triazine intermediate 70, setting the stage for a triazine-driven bisaryl ether synthesis, which afforded macrocycle 71 (Scheme 12) in 67% yield and as a 1:1 ratio of atropisomers. Developed in response to the challenge presented by vancomycin,81d,86 this reaction was templated by copper(I), which is thought to bind to the strategically placed triazine moiety, templating the substrate to bring about the desired macrocyclic bisaryl ether formation under exceptionally mild conditions while avoiding epimerization of the highly sensitive arylglycine groups. The desired atropisomer was then advanced to intermediate 72, at which point a second triazine-driven bisaryl ether formation was employed to forge the remaining macrocyclic domain of the molecule, providing predominantly the undesired atropisomer (73). Thermal equilibration of 73 gave access to the desired atropisomer (74), which was then carried forward to complete the total syntheses of vancomycin aglycon and vancomycin (7). These highlights give a few examples of how natural product total synthesis can drive the development of synthetic technologies.
Scheme 11.
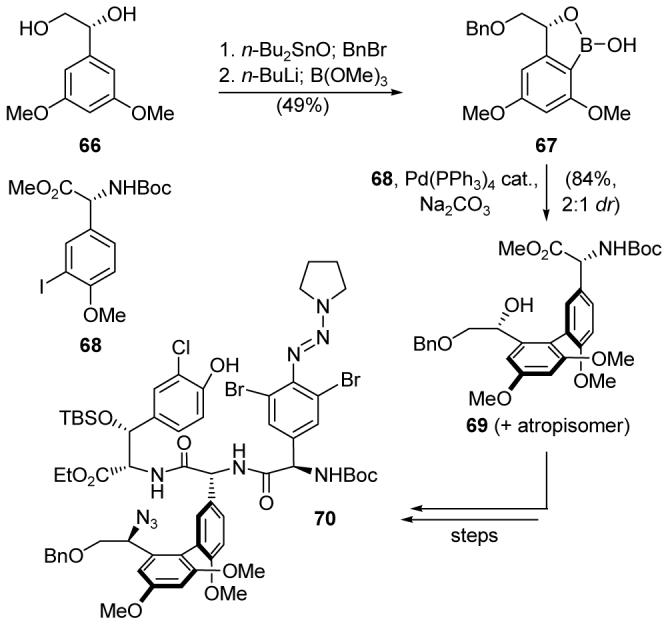
Application of an atropselective Suzuki cross-coupling to the construction of triazine 70 (Nicolaou et al., 1998).81
Scheme 12.
Application of the triazine-driven bisaryl ether synthesis to the total synthesis of vancomycin aglycon and vancomycin (7) (Nicolaou et al., 1998-1999).81,83
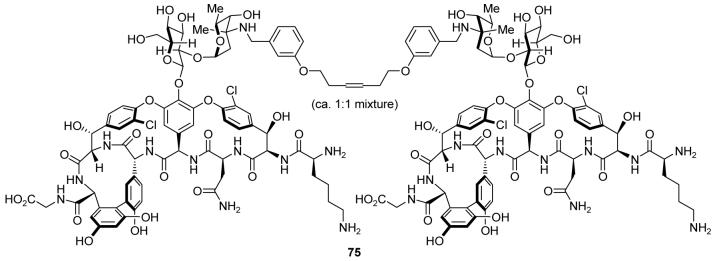
Having completed the total synthesis of vancomycin, we proceeded to design and synthesize a number of vancomycin structures for biological evaluation.87 These studies culminated in the discovery of several vancomycin dimers, such as 75 (Figure 10), that exhibited highly potent activity against drug-resistant bacteria, including vancomycin-resistant strains. Using dynamically generated virtual combinatorial libraries, a concept first introduced by Lehn and coworkers,88 a greater number of potential dimeric pairs were evaluated than could readily be prepared individually. In short, mixtures of monomeric units were allowed to interact with a target containing an l-Lys-d-Ala-d-Ala moiety, and subsequent dimerization of the preorganized monomeric units preferentially dimerized the tightest-binding pairs. These dimeric compounds exhibited not only enhanced potency, but also the ability to overcome the most common mechanism of bacterial resistance to vancomycin.89 Many resistant strains alter the vancomycin binding site into an l-Lys-d-Ala-d-Lac ester linkage, disrupting a critical hydrogen bond to vancomycin. The tighter binding of these dimeric analogs evidently allows them to retain high potency even against such strains. Therefore, vancomycin dimers such as 75 represent promising lead compounds for the development of next-generation vancomycin-based antibiotics.
Figure 10.
Molecular structure of a highly potent dimeric vancomycin analog active against vancomycin-resistant bacteria (Nicolaou et al., 2001).87
8. Other Promising Bioactive Molecules
The development of clinically useful drugs is a long and tedious process that can take many years to come to fruition. Experience, however, teaches us that today’s basic discoveries may be the foundation of tomorrow’s medicines. The sections above highlight our contributions to the chemistry and biology of some molecules successful in human medicine. This section will briefly highlight selected examples of our studies on a number of naturally occurring substances that possess promising biological activities but are not, at least as yet, in the clinic.
Following our research on calicheamicin γ1I, our work on enediynes has expanded to include investigations of the chemistry and biology of other enediyne natural products and designed analogs. During the 1990s, a series of analogs of dynemicin A (8, Figure 11)90 were designed and synthesized, and their biological properties evaluated.91,92 Dynemicin analog 76 was especially interesting, exhibiting potent broad-spectrum activity against tumor cells, in particular leukemia cell lines. In 2007, we accomplished a racemic synthesis and stereochemical assignment of uncialamycin (9, Figure 11),93,94 an enediyne recently discovered in minute quantities from an incompletely characterized strain of Streptomycete. Our subsequent enantioselective synthesis of uncialamycin (9) and 26-epi-uncialamycin (77, Figure 11) enabled extensive in vitro biological investigations of these molecules, which we demonstrated to be highly potent DNA cleaving agents with promising antibiotic and antitumor properties.95
Figure 11.
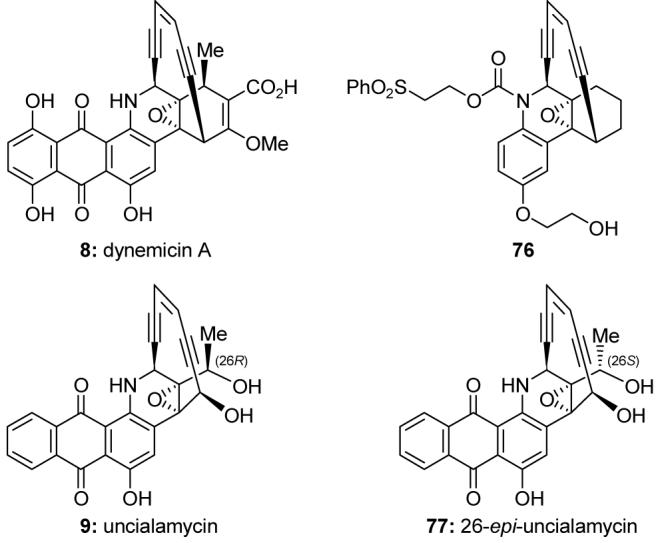
Molecular structures of selected natural and designed enediyne compounds (Nicolaou et al., 1990-1992; 2007-2008).91,94,95
Eleutherobin (10, Figure 12)96 is a cytotoxic microtubule stabilizing agent isolated from an Eleutherobia species of soft coral collected from a coastal area of western Australia and disclosed in 1995. Because of the limited supply of this promising but extremely scarce bioactive substance and of the closely related natural products eleuthosides A (78, Figure 12) and B (79, Figure 12)97 and sarcodictyins A (11, Figure 12) and B (80, Figure 12)98 and their perceived importance as potential anticancer agents,99 we set out to synthesize them in the laboratory, a goal that was achieved in 1997.100,101 In addition to supplying enough material for further biological evaluation, the synthetic technology that was developed was also adapted to the solution and solid phase synthesis of a combinatorial library of these novel structures.102 The evaluation of these analogs defined the SARs for this class of molecules and identified compound 81 (Figure 12) as a potent cytotoxic agent that retains activity against Taxol®-resistant cell lines.
Figure 12.
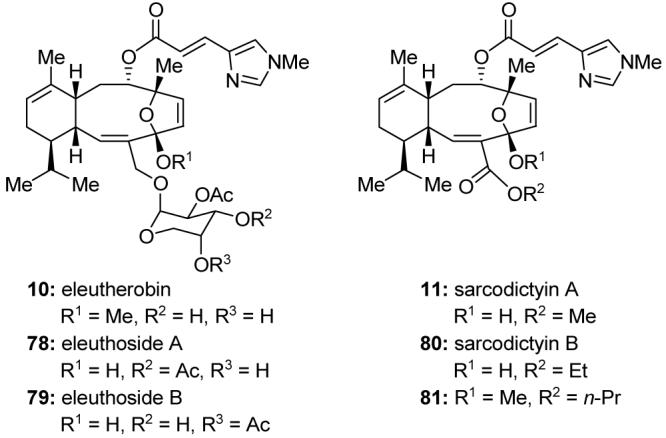
Molecular structures of eleutherobin (10) and related compounds (Nicolaou et al., 1997-1998).100,102
Azaspiracid-1 (12, Figure 13) is a marine toxin found in contaminated mussels. Isolated in 1998,103 it is responsible for a toxic syndrome known as azaspiracid poisoning (AZP).104 Because of the importance of this toxin in human health, we pursued the total synthesis of the published structure of the azaspiracid-1 (four possible isomers were proposed, one of which was structure 82, Figure 13) only to find, in 2003, that none of the proposed structures were correct.105 Careful investigation of the structural discrepancies and de novo synthesis of multiple fragments and analogs for spectroscopic comparisons with the natural substance and its degradation products ultimately revealed the true structure of azaspiracid-1 to be 12 (Figure 13).106,107 An improved synthesis of this molecule108 also provided access to its siblings, azaspiracids-2 and -3, which enabled biological investigations that were previously unattainable due to the natural scarcity of these neurotoxins.109
Figure 13.
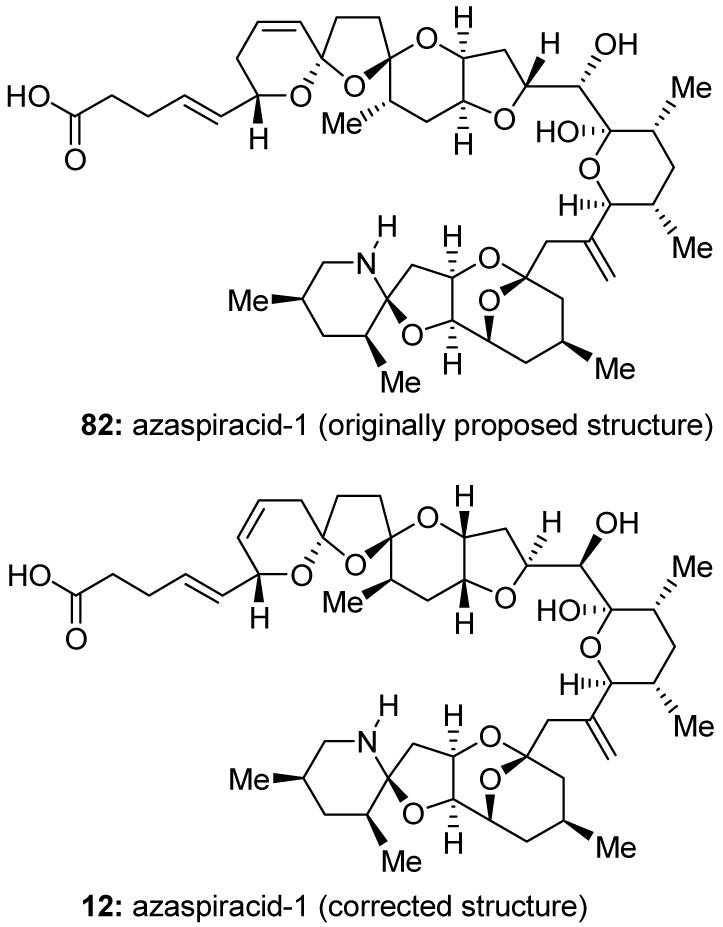
Originally proposed (82) and corrected (12) molecular structures of azaspiracid-1 (Nicolaou et al., 2003-2004).105,106
Discovered in 1954, thiostrepton (13, Figure 14)110 is the flagship member of the thiopeptide class of antibiotics.111 Thiostrepton possesses antibacterial, antiparasitic, and immunosuppressant activities. Though it has not been used in human medicine, thiostrepton is used as a veterinary antibiotic. In 2004, we completed a total synthesis of thiostrepton featuring a biomimetic aza-Diels-Alder cycloaddition to forge the dehydropiperidine core of the molecule.112,113 Subsequent chemical biology studies revealed significant antibacterial and cytotoxic properties for the simplified compound 83 (Figure 14).114
Figure 14.
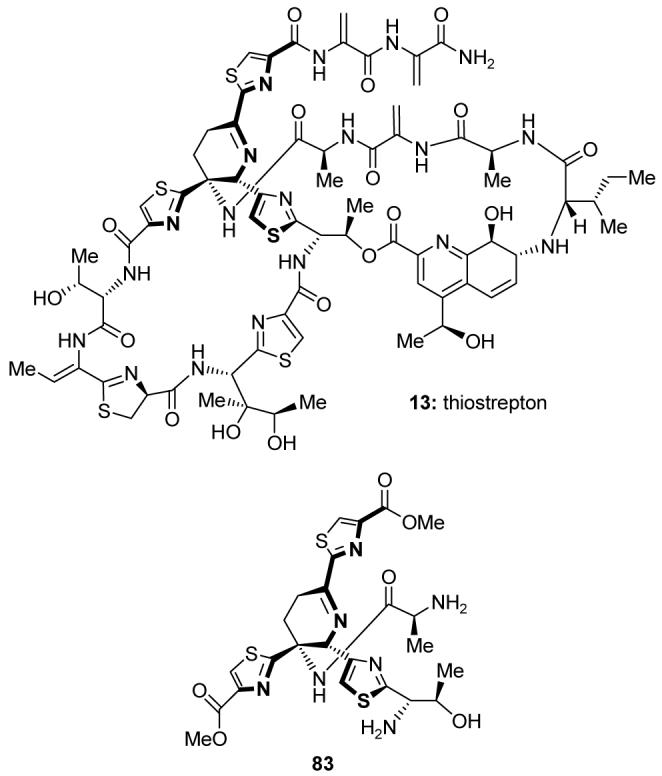
Molecular structures of thiostrepton (13) and a simplified bioactive analog (83) (Nicolaou et al., 2004-2005).112,114
Reported in 2004 from the rare actinomycete Verrucosispora strain AB18-032, abyssomicin C (14, Scheme 13) is the first natural product discovered to inhibit the biosynthesis of p-aminobenzoic acid.115 This enzymatic pathway is essential to many microorganisms, but absent in humans, making it an attractive target for intervention as a means to develop new antibiotics.116 Our total synthesis of abyssomicin C117,118 resulted in the serendipitous discovery of the more potent atrop-abyssomicin C (84, Scheme 13) and a facile acid-promoted interconversion between the two atropisomers (14 and 84). Careful investigation of the chemistry of abyssomicin C and atrop-abyssomicin C also shed light on the possible biosynthetic origin of abyssomicin D (86, Scheme 13) and uncovered the previously undescribed iso-abyssomicin D (85, Scheme 13). Interestingly, atrop-abyssomicin C (84) was subsequently identified as the primary abyssomicin metabolite of Verrucosispora strain AB18-032.119
Scheme 13.
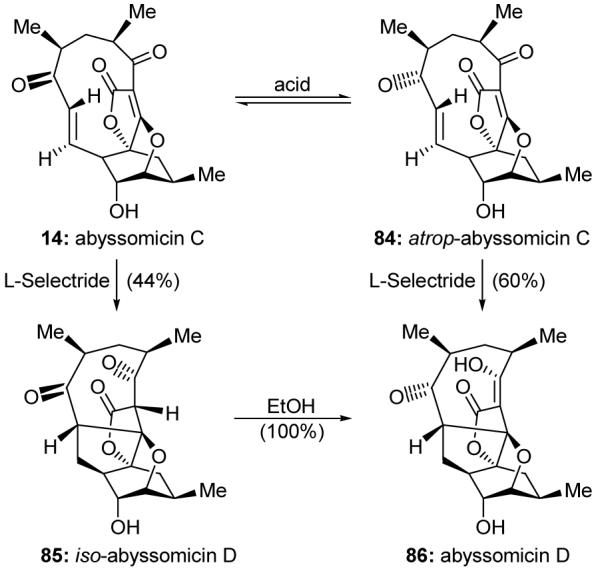
Highlights of selected reactions of the abyssomicins (Nicolaou and Harrison, 2006-2007).117
Platensimycin (15, Figure 15)120 and platencin (16, Figure 15)121 were reported in 2006 and 2007, respectively, as novel fatty acid biosynthesis inhibitors.122 Platensimycin is a selective inhibitor of FabF, and platencin is an inhibitor of FabF and FabH, enzymes in the bacterial type II fatty acid biosynthesis (FAS II) pathway. These antibiotics display broad-spectrum activity against Gram-positive bacteria, including MRSA and vancomycin-intermediate S. aureus (VISA). We reported the first total synthesis of racemic platensimycin123 as well as the first asymmetric synthesis of both platensimycin124,125 and platencin.126 Our laboratory also designed and synthesized the platensimycin analogs adamantaplatensimycin (87, Figure 15)127 and carbaplatensimycin (88, Figure 15),128 and reported their potent antibacterial properties.
Figure 15.
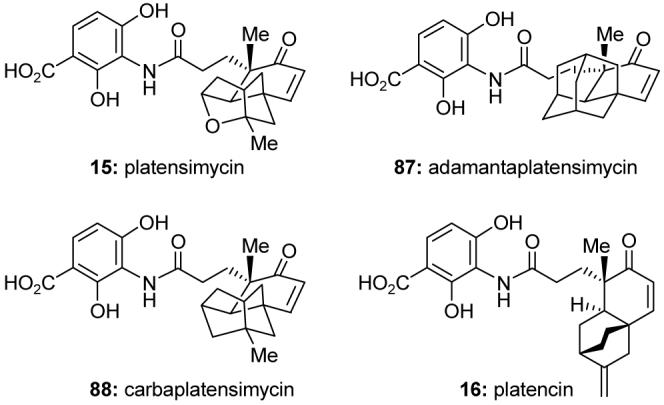
Molecular structures of platensimycin (15), platencin (16), and designed platensimycin analogs (Nicolaou et al., 2007-2008).123,124,126-128
Isolated from the Antarctic tunicate Synoicum adareanum and disclosed in 2006, palmerolide A (17, Figure 16) was claimed to possess potent cytotoxicity against melanoma cell lines and impressive selectivity in the National Cancer Institute (NCI) 60 human tumor cell line assay.129 Prompted by this promising biological profile, the Nicolaou-Chen group in Singapore developed an enantioselective synthesis of palmerolide A which not only contributed to the structural revision of this natural product from 89 (Figure 16) to 17 (Figure 16), but also rendered palmerolide A and several of its analogs readily available for biological investigations.130,131
Figure 16.
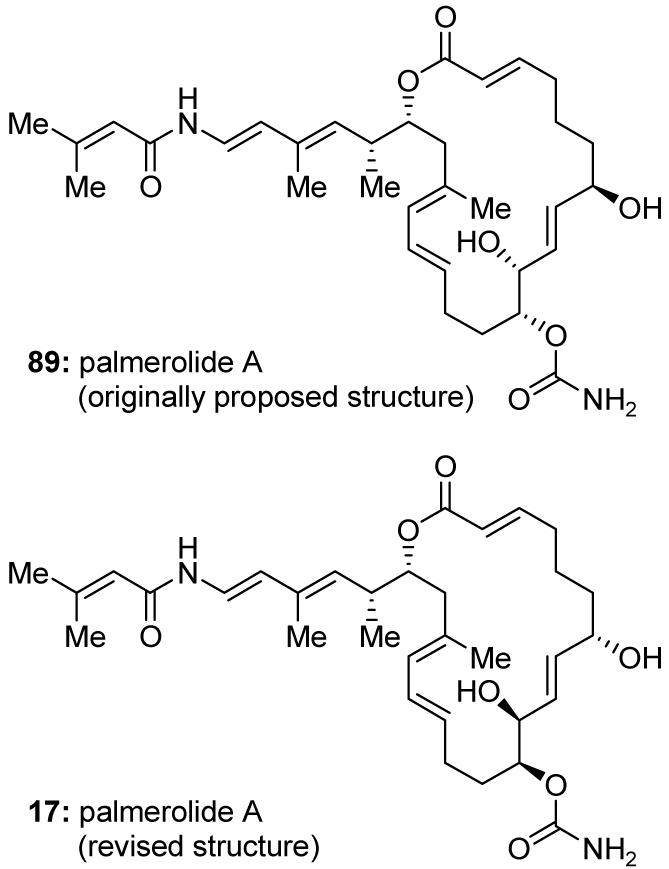
Originally proposed (89) and revised (17) molecular structures of palmerolide A (Nicolaou, Chen et al., 2007).130
9. Conclusion and Future Perspectives
As demonstrated in this article, chemistry not only plays an important role in isolating and structurally characterizing bioactive secondary metabolites, but also contributes decisively to understanding and further developing these natural products into useful drugs. In particular, chemical synthesis, whether semi-synthesis or total synthesis, often plays a pivotal role in supplying the natural substance in large amounts and in delivering novel analogs for biological investigations, clarifying the mechanism of action, and providing useful intelligence on their SARs. In addition, these studies frequently provide useful insights into the intrinsic chemical and physical properties of such molecules that enable their eventual development as medicines. The story of Aspirin®2,132 continues to be a brilliant paradigm for drug discovery, demonstrating the value of folk medicine, isolation and structural chemistry, medicinal chemistry on the originally-isolated molecule, and chemical synthesis as a means to manufacture the active ingredient of the drug. The intervening successes since the introduction of this important medication bode well for further discoveries in the future through the same avenue, the one that starts with nature.
Acknowledgements
We acknowledge the contributions of the many coworkers and collaborators with whom we have the privilege of working and whose names appear in the cited papers. Without them, this review would not have been written. We thank the National Institutes of Health and the Skaggs Institute for Chemical Biology for providing financial support for our research.
References and Notes
- 1.Nicolaou KC, Montagnon T. Molecules That Changed the World. Wiley-VCH; Weinheim: 2008. p. 366. [Google Scholar]
- 2.Nicolaou KC, Guy RK, Gunzner JL. Medchem News. 1997;3:12. [Google Scholar]
- 3.Isolation:Vandeputte J, Watchtel JL, Stiller ET. Antibiot. Annu. 1956:587.structure determination:Mechinski W, Shaffner CP, Ganis P, Avitabile G. Tetrahedron Lett. 1970;11:3873.Ganis P, Avitabile G, Mechinski W, Shaffner CP. J. Am. Chem. Soc. 1971;93:4560. doi: 10.1021/ja00747a037.
- 4.(a) Omura S, editor. Macrolide Antibiotics, Chemistry, Biology and Practice. Academic; New York: 1984. p. 635. [Google Scholar]; (b) Tereshin IM. Polyene Antibiotics-Present and Future. University of Tokyo; Tokyo: 1976. p. 160. [Google Scholar]
- 5.(a) Lemke A, Kiderlen AF, Kayser O. Appl. Microbiol. Biotechnol. 2005;68:151. doi: 10.1007/s00253-005-1955-9. [DOI] [PubMed] [Google Scholar]; (b) Gallis HA, Drew RH, Pickard WW. Rev. Infect. Dis. 1990;12:308. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]; (c) Ostrosky-Zeichner L, Marr KA, Rex JH, Cohen SH. Clin. Infect. Dis. 2003;37:415. doi: 10.1086/376634. [DOI] [PubMed] [Google Scholar]
- 6.Ellis D. J. Antimicrob. Chemother. 2002;49(S1):7. doi: 10.1093/jac/49.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 7.(a) Hartsel S, Bolard J. Trends Pharmacol. Sci. 1996;17:445. doi: 10.1016/s0165-6147(96)01012-7. [DOI] [PubMed] [Google Scholar]; (b) Barrett JP, Vardulaki KA, Conlon C, Cooke J, Daza-Ramirez P, Evans EG, Hawkey PM, Herbrecht R, Marks DI, Moraleda JM, Park GR, Senn SJ, Viscoli C. Clin. Ther. 2003;25:1295. doi: 10.1016/s0149-2918(03)80125-x. [DOI] [PubMed] [Google Scholar]
- 8.(a) de Kruijff B, Demel RA. Biochim. Biophys. Acta. 1974;339:57. doi: 10.1016/0005-2736(74)90332-0. [DOI] [PubMed] [Google Scholar]; (b) Andreoli TE. Ann. New York Acad. Sci. 1974;234:448. doi: 10.1111/j.1749-6632.1974.tb43283.x. [DOI] [PubMed] [Google Scholar]
- 9.Palacios DS, Anderson TM, Burke MD. J. Am. Chem. Soc. 2007;129:13804. doi: 10.1021/ja075739o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Nicolaou KC, Daines RA, Uenishi J, Li WS, Papahatjis DP, Chakraborty TK. J. Am. Chem. Soc. 1987;109:2205. [Google Scholar]; (b) Nicolaou KC, Daines RA, Chakraborty TK. J. Am. Chem. Soc. 1987;109:2208. [Google Scholar]; (c) Nicolaou KC, Daines RA, Chakraborty TK, Ogawa Y. J. Am. Chem. Soc. 1987;109:2821. [Google Scholar]; (d) Nicolaou KC, Chakraborty TK, Ogawa Y, Daines RA, Simpkins NS, Furst GT. J. Am. Chem. Soc. 1988;110:4660. [Google Scholar]; (e) Nicolaou KC, Daines RA, Uenishi J, Li WS, Papahatjis DP, Chakraborty TK. J. Am. Chem. Soc. 1988;110:4672. [Google Scholar]; (f) Nicolaou KC, Daines RA, Chakraborty TK, Ogawa Y. J. Am. Chem. Soc. 1988;110:4685. [Google Scholar]; (g) Nicolaou KC, Daines RA, Ogawa Y, Chakraborty TK. J. Am. Chem. Soc. 1988;110:4696. [Google Scholar]
- 11.(a) Nicolaou KC, Seitz SP, Pavia MR, Petasis NA. J. Org. Chem. 1979;44:4011. [Google Scholar]; (b) Nicolaou KC, Pavia MP, Seitz SP. J. Am. Chem. Soc. 1981;103:1224. [Google Scholar]; (c) Nicolaou KC, Seitz SP, Pavia MP. J. Am. Chem. Soc. 1982;104:2030. [Google Scholar]
- 12.(a) Buechi G, Wueest H. Helv. Chim. Acta. 1979;62:2661. [Google Scholar]; (b) Still WC, Novack VJ. J. Am. Chem. Soc. 1984;106:1148. [Google Scholar]
- 13.For selected examples of the use of ketophosphonate-aldehyde macrocyclizations in complex molecule synthesis, see:Schlessinger RH, Poss MA, Richardson S. J. Am. Chem. Soc. 1986;108:3112.Nakajima N, Hamada T, Tanaka T, Oikawa Y, Yonemitsu O. J. Am. Chem. Soc. 1986;108:4645.Kozikowski AP, Xia Y. J. Org. Chem. 1987;52:1375.Akita H, Yamada H, Matsukura H, Nakata T, Oishi T. Tetrahedron Lett. 1990;31:1735.Duplantier AJ, Masamune S. J. Am. Chem. Soc. 1990;112:7079.Nishida A, Kikuno Y, Kawahara N, Nishida M, Yonemitsu O. Tetrahedron Lett. 1995;36:3215.Elliott MR, Dhimane AL, Hamon L, Malacria M. Eur. J. Org. Chem. 2000;1:155.Taillier C, Bellosta V, Cossy J. Org. Lett. 2004;6:2149. doi: 10.1021/ol049433n.Packard GK, Hu Y, Vescovi A, Rychnovsky SD. Angew. Chem., Int. Ed. 2004;43:2822. doi: 10.1002/anie.200453697.Seo S-Y, Jung J-K, Paek S-M, Lee Y-S, Kim S-H, Suh Y-G. Tetrahedron Lett. 2006;47:6527.
- 14.(a) Lee MD, Dunne TS, Siegel MM, Chang CC, Morton GO, Borders DB. J. Am. Chem. Soc. 1987;109:3464. [Google Scholar]; (b) Lee MD, Dunne TS, Chang CC, Ellestad GA, Siegel MM, Morton GO, McGahren WJ, Borders DB. J. Am. Chem. Soc. 1987;109:3466. [Google Scholar]; (c) Lee MD, Dunne TS, Chang CC, Siegel MM, Morton GO, Ellestad GA, McGahren WJ, Borders DB. J. Am. Chem. Soc. 1992;114:985. [Google Scholar]
- 15.(a) Borders DB, Doyle TW, editors. Enediyne Antibiotics as Antitumor Agents. Marcel Dekker; New York: 1995. p. 459. [Google Scholar]; (b) Smith AL, Nicolaou KC. J. Med. Chem. 1996;39:2103. doi: 10.1021/jm9600398. [DOI] [PubMed] [Google Scholar]; (c) Jones GB, Fouad FS. Curr. Pharm. Des. 2002;8:2415. doi: 10.2174/1381612023392810. [DOI] [PubMed] [Google Scholar]
- 16.Thorson JS, Sievers EL, Ahlert J, Shepard E, Whitwam RE, Onwueme KC, Ruppen M. Curr. Pharm. Des. 2000;6:1841. doi: 10.2174/1381612003398564. [DOI] [PubMed] [Google Scholar]
- 17.(a) Jones RR, Bergman RG. J. Am. Chem. Soc. 1972;94:660. [Google Scholar]; (b) Bergman RG. Acc. Chem. Res. 1973;6:25. [Google Scholar]
- 18.Upon activation of structurally related esperamicins, radical intermediates have been observed by EPR, see:Golik J. In: Enediyne Antibiotics as Antitumor Agents. Borders DB, Doyle TW, editors. Marcel Dekker; New York: 1995. pp. 187–216.
- 19.(a) Nicolaou KC, Ogawa Y, Zuccarello G, Kataoka H. J. Am. Chem. Soc. 1988;110:7247. [Google Scholar]; (b) Nicolaou KC, Zuccarello G, Riemer C, Estevez VA, Dai W-M. J. Am. Chem. Soc. 1992;114:7360. [Google Scholar]
- 20.Paquette LA. Org. React. 1977;25:1. [Google Scholar]
- 21.(a) Nicolaou KC, Schreiner EP, Iwabuchi Y, Suzuki T. Angew. Chem., Int. Ed. Engl. 1992;31:340. [Google Scholar]; (b) Gomez-Paloma L, Smith JA, Chazin WJ, Nicolaou KC. J. Am. Chem. Soc. 1994;116:3697. [Google Scholar]; (c) Li T, Zeng Z, Estevez VA, Baldenius K-U, Nicolaou KC, Joyce GF. J. Am. Chem. Soc. 1994;116:3709. [Google Scholar]; (d) Liu C, Smith BM, Ajito K, Komatsu H, Gomez-Paloma L, Li T, Theodorakis EA, Nicolaou KC, Vogt PK. Proc. Natl. Acad. Sci. U.S.A. 1996;93:940. doi: 10.1073/pnas.93.2.940. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Nicolaou KC, Ajito K, Komatsu H, Smith BM, Bertinato P, Gomez-Paloma L. J. Chem. Soc., Chem. Commun. 1996:1495. [Google Scholar]; (f) Nicolaou KC, Smith BM, Ajito K, Komatsu H, Gomez-Paloma L, Tor Y. J. Am. Chem. Soc. 1996;118:2303. [Google Scholar]; (g) Bifulco G, Galeone A, Gomez-Paloma L, Nicolaou KC, Chazin WJ. J. Am. Chem. Soc. 1996;118:8817. [Google Scholar]; (h) Nicolaou KC, Smith BM, Pastor J, Watanabe Y, Weinstein DS. Synlett. 1997:401. [Google Scholar]; (i) Bifulco G, Galeone A, Nicolaou KC, Chazin WJ, Gomez-Paloma L. J. Am. Chem. Soc. 1998;120:7183. [Google Scholar]
- 22.(a) Nicolaou KC, Groneberg RD, Miyazaki T, Stylianides NA, Schulze TJ, Stahl W. J. Am. Chem. Soc. 1990;112:8193. [Google Scholar]; (b) Smith AL, Hwang C-K, Pitsinos E, Scarlato G, Nicolaou KC. J. Am. Chem. Soc. 1992;114:3134. [Google Scholar]; (c) Nicolaou KC, Hummel CW, Pitsinos EN, Nakada M, Smith AL, Shibayama K, Saimoto H. J. Am. Chem. Soc. 1992;114:10082. [Google Scholar]; (d) Groneberg RD, Miyazaki T, Stylianides NA, Schulze TJ, Stahl W, Schreiner EP, Suzuki T, Iwabuchi Y, Smith AL, Nicolaou KC. J. Am. Chem. Soc. 1993;115:7593. [Google Scholar]; (e) Smith AL, Pitsinos EN, Hwang C-K, Mizuno Y, Saimoto H, Scarlato GR, Suzuki T, Nicolaou KC. J. Am. Chem. Soc. 1993;115:7612. [Google Scholar]; (f) Nicolaou KC, Hummel CW, Nakada M, Shibayama K, Pitsinos EN, Saimoto H, Mizuno Y, Baldenius K-U, Smith AL. J. Am. Chem. Soc. 1993;115:7625. [Google Scholar]; (g) Nicolaou KC. Angew. Chem., Int. Ed. Engl. 1993;32:1377. [Google Scholar]
- 23.Mitsunobu O. Synthesis. 1981:1. [Google Scholar]
- 24.(a) Hitchcock SA, Boyer SH, Chu-Moyer MY, Olson SH, Danishefsky SJ. Angew. Chem., Int. Ed. 1994;33:858. [Google Scholar]; (b) Halcomb RL, Boyer SH, Wittman MD, Olson SH, Denhart DJ, Liu KKC, Danishefsky SJ. J. Am. Chem. Soc. 1995;117:5720. [Google Scholar]; (c) Hitchcock SA, Chu-Moyer MY, Boyer SH, Olson SH, Danishefsky SJ. J. Am. Chem. Soc. 1995;117:5750. [Google Scholar]; (d) Danishefsky SJ, Shair MD. J. Org. Chem. 1996;61:16. [Google Scholar]
- 25.(a) Dumont FJ, Su Q. Life Sci. 1996;58:373. doi: 10.1016/0024-3205(95)02233-3. [DOI] [PubMed] [Google Scholar]; (b) Law BK. Crit. Rev. Oncol. Hematol. 2005;56:47. doi: 10.1016/j.critrevonc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 26.(a) Vezina C, Kudelski A, Sehgal SN. J. Antibiot. 1975;28:721. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]; (b) Sehgal SN, Baker H, Vezina C. J. Antibiot. 1975;28:727. doi: 10.7164/antibiotics.28.727. [DOI] [PubMed] [Google Scholar]
- 27.Martel RR, Klicius J, Galet S. Can. J. Physiol. Pharmacol. 1977;55:48. doi: 10.1139/y77-007. [DOI] [PubMed] [Google Scholar]
- 28.(a) Rosen MK, Schreiber SL. Angew. Chem., Int. Ed. Engl. 1992;31:384. [Google Scholar]; (b) Schreiber SL. Science. 1991;251:283. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]; (c) Schreiber SL, Liu J, Albers MW, Karmacharya R, Koh E, Martin PK, Rosen MK, Standaert RF, Wandless TJ. Transplantation Proc. 1991;23:2839. [PubMed] [Google Scholar]; (d) Snyder SH, Sabatini DM. Nature Med. 1995;1:32. doi: 10.1038/nm0195-32. [DOI] [PubMed] [Google Scholar]
- 29.(a) Dumont FJ, Melino MR, Staruch MJ, Koprak SL, Fischer PA, Sigal NH. J. Immunol. 1990;114:1418. [PubMed] [Google Scholar]; (b) Bierer BE, Mattila PS, Standaert RF, Herzenberg LA, Burakoff SJ, Crabtree G, Schreiber SL. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9231. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsang CK, Qi H, Liu LF, Zheng XFS. Drug Disc. Today. 2007;12:112. doi: 10.1016/j.drudis.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 31.(a) Nicolaou KC, Chakraborty TK, Piscopio AD, Minowa N, Bertinato P. J. Am. Chem. Soc. 1993;115:4419. [Google Scholar]; (b) Nicolaou KC, Piscopio AD, Bertinato P, Chakraborty TK, Minowa N, Koide K. Chem. Eur. J. 1995;1:318. [Google Scholar]
- 32.For selected reviews on palladium-catalyzed cross-coupling reactions, see:Hegedus LS. Transition Metals in the Synthesis of Complex Organic Molecules. 2nd ed. University Science Books; Sausalito: 1999. p. 352.Negishi E, editor. Handbook of Organopalladium Chemistry for Organic Synthesis. Wiley Interscience; New York: 2002. p. 3350.de Meijere A, Diederich F, editors. Metal-Catalyzed Cross-Coupling Reactions. 2nd ed. Wiley-VCH; Weinheim: 2004. p. 938.Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem., Int. Ed. 2005;44:4442. doi: 10.1002/anie.200500368.
- 33.For another example of “double stitching” ring cyclization, see:Shair MD, Yoon TY, Danishefsky SJ. Angew. Chem., Int. Ed. Engl. 1995;34:1721.Shair MD, Yoon TY, Mosny KK, Chou TC, Danishefsky SJ. J. Am. Chem. Soc. 1996;118:9509.(c) ref. 24d.
- 34.Romo D, Meyer SD, Johnson DD, Schreiber SL. J. Am. Chem. Soc. 1993;115:7906. [Google Scholar]
- 35.Hayward CM, Yohannes D, Danishefsky SJ. J. Am. Chem. Soc. 1993;115:9345. [Google Scholar]
- 36.(a) Smith AB, Condon SM, McCauley JA, Leazer JL, Leahy JW, Maleczka RE. J. Am. Chem. Soc. 1995;117:5407. [Google Scholar]; (b) Smith AB, Condon SM, McCauley JA, Leazer JL, Leahy JW, Maleczka RE. J. Am. Chem. Soc. 1997;119:947. [Google Scholar]; (c) Smith AB, Condon SM, McCauley JA, Leazer JL, Leahy JW, Maleczka RE. J. Am. Chem. Soc. 1997;119:962. [Google Scholar]; (d) Smith AB, Condon SM, McCauley JA. Acc. Chem. Res. 1998;31:35. [Google Scholar]
- 37.Maddess ML, Tackett MN, Watanabe H, Brennan PE, Spilling CD, Scott JS, Osborn DP, Ley SV. Angew. Chem., Int. Ed. 2007;46:591. doi: 10.1002/anie.200604053. [DOI] [PubMed] [Google Scholar]
- 38.(a) Goodman J, Walsh V. The Story of Taxol. Cambridge University Press; Cambridge: 2001. p. 282. [Google Scholar]; (b) Suffness M, editor. Taxol: Science and Applications. CRC Press; Boca Raton: 1995. p. 448. [Google Scholar]; (c) Nicolaou KC, Guy RK, Potier P. Scientific American. 1996;274:94. doi: 10.1038/scientificamerican0696-94. [DOI] [PubMed] [Google Scholar]
- 39.Wani MC, Taylor HL, Wall ME, Coggin P, McPhail AT. J. Am. Chem. Soc. 1971;93:2325. doi: 10.1021/ja00738a045.For a review on the discovery of Taxol®, see:Oberlies NH, Kroll DJ. J. Nat. Prod. 2004;67:129. doi: 10.1021/np030498t.
- 40.(a) Schiff PB, Fant J, Horwitz SB. Nature. 1979;277:665. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]; (b) Schiff PB, Horwitz SB. Proc. Natl. Acad. Sci. U.S.A. 1980;77:1561. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.(a) Kingston DGI. Chem. Commun. 2001:867. [Google Scholar]; (b) Kingston DGI. Phytochemistry. 2007;68:1844. doi: 10.1016/j.phytochem.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cragg GM, Schepartz SA, Suffness M, Grever MR. J. Nat. Prod. 1993;56:1657. doi: 10.1021/np50100a001. [DOI] [PubMed] [Google Scholar]
- 43.(a) Denis JN, Green AE, Guénard D, Guéritte-Voegelein F, Mangatal L, Potier P. J. Am. Chem. Soc. 1988;110:5917. [Google Scholar]; (b) Guénard D, Guéritte-Voegelein F, Potier P. Acc. Chem. Res. 1993;26:160. [Google Scholar]
- 44.Frense D. Appl. Micribiol. Biotechnol. 2007;73:1233. doi: 10.1007/s00253-006-0711-0. [DOI] [PubMed] [Google Scholar]
- 45.(a) Nicolaou KC, Yang Z, Liu J-J, Ueno H, Nantermet PG, Guy RK, Claiborne CF, Renaud J, Couladouros EA, Paulvannan K, Sorensen EJ. Nature. 1994;367:630. doi: 10.1038/367630a0. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Nantermet PG, Ueno H, Guy RK, Couladouros EA, Sorensen EJ. J. Am. Chem. Soc. 1995;117:624. [Google Scholar]; (c) Nicolaou KC, Liu J-J, Yang Z, Ueno H, Sorensen EJ, Claiborne CF, Guy RK, Hwang C-K, Nakada M, Nantermet PG. J. Am. Chem. Soc. 1995;117:634. [Google Scholar]; (d) Nicolaou KC, Yang Z, Liu J-J, Nantermet PG, Claiborne CF, Renaud J, Guy RK, Shibayama K. J. Am. Chem. Soc. 1995;117:645. [Google Scholar]; (e) Nicolaou KC, Ueno H, Liu J-J, Nantermet PG, Yang Z, Renaud J, Paulvannan K, Chadha R. doi: 10.1038/367630a0. [DOI] [PubMed] [Google Scholar]; (f) Nicolaou KC, Guy RK. Angew. Chem., Int. Ed. Engl. 1995;34:2079. [Google Scholar]
- 46.For selected reviews on Diels-Alder cycloadditions, see:Corey EJ. Angew. Chem., Int. Ed. 2002;41:1650. doi: 10.1002/1521-3773(20020517)41:10<1650::aid-anie1650>3.0.co;2-b.Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew. Chem., Int. Ed. 2002;41:1668. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z.
- 47.Narasaka K, Shimada S, Osoka K, Iwasawa N. Synthesis. 1991:1171. [Google Scholar]
- 48.(a) Shapiro RH. Org. React. 1976;23:405. [Google Scholar]; (b) Chamberlin AR, Bloom SH. Org. React. 1990;39:1. [Google Scholar]
- 49.(a) McMurry JE. Acc. Chem. Res. 1983;16:405. [Google Scholar]; (b) McMurry JE. Chem. Rev. 1989;89:1513. [Google Scholar]
- 50.(a) Holton RA, Somoza C, Kim H-B, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S, Nadizadeh H, Suzuki Y, Tao C, Vu P, Tang S, Zhang P, Murthi KK, Gentile LN, Liu JH. J. Am. Chem. Soc. 1994;116:1597. [Google Scholar]; (b) Holton RA, Kim H-B, Somoza C, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S, Nadizadeh H, Suzuki Y, Tao C, Vu P, Tang S, Zhang P, Murthi KK, Gentile LN, Liu JH. J. Am. Chem. Soc. 1994;116:1599. [Google Scholar]; (c) Holton RA, Somoza C, Kim H-B, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S, Nadizadeh H, Suzuki Y, Tao C, Vu P, Tang S, Zhang P, Murthi KK, Gentile LN, Liu JH. ACS Symp. Ser. 1995;583:288. [Google Scholar]
- 51.(a) Masters JJ, Link JT, Snyder LB, Young WB, Danishefsky SJ. Angew. Chem., Int. Ed. Engl. 1995;34:1723. [Google Scholar]; (b) Danishefsky SJ, Masters JJ, Young WB, Link JT, Snyder LB, Magee TV, Jung DK, Isaacs RCA, Bornmann WG, Alaimo CA, Coburn CA, Di Grandi MJ. J. Am. Chem. Soc. 1996;118:2843. [Google Scholar]
- 52.(a) Wender PA, Badham NF, Conway SP, Floreancig PE, Glass TE, Gränicher C, Houze JB, Jänichen J, Lee D, Marquess DG, McGrane PL, Meng W, Mucciaro TP, Mühlebach M, Natchus MG, Paulsen H, Rawlins DB, Satkofsky J, Shuker AJ, Sutton JC, Taylor RE, Tomooka K. J. Am. Chem. Soc. 1997;119:2755. [Google Scholar]; (b) Wender PA, Badham NF, Conway SP, Floreancig PE, Glass TE, Houze JB, Krauss NE, Lee D, Marquess DG, McGrane PL, Meng W, Natchus MG, Shuker AJ, Sutton JC, Taylor RE. J. Am. Chem. Soc. 1997;119:2757. [Google Scholar]
- 53.(a) Morihira K, Hara R, Kawahara S, Nishimori T, Nakamura N, Kusama H, Kuwajima I. J. Am. Chem. Soc. 1998;120:12980. [Google Scholar]; (b) Kuwajima I, Kusama H. Synlett. 2000:1385. [Google Scholar]
- 54.(a) Shiina I, Iwadare H, Sakoh H, Hasegawa M, Tani Y-I, Mukaiyama T. Chem. Lett. 1998:1. [Google Scholar]; (b) Shiina I, Saitoh K, Frechard-Ortuno I, Mukaiyama T. Chem. Lett. 1998:3. [Google Scholar]; (c) Mukaiyama T, Shiina I, Iwadare H, Saitoh M, Nishimura T, Ohkawa N, Sakoh H, Nishimura K, Tani Y-I, Hasegawa M, Yamada K, Saitoh K. Chem. Eur. J. 1999;5:121. [Google Scholar]
- 55.Nicolaou KC, Couladouros EA, Nantermet PG, Renaud J, Guy RK, Wrasidlo W. Angew. Chem., Int. Ed. Engl. 1994;33:1581. [Google Scholar]
- 56.(a) Nicolaou KC, Riemer C, Kerr MA, Rideout D, Wrasidlo W. Nature. 1993;364:464. doi: 10.1038/364464a0. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Guy RK, Pitsinos EN, Wrasidlo W. Angew. Chem., Int. Ed. Engl. 1994;33:1583. [Google Scholar]
- 57.(a) Nicolaou KC, Claiborne CF, Nantermet PG, Couladouros EA, Sorensen EJ. J. Am. Chem. Soc. 1994;116:1591. [Google Scholar]; (b) Nicolaou KC, Claiborne CF, Paulvannan K, Postema MHD, Guy RK. Chem. Eur. J. 1997;3:399. [Google Scholar]
- 58.Guy RK, Scott ZA, Sloboda RD, Nicolaou KC. Chem. Biol. 1996;3:1021. doi: 10.1016/s1074-5521(96)90168-4. [DOI] [PubMed] [Google Scholar]
- 59.(a) Nicolaou KC, Roschangar F, Vourloumis D. Angew. Chem., Int. Ed. 1998;37:2014. doi: 10.1002/(SICI)1521-3773(19980817)37:15<2014::AID-ANIE2014>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]; (b) He L, Orr GA, Horwitz SB. Drug Disc. Today. 2001;6:1153. doi: 10.1016/s1359-6446(01)02038-4. [DOI] [PubMed] [Google Scholar]; (c) Borzilleri RM, Vite GD. Drugs Future. 2002;27:1149. [Google Scholar]; (d) Wartmann M, Altmann K-H. Curr. Med. Chem. Anticancer Agents. 2002;2:123. doi: 10.2174/1568011023354489. [DOI] [PubMed] [Google Scholar]; (e) Altaha R, Fojo T, Reed E, Abraham J. Curr. Pharm. Des. 2002;8:1707. doi: 10.2174/1381612023394043. [DOI] [PubMed] [Google Scholar]; (f) Altmann K-H. Mini-Rev. Med. Chem. 2003;3:149. doi: 10.2174/1389557033405269. [DOI] [PubMed] [Google Scholar]; (g) Altmann K-H. Curr. Pharm. Des. 2005;11:1595. doi: 10.2174/1381612053764715. [DOI] [PubMed] [Google Scholar]; (h) Altmann K-H, Pfeiffer B, Arseniyadis S, Pratt BA, Nicolaou KC. ChemMedChem. 2007;2:396. doi: 10.1002/cmdc.200600206. [DOI] [PubMed] [Google Scholar]
- 60.Höfle G, Reichenbach H. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, editors. CRC Press; Boca Raton: 2005. pp. 413–450. [Google Scholar]
- 61.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M, Lazarides E, Woods CM. Cancer Res. 1995;55:2325. [PubMed] [Google Scholar]
- 62.Kowalski RJ, Giannakakou P, Hamel E. J. Biol. Chem. 1997;272:2534. doi: 10.1074/jbc.272.4.2534. [DOI] [PubMed] [Google Scholar]
- 63.(a) Wolff A, Technau A, Brandner G. Int. J. Oncol. 1997;11:123. doi: 10.3892/ijo.11.1.123. [DOI] [PubMed] [Google Scholar]; (b) Altmann K-H, Wartmann M, O’Reilly T. Biochim. Biophys. Acta. 2000;1470:M79. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 64.(a) Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Pot P, Sackett G, Nicolaou KC, Fojo T. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2904. doi: 10.1073/pnas.040546297. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) He L, Yang C-P, Horwitz SB. Mol. Cancer Ther. 2001;1:3. [PubMed] [Google Scholar]
- 65.(a) Harris CR, Danishefsky SJ. J. Org. Chem. 1999;64:8434. [Google Scholar]; (b) Mulzer J, Martin HJ, Berger M. J. Heterocycl. Chem. 1999;36:1421. [Google Scholar]; (c) Nicolaou KC, Ritzen A, Namoto K. Chem. Commun. 2001;17:1523. doi: 10.1039/b104949f. [DOI] [PubMed] [Google Scholar]; (d) Altmann K-H. Org. Biomol. Chem. 2004;2:2137. doi: 10.1039/b405839a. [DOI] [PubMed] [Google Scholar]; (e) Watkins EB, Chittiboyina AG, Jung J-C, Avery MA. Curr. Pharm. Des. 2005;11:1615. doi: 10.2174/1381612053764742. [DOI] [PubMed] [Google Scholar]; (f) Watkins EB, Chittiboyina AG, Avery MA. Eur. J. Org. Chem. 2006:4071. [Google Scholar]
- 66.(a) Balog A, Meng D, Kamenecka T, Bertinato P, Su D-S, Sorensen EJ, Danishefsky SJ. Angew. Chem., Int. Ed. Engl. 1996;35:2801. [Google Scholar]; (b) Su D-S, Meng D, Bertinato P, Balog A, Sorensen EJ, Danishefsky SJ, Zheng Y-H, Chou T-C, He L, Horwitz SB. Angew. Chem., Int. Ed. Engl. 1997;36:757. [Google Scholar]; (c) Meng DF, Bertinato P, Balog A, Su DS, Kamenecka T, Sorensen EJ, Danishefsky SJ. J. Am. Chem. Soc. 1997;119:10073. [Google Scholar]
- 67.Macrolactonization strategy:Nicolaou KC, Sarabia F, Ninkovic S, Yang Z. Angew. Chem., Int. Ed. Engl. 1997;36:525.Nicolaou KC, Winssinger N, Pastor J, Ninkovic S, Sarabia F, He Y, Vourloumis D, Yang Z, Li T, Giannakakou P, Hamel E. Nature. 1997;387:268. doi: 10.1038/387268a0.Nicolaou KC, Ninkovic S, Sarabia F, Vourloumis D, He Y, Vallberg H, Finlay MRV, Yang Z. J. Am. Chem. Soc. 1997;119:7974.
- 68.Ring closing metathesis strategy:Yang Z, He Y, Vourloumis D, Vallberg H, Nicolaou KC. Angew. Chem., Int. Ed. Engl. 1997;36:166.(b) ref. 67b;Nicolaou KC, He Y, Vourloumis D, Vallberg H, Roschangar F, Sarabia F, Ninkovic S, Yang Z, Trujillo JI. J. Am. Chem. Soc. 1997;119:7960.
- 69.(a) Schinzer D, Limberg A, Bauer A, Böhm OM, Cordes M. Angew. Chem., Int. Ed. Engl. 1997;36:523. [Google Scholar]; (b) Schinzer D, Bauer A, Böhm OM, Limberg A, Cordes M. Chem. Eur. J. 1999;5:2483. [Google Scholar]; (c) Schinzer D, Bauer A, Böhm OM, Limberg A, Cordes M. Chem. Eur. J. 1999;5:2492. [Google Scholar]
- 70.(a) Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]; (b) Trnka TM, Morgan JP, Sanford MS, Wilhelm TE, Scholl M, Choi T-L, Ding S, Day MW, Grubbs RH. J. Am. Chem. Soc. 2003;125:2546. doi: 10.1021/ja021146w. [DOI] [PubMed] [Google Scholar]
- 71.For selected reviews on the olefin metathesis reaction, see:Grubbs RH, Chang S. Tetrahedron. 1998;54:4413.Fürstner A. Angew. Chem., Int. Ed. 2000;39:3012.Schmidt B, Hermanns J. Top. Organomet. Chem. 2004;13:223.Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem., Int. Ed. 2005;44:4490. doi: 10.1002/anie.200500369.Schrock RR, Czekelius C. Adv. Synth. Catal. 2007;349:55.Mori M. Adv. Synth. Catal. 2007;349:121.Holub N, Blechert S. Chem. Asian J. 2007;2:1064. doi: 10.1002/asia.200700072.
- 72.(a) Nicolaou KC, Sarabia F, Ninkovic S, Finlay M,R, Boddy CNC. Angew. Chem., Int. Ed. 1998;37:81. [Google Scholar]; (b) Nicolaou KC, Scarpelli R, Bollbuck B, Werschkun B, Pereira MMA, Wartmann M, Altmann K-H, Zaharevitz D, Gussio R, Giannakakou P. Chem. Bio. 2000;7:593. doi: 10.1016/s1074-5521(00)00006-5. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Namoto K, Li J, Ritzen A, Ulven T, Shoji M, Zaharevitz D, Gussio R, Sackett DL, Ward RD, Hensler A, Fojo T, Giannakakou P. ChemBioChem. 2001;2:69. doi: 10.1002/1439-7633(20010105)2:1<69::AID-CBIC69>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Namoto K, Ritzen A, Ulven T, Shoji M, Li J, D’Amico G, Liotta D, French CT, Wartmann M, Altmann K-H, Giannakakou P. J. Am. Chem. Soc. 2001;123:9313. doi: 10.1021/ja011338b. [DOI] [PubMed] [Google Scholar]; (e) Nicolaou KC, Ritzen A, Namoto K, Buey RM, Diaz JF, Andreu JM, Wartmann M, Altmann K-H, O’Brate A, Giannakakou P. Tetrahedron. 2002;58:6413. [Google Scholar]
- 73.Nicolaou KC, Pratt BA, Arseniyadis S, Wartmann M, O’Brate A, Giannakakou P. ChemMedChem. 2006;1:41. doi: 10.1002/cmdc.200500056. [DOI] [PubMed] [Google Scholar]
- 74.(a) Nagarajan R, editor. Glycopeptide Antibiotics. Marcel Dekker; New York: 1994. [Google Scholar]; (b) Yao RC, Crandall LW. Drugs Pharm. Sci. 1994;63:1. [Google Scholar]; (c) Rao AVR, Gurjar MK, Reddy KL, Rao AS. Chem. Rev. 1995;95:2135. [Google Scholar]; (d) Nicolaou KC, Boddy CNC, Bräse S, Winssinger N. Angew. Chem., Int. Ed. 1999;38:2096. doi: 10.1002/(sici)1521-3773(19990802)38:15<2096::aid-anie2096>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 75.McCormick MH, Stark WM, Pittenger GE, Pittenger RC, McGuire JM. Antibiot. Annu. 19551956:606. [PubMed] [Google Scholar]
- 76.For a thematic issue on antibiotic resistance, see:Walsh CT, Wright G, editors. Chem. Rev. 2005;105:391–774. doi: 10.1021/cr030100y.
- 77.Hiramatsu K. Drug Resist. Updates. 1998;1:135. doi: 10.1016/s1368-7646(98)80029-0. [DOI] [PubMed] [Google Scholar]
- 78.Williams DH. Nat. Prod. Rep. 1996;13:469. doi: 10.1039/np9961300469. [DOI] [PubMed] [Google Scholar]
- 79.Harris CM, Harris TM. J. Am. Chem. Soc. 1982;104:4293. [Google Scholar]
- 80.(a) Evans DA, Wood MR, Trotter BW, Richardson TI, Barrow JC, Katz JL. Angew. Chem., Int. Ed. 1998;37:2700. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2700::AID-ANIE2700>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]; (b) Evans DA, Dinsmore CJ, Watson PS, Wood MR, Richardson TI, Trotter BW, Katz JL. Angew. Chem., Int. Ed. 1998;37:2704. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2704::AID-ANIE2704>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 81.(a) Nicolaou KC, Nataranjan S, Li H, Jain NF, Hughes R, Solomon ME, Ramanjulu JM, Boddy CNC, Takayanagi M. Angew. Chem., Int. Ed. 1998;37:2708. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2708::AID-ANIE2708>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Jain NF, Nataranjan S, Hughes R, Solomon ME, Li H, Ramanjulu JM, Takayanagi M, Koumbis AE, Bando T. Angew. Chem., Int. Ed. 1998;37:2714. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2714::AID-ANIE2714>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Takayanagi M, Jain NF, Nataranjan S, Koumbis AE, Bando T, Ramanjulu JM. Angew. Chem., Int. Ed. 1998;37:2717. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2717::AID-ANIE2717>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Li H, Boddy CNC, Ramanjulu JM, Yue T-Y, Natarajan S, Chu X-J, Bräse S, Rübsam F. Chem. Eur. J. 1999;5:2584. [Google Scholar]; (e) Nicolaou KC, Boddy CNC, Li H, Koumbis AE, Hughes R, Natarajan S, Jain NF, Ramanjulu JM, Bräse S, Solomon ME. Chem. Eur. J. 1999;5:2602. [Google Scholar]; (f) Nicolaou KC, Koumbis AE, Takayanagi M, Natarajan S, Jain NF, Bando T, Li H, Hughes R. Chem. Eur. J. 1999;5:2622. [Google Scholar]
- 82.(a) Boger DL, Miyazaki S, Kim SH, Wu JH, Loiseleur O, Castle SL. J. Am. Chem. Soc. 1999;121:3226. [Google Scholar]; (b) Boger DL, Miyazaki S, Kim SH, Castle SL, Wu JH, Loiseleur O, Jin Q. J. Am. Chem. Soc. 1999;121:10004. [Google Scholar]; (c) Boger DL. Med. Res. Rev. 2001;21:356. doi: 10.1002/med.1014. [DOI] [PubMed] [Google Scholar]
- 83.(a) Nicolaou KC, Mitchell HJ, Jain NF, Winssinger N, Hughes R, Bando T. Angew. Chem., Int. Ed. 1999;38:240. [Google Scholar]; (b) Nicolaou KC, Mitchell HJ, Jain NF, Bando T, Hughes R, Winssinger N, Natarajan S, Koumbis AE. Chem. Eur. J. 1999;5:2648. [Google Scholar]
- 84.(a) Boger DL, Kim SH, Miyazaki S, Strittmatter H, Weng J-H, Mori Y, Rogel O, Castle SL, McAtee JJ. J. Am. Chem. Soc. 2000;122:7416. [Google Scholar]; (b) Boger DL, Kim SH, Mori Y, Weng J-H, Rogel O, Castle SL, McAtee JJ. J. Am. Chem. Soc. 2001;123:1862. doi: 10.1021/ja003835i. [DOI] [PubMed] [Google Scholar]
- 85.Evans DA, Katz JL, Peterson GS, Hintermann T. J. Am. Chem. Soc. 2001;123:12411. doi: 10.1021/ja011943e. [DOI] [PubMed] [Google Scholar]
- 86.Nicolaou KC, Boddy CNC, Natarajan S, Yue T-Y, Li H, Bräse S, Ramanjulu JM. J. Am. Chem. Soc. 1997;119:3421. [Google Scholar]
- 87.(a) Nicolaou KC, Winssinger N, Hughes R, Smethurst C, Cho SY. Angew. Chem., Int. Ed. 2000;39:1084. doi: 10.1002/(sici)1521-3773(20000317)39:6<1084::aid-anie1084>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Hughes R, Cho SY, Winssinger N, Smethurst C, Labischinski H, Endermann R. Angew. Chem., Int. Ed. 2000;39:3823. doi: 10.1002/1521-3773(20001103)39:21<3823::AID-ANIE3823>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Cho SY, Hughes R, Winssinger N, Smethurst C, Labsichinski H, Endermann R. Chem. Eur. J. 2001;7:3798. doi: 10.1002/1521-3765(20010903)7:17<3798::aid-chem3798>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Hughes R, Cho SY, Winssinger N, Labischinski H, Endermann R. Chem. Eur. J. 2001;7:3824. doi: 10.1002/1521-3765(20010903)7:17<3824::aid-chem3824>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 88.(a) Hasenknopf B, Lehn J-M, Kneisel BO, Baum G, Fenske D. Angew. Chem., Int. Ed. Engl. 1996;35:1838. [Google Scholar]; (b) Hasenknopf B, Lehn J-M, Boumediene N, Doupot-Gervais A, Van Dorsselaer B, Kneisel BO, Fenske D. J. Am. Chem. Soc. 1997;119:10956. [Google Scholar]; (c) Huc I, Lehn J-M. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2106. doi: 10.1073/pnas.94.6.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lehn J-M. Chem. Eur. J. 1999;5:2455. [Google Scholar]; (e) Berl V, Huc I, Lehn J-M, DeCian A, Fischer J. Eur. J. Org. Chem. 1999:3089. [Google Scholar]; (f) Ramstorm O, Lehn J-M. ChemBioChem. 2000;1:41. doi: 10.1002/1439-7633(20000703)1:1<41::AID-CBIC41>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]; (g) Lehn J-M, Eliseev AV. Science. 2001;291:2331. doi: 10.1126/science.1060066. [DOI] [PubMed] [Google Scholar]
- 89.(a) Woodford N, Johnson AP, Morrison D, Speller DCE. Clin. Microbiol. Rev. 1995;8:561. doi: 10.1128/cmr.8.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Murray BE. Am. J. Med. 1997;102:284. doi: 10.1016/S0002-9343(99)80270-8. [DOI] [PubMed] [Google Scholar]; (c) Williams DH, Bardslet B. Angew. Chem., Int. Ed. 1999;38:1172. doi: 10.1002/(SICI)1521-3773(19990503)38:9<1172::AID-ANIE1172>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 90.(a) Konishi M, Ohkuma H, Matsumoto K, Kamei H, Miyaki T, Oki T, Kawaguchi H, VanDuyne GD, Clardy J. J. Antibiot. 1989;42:1449. doi: 10.7164/antibiotics.42.1449. [DOI] [PubMed] [Google Scholar]; (b) Konishi M, Ohkuma H, Tsuno T, Oki T, VanDuyne GD, Clardy J. J. Am. Chem. Soc. 1990;112:3715. [Google Scholar]
- 91.(a) Nicolaou KC, Hwang C-K, Smith AL, Wendeborn SV. J. Am. Chem. Soc. 1990;112:7416. [Google Scholar]; (b) Nicolaou KC, Dai W-M. Angew. Chem., Int. Ed. Engl. 1991;30:1387. [Google Scholar]; (c) Nicolaou KC, Dai W-M, Tsay S-C, Estevez VA, Wrasidlo W. Science. 1992;256:1172. doi: 10.1126/science.256.5060.1172. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Maligres P, Suzuki T, Wendeborn SV, Dai W-M, Chadha RK. J. Am. Chem. Soc. 1992;114:8890. [Google Scholar]; (e) Nicolaou KC, Smith AL, Yue EW. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5881. doi: 10.1073/pnas.90.13.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.For total syntheses of dynemicin A, see:Myers AG, Fraley ME, Tom NJ, Cohen SB, Madar DJ. Chem. Biol. 1995;2:33. doi: 10.1016/1074-5521(95)90078-0.Myers AG, Tom NJ, Fraley ME, Cohen SB, Madar DJ. J. Am. Chem. Soc. 1997;119:6072.(c) ref. 33.
- 93.Uncialamycin isolation:Davies J, Wang H, Taylor T, Warabi K, Huang X-H, Andersen RJ. Org. Lett. 2005;7:5233. doi: 10.1021/ol052081f.
- 94.Nicolaou KC, Zhang H, Chen JS, Crawford JJ, Pasunoori L. Angew. Chem., Int. Ed. 2007;46:4704. doi: 10.1002/anie.200700917. [DOI] [PubMed] [Google Scholar]
- 95.Nicolaou KC, Chen JS, Zhang H, Montero A. Angew. Chem., Int. Ed. 2008;47:185. doi: 10.1002/anie.200704577. [DOI] [PubMed] [Google Scholar]
- 96.(a) Fenical W-H, Hensen PR, Lindel T. 5,473,057. U.S. Patent. 1995; Chem. Abstr. 1996;124:194297. [Google Scholar]; (b) Lindel T, Jensen PR, Fenical W, Long BH, Casazza AM, Carboni J, Fairchild CR. J. Am. Chem. Soc. 1997;119:8744. [Google Scholar]; (c) Long BH, Carboni JM, Wasserman AJ, Cornell LA, Casazza AM, Jensen PR, Lindel T, Fenical W-H, Fairchild CR. Cancer Res. 1998;58:1111. [PubMed] [Google Scholar]
- 97.Ketzinel S, Rudi A, Schleyer M, Benayahu Y, Kashman Y. J. Nat. Prod. 1996;59:873. [Google Scholar]
- 98.(a) D’Ambrosio M, Guerriero A, Pietra F. Helv. Chim. Acta. 1987;70:2019. [Google Scholar]; (b) D’Ambrosio M, Guerriero A, Pietra F. Helv. Chim. Acta. 1988;71:964. [Google Scholar]
- 99.For selected reviews on this class of molecules, see:Nicolaou KC, Pfefferkorn J, Xu J, Winssinger N, Ohshima T, Kim S, Hosokawa S, Vourloumis D, van Delft F, Li T. Chem. Pharm. Bull. 1999;47:1199. doi: 10.1248/cpb.47.1199.Stachel SJ, Biswas K, Danishefsky SJ. Curr. Pharm. Des. 2001;7:1277. doi: 10.2174/1381612013397410.He L, Orr GA, Horwitz SB. Drug Disc. Today. 2001;6:1153. doi: 10.1016/s1359-6446(01)02038-4.
- 100.(a) Nicolaou KC, van Delft F, Ohshima T, Vourloumis D, Xu J, Hosokawa S, Pfefferkorn J, Kim S, Li T. Angew. Chem., Int. Ed. Engl. 1997;36:2520. [Google Scholar]; (b) Nicolaou KC, Xu J-Y, Kim S, Ohshima T, Hosokawa S, Pfefferkorn J. J. Am. Chem. Soc. 1997;119:11353. [Google Scholar]; (c) Nicolaou KC, Kim A, Pfefferkorn J, Xu J-Y, Ohshima T, Hosokawa S, Vouloumis D, Li T. Angew. Chem., Int. Ed. 1998;37:1418. doi: 10.1002/(SICI)1521-3773(19980605)37:10<1418::AID-ANIE1418>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Xu JY, Kim S, Pfefferkorn J, Ohshima T, Vourloumis D, Hosokawa S. J. Am. Chem. Soc. 1998;120:8661. [Google Scholar]; (e) Nicolaou KC, Ohshima T, Hosokawa S, van Delft FL, Vourloumis D, Xu JY, Pfefferkorn J, Kim S. J. Am. Chem. Soc. 1998;120:8674. doi: 10.1248/cpb.47.1199. [DOI] [PubMed] [Google Scholar]
- 101.For other total syntheses of these molecules, see:Chen X-T, Zhou B, Bhattacharya SK, Gutteridge CE, Pettus TRR, Danishefsky SJ. Angew. Chem., Int. Ed. 1998;37:789. doi: 10.1002/(SICI)1521-3773(19980403)37:6<789::AID-ANIE789>3.0.CO;2-3.Chen X-T, Bhattacharya SK, Zhou B, Gutteridge CE, Pettus TRR, Danishefsky SJ. J. Am. Chem. Soc. 1999;121:6563.Ritter N, Metz P. Synlett. 2003:2422.Castoldi D, Caggiano L, Panigada L, Sharon O, Costa AM, Gennari C. Angew. Chem., Int. Ed. 2005;44:588. doi: 10.1002/anie.200461767.Castoldi D, Caggiano L, Panigada L, Sharon O, Costa AM, Gennari C. Chem. Eur. J. 2006;12:51. doi: 10.1002/chem.200500749.Gennari C, Castoldi D, Sharon O. Pure Appl. Chem. 2007;79:173.
- 102.Nicolaou KC, Winssinger N, Vourloumis D, Ohshima T, Kim S, Pfefferkorn J, Xu J-Y, Li T. J. Am. Chem. Soc. 1998;120:10814. [Google Scholar]
- 103.Satake M, Ofuji K, Naoki H, James KJ, Furey A, McMahon T, Silke J, Yasumoto T. J. Am. Chem. Soc. 1998;120:9967. [Google Scholar]
- 104.(a) McMahon T, Silke J. Harmful Algae News. 1996;14:2. [Google Scholar]; (b) Ito E, Satake M, Ofuji K, Higashi M, Harigaya K, McMahon T, Yasumoto T. Toxicon. 2002;40:193. doi: 10.1016/s0041-0101(01)00226-4. [DOI] [PubMed] [Google Scholar]
- 105.(a) Nicolaou KC, Li Y, Uesaka N, Koftis TV, Vyskocil S, Ling T, Govindasamy M, Qian W, Bernal F, Chen DY-K. Angew. Chem., Int. Ed. 2003;42:3643. doi: 10.1002/anie.200351825. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Chen DY-K, Li Y, Qian W, Ling T, Vyskocil S, Koftis TV, Govindasamy M, Uesaka N. Angew. Chem., Int. Ed. 2003;42:3649. doi: 10.1002/anie.200351826. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Pihko PM, Bernal F, Frederick MO, Qian W, Uesaka N, Diedrichs N, Hinrichs J, Koftis TV, Loizidou E, Petrovic G, Rodriquez M, Sarlah D, Zou N. J. Am. Chem. Soc. 2006;128:2244. doi: 10.1021/ja0547477. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Chen DY-K, Li Y, Uesaka N, Petrovic G, Koftis TV, Bernal F, Frederick MO, Govindasamy M, Ling T, Pihko PM, Tang W, Vyskocil S. J. Am. Chem. Soc. 2006;128:2258. doi: 10.1021/ja054748z. [DOI] [PubMed] [Google Scholar]
- 106.(a) Nicolaou KC, Vyskocil S, Koftis TV, Yamada YMA, Ling T, Chen DY-K, Tang W, Petrovic G, Frederick MO, Li Y, Satake M. Angew. Chem., Int. Ed. 2004;43:4312. doi: 10.1002/anie.200460695. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Koftis TV, Vyskocil S, Petrovic G, Ling T, Yamada YMA, Tang W, Frederick MO. Angew. Chem., Int. Ed. 2004;43:4318. doi: 10.1002/anie.200460696. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Koftis TV, Vyskocil S, Petrovic G, Tang W, Frederick MO, Chen DY-K, Li Y, Ling T, Yamada YMA. J. Am. Chem. Soc. 2006;128:2859. doi: 10.1021/ja054750q. [DOI] [PubMed] [Google Scholar]
- 107.For a total synthesis of ent-azaspiracid-1, see:Evans DA, Kvaernø L, Mulder JA, Raymer B, Dunn TB, Beauchemin A, Olhava EJ, Juhl M, Kagechika K. Angew. Chem., Int. Ed. 2007;46:4693. doi: 10.1002/anie.200701515.Evans DA, Dunn TB, Kvaernø L, Beauchemin A, Raymer B, Olhava EJ, Mulder JA, Juhl M, Kagechika K, Favor DA. Angew. Chem., Int. Ed. 2007;46:4698. doi: 10.1002/anie.200701520.
- 108.(a) Nicolaou KC, Frederick MO, Petrovic G, Cole KP, Loizidou E. Angew. Chem., Int. Ed. 2006;45:2609. doi: 10.1002/anie.200600295. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Frederick MO, Louizidou EZ, Petrovic G, Cole KP, Koftis TV, Yamada YMA. Chem. Asian J. 2006;1-2:245. doi: 10.1002/asia.200600059. [DOI] [PubMed] [Google Scholar]
- 109.For selected examples of biological investigations made possible by synthetic azaspiracids, see:Ito E, Frederick MO, Koftis TV, Tang W, Petrovic G, Ling T, Nicolaou KC. Harmful Algae. 2006;5:586.Alfonso A, Vieytes MR, Ofuji K, Satake M, Nicolaou KC, Frederick MO, Botana LM. Biochem. Biophys. Res. Commun. 2006;346:1091. doi: 10.1016/j.bbrc.2006.06.019.Vilarino N, Nicolaou KC, Frederick MO, Cagide E, Ares IM, Louzao MC, Vieytes MR, Botana LM. Chem. Res. Toxicol. 2006;19:1459. doi: 10.1021/tx060131z.Vale C, Nicolaou KC, Frederick MO, Gomez-Limia B, Alfonso A, Vieytes MR, Botana LM. J. Med. Chem. 2007;50:356. doi: 10.1021/jm061063g.Vilarino N, Nicolaou KC, Frederick MO, Vieytes MR, Botana LM. Biochem. Pharm. 2007;74:327. doi: 10.1016/j.bcp.2007.04.004.Vale C, Gomez-Limia B, Nicolaou KC, Frederick MO, Vieytes MR, Botana LM. Cell. Phys. Biochem. 2007;20:957. doi: 10.1159/000110456.
- 110.(a) Pagano JF, Weinstein MJ, Stout HA, Donovick R. Antibiot. Annu. 19551956:554. [PubMed] [Google Scholar]; (b) Vandeputte J, Dutcher JD. Antibiot. Annu. 19551956:560. [PubMed] [Google Scholar]; (c) Steinberg BA, Jambor WP, Suydam LO. Antibiot. Annu. 19551956:562. [PubMed] [Google Scholar]
- 111.(a) Bagley MC, Dale JW, Merritt EA, Xiong X. Chem. Rev. 2005;105:685. doi: 10.1021/cr0300441. [DOI] [PubMed] [Google Scholar]; (b) Hughes RA, Moody CJ. Angew. Chem., Int. Ed. 2007;46:7930. doi: 10.1002/anie.200700728. [DOI] [PubMed] [Google Scholar]
- 112.(a) Nicolaou KC, Safina BS, Zak M, Estrada AA, Lee SH. Angew. Chem., Int. Ed. 2004;43:5087. doi: 10.1002/anie.200461340. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Zak M, Safina BS, Lee SH, Estrada AA. Angew. Chem., Int. Ed. 2004;43:5092. doi: 10.1002/anie.200461341. [DOI] [PubMed] [Google Scholar]; (c) Nicolaou KC, Safina BS, Zak M, Lee SH, Nevalainen M, Bella M, Estrada AA, Funke C, Zécri FJ, Bulat S. J. Am. Chem. Soc. 2005;127:11159. doi: 10.1021/ja0529337. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Zak M, Safina BS, Estrada AA, Lee SH, Nevalainen M. J. Am. Chem. Soc. 2005;127:11176. doi: 10.1021/ja052934z. [DOI] [PubMed] [Google Scholar]
- 113.For a total synthesis of siomycin A, a related natural product, see:Mori T, Higashibayashi S, Goto T, Kohno M, Satouchi Y, Shinko K, Suzuki K, Suzuki S, Tohmiya H, Hashimoto K, Nakata M. Tetrahedron Lett. 2007;48:1331.
- 114.Nicolaou KC, Zak M, Rahimipour S, Estrada AA, Lee SH, O’Brate A, Giannakakou P, Ghadiri MR. J. Am. Chem. Soc. 2005;127:15042. doi: 10.1021/ja0552803. [DOI] [PubMed] [Google Scholar]
- 115.(a) Bister B, Bischoff D, Ströbele M, Riedlinger J, Riecke A, Wolter F, Bull AT, Zähner H, Fiedler H-P, Süssmuth RD. Angew. Chem., Int. Ed. 2004;43:2574. doi: 10.1002/anie.200353160. [DOI] [PubMed] [Google Scholar]; (b) Riedlinger J, Reicke A, Zähner H, Krismer B, Bull AT, Maldonado LA, Ward AC, Goodfellow M, Bister B, Bischoff D, Süssmuth RD, Fiedler H-P. J. Antibiot. 2004;57:271. doi: 10.7164/antibiotics.57.271. [DOI] [PubMed] [Google Scholar]
- 116.Walsh CT, Liu J, Rusnak F, Sakaitani M. Chem. Rev. 1990;90:1105. [Google Scholar]
- 117.(a) Nicolaou KC, Harrison ST. Angew. Chem., Int. Ed. 2006;45:3256. doi: 10.1002/anie.200601116. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Harrison ST. J. Am. Chem. Soc. 2007;129:429. doi: 10.1021/ja067083p. [DOI] [PubMed] [Google Scholar]
- 118.For the first total synthesis of abyssomicin C, see:Zapf CW, Harrison BA, Drahl C, Sorensen EJ. Angew. Chem., Int. Ed. 2005;44:6533. doi: 10.1002/anie.200502119.for formal total syntheses, see:Snider BB, Zou Y. Org. Lett. 2005;7:4939. doi: 10.1021/ol0518941.Couladouros EA, Bouzas EA, Magos AD. Tetrahedron. 2006;62:5272.for a review, see:Peters R, Fischer DF. Angew. Chem., Int. Ed. 2006;45:5736. doi: 10.1002/anie.200602409.
- 119.Keller S, Nicholson G, Drahl C, Sorensen EJ, Fiedler H-P, Süssmuth RD. J. Antibiot. 2007;60:391. doi: 10.1038/ja.2007.54. [DOI] [PubMed] [Google Scholar]
- 120.(a) Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio Á, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, Singh SB. Nature. 2006;441:358. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]; (b) Singh SB, Jayasuriya H, Ondeyka JG, Herath KB, Zhang C, Zink DL, Tsou NN, Ball RG, Basilio A, Genilloud O, Diez MT, Vicente F, Pelaez F, Young K, Wang J. J. Am. Chem. Soc. 2006;128:11916. doi: 10.1021/ja062232p. [DOI] [PubMed] [Google Scholar]
- 121.(a) Wang J, Kodali S, Lee SH, Galgoci A, Painter R, Dorso K, Racine F, Motyl M, Hernandez L, Tinney E, Colletti SL, Herath K, Cummings R, Salazar O, González I, Basilio A, Vicente F, Genilloud O, Pelaez F, Jayasuriya H, Young K, Cully D, Singh SB. Proc. Natl. Acad. Sci. U.S.A. 2007;104:7612. doi: 10.1073/pnas.0700746104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jayasuriya H, Herath KB, Zhang C, Zink DL, Basilio A, Genilloud O, Diez MT, Vicente F, Gonzalez I, Salazar O, Pelaez F, Cummings R, Ha S, Wang J, Singh SB. Angew. Chem., Int. Ed. 2007;46:4684. doi: 10.1002/anie.200701058. [DOI] [PubMed] [Google Scholar]
- 122.For a review of fatty acid biosynthesis, see:White SW, Zheng J, Zhang Y-M, Rock CO. Annu. Rev. Biochem. 2005;74:791. doi: 10.1146/annurev.biochem.74.082803.133524.
- 123.Nicolaou KC, Li A, Edmonds DJ. Angew. Chem., Int. Ed. 2006;45:7086. doi: 10.1002/anie.200603892. [DOI] [PubMed] [Google Scholar]
- 124.Nicolaou KC, Edmonds DJ, Li A, Tria GS. Angew. Chem., Int. Ed. 2007;46:3942. doi: 10.1002/anie.200700586. [DOI] [PubMed] [Google Scholar]
- 125.Many synthetic approaches to platensimycin have been reported. For reviews, see:Tiefenbacher K, Mulzer J. Angew. Chem., Int. Ed. 2008;47:2548. doi: 10.1002/anie.200705303.Nicolaou KC, Chen JS, Edmonds DJ, Estrada AA. submitted.
- 126.Nicolaou KC, Tria GS, Edmonds DJ. Angew. Chem., Int. Ed. 2008;47:1780. doi: 10.1002/anie.200800066. [DOI] [PubMed] [Google Scholar]
- 127.Nicolaou KC, Lister T, Denton RM, Montero A, Edmonds DJ. Angew. Chem., Int. Ed. 2007;46:4712. doi: 10.1002/anie.200701548. [DOI] [PubMed] [Google Scholar]
- 128.Nicolaou KC, Tang Y, Wang J, Stepan AF, Li A, Montero A. J. Am. Chem. Soc. 2007;129:14850. doi: 10.1021/ja076126e. [DOI] [PubMed] [Google Scholar]
- 129.Diyabalanage T, Amsler CD, McClintock JB, Baker BJ. J. Am. Chem. Soc. 2006;128:5630. doi: 10.1021/ja0588508. [DOI] [PubMed] [Google Scholar]
- 130.(a) Nicolaou KC, Guduru R, Sun Y-P, Banerji B, Chen DY-K. Angew. Chem., Int. Ed. 2007;46:5896. doi: 10.1002/anie.200702243. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Sun Y-P, Guduru R, Banerji B, Chen DY-K. J. Am. Chem. Soc. 2008;130:3633. doi: 10.1021/ja710485n. [DOI] [PubMed] [Google Scholar]
- 131.For the first structural revision of palmerolide A and the total synthesis of ent-palmerolide A, see:Jiang X, Liu B, Lebreton S, De Brabander JK. J. Am. Chem. Soc. 2007;129:6386. doi: 10.1021/ja0715142.see also a structural revision from degradative studies:Lebar MD, Baker BJ. Tetrahedron Lett. 2007;48:8009.
- 132.Nicolaou KC, Montagnon T. Molecules That Changed the World. Wiley-VCH; Weinheim: 2008. pp. 21–28. [Google Scholar]