Abstract
Background: In the 1990s, oxidation was found to occur in ultra-high molecular weight polyethylene total joint replacement components following gamma irradiation and prolonged shelf aging in air. Orthopaedic manufacturers developed barrier packaging to reduce oxidation during and after radiation sterilization. The present study explores the hypothesis that polyethylene components sterilized in a low-oxygen environment undergo similar in vivo oxidative mechanisms as inserts sterilized in air. In addition, the potential influence of the different sterilization processes on the wear performance of the polyethylene components was examined.
Methods: An analysis of oxidation, wear, and surface damage was performed for forty-eight acetabular liners and 123 tibial inserts. The mean implantation time was 12.3 ± 3.7 years for thirty-one acetabular liners that had been gamma sterilized in air and 4.0 ± 2.5 years for the seventeen acetabular liners that had been gamma sterilized in inert gas. The mean implantation time was 11.0 ± 3.2 years for the twenty-six tibial inserts that had been sterilized in air and 2.8 ± 2.2 years for the ninety-seven tibial inserts that had been gamma sterilized in inert gas. Oxidation and hydroperoxide levels were characterized in loaded and unloaded regions of the inserts.
Results: Measurable oxidation and oxidation potential were observed in all cohorts. The oxidation and hydroperoxide levels were regional. Surfaces with access to body fluids were more heavily oxidized than protected bearing surfaces were. This variation appeared to be greater in historical (gamma-in-air-sterilized) components. Regarding wear performance, historical and conventional acetabular liners showed similar wear penetration rates, whereas a low incidence of delamination was confirmed for the conventional tibial inserts in the first decade of implantation.
Conclusions: The present study explores the impact of industry-wide changes in sterilization practices for polyethylene. We found lower oxidation and oxidation potential in the conventional acetabular liners and tibial inserts that had been gamma sterilized in inert gas as compared with the historical components that had been gamma sterilized in air. However, we also found strong evidence that conventional components undergo mechanisms of in vivo oxidation similar to those observed following gamma irradiation in air. In addition, gamma sterilization in inert gas did not provide polyethylene with a significant improvement in terms of wear resistance as compared with gamma sterilization in air, except for a lower incidence of delamination in the first decade of implantation for tibial inserts.
Clinical Relevance: Our research demonstrates that gamma inert sterilization may have improved, but not completely solved, the problem of polyethylene oxidation for hip and knee arthroplasty.
For the past three decades, gamma irradiation has been the method most commonly used to sterilize ultra-high molecular weight polyethylene for use in both total hip and knee arthroplasty components1. However, oxidative degradation was recognized to occur following gamma radiation sterilization in air and prolonged shelf storage in air2,3. Furthermore, post-irradiation oxidation during long-term shelf aging was linked to a substantial loss in mechanical properties (as much as 90%) and, in some cases, more rapid polyethylene wear, especially in patients managed with knee arthroplasty4-7. In response to the findings of oxidation during shelf aging in historical (gamma-in-air-sterilized) polyethylene components, the major orthopaedic manufacturers in the United States discontinued gamma sterilization in air during the middle to late 1990s and introduced gamma sterilization in a low-oxygen or inert gas environment in combination with barrier packaging to reduce, and hopefully avoid, oxidation prior to implantation1. In vivo degradation was first studied by Eyerer, Grood, and colleagues in the 1980s8,9. However, until recently, historical polyethylene acetabular liners were not widely recognized to be susceptible not only to shelf aging but also to in vivo oxidation associated with a characteristic regional variation pattern10,11.
Despite the current widespread use of conventional (gamma-inert-sterilized) polyethylene, some concerns still remain because radiation sterilization of polyethylene results in the generation of macroradicals (precursors of oxidation) within the polymer, regardless of the irradiation environment6. As conventional gamma-inert-sterilized polyethylene still contains free radicals, it is expected to be susceptible to in vivo oxidation11. In this regard, the potential extent and clinical importance of in vivo degradation in conventional hip and knee components are poorly understood. On the other hand, gamma inert sterilization is believed by some researchers to impart a slightly higher wear resistance to polyethylene components than gamma sterilization in air does12. However, the expected improvement in clinical wear performance has not been found to be clinically important in radiographic studies of hip implants13,14. Thus, additional retrieval data are needed to quantify the hypothesized clinical benefit of gamma inert sterilization in terms of oxidative and wear properties. To date, published studies have approached the in vivo oxidation problem with use of a combined analysis of acetabular liners and tibial inserts15 or have focused only on acetabular liners produced from a single resin10,11.
The primary objective of the present study was to investigate the hypothesis that polyethylene acetabular liners and tibial inserts that had been radiation-sterilized in a low-oxygen environment would undergo similar in vivo oxidative mechanisms as polyethylene components that had been radiation-sterilized in air. In addition, the potential influence of both resin type and conversion method on in vivo oxidation was evaluated for both historical (gamma-air-sterilized) and conventional (gamma-inert-sterilized) retrieved polyethylene hip and knee components. Finally, we also investigated the hypothesis that polyethylene components sterilized in a low-oxygen environment would have similar wear mechanisms as those sterilized in air.
Materials and Methods
We performed oxidation, wear, and surface-damage analyses of forty-eight retrieved acetabular liners and 123 retrieved tibial inserts. The mean implantation time (and standard deviation) was 12.3 ± 3.7 years for thirty-one acetabular components that had been gamma sterilized in air and 4.0 ± 2.5 years for the seventeen acetabular components that had been gamma sterilized in inert gas. The historical (gamma-air-sterilized) acetabular liners had been produced in five different designs by two manufacturers: Harris-Galante I (Zimmer, Warsaw, Indiana) (n = 5), Harris-Galante II (Zimmer) (n = 13), Hexloc (Biomet, Warsaw, Indiana) (n = 7), Ringloc (Biomet) (n = 2), and Trilogy (Zimmer) (n = 4). The conventional (gamma-inert-sterilized) acetabular liners had been produced in two designs: Harris-Galante II (n = 3) and Trilogy (n = 14). The femoral head sizes were 22 (n = 3), 26 (n = 2), 28 (n = 36), and 32 (n = 7) mm in diameter. The primary reasons for revision of the thirty-one historical acetabular liners included polyethylene wear (twelve; 39%), loosening (ten; 32%), instability (four; 13%), osteolysis (two; 6%), a benign acetabular cyst (one; 3%), fracture of the liner (one; 3%), and locking mechanism failure (one; 3%). The primary reasons for revision in the group of the seventeen conventional hip acetabular liners were loosening (eight; 47%) and instability (six; 35%); only three of the seventeen conventional acetabular liners were revised because of polyethylene wear (one; 6%), periprosthetic fracture (one; 6%), or infection (one; 6%).
The mean implantation time was 11.0 ± 3.2 years for the twenty-six tibial inserts that had been gamma sterilized in air and 2.8 ± 2.2 years for the ninety-seven tibial inserts that had been gamma sterilized in inert gas. The historical tibial inserts were produced in seven different designs: Insall-Burstein II (Zimmer) (n = 10), Miller-Galante II (Zimmer) (n = 8), Miller-Galante I (Zimmer) (n = 3), AGC (Biomet) (n = 2), Kinemax (Stryker Orthopaedics, Mahwah, New Jersey) (n = 1), Maxim Primary (Biomet) (n = 1), and NexGen (Zimmer) (n = 1). The conventional tibial inserts were produced in nine different designs: NexGen (n = 34), Scorpio (Stryker Orthopaedics) (n = 24); Insall-Burstein II (n = 17); AGC (n = 7), Maxim (n = 5), Scorpio NRG (Stryker Orthopaedics) (n = 4), Ascent (Biomet) (n = 3), Miller-Galante II (n = 2), and Miller-Galante I (n = 1). The primary reasons for revision of the twenty-six historical tibial inserts included loosening (ten; 38.5%), polyethylene wear (five; 19.2%), infection (five; 19.2%), and instability (four; 15.4%); only two historical tibial inserts were revised because of knee effusion (one; 3.8%) or patellar complications (one; 3.8%). Similarly, the primary reasons for revision of the ninety-seven conventional tibial inserts included loosening (thirty-three; 34%), infection (twenty-nine; 30%), instability (twelve; 12%), arthrofibrosis (four; 4%), and polyethylene wear (three; 3%); other conditions, including pain, fracture, contracture, inflammation and mechanical complications, accounted for the remaining sixteen cases (16%).
The sterilization method and date as well as the revision date were traced in all cases. The average shelf life (and standard deviation) was 0.6 ± 0.7 years and 0.8 ± 0.7 years for the historical and conventional acetabular liners, respectively. Similarly, the average shelf life was 1.1 ± 1.3 and 1.1 ± 1.5 years for the historical and conventional tibial inserts, respectively. In addition, the conversion method (extrusion, bulk compression molded, or direct compression molded) and the type of ultra-high molecular weight polyethylene resin (GUR 4150, GUR 1020, GUR 1050, or Montell 1900H) were known (Tables I and II). After removal, the implants were stored in freezers (−80°C) to minimize ex vivo changes. The average time in cryogenic storage before testing was 0.2 ± 0.5 year.
TABLE I.
Shelf Lives (Prior to Implantation) and Implantation Times for the Historical and Conventional Hip Retrievals Classified According to Resin and Processing Route
| Processing Conditions | N | Shelf Life*(yr) | In Vivo Time*(yr) |
|---|---|---|---|
| Gamma-air, extruded, GUR 4150 | 31 | 0.6 ± 0.7 | 12.3 ± 3.7 |
| Gamma-inert, molded, GUR 1050 | 17 | 0.8 ± 0.7 | 4.0 ± 2.5 |
The values are given as the average and the standard deviation.
TABLE II.
Shelf Lives (Prior to Implantation) and Implantation Times for the Historical and Conventional Knee Retrievals Classified According to Resin and Processing Route
| Processing Conditions | N | Shelf Life*(yr) | In Vivo Time*(yr) |
|---|---|---|---|
| Gamma-air, direct compression molded, 1900H | 4 | 0.7 ± 0.3 | 13.5 ± 0.7 |
| Gamma-air, extruded, GUR 4150 | 19 | 1.2 ± 1.5 | 10.9 ± 3.3 |
| Gamma-air, molded, GUR 1050 | 3 | 1.2 ± 1.4 | 8.7 ± 2.8 |
| Gamma-inert, molded, 1900H | 3 | 0.6 ± 0.2 | 4.5 ± 2.9 |
| Gamma-inert, direct compression molded, 1900H | 34 | 1.3 ± 1.8 | 3.4 ± 2.3 |
| Gamma-inert, molded, GUR 1050 | 32 | 1.4 ± 1.5 | 3.0 ± 2.2 |
| Gamma-inert, molded, GUR 1020 | 28 | 0.7 ± 0.8 | 1.7 ± 1.4 |
The values are given as the average and the standard deviation.
Wear was assessed directly on the intact acetabular liners by using a calibrated digital micrometer (accuracy, 0.001 mm) to measure the thickness of the liners. Three measurements were made in both the superior and inferior portions of the acetabular liner and then were averaged. The superior and inferior locations were identified by examining the articulating surface under a stereomicroscope for the presence of contact damage, such as the presence of scratches and the removal of machining marks. Femoral head penetration was calculated by subtracting the thickness of the liner in the superior and inferior regions. The penetration rate was then calculated by dividing the penetration by the implantation time.
Surface damage was evaluated in the intact tibial inserts with use of the Hood damage-scoring technique1,16. Both the medial and lateral articulating surfaces were divided into quadrants and were scored on a scale (from 0 to 3) for the presence and extent of seven damage modes (pitting, embedded debris, scratching, delamination, surface deformation, burnishing, and abrasion), corresponding to a maximum score of 24 for each damage mode on the articulating surfaces. The inferior surface was also divided into quadrants and was scored with use of the same criteria, corresponding to a maximum score of 12 for each damage mechanism. For posterior stabilized inserts, the anterior, posterior, medial, and lateral surfaces of the post were scored independently, resulting in a maximum score of 12 for each wear damage mode. We paid special attention to total delamination scores of >10 because delamination has been correlated with elevated in vivo oxidation17.
Oxidation was assessed in 200-μm thin sections cut from both retrieved acetabular liners and tibial inserts with the use of a microtome. In the case of the hip liners the sections were obtained from the superior-inferior axis, whereas in the case of the tibial inserts the sections were obtained from two locations in the sagittal plane: (1) the medial articulating surface and (2) the unloaded central spine (including the post in posterior stabilized designs). Prior to the oxidation analysis, darkfield optical micrographs of the sections were made to inspect for the presence of white bands (high-oxidation areas). Then, all of the sections were soaked in boiling heptane for six hours to extract absorbed lipids, which otherwise might interfere with the oxidation analysis18. The extracted polyethylene sections were then scanned through the thickness in 0.1-mm depth increments (thirty-two repeat scans per sampled location) with use of Fourier transform infrared spectroscopy. The maximum oxidation index was calculated from the infrared spectra in accordance with ASTM International (ASTM) Standard F2102-01. Regions of interest in the sections of acetabular liners included the rim, bearing, and backside surfaces in both the superior and inferior regions of the component. Regions of interest in the sections of tibial inserts included the superior and inferior (backside) surfaces of the medial plateau, the anterior and posterior faces of the inserts, and the posterior stabilizing post (when applicable). ASTM oxidation indices may be interpreted as low (<1), moderate to severe (1 to 3), and critical (>3), consistent with a recently published study19. The evolution of oxidation with total time (shelf life and implantation time) was modeled statistically by an exponential growth model as proposed by Currier et al.15. Exponential models also appeared to fit the evolution of oxidation better than quadratic or biquadratic models for artificially aged polyethylene20,21.
Hydroperoxide content was also measured in the retrieved polyethylene component sections and was conducted following Fourier transform infrared analysis. Hydroperoxides are intermediate oxidation products that eventually convert into carbonyl groups (final oxidation products) and thus represent the oxidation potential of polyethylene22. The study of the hydroperoxide levels required the exposure of the sections to nitric oxide for at least sixteen hours to convert hydroperoxides to nitrates, which are easily detected with Fourier transform infrared spectroscopy23. The same regions of interest used for the oxidation analysis were used for the assessment of hydroperoxides. A hydroperoxide index was calculated as the area under the peak between 1600 and 1670 cm−1, divided by the area of the reference peak between 1330 and 1396 cm−1.
Student t tests or Wilcoxon tests were conducted to assess differences in oxidation, oxidation potential, and wear results between material groups in both acetabular liner and tibial insert retrievals. We paid special attention to potential influences of the sterilization method, polyethylene resin type, and conversion methods on oxidation. In addition, regional variations were considered within the acetabular liners and tibial inserts by means of paired t tests. In some cases, linear models with different variables as covariates (e.g., shelf life, implantation time) were used to examine the corresponding influence on oxidation and wear performance. The effect of the total time of exposure to oxygen on the oxidation levels of gamma-irradiated polyethylene components was examined with use of an exponential regression fit of the data. Probability values of <0.05 were considered to indicate significance.
Source of Funding
This research was funded in part by a National Institutes of Health R01 research grant (AR47904) and by Stryker Orthopaedics, Inc., Sulzer, and Zimmer, Inc. The orthopaedic manufacturers traced the polyethylene sterilization and manufacturing details for the implants analyzed in this study.
Results
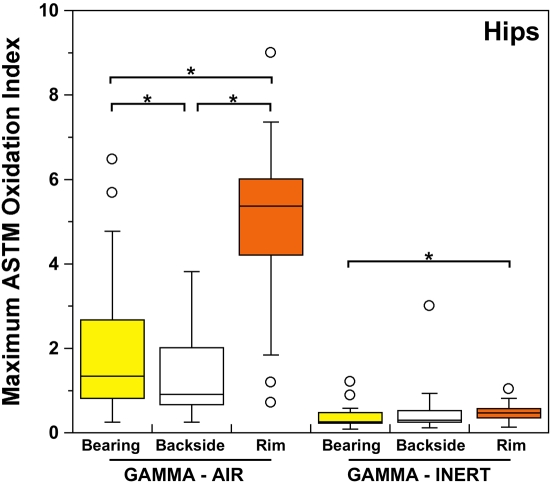
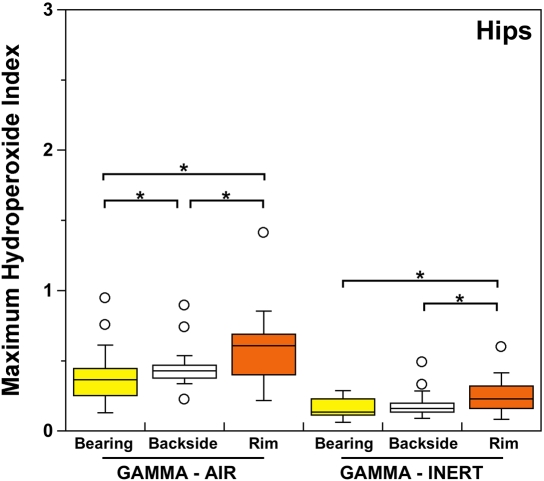
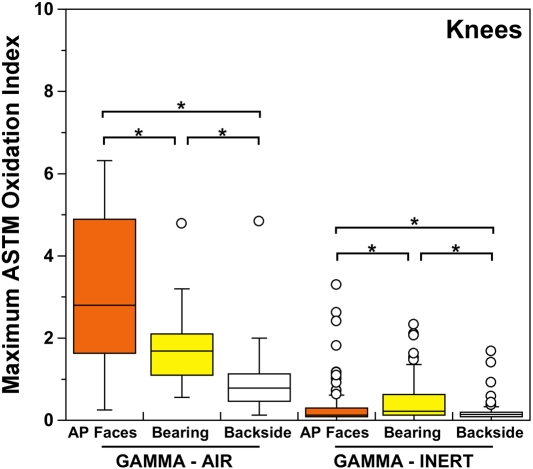
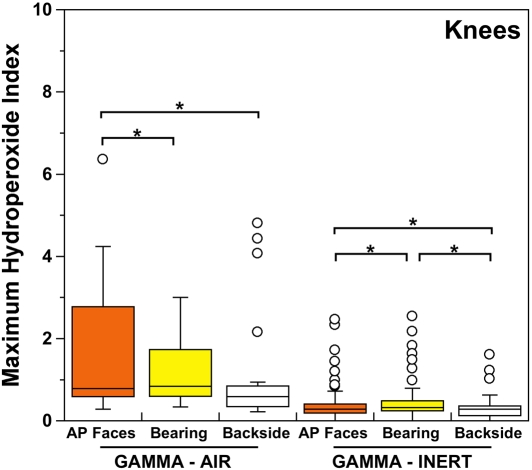
Both historical and conventional retrievals were observed to oxidize, regardless of the processing route and resin type. However, historical (gamma-air-sterilized) components had much greater oxidation and hydroperoxide levels than did conventional (gamma-inert-sterilized) inserts in both the hip and knee retrieval cohorts. Subsurface white bands (regions of high oxidation) were also apparent in microtomed sections of historical components examined with darkfield optical microscopy, especially at the rim area of acetabular liners and the anterior and posterior faces of tibial inserts (see Appendix). Moreover, there was substantial regional variation in the oxidation and hydroperoxide contents of the historical retrievals (Figs. 1-A through 2-B). In particular, the rim area of historical hip liners showed significantly greater oxidation (mean and standard deviation, 5.1 ± 1.7) than did the bearing (1.9 ± 1.6; p < 0.0001) and backside surfaces (1.4 ± 1.0; p < 0.0001) (Fig. 1-A). The articulating surface also had significantly higher oxidation levels than the backside (p = 0.01). Regarding regional variation in knee retrievals, the anterior and posterior faces of historical tibial inserts were usually significantly more oxidized (mean and standard deviation, 3.1 ± 1.9) than were the articulating (1.8 ± 1.0; p = 0.0001) and inferior surfaces (1.0 ± 0.9; p < 0.0001) (Fig. 2-A). The articulating surface also had greater oxidation than did the backside (p = 0.0008). Hydroperoxide results reflected a similar regional variation pattern in historical acetabular liners, with significantly higher oxidation potential in the rim region (mean and standard deviation, 0.6 ± 0.3) than in the bearing (0.4 ± 0.2; p = 0.0003) and backside surfaces (0.5 ± 0.2; p = 0.005) (Fig. 1-B). Hydroperoxide levels were significantly higher at the faces of the retrieved tibial inserts (mean and standard deviation, 1.7 ± 1.6) than at the articular (1.1 ± 0.7; p = 0.01) and backside surfaces (1.0 ± 1.3; p = 0.01) (Fig. 2-B).
Fig. 1-A Fig. 1-B.
ASTM (formerly American Society for Testing and Materials) oxidation indices (Fig. 1-A) and hydroperoxide contents (Fig. 1-B) for the historical (gamma-air) and conventional (gamma-inert) acetabular liners. Substantial regional variation was observed in the maximum oxidation and oxidation potential of retrieved liners. The asterisks (*) indicate significant differences in paired t tests. The horizontal lines within the boxes indicate the median values, and the circles represent outliers.
Fig. 2-A Fig. 2-B.
ASTM (formerly American Society for Testing and Materials) oxidation indices (Fig. 2-A) and hydroperoxide contents (Fig. 2-B) for the historical (gamma-air) and conventional (gamma-inert) knee retrievals. Substantial regional variation was observed in the maximum oxidation and oxidation potential of retrieved tibial inserts. The asterisks (*) indicate significant differences in paired t tests. The horizontal lines within the boxes indicate the median values, and the circles represent outliers. AP = anterior and posterior.
Even though conventional components presented lower and more homogeneous levels of oxidation and oxidation potential than historical components did, regional variations could still be detected. Thus, microtomed thin sections of conventional acetabular liners and tibial inserts occasionally showed subsurface white band formation, especially in areas exposed to body fluids (see Appendix). Oxidation and hydroperoxide contents were typically higher in the rim of conventional (gamma-inert-sterilized) acetabular components (mean and standard deviation, 0.5 ± 0.2 and 0.3 ± 0.1 for oxidation and hydroperoxide levels, respectively) with respect to the bearing (0.4 ± 0.3 [p < 0.02] and 0.2 ± 0.1 [p < 0.0002], respectively) and inferior surfaces (0.5 ± 0.7 [p > 0.05] and 0.2 ± 0.1 [p < 0.05], respectively) (Figs. 1-A and 1-B). In contrast with the historical tibial inserts, the most oxidized region in conventional (gamma-inert-sterilized) tibial inserts was the articulating surface, which exhibited greater oxidation (mean and standard deviation, 0.5 ± 0.5) and oxidation potential (0.4 ± 0.4) than the anterior and posterior faces (0.3 ± 0.5 [p < 0.001] and 0.4 ± 0.4 [p = 0.02], respectively) and the backside (0.2 ± 0.2 [p < 0.0001] and 0.3 ± 0.2 [p = 0.0002]) (Figs. 2-A and 2-B). Nevertheless, the oxidation and hydroperoxide contents in the anterior and posterior faces of the tibial inserts were still significantly higher than those in the backside (p = 0.0004 and p = 0.003, respectively).
The temporal evolution of oxidation in the acetabular liner and tibial insert cohorts was consistent with an exponential growth trend with total time (i.e., the sum of shelf life, in vivo time, and ex vivo life) as well as the aforementioned regional variations (Figs. 3-A through 4-C). Thus, historical components reached elevated oxidation levels (∼7) after fifteen to twenty years of exposure to oxygen, whereas conventional inserts attained oxidation indices of as high as 3 after almost ten years (Figs. 3-A and 4-A, respectively). R2 values ranged from 0.21 to 0.54 (depending on the region) in the case of acetabular liners and from 0.34 to 0.55 in the case of tibial inserts. As conventional polyethylene components are not expected to oxidize during shelf storage, the oxidation data corresponding to the articulating region of only conventional tibial inserts is represented against implantation time in Figure 5. Linear models with shelf life, implantation time, and processing method as covariates also revealed a significant influence of the in vivo period on the oxidation levels of the anterior and posterior (p = 0.0005) and bearing surfaces (p = 0.0007) of conventional knee components.
Fig. 3-A Fig. 3-B Fig. 3-C.
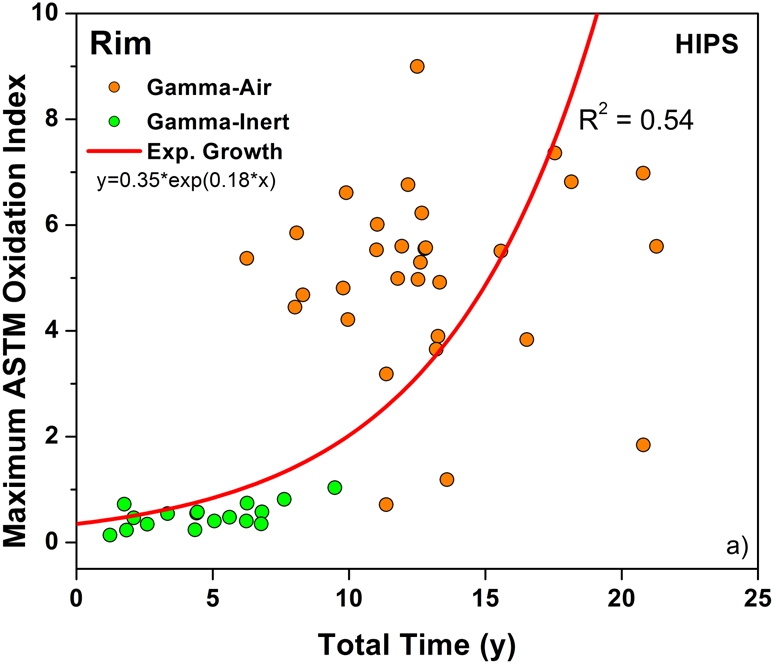
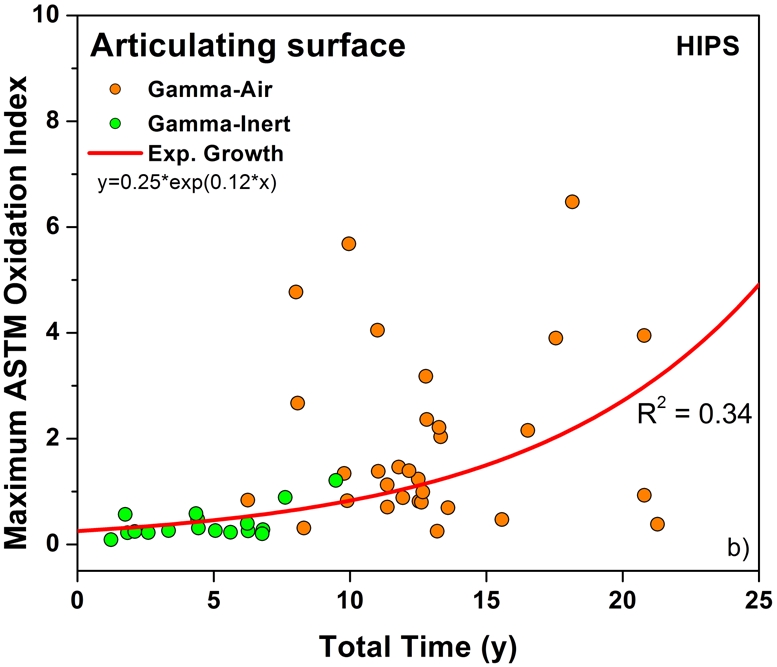
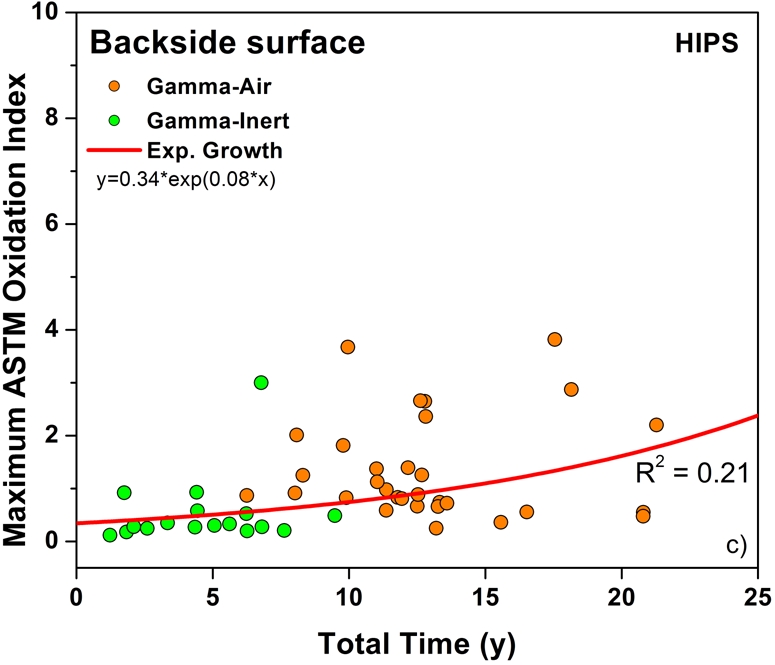
Oxidation rate plots, by region, for the rim (Fig. 3-A) (r2 = 0.54, p < 0.0001), bearing (Fig. 3-B) (r2 = 0.34, p < 0.0001), and backside (Fig. 3-C) (r2 = 0.21, p < 0.001) of the retrieved acetabular liners. ASTM = ASTM International.
Fig. 4-A Fig. 4-B Fig. 4-C.
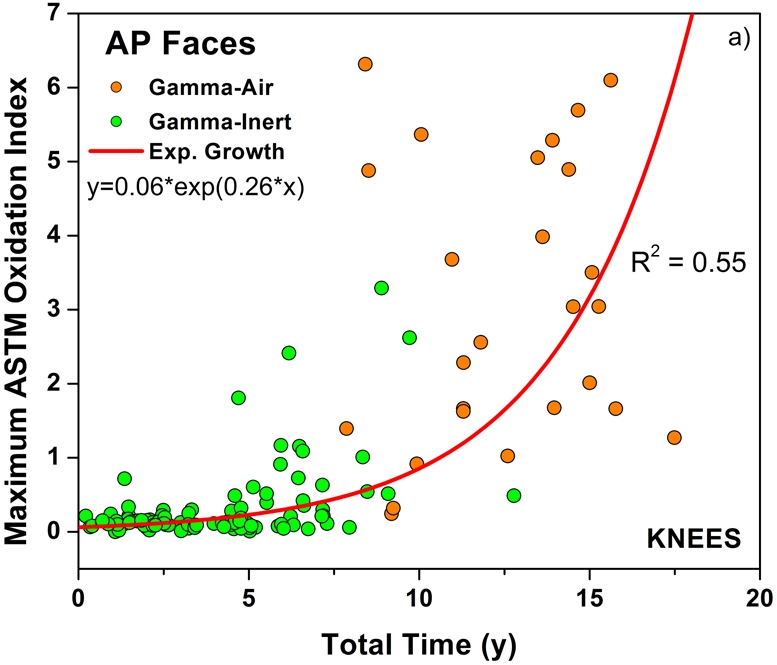
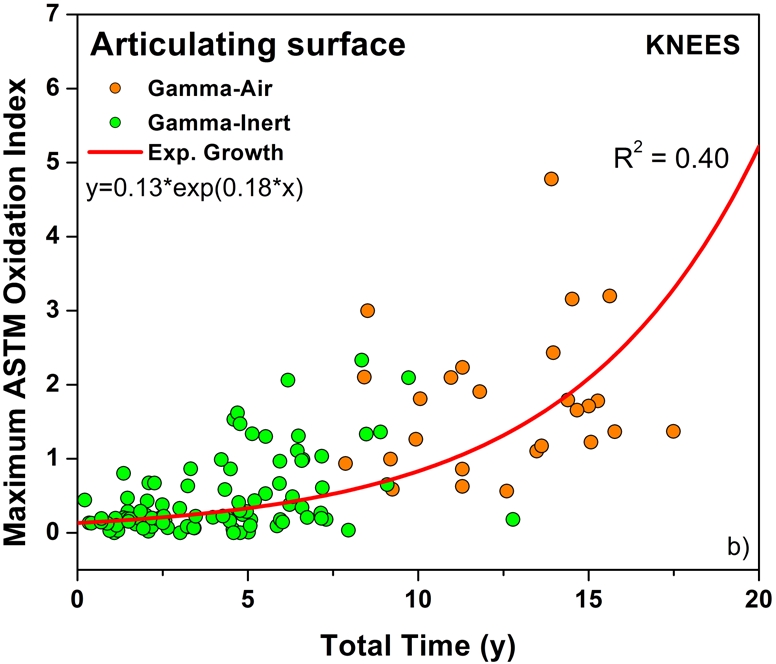
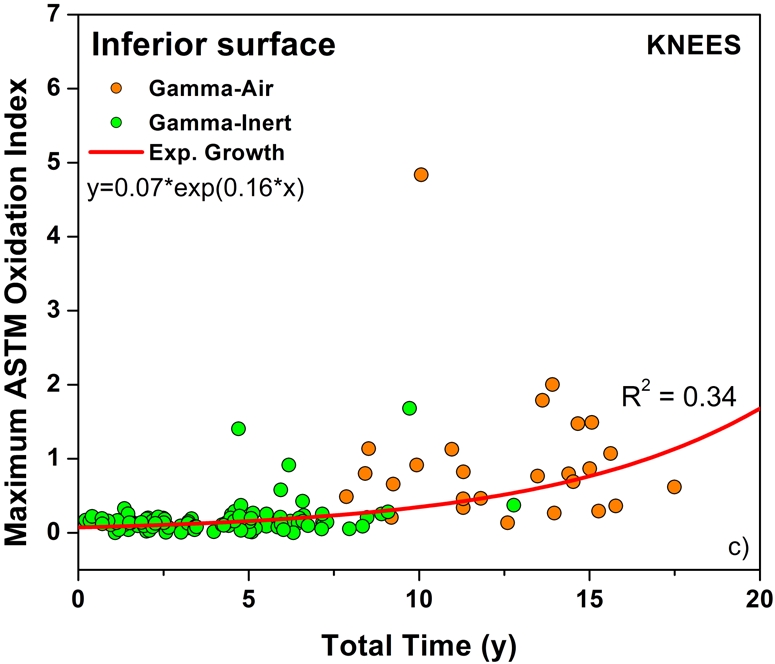
Oxidation rate plots, by region, for the anterior and posterior (AP) faces (Fig. 4-A) (r2 = 0.55, p < 0.0001), articulating surface (Fig. 4-B) (r2 = 0.40, p < 0.0001), and inferior surface (Fig. 4-C) (r2 = 0.34, p < 0.0001) for the retrieved tibial inserts. ASTM = ASTM International.
Fig. 5.
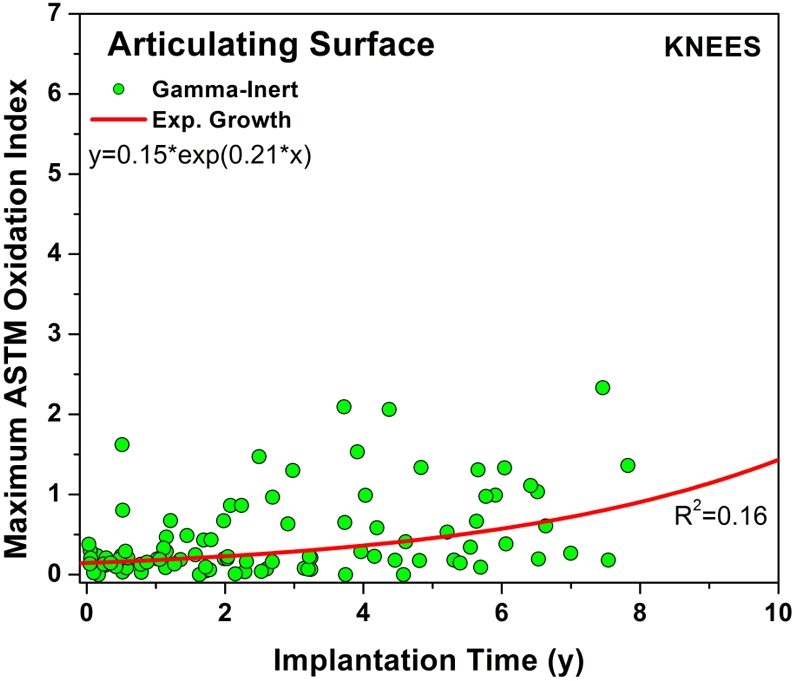
Plot of maximum oxidation against implantation time for the conventional tibial inserts at the bearing surface. The exponential growth curve fit (r2 = 0.16, p < 0.0001) predicts an oxidation index of >1.0 after ten years of implantation for the conventional inserts. ASTM = ASTM International.
We observed comparable findings in terms of the wear penetration rate for the historical and conventional acetabular liners (mean and standard deviation, 0.16 ± 0.11 compared with 0.17 ± 0.18 mm/year; p = 0.49). However, post hoc analysis confirmed that the present sample size did not provide sufficient power (5%) to detect a 0.01-mm/year difference in penetration rates. In addition, we found no significant correlations between wear rate and shelf life (p = 0.5), implantation period (p = 0.1), femoral head size (p = 0.9; power, 9%), or oxidation at the articular surface (p = 0.5). Nevertheless, two of the most oxidized historical acetabular liners showed signs of wear-through, which was not observed in the conventional acetabular liners (see Appendix).
With regard to retrieved knee components, the Hood total damage scores confirmed that historical tibial inserts accumulated significantly more surface damage than conventional bearings did (mean and standard deviation, 68 ± 28 compared with 55 ± 23; p = 0.01) (Table III). As far as consolidation method and resin type are concerned, linear models confirmed no significant influence (p > 0.05) on total surface damage scores for retrievals that underwent the same sterilization procedure (Table IV). However, the powers provided by both historical and conventional retrievals were below the typical standard for acceptable power (80%), especially for the former (13% compared with 63%). In contrast, total surface damage scores were sensitive to oxidation at the anterior and posterior faces of conventional tibial inserts (p < 0.04), according to linear models. In general, scratching, burnishing, and pitting were the predominant surface damage modes, and no significant differences were found between historical and conventional retrieved tibial inserts (p > 0.3 in all cases). Surface deformation (p < 0.05), embedded debris (p = 0.0002), and particularly delamination (p < 0.0001) were significantly higher in historical bearings as compared with conventional bearings. Delamination scores and the scores associated with abrasive and adhesive damage modes (i.e., the sum of scratching, burnishing, abrasion, and embedded debris damage scores) are also shown in Table III for the retrieved knee components. Abrasive/adhesive damage scores of historical and conventional tibial inserts were statistically indistinguishable in this study (p = 0.8, t test; power = 10%). It was observed, however, that only 6% of conventional knee components scored >10 in delamination, as compared with 58% of historical tibial inserts. Moreover, linear models with sterilization method, shelf life, implantation time, and oxidation at the different regions as covariates corroborated that delamination was strongly dependent on oxidation at the anterior and posterior faces (p < 0.0001) and the articulating surface (p < 0.03). Even though extensive delamination generally occurred much more frequently in historical tibial inserts after long-term implantation, a few conventional components still showed extensive delamination, especially after long-term storage on the shelf (see Appendix).
TABLE III.
Total Damage Scores per Region and Total Delamination and Abrasive/Adhesive Damage Scores for the Historical and Conventional Knee Retrievals*
| Sterilization Method | Condylar Damage Score | Backside Damage Score | Post Damage Score | Total Delamination Score | Total Abrasive/Adhesive Score | Total Surface Damage Score |
|---|---|---|---|---|---|---|
| Gamma-air | 48 ± 19 | 15 ± 7 | 17 ± 7 | 12 ± 9 | 34 ± 15 | 68 ± 28 |
| Gamma-inert | 34 ± 14 | 11 ± 7 | 12 ± 7 | 2 ± 5 | 36 ± 17 | 55 ± 23 |
The values are given as the average and the standard deviation.
TABLE IV.
Regional and Total Surface Damage Scores for the Retrieved Tibial Inserts Classified According to Processing Conditions (Sterilization Procedure, Consolidation Method, and Polyethylene Resin Type)*
| Processing Conditions | Condylar Damage Score | Backside Damage Score | Post Damage Score† | Total Surface Damage Score |
|---|---|---|---|---|
| Gamma-air, direct compression molded, 1900H | 48 ± 27 | 17 ± 8 | NA | 56 ± 36 |
| Gamma-air, extruded, GUR 4150 | 48 ± 18 | 14 ± 7 | 19 ± 7 | 69 ± 24 |
| Gamma-air, molded, GUR 1050 | 49 ± 27 | 20 ± 11 | 15 ± 9 | 84 ± 46 |
| Gamma-inert, molded, 1900H | 23 ± 7 | 10 ± 9 | 14 ± 8 | 47 ± 2 |
| Gamma-inert, direct compression molded, 1900H | 35 ± 19 | 10 ± 6 | 12 ± 7 | 54 ± 27 |
| Gamma-inert, molded, GUR 1050 | 37 ± 11 | 16 ± 7 | 14 ± 8 | 64 ± 22 |
| Gamma-inert, molded, GUR 1020 | 31 ± 10 | 8 ± 5 | 9 ± 4 | 45 ± 16 |
The values are given as the average and the standard deviation.
NA = Not applicable (nonmodular designs, direct compression-molded onto metal backing, cruciate-retaining designs).
Discussion
These oxidation and oxidation potential results support our hypothesis that conventional acetabular liners and tibial inserts, like historical implants, undergo oxidative degradation in vivo. However, the overall severity of degradation reached in the conventional components within a decade of service was lower than that reached in historical inserts, and regional variations appeared to be less pronounced as well. Thus, the most severe manifestations of oxidation usually occurred in the unloaded regions of historical components, namely the rim and the anterior and posterior faces of acetabular liners and tibial inserts, respectively, whereas in conventional knee components the loaded regions attained greater oxidation than the remaining areas. These patterns of regional variation suggest that greater access to oxygen from body fluids and/or adjacent tissues may be a key factor in the development of in vivo oxidation in both historical and conventional components10,11. In support of this hypothesis, regions protected by metal components from direct contact with the in vivo environment, such as the backside of acetabular liners and tibial inserts, typically had mild to moderate in vivo oxidation and oxidation potential.
Our data further suggest that a mechano-oxidative effect may be occurring at the articulating surface of tibial inserts as a result of the high-stress cyclic loading, accentuating the rate of oxidation (Fig. 4-B)24. In addition, the relative nonconformity of tibial inserts with respect to acetabular liners might allow for easier access of oxygen-containing body fluids to the articulating surface in total knee replacements. Despite the differences in regional variations between historical and conventional tibial inserts, it is still unknown whether the conventional components will maintain the currently observed patterns after long-term implantation or will evolve to the regional variations observed in historical tibial inserts.
It is remarkable that neither the mechanism of oxidation nor its eventual progression was significantly affected by the processing route or resin type in the present study (as demonstrated by the comparisons between GUR components and 1900H components). In the case of historical tibial inserts, 1900H components appeared to oxidize at a slower, but not significantly different, rate. Nevertheless, considerable mechanical degradation has been reported to occur at lower oxidation levels for historical polyethylene inserts produced from 1900H resins with respect to those manufactured from GUR resins15. Moreover, although historical direct-compression-molded 1900H components have been reported to be oxidation and damage-resistant25-28, recent studies also have demonstrated no significant difference in the wear performance of conventional polyethylene components and non-irradiated specimens manufactured from different resins and with various consolidation methods29,30, 1900H and direct compression molding among them.
Even though our available data clearly confirm that conventional components undergo in vivo oxidation, the clinical relevance of this finding appears to be low in the first decade after implantation. Generally, our retrieved conventional acetabular liners were revised for reasons not related to polyethylene wear, and only 6% of the retrieved knee components demonstrated extensive delamination (damage scores for this mode, >10). Conventional components were irradiated and presumably were stored in low-oxygen packaging before implantation in order to minimize oxidation, and hence the oxidation rate during shelf aging was likely much lower than that for historical components. However, the oxidation potential results suggest that in vivo degradation will increase over time as hydroperoxides are considered to decompose into the final oxidation products22,31. Also, the exponential growth fit of the oxidation rate data (Figs. 3-A through 4-C) suggests that the second decade of implantation could result in severe in vivo oxidation for conventional components, assuming that their long-term behavior follows the same exponential relationship as historical components. On the basis of extrapolation of our data, in vivo oxidation is, on the average, predicted to result in an oxidation index of >1.0 at the bearing surface after ten years of implantation. It remains unknown whether the actual oxidation will be delayed compared with historical components in the second decade of implantation.
Shelf life was recognized to negatively affect the oxidation level and mechanical properties of historical acetabular liners and tibial inserts2,7, as previously mentioned. This motivated the introduction of low-oxygen, barrier packaging for gamma radiation sterilization of polyethylene components. Even so, totally oxygen-free atmospheres were not necessarily achieved. In this regard, changes in the distribution of oxidation and hydroperoxides in irradiated polyethylene components recently have been associated with differences in the irradiation and packaging conditions, namely, radiation dose rate, irradiation temperature, packaging type, and packaging environment, even in the cases of conventional and highly crosslinked polyethylenes32. Orthopaedic manufacturers improved the first-generation packaging technology that had been developed for gamma inert sterilization of polyethylene components and introduced second-generation packaging to improve isolation from oxygen11. For example, one manufacturer incorporated a glass barrier into their first-generation argon packaging in November 199511. In this regard, one of our conventional knee retrievals was stored in a first-generation barrier package on the shelf for six years, and it showed high oxidation values and extensive delamination after only 3.7 years in vivo (Fig. 5) (see Appendix). Therefore, orthopaedic surgeons should be aware that long periods of shelf storage might be still deleterious for conventional polyethylene components, depending on the type of barrier packaging used by a particular manufacturer32.
A desirable effect of irradiation is the creation of crosslinks (covalent bonds) within the polymer as crosslinking increases the resistance to abrasive and adhesive wear1,33,34. Accordingly, highly crosslinked polyethylenes have been developed and introduced for clinical use in total joint replacement1. Neither historical nor conventional polyethylenes belong to the category of highly crosslinked polyethylenes as they receive a relatively low dose (25 to 40 kGy) of gamma radiation for sterilization purposes. Nevertheless, a slightly higher surface crosslinking level is attributed to conventional components as compared with historical components12. For that reason, better wear performance might be expected for conventional components as compared with historical components. However, the evaluation of penetration and surface damage in our retrieval cohorts did not support a significant improvement in wear resistance for conventional components in comparison with historical components, except for delamination damage in tibial inserts. Historical and conventional hip liners showed comparable penetration rates, and likewise tibial inserts had similar abrasive/adhesive damage scores, regardless of sterilization method (Table III).
On the other hand, delamination represents a damage mechanism that is very different from damage due to the abrasive and adhesive wear modes. Delamination is a fatigue-related damage mechanism that is exacerbated by subsurface oxidation27. The conventional tibial inserts in the present study demonstrated a much lower incidence of delamination than the historical knee components did. However, severe delamination was observed in six of the ninety-seven conventional knee components, and hence in vivo oxidation cannot be ruled out as a potential cause of delamination in these conventional tibial inserts. Overall, according to our findings to date, gamma inert sterilization succeeds in diminishing in vivo oxidation and the incidence of early delamination in short-term implanted tibial inserts, but it does not contribute to significantly enhanced abrasive/adhesive wear properties in comparison with gamma sterilization in air. The effect of resin type and consolidation method on the clinical wear resistance of polyethylene bearings appears to be marginal in the present study, although larger sample size may help to corroborate the current results. Our findings are consistent with recent clinical wear data reported by Sychterz et al. that indicate that the wear rates of historical and conventional acetabular liners are indistinguishable14. Patient factors such as sex, weight, level of activity, and age may account for a much greater variability in clinical wear performance than sterilization or processing factors, as reported by Faris and colleagues13.
In light of the likely progression of in vivo oxidation in conventional polyethylene components during the second decade of implantation, other sterilization methods or strategies to prevent or greatly inhibit oxidation should be considered. However, some of the current alternate sterilization methods have important disadvantages. Nonradiative sterilization methods, such as ethylene oxide or gas-plasma sterilization, do not introduce free radicals (precursors of oxidation) in the polymer, but they also do not promote crosslinking, and, therefore, the beneficial improvement in wear resistance associated with radiation-induced crosslinking is lost14,34. On the other hand, certain types of first-generation highly crosslinked polyethylenes undergo post-irradiation remelting, which eliminates residual free radicals at the expense of mechanical properties, before final sterilization without radiation. The blending of vitamin E as an antioxidant in polyethylene prior to consolidation is a promising alternative strategy to provide oxidative stability to both conventional gamma-inert sterilized and highly crosslinked polyethylenes while better preserving mechanical properties35-37.
In conclusion, lower oxidation and oxidation potential levels were found for conventional (gamma-inert-sterilized) acetabular liners and tibial inserts as compared with historical (gamma-air-sterilized) liners and inserts. In addition, both conventional and historical components showed comparable abrasive/adhesive wear performance. Nevertheless, the incidence of delamination was significantly reduced in the first decade of implantation in conventional knee components in comparison with historical components. Although gamma sterilization and storage in inert environments are effective for preventing oxidation, polyethylene components might still oxidize during shelf storage, depending on the type of barrier packaging. In addition, our findings to date suggest that conventional (gamma-inert-sterilized) polyethylene components undergo in vivo oxidation, with degradation mechanisms similar to those observed in historical components. It is unknown, however, whether the in vivo oxidation rate of the conventional components will be the same as for historical components during the second decade of implantation. Therefore, there is concern about the potential impact of oxidative degradation on their long-term clinical performance. Overall, gamma sterilization in an inert environment was confirmed as a beneficial sterilization method over gamma sterilization in air, but it has not yet completely solved the problem of in vivo oxidation for hip and knee arthroplasty.
Supplementary Material
Acknowledgments
Note: Special thanks are due to Rebecca Moore for her assistance.
Appendix
Figures showing typical photographs of bearing surfaces as well as darkfield optical micrographs of microtomed sections corresponding to historical and conventional retrievals are available with the electronic versions of this article, on our web site at jbjs.org (go to the article citation and click on “Supplementary Material”) and on our quarterly CD/DVD (call our subscription department, at 781-449-9780, to order the CD or DVD). 
Disclosure: In support of their research for or preparation of this work, one or more of the authors received, in any one year, outside funding or grants in excess of $10,000 from the National Institutes of Health (NIH R01 AR47904), Stryker Orthopaedics, Zimmer, and Sulzer. In addition, one or more of the authors or a member of his or her immediate family received, in any one year, payments or other benefits in excess of $10,000 or a commitment or agreement to provide such benefits from a commercial entity (Zimmer). Also, commercial entities (Stryker and Zimmer) paid or directed in any one year, or agreed to pay or direct, benefits in excess of $10,000 to a research fund, foundation, division, center, clinical practice, or other charitable or nonprofit organization with which one or more of the authors, or a member of his or her immediate family, is affiliated or associated.
Investigation performed at the Implant Research Center, School of Biomedical Engineering, Science and Health Systems, Drexel University, Philadelphia, Pennsylvania
References
- 1.Kurtz SM, editor. The UHMWPE biomaterials handbook: ultra high molecular weight polyethylene in total joint replacement and medical devices. 2nd ed. Burlington, MA: Academic Press; 2009.
- 2.Currier BH, Currier JH, Collier JP, Mayor MB, Scott RD. Shelf life and in vivo duration. Impacts on performance of tibial bearings. Clin Orthop Relat Res. 1997;342:111-22. [PubMed] [Google Scholar]
- 3.Premnath V, Harris WH, Jasty M, Merrill EW. Gamma sterilization of UHMWPE articular implants: an analysis of the oxidation problem. Biomaterials. 1996;17:1741-53. [DOI] [PubMed] [Google Scholar]
- 4.Collier JP, Sperling DK, Currier JH, Sutula LC, Saum KA, Mayor MB. Impact of gamma sterilization on clinical performance of polyethylene in the knee. J Arthroplasty. 1996;11:377-89. [DOI] [PubMed] [Google Scholar]
- 5.Puolakka TJ, Keränen JT, Juhola KA, Pajamäki KJ, Halonen PJ, Nevalainen JK, Saikko V, Lehto MU, Järvinen M. Increased volumetric wear of polyethylene liners with more than 3 years of shelf-life time. Int Orthop. 2003;27:153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene. A hip-simulator study. J Bone Joint Surg Am. 2000;82:1708-25. [DOI] [PubMed] [Google Scholar]
- 7.Sutula LC, Collier JP, Saum KA, Currier BH, Currier JH, Sanford WM, Mayor MB, Wooding RE, Sperling DK, Williams IR, Kasprzak DJ, Surprenant VA. Impact of gamma sterilization on clinical performance of polyethylene in the hip. Clin Orthop Relat Res. 1995;319:28-40. [PubMed] [Google Scholar]
- 8.Eyerer P, Ke YC. Property changes of UHMW polyethylene hip cup endoprostheses during implantation. J Biomed Mater Res. 1984;18:1137-51. [DOI] [PubMed] [Google Scholar]
- 9.Grood ES, Shastri R, Hopson CN. Analysis of retrieved implants: crystallinity changes in ultrahigh molecular weight polyethylene. J Biomed Mater Res. 1982;16:399-405. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz SM, Rimnac CM, Hozack WJ, Turner J, Marcolongo M, Goldberg VM, Kraay MJ, Edidin AA. In vivo degradation of polyethylene liners after gamma sterilization in air. J Bone Joint Surg Am. 2005;87:815-23. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz SM, Hozack WJ, Purtill JJ, Marcolongo M, Kraay MJ, Goldberg VM, Sharkey PF, Parvizi J, Rimnac CM, Edidin AA. Significance of in vivo degradation for polyethylene in total hip arthroplasty. Clin Orthop Relat Res. 2006;453:47-57. [DOI] [PubMed] [Google Scholar]
- 12.Bargmann LS, Bargmann BC, Collier JP, Currier BH, Mayor MB. Current sterilization and packaging methods for polyethylene. Clin Orthop Relat Res. 1999;369:49-58. [DOI] [PubMed] [Google Scholar]
- 13.Faris PM, Ritter MA, Pierce AL, Davis KE, Faris GW. Polyethylene sterilization and production affects wear in total hip arthroplasties. Clin Orthop Relat Res. 2006;453:305-8. [DOI] [PubMed] [Google Scholar]
- 14.Sychterz CJ, Orishimo KF, Engh CA. Sterilization and polyethylene wear: clinical studies to support laboratory data. J Bone Joint Surg Am. 2004;86:1017-22. [PubMed] [Google Scholar]
- 15.Currier BH, Currier JH, Mayor MB, Lyford KA, Van Citters DW, Collier JP. In vivo oxidation of gamma-barrier-sterilized ultra-high-molecular-weight polyethylene bearings. J Arthroplasty. 2007;22:721-31. [DOI] [PubMed] [Google Scholar]
- 16.Hood RW, Wright TM, Burstein AH. Retrieval analysis of total knee prostheses: a method and its application to 48 total condylar prostheses. J Biomed Mater Res. 1983;17:829-42. [DOI] [PubMed] [Google Scholar]
- 17.Kurtz S, Parvizi J, Purtill J, Sharkey P, MacDonald D, Shah P, Brenner E, Kraay M, Goldberg V, Rimnac C. In vivo oxidation contributes to delamination but not pitting in TKA. In: Transactions of the 53rd Annual Meeting of the Orthopaedic Research Society; 2007. Feb 11-14; San Diego, CA. Poster no 1891.
- 18.Shen FW, Yu YJ, McKellop H. Potential errors in FTIR measurement of oxidation in ultrahigh molecular weight polyethylene implants. J Biomed Mater Res. 1999;48:203-10. [DOI] [PubMed] [Google Scholar]
- 19.Currier BH, Currier JH, Mayor MB, Lyford KA, Collier JP, Van Citters DW. Evaluation of oxidation and fatigue damage of retrieved Crossfire polyethylene acetabular cups. J Bone Joint Surg Am. 2007;89:2023-9. [DOI] [PubMed] [Google Scholar]
- 20.Gugumus F. Thermooxidative degradation of polyolefins in the solid state: part 1. Experimental kinetics of functional group formation. Polymer Degrad Stabil. 1996;52:131-44. [Google Scholar]
- 21.Gugumus F. Thermooxidative degradation of polyolefins in the solid state: part 5. Kinetics of functional group formation in PE-HD and PE-LLD. Polymer Degrad Stabil. 1997;55:21-43. [Google Scholar]
- 22.Costa L, Luda MP, Trossarelli L, Brach del Prever EM, Crova M, Gallinaro P. Oxidation in orthopaedic UHMWPE sterilized by gamma-radiation and ethylene oxide. Biomaterials. 1998;19:659-68. [DOI] [PubMed] [Google Scholar]
- 23.Bracco P, Brach del Prever EM, Cannas M, Luda MP, Costa L. Oxidation behaviour in prosthetic UHMWPE components sterilised with high energy radiation in a low oxygen environment. Polymer Degrad Stabil. 2006;91:2030-8. [Google Scholar]
- 24.Costa L, Luda MP, Trossarelli L. Ultra-high molecular weight polyethylene:1. Mechano-oxidative degradation. Polymer Degrad Stabil. 1997;55:329-38. [Google Scholar]
- 25.Berzins A, Jacobs JJ, Berger R, Ed C, Natarajan R, Andriacchi T, Galante JO. Surface damage in machined ram-extruded and net-shape molded retrieved polyethylene tibial inserts of total knee replacements. J Bone Joint Surg Am. 2002;84:1534-40. [DOI] [PubMed] [Google Scholar]
- 26.Ritter MA. Direct compression molded polyethylene for total hip and knee replacements. Clin Orthop Relat Res. 2001;393:94-100. [DOI] [PubMed] [Google Scholar]
- 27.Won CH, Rohatgi S, Kraay MJ, Goldberg VM, Rimnac CM. Effect of resin type and manufacturing method on wear of polyethylene tibial components. Clin Orthop Relat Res. 2000;376:161-71. [DOI] [PubMed] [Google Scholar]
- 28.Benson LC, DesJardins JD, LaBerge M. Effects of in vitro wear of machined and molded UHMWPE tibial inserts on TKR kinematics. J Biomed Mater Res. 2001;58:496-504. [DOI] [PubMed] [Google Scholar]
- 29.Gul RM, McGarry FJ, Bragdon CR, Muratoglu OK, Harris WH. Effect of consolidation on adhesive and abrasive wear of ultra high molecular weight polyethylene. Biomaterials. 2003;24:3193-9. [DOI] [PubMed] [Google Scholar]
- 30.Lancin P, Essner A, Yau SS, Wang AG. Wear performance of 1900 direct compression molded, 1020 direct compression molded, and 1020 sheet compression molded UHMWPE under knee simulator testing. Wear. 2007;263:1030-3. [Google Scholar]
- 31.Costa L, Luda MP, Trossarelli L. Ultra high molecular weight polyethylene—II. Thermal- and photo-oxidation. Polymer Degrad Stabil. 1997;58:41-54. [Google Scholar]
- 32.Costa L, Bracco P, Brach Del Prever EM, Kurtz SM, Gallinaro P. Oxidation and oxidation potential in contemporary packaging for polyethylene total joint replacement components. J Biomed Mater Res B Appl Biomater. 2006;78:20-6. [DOI] [PubMed] [Google Scholar]
- 33.Muratoglu OK, Bragdon CR, O'Connor DO, Jasty M, Harris WH, Gul R, McGarry F. Unified wear model for highly crosslinked ultra-high molecular weight polyethylenes (UHMWPE). Biomaterials. 1999;20:1463-70. Erratum in: Biomaterials. 2003;24:1527. [DOI] [PubMed] [Google Scholar]
- 34.Kurtz SM, Muratoglu OK, Evans M, Edidin AA. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials. 1999;20:1659-88. [DOI] [PubMed] [Google Scholar]
- 35.Oral E, Greenbaum ES, Malhi AS, Harris WH, Muratoglu OK. Characterization of irradiated blends of alpha-tocopherol and UHMWPE. Biomaterials. 2005;26:6657-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shibata N, Kurtz SM, Tomita N. Recent advances of mechanical performance and oxidation stability in ultrahigh molecular weight polyethylene for total joint replacement: highly crosslinked and alpha-tocopherol doped. J Biomed Sci Eng. 2006;1:107-23. [Google Scholar]
- 37.Kurtz SM, Dumbleton J, Siskey RS, Wang A, Manley M. Trace concentrations of vitamin E protect radiation crosslinked UHMWPE from oxidative degradation. J Biomed Mater Res A. 2008. Jun 18 [Epub ahead of print]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.