Abstract
This investigation considered possible health-related neurobiological processes associated with “emotional approach coping” (EAC), or intentional efforts to identify, process, and express emotions surrounding stressors. It was hypothesized that higher dispositional use of EAC strategies would be related to neural activity indicative of greater trait approach motivational orientation and to lower proinflammatory cytokine and cortisol responses to stress. To assess these relationships, 46 healthy participants completed a questionnaire assessing the two components of EAC (i.e., emotional processing and emotional expression), and their resting frontal cortical asymmetry was measured using electroencephalography (EEG). A subset (N = 22) of these participants’ levels of the soluble receptor for tumor necrosis factor-alpha (sTNFαRII), interleukin-6 (IL-6), and cortisol (all obtained from oral fluids) were also assessed before and after exposure to an acute laboratory stressor. Consistent with predictions, higher reported levels of emotional expression were significantly associated with greater relative left-sided frontal EEG asymmetry, indicative of greater trait approach motivation. Additionally, people who scored higher on EAC, particularly the emotional processing component, tended to show a less-pronounced TNF-α stress response. EAC was unrelated to levels of IL-6 and cortisol. Greater left-sided frontal EEG asymmetry was significantly related to lower baseline levels of IL-6 and to lower stress-related levels of sTNFαRII, and was marginally related to lower stress-related levels of IL-6. The findings suggest that the salubrious effects of EAC strategies for managing stress may be linked to an approach-oriented neurocognitive profile and to well-regulated proinflammatory cytokine responses to stress.
Keywords: emotional approach coping, frontal asymmetry, EEG, approach motivation, Trier Social Stress Test, cortisol, proinflammatory cytokines, sTNFαRII, IL-6
According to functionalist views of emotions, emotions are complex systems that developed over the course of human evolution to coordinate adaptive responses to the demands of physical and social stimuli and challenges in the environment (cf., Keltner and Gross, 1999). This view conflicts with more traditional accounts that view emotions as maladaptive and disruptive to rational thought and other cognitive abilities (see Damasio, 1994). In terms of stress-related coping processes, this discrepancy between views of emotions as adaptive versus maladaptive is an important one, as the functionalist view suggests that coping via intentional efforts to process and express emotions is beneficial, whereas the more traditional view contends that focusing coping efforts on emotions is detrimental to successful coping (Stanton et al., 1994). This debate is of relevance to health, as one’s mental and physical well-being are linked, in part, to how effectively one copes with stress (Penley et al., 2002; Taylor and Stanton, 2007). The present investigation sought to shed some light on this matter by considering relationships between emotion-focused coping strategies and health-related neurobiological processes.
Assessing the Role of Emotional Approach Coping
Some prior research has found that an individual’s tendency to engage in emotion-focused coping (i.e., coping aimed at regulating the negative emotional consequences of a stressor), particularly through emotional expression, is associated with distress and dysfunction (see Stanton et al., 1994; Stanton et al., 2002 for reviews). However, in their review of the coping literature, Stanton and colleagues (1994) observed that many items on emotion-oriented coping scales are confounded with distress or self-deprecation, potentially creating a spurious relationship in these prior studies between emotion-focused coping and maladjustment. In light of this confound and clinical and empirical research citing benefits of processing and expressing emotions associated with stressful events (e.g., Horowitz, 1976; Mendolia and Kleck, 1993; Pennebaker et al., 1988), Stanton and colleagues (1994; Stanton et al., 2000b) developed and validated self-report measures of Emotional Approach Coping (EAC), which are unconfounded with distress and self-deprecation. Emotional processing refers to active efforts to acknowledge one’s emotions and to explore their meanings, in order to gain a better understanding of one’s emotional response to a stressor. Emotional expression involves active verbal and/or nonverbal attempts to communicate or symbolize one’s emotional responses to a stressor.
A growing body of research shows that greater use of emotion-oriented coping strategies, as assessed with the EAC scales, is associated with more adaptive psychological and physical health outcomes in young adults (see Austenfeld and Stanton, 2004 for a review) and medical patient samples. For instance, greater emotional expression predicted decreased distress, improved self-perceived physical health status, increased vigor, and fewer medical appointments for cancer-related morbidities among women completing treatment for breast cancer (Stanton et al., 2000a). In a study of couples undergoing an insemination treatment for infertility, higher emotional processing and expression scores prior to the insemination attempt predicted less distress in both women and men one week after receiving a negative pregnancy result (Berghuis and Stanton, 2002). Higher EAC scores (both emotional processing and expression) were also associated with lower affective pain and depressive symptoms in chronic myofascial pain patients, and for men, the emotional expression component of EAC was uniquely related to lower sensory pain and physical impairment (Smith et al., 2002).
Findings demonstrating health correlates of EAC are important, not only because they contradict the conclusions drawn from research using the confounded emotional coping measures, but also because they suggest possible methods for coping with stress that may improve people’s mental and physical health outcomes. Despite the evidence that a focus on emotions is adaptive when dealing with stress, the specific mechanisms through which EAC contributes to positive health outcomes are not well-understood. Although much recent research has revealed important connections between neural and physiological processes and health outcomes, the relation between EAC strategies and neurobiological measures associated with well-being have not yet been examined. The present investigation was designed to address this gap in the literature. Specifically, the associations of EAC strategies with neural profiles of approach/avoidance motivational tendencies and with proinflammatory cytokine and neuroendocrine responses to stress were examined.
Neurocognitive and Biological Processes Associated with EAC
Neurocognitive correlates of EAC
A critical component of the EAC strategy is the tendency to adopt an approach-oriented response when coping with stress. However, the relation between EAC and basic neurocognitive mechanisms of approach motivation have not previously been examined, and elucidating this relationship was a primary concern of the present investigation. In the psychophysiology literature, individual differences in approach motivation have been associated with greater baseline activation of the left prefrontal cortex (PFC), a region involved in self-regulation and the coordination of goal-directed action (Amodio et al., 2004; Miller and Cohen, 2001), among other related functions. Much of the research linking left PFC activity to approach motivation has used baseline electroencephalography (EEG) to assess left vs. right asymmetries in frontal cortical activity, which have been localized to dorsolateral regions of the PFC (Pizzagalli et al., 2005). This body of research has demonstrated consistent links between greater left frontal asymmetry and both state and trait forms of approach motivation (e.g., Amodio et al., 2008; Coan and Allen, 2003; Harmon-Jones and Sigelman, 2001), independent of the objective valence (i.e., positivity or negativity) of the approach motivation (e.g., Harmon-Jones, 2003).
A link between EAC and frontal asymmetry would be significant because frontal asymmetry has been associated with a range of psychological profiles and biological processes associated with health. For instance, individuals with greater resting left-frontal asymmetry perceive environmental stress as less aversive, are less likely to suffer from depression, and are less likely to show negative affect in response to certain stressors (Davidson, 1992; Henriques and Davidson, 1990, 1991; Tomarken et al., 1990; Tomarken et al., 1992a). Similarly, manipulated decreases in left frontal asymmetry through neurofeedback can cause depressed mood (Allen et al., 2001). Also, a recent study reported that that a naturalistic stressor, examination stress, was associated with a shift from relatively greater left frontal activity in students during the low examination stress period to relatively greater right frontal activity during the high stress examination period (Lewis et al., 2007).
Increased activity in the left neural hemisphere has also been related to increased immunocompetence and to cortisol levels. For example, women with extreme left frontal asymmetry had higher levels of natural killer (NK) cell function than those with extreme right frontal activation (Kang et al., 1991), and participants with greater relative left-sided activation had a smaller decrement in NK cell activity in vitro after an emotional stressor (Davidson et al., 1999). A study using an in vivo immune measure found that individuals displaying greater relative left-sided activity produced a larger antibody titer rise (i.e., a healthier immune response) to influenza vaccination (Rosenkranz et al., 2003). The right hemisphere also appears to be more involved in cortisol release than is the left hemisphere (Wittling, 1995), and relatively greater left frontal activity has been related to lower cortisol levels (Buss et al., 2003; Kalin et al., 1998).
Although underlying mechanisms are not yet fully understood, these findings suggest that, in addition to its relationship with approach motivation, greater left-sided frontal EEG activity is associated with healthier psychological and biological profiles. If a stronger tendency to engage in EAC reflects a stronger underlying approach motivational orientation, a relationship between EAC and resting frontal EEG asymmetry would be expected in this study. If found, this relationship would thus suggest one way in which EAC may be related to health.
Biological correlates of EAC
This study also assessed the direct relationships between EAC and biological stress responses. Increases in proinflammatory cytokine activity are often seen in response to stressful events, such as laboratory stressors and some psychological stressors (e.g., examinations, public speaking) (Ackerman et al., 1998; Brydon et al., 2004; Dickerson et al., 2004; Maes et al., 1998; Pace et al., 2006; von Känel et al., 2006; see Steptoe et al., 2007 for a review). Proinflammatory cytokine activity, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), has been tied to negative emotional states and ultimately to adverse changes in physical and mental health, and there is evidence indicating that proinflammatory cytokines may cause depressive illness (Das, 2007). The hypothalamic-pituitary-adrenal (HPA) axis is also commonly activated in response to stress, leading to the production of glucocorticoids, including cortisol (Sapolsky et al., 2000). Although this response is protective in the short term, chronic or recurrent activation can cause dysregulation of the HPA axis and of cortisol, leading to deleterious effects with implications for health (e.g., Cannon, 1932; Seeman and McEwen, 1996; Uchino et al., 1996). If, as the functionalist view of emotions posits, EAC aids a person in managing stressful situations, biological stress responses may be more well-regulated for people reporting higher EAC. To assess these relationships in the present study, proinflammatory cytokine and cortisol responses to an acute laboratory stressor were assessed.
Study Overview
The present research examined the relation of EAC with resting frontal EEG asymmetry in order to test the hypothesis that EAC is associated with neural processes involved in approach motivation. It also assessed the relationships between EAC and proinflammatory cytokine and cortisol responses to an acute laboratory stressor to assess the implications of an EAC style for biological stress reactivity. It was hypothesized that higher EAC scores would be associated with greater left-sided frontal EEG asymmetry and with lower proinflammatory cytokine (specifically sTNFαRII1 and IL-6) and cortisol responses to the stressor. Given previous work relating EEG asymmetry to immune and neuroendocrine factors, the direct relationships between EEG asymmetry and proinflammatory cytokines and cortisol were examined in ancillary analyses.
Methods
Participants
Fifty-four undergraduate students or recent graduates (34 women, 20 men), ages 18 to 24 (M = 20.1, SD = 1.45)2, participated individually in exchange for cash or extra course credit. Participants were all right-handed to control for handedness effects on EEG asymmetry. All participants completed the EAC scales and underwent EEG recording. Twenty-six of these participants (20 women, 6 men) had also previously participated in a separate stress-reactivity study, thus providing the added opportunity to look for relationships between EAC, frontal cortical asymmetry, and biological stress responses. Prospective participants for the stress study had been prescreened to exclude anyone with serious physical or mental health problems (e.g., depression, diagnosis of PTSD), use of medications affecting cardiovascular or endocrine functions (e.g., corticosteroids), current use of mental health-related medications (e.g., Prozac), or women who were pregnant or lactating.
Of the larger sample, data from eight participants were excluded due to: excessive EEG artifact (4), failure to follow task instructions (2), or because scores on one or more measures were considered an outlier (SD > 3; 2). Thus, the final sample consisted of 46 participants (30 women, 16 men), with a sub-sample size of 22 (18 women, 4 men) who had also completed the stress-reactivity protocol3.
Assessment of Frontal EEG Asymmetry
EEG recording
Upon arrival to the EEG lab, participants provided informed consent, were fitted with a stretch-lycra electrode cap, and were seated in a dimly-lit, sound-proofed room in a comfortable chair. EEG was collected from 28 scalp sites using Ag/AgCl electrodes and Electro-Gel (Eaton, OH) as the conductive medium, positioned according to the 10–20 system. These sites included midline, frontal, central, temporal, parietal, and occipital locations, referenced to the left earlobe. A ground electrode was placed on the forehead, and EEG was also recorded from the right earlobe. Vertical and horizontal electrooculogram (EOG) was collected to assess eye movements and to facilitate artifact rejection. Impedances were below 5kΩ at each scalp site (below 10kΩ at EOG sites). EEG was recorded with a .1 – 100 Hz bandpass filter and digitized at 1000 Hz using a Synamps amplifier (Neuroscan Labs, El Paso, TX). Offline, EEG was manually scored for movement artifact and rereferenced to average earloabe activity.
Resting EEG measurement
Participants were instructed to sit with their head and body still while eight, 1-min intervals of EEG were recorded. Participants were instructed to keep their eyes open for four intervals and closed for four intervals, in an order that was counterbalanced across participants. The experimenter gave instructions via intercom from an adjacent room.
Frontal EEG asymmetry processing
For analysis of spectral power, an eyeblink-rejection algorithm was applied in which EOG deflections exceeding +/− 75 mV were removed. Additional movement artifacts were removed manually. All artifact-free 2.048-ms epochs were extracted through a Hamming window to prevent spurious estimates of spectral power. Contiguous epochs were overlapped by 75% to minimize loss of data due to Hamming window extraction, and power spectra were calculated via fast Fourier transform (Davidson et al., 2000). These power values (in µV2) were averaged across epochs within each 1-min resting trial. Because alpha power is inversely related to cortical activity (Lindsley and Wicke, 1974), total power within the alpha frequency range (8–13 Hz) was obtained for analysis. The power values at each site were natural log transformed (to reduce skew) and averaged. Alpha asymmetry scores were computed for frontal, temporal, parietal, and occipital regions of the scalp by subtracting left log-alpha power from right log-alpha power, such that higher scores indicated greater left-sided EEG activity. Frontal asymmetry was quantified as the difference between activity at frontal sites Fp2 and Fp1, and the frontal EEG asymmetry scores used for all reported analyses were computed to be the standardized residuals that resulted from a regression analysis in which frontal asymmetry was the dependent variable and asymmetry scores at the parietal, temporal, and occipital regions were the predictors.
Self-Report Measures
Emotional approach coping measurement
Following EEG recording, participants completed Stanton et al.’s (2000b) self-report measure of dispositional EAC. This measure was embedded in a set of short personality and attitudes surveys and was completed in privacy. The EAC questionnaire asked participants to indicate what they “generally do and feel when you experience stressful events.” Participants responded to the 16 items using a scale ranging from 1 (“I usually don’t do this at all”) to 4 (“I usually do this a lot”). The emotional processing and emotional expression components of EAC were each assessed with 8 intermixed items4. Examples of items measuring emotional processing included, “I take the time to figure out what I’m really feeling” and “I delve into my feelings to get a thorough understanding of them.” Examples of items measuring coping through emotional expression included, “I take time to express my emotions” and “I let my feelings come out freely.” For each participant, an average score for each scale was computed, as was an average overall score based on all 16 items. Cronbach’s alphas for the emotional processing and emotional expression scales were .89 and .86, respectively, and for the overall scale was .89. The correlation between the two EAC scales in this study was r (44) = .36, p = .01, as is typically observed (Stanton et al., 2000b).
Behavioral inhibition and activation
All participants also completed Carver and White’s (1994) BIS and BAS scales in order to assess the relationships between the EAC scales and a broader individual difference measure of dispositional approach (i.e., BAS) . BIS items (7) and BAS items (13) were intermixed, and average scores were computed separately for the BIS and BAS scales. In this sample, BIS and BAS were uncorrelated, r (44) = −.04, p = ns, as in previous research (e.g., Carver & White, 1994).
Depressive symptoms
In the sub-sample of participants that participated in the stress-reactivity study, depressive symptoms were assessed via the Beck Depression Inventory (BDI; Beck et al., 1961). The 21-item BDI assesses the cognitive, affective, and vegetative symptoms of depression rated on a 4-point scale from 0 to 3. The BDI is a well validated and widely used measure of depression, and it was included in the present investigation in order to assess and control for relationships between depressive symptomatology and proinflammatory cytokine levels, given their well-documented association.
Health behaviors
The participants in the sub-sample also completed a questionnaire to assess health behaviors on the day of the stress-reactivity study that are known to affect the immune system, including coffee and alcohol consumption, number of cigarettes smoked, prescription drug use, amount of exercise that day, and hours of sleep the previous night.
Stress Task Protocol
Baseline assessments
Laboratory visits were scheduled for the afternoon between 2:30 and 4:30 PM to minimize variability due to the circadian rhythm of cortisol (Kirschbaum and Hellhammer, 1989). Participants were asked not to consume caffeine or eat anything for at least 1 hour before their session.
Ten min after entering the laboratory and after informed consent was obtained, baseline samples of oral mucosal transudate (OMT), for analysis of proinflammatory cytokines (sTNFαRII and IL-6), and of saliva, for cortisol analysis, were collected. Saliva contains enzymes and other particles form the parotid and salivary glands, and it provides an established method for assessing cortisol (Kirschbaum and Hellhammer, 1989). OMT is a filtrate of blood plasma and has been validated for assessing certain markers of immune activation (Nishanian et al., 1998). Specifically, Nishanian and colleagues (1998) found that levels of sTNFαRII in OMT were significantly and highly correlated with those obtained from plasma (p < .026). In addition, Dickerson et al. (2004) used OMT for measurement of sTNFαRII in healthy participants and found significant increases in sTNFαRII 30 min after the onset of a stressor, an effect that was replicated across three experimental sessions on separate days. Thus, oral fluids provide a non-invasive and easily repeatable way to reliably measure neuroendocrine and immune activity.
To collect OMT samples, an Orasure collective device (Epitope, Beaverton, OR) was placed between the lower cheek and gum. To collect saliva samples, participants rolled a sterile cotton swab in their mouth for 3 min and then placed the swab in a Salivette® salivary collection tube (Sarstedt, Inc., North Carolina). After completing a set of interview questions about their home life, friendships, romantic relationships, work and hobbies (material that is not part of the present analyses), participants were escorted to the laboratory for the stress challenge tasks.
Rest and stress challenge tasks
Participants sat at a table and rested for 10 min in order to relax and allow physiological function to approach resting levels. They then completed the Trier Social Stress Test (TSST; Kirschbaum et al., 1993), a widely-used laboratory stress challenge known to elicit biological stress responses. In brief, the TSST consists of preparation (5 min) and delivery (5 min) of a speech to an unresponsive audience, followed by 5 min of critiqued mental arithmetic. Approximately 25 min after the onset of the TSST, second samples of OMT and saliva were collected (Post-Stress assessment 1; PS1). Following an additional 30 min of recovery time, during which additional questionnaires (e.g., demographics and the health behavior survey) were completed, the third set of samples of OMT and saliva were collected (Post-Stress assessment 2; PS2). The PS1 and PS2 samples approximately correspond to peak and recovery levels of cortisol, respectively (Dickerson and Kemeny, 2004). Although, like cortisol, proinflammatory cytokine increases to acute psychological laboratory stressors appear after a delay, they have been shown to continue to increase for up to 2 hours or more post-stress (Steptoe et al., 2007). Thus, assessments at both PS1 and PS2 of sTNFαRII and IL-6 were intended to measure stress-induced increases in these proinflammatory cytokines.
Assay Procedures
Vials containing OMT and saliva samples were refrigerated immediately after collection, and within 16 hours transferred to a −80°C freezer for storage. Saliva samples were shipped for overnight delivery on dry ice to the Behavioral Endocrinology Laboratory at Pennsylvania State University where the cortisol assays were conducted. Salivary cortisol levels were determined from a 25-µl sample, which was assayed in duplicate by radioimmunoassay using the HS-cortisol High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit (Salimetrics LLC, State College, PA). All samples were within a pH range of 3.5 – 9.0, which allows for robust results when using the HS-Cortisol Assay. The inter- and intra-assay coefficients of variation were less than 15% and 10%, respectively. Due to a processing error, cortisol data at time points PS1 and PS2 were missing for one participant. For the data analyses, cortisol measurements from each time point (baseline, PS1, and PS2) were log-transformed to correct for non-normality (prior to the transformation, a constant of 1 was added to each measurement so that all log-transformed values would be positive).
The proinflammatory cytokine assays were conducted at the Center for Interdisciplinary Research in Immunology and Disease (CIRID) at the University of California, Los Angeles. sTNFαRII was measured using the Quantikine Human sTNFαRII enzyme immunoassay kit manufactured by R&D Systems (Minneapolis, MN), and IL-6 was measured using the IMx automated microparticle enzyme immunoassay system (Abbot, Abbott Park, IL). The inter- and intra-assay coefficients of variation were less than or equal to 4.1% and 7.5%, respectively, for sTNFαRII and were less than 9.0% and 3.3%, respectively, for IL-6. Protein in oral fluids was quantified by the Bradford method using the Bio-Rad protein assay kit with bovine plasma albumin as the standard. All sTNFαRII and IL-6 results are reported using analyte-to-protein ratios, or the ratio of the experimental value for the analyte to the protein concentration in the same test sample. Because the analyte-to-protein ratio controls for differences in salivary flow rate, which could be altered by the experimental procedures or vary between individuals, the ratio values are more reliable than the analyte values alone (Dickerson et al., 2004; Nishanian et al., 1998). As with the cortisol values, the IL-6/protein ratios for each of the collection time points (baseline, PS1, and PS2) were log-transformed (after a constant of 1 was added to each measurement) to correct for non-normality. The sTNFαRII/protein ratios for each of the three collection time points were not transformed, as values on this measure were normally distributed.
Results
Relation of EAC to Resting Frontal EEG Asymmetry and to BAS
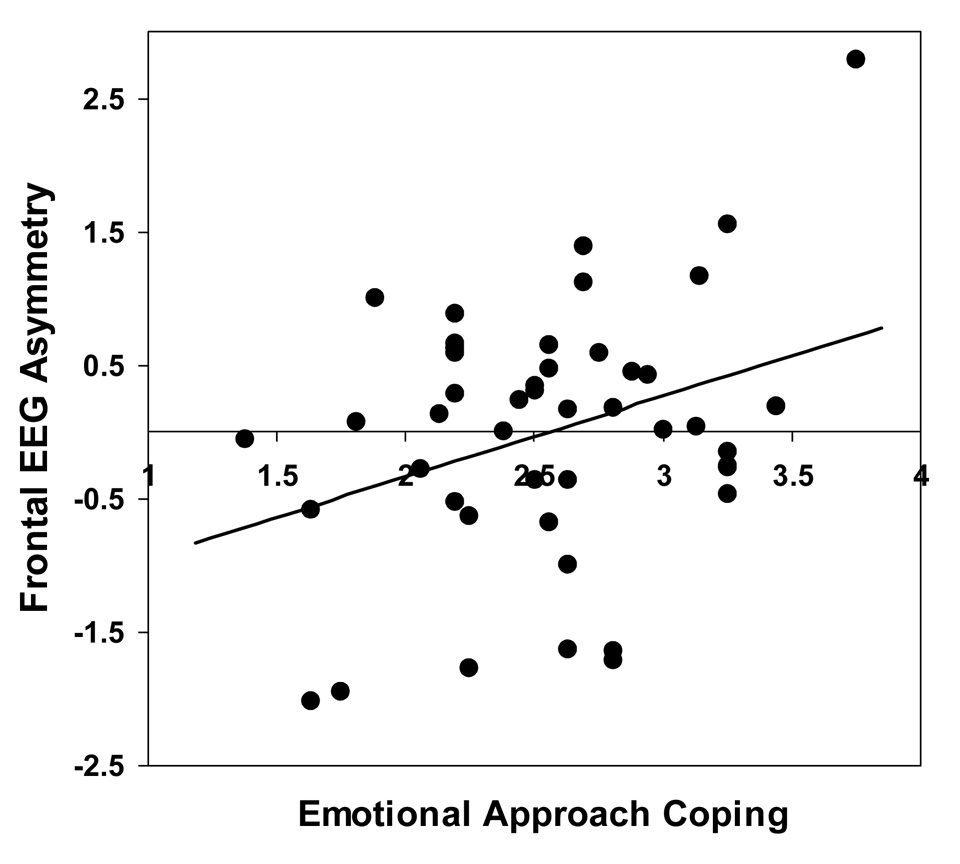
To test the primary hypothesis that higher EAC scores would be related to greater left-sided frontal EEG asymmetry, correlations were conducted between EAC scores and frontal EEG asymmetry scores. Consistent with the hypothesis, greater left-sided frontal EEG asymmetry was significantly positively correlated with total EAC score, r (44) = .33, p = .03 (see Fig. 1). Additional analyses examined the relationship between frontal EEG asymmetry and the separate EAC scales. These revealed that frontal EEG asymmetry was significantly correlated with emotional expression, r (44) = .39, p < .01, but not with emotional processing, r (44) = .18, p = ns5. Thus, as predicted, individuals who reported engaging in more emotional approach coping, particularly through emotional expression, evinced neural activity indicative of greater approach motivation.
Figure 1.
Relation between emotional approach coping and frontal EEG asymmetry. Higher overall EAC scores (displayed on the abscissa) were associated with greater left-sided frontal EEG asymmetry. The ordinate displays the frontal EEG asymmetry scores, which represent residualized right- minus left-sided alpha power at frontal scalp sites, controlling for asymmetry at other scalp regions. Higher frontal EEG asymmetry scores indicate greater left-sided frontal cortical activity, which is indicative of greater trait approach orientation.
Because a tendency to engage in EAC may simply be a proxy for more generalized dispositional approach orientation (e.g., BAS), we assessed the correlations between the EAC variables and BIS/BAS scores. None of these correlations were significant (all p’s > .34), and controlling for BAS did not alter the significant relationships found between frontal EEG asymmetry and total EAC scores or emotional expression scores.
Preliminary Analyses of Proinflammatory Cytokine and Cortisol Data
As expected, proinflammatory cytokine levels increased from baseline to PS1 and from PS1 to PS2 for both sTNFαRII and IL-6, and when controlling for health behaviors known to affect the immune system, the effect of time was significant for sTNFαRII, F (2, 36) = 4.76, p = .02, and was marginally significant for IL-6, F (2, 36) = 2.33, p = .11. Cortisol also displayed the expected pattern, as PS1 (peak) levels of cortisol were higher than both baseline and PS2 (recovery) levels, and this quadratic effect of time was significant, F (1, 20) = 7.81, p = .01.
Preliminary analyses also assessed relationships between baseline levels of cortisol and baseline levels of sTNFαRII and IL-6, and between changes in the levels of these parameters from baseline to PS1, and, in the case of the proinflammatory cytokines, from baseline to PS2. The significant relationships were as follows (when controlling for health behaviors): baseline levels of cortisol were marginally inversely correlated with baseline levels of both sTNFαRII, r = −.44, p = .07, and IL-6, r = −.41, p = .09, and baseline levels of sTNFαRII and IL-6 were highly positively correlated with each other, r = .80, p < .001. Changes in sTNFαRII from baseline to PS1 were significantly and positively related to changes in IL-6 from baseline to PS1, r = .53, p = .02, and changes in sTNFαRII from baseline to PS2 were significantly and positively related to changes in IL-6 from baseline to PS2, r = .59, p = .01.
Relation of EAC to Proinflammatory Cytokine and Cortisol Reactivity
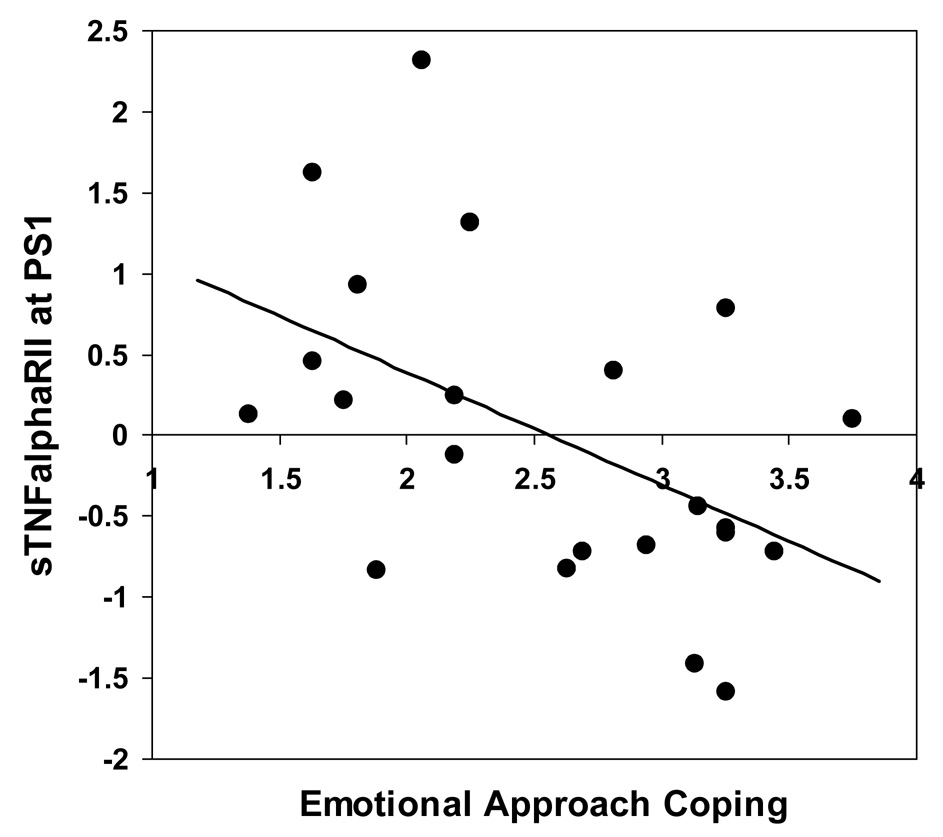
It was hypothesized that higher dispositional EAC would be associated with lower proinflammatory cytokine (i.e., sTNFαRII and IL-6) and cortisol reactivity to the TSST. Consistent with predictions, in partial correlations controlling for baseline levels of sTNFαRII, sTNFαRII at time PS1 was significantly negatively correlated with total EAC score, r (19) = − .50, p = .02 (see Fig. 2), and with the emotional processing component of EAC, r (19) = −.48, p = .03, and it was marginally related to the emotional expression component of EAC, r (19) = −.41, p = .06, at this time point6. That is, people who reported that they were more likely to cope with stressors by actively processing and expressing their emotions exhibited lower sTNFαRII responses to the TSST 25 min after the onset of the stressor than people who were lower on emotional approach. A similar trend was found at assessment PS2, although not significantly so (partial correlations between EAC scores and sTNFαRII levels at PS2, controlling for baseline levels of sTNFαRII, produced r’s that ranged from −.32 to −.28, and p’s ranged from .16 to .22). When controlling for health behaviors in addition to baseline levels of sTNFαRII, the relationships of sTNFαRII at time PS1 to total EAC score and to the emotional processing component of EAC were marginally significant (r = −.43, p = .08 and r = −.40, p = .10, respectively), and the relationship of sTNFαRII at time PS1 to the emotional expression component of EAC became nonsignificant (r = −.34, p = ns).
Figure 2.
Relation between emotional approach coping and post-stress sTNFαRII. Overall EAC scores (displayed on the abscissa) were negatively correlated with stress-responsive levels of the soluble receptor for tumor necrosis factor-alpha (sTNFαRII) at 25 min after the onset of the stressor. The scores displayed on the ordinate represent residualized levels of sTNFαRII at the PS1 assessment, controlling for baseline levels of sTNFαRII.
In partial correlations controlling for baseline levels of IL-6 and cortisol, respectively, EAC scores were not significantly related to PS1 levels of IL-6 and cortisol, or to PS2 levels of IL-6. Also, no significant relationships were found between EAC scores and baseline levels of sTNFαRII, IL-6, or cortisol. The relationship between EAC scores and rate of recovery of cortisol stress responses, as assessed by computing the partial correlation between EAC scores and PS2 cortisol levels controlling for PS1 cortisol levels, was also not significant. Similar findings were obtained when controlling for health behaviors.
Given that depression has been shown in a number of studies to relate to proinflammatory cytokine levels, the relationships between depressive symptomatology measured by the BDI and proinflammatory cytokine levels in the present sample were assessed. No significant relationships were found between BDI scores and baseline levels or changes in levels of sTNFαRII and IL-6. Nonetheless, the previous analyses involving proinflammatory cytokines were re-conducted controlling for BDI scores, and nearly identical results were obtained. Also, controlling for BAS scores did not affect the results of any of these analyses.
Relation of Resting Frontal EEG Asymmetry to Biological Stress Responses
On the basis of previous research (e.g., Buss et al., 2003; Davidson et al., 1999; Kang et al., 1991), it was postulated that greater left-sided frontal asymmetry would be associated with lower baseline and stress-related levels of proinflammatory cytokines and cortisol. In line with this prediction, a marginally significant relationship was found between greater left-sided frontal asymmetry and lower levels of sTNFαRII at time PS1 (controlling for baseline sTNFαRII levels), r (19) = −.40, p = .07. This trend was also seen at PS2, r (19) = −.35, p = .12. When controlling for health behaviors, however, statistically significant relationships emerged between frontal asymmetry scores and proinflammatory cytokine levels. Specifically, greater left-sided frontal asymmetry was significantly related to lower baseline levels of IL-6, r = −.58, p < .01, and to lower levels of sTNFαRII at time PS2 (controlling for baseline levels of sTNFαRII), r = −.56, p = .02. Greater left-sided frontal asymmetry was marginally correlated with lower levels of IL-6 at time PS2 (controlling for baseline levels of IL-6), r = −.39, p = .11. Controlling for BDI scores did not affect any of these results. Thus, participants with greater left-sided frontal asymmetry had lower baseline levels of IL-6 and lower post-stress sTNFαRII levels, and tended to have lower post-stress IL-6 levels, compared to individuals scoring lower on the frontal asymmetry measure. No other significant relationships were found between frontal asymmetry and baseline, PS1, or PS2 levels of sTNFαRII, IL-6, or cortisol.
Discussion
Accumulating evidence reveals that a focus on emotions is adaptive when dealing with stressors, and the present investigation considered neurobiological processes by which emotional approach coping may be related to enhanced health. The primary hypothesis that a dispositional tendency to cope via emotional approach would be related to established neural correlates of approach motivational orientation was supported. That is, participants higher in EAC, and particularly in the emotional expression component, showed greater relative left-sided resting frontal EEG activity, which is indicative of a greater dispositional approach motivational orientation.
Although not predicted, it is not surprising that frontal asymmetry was significantly related only to the emotional expression component of EAC, and not to emotional processing, as emotional expression involves active verbal and/or nonverbal attempts to communicate or symbolize one’s emotional experience regarding a stressor. As such, emotional expression entails a behavioral component in which one engages his/her emotions and acts upon them, which conceptually would be expected to relate to one’s dispositional approach/avoidance tendencies. Emotional processing, by contrast, may take on different forms for different people. Whereas for some people emotional processing involves approach towards, and engagement and exploration of, one’s emotions, it may be more likely to take the form of rumination or negative cyclic thinking for others. Rumination has been conceptualized by Martell and colleagues (2001) as an escape or avoidance behavior that keeps an individual separated from others and may prevent problem solving, and, indeed, it has been found that individuals who are more likely to engage in behavioral avoidance are more likely to ruminate (Moulds et al., 2007). Thus, the relationship between emotional processing and approach/avoidance tendencies is not a straightforward one.
On the whole, the frontal asymmetry findings support the contention that a person’s tendency to cope via emotional approach may be a reflection of a more centrally-mediated neurocognitive profile associated with approach motivation. Because there is evidence that frontal EEG asymmetry is related to affect and depression (Davidson, 1992; Henriques and Davidson, 1990, 1991) and to immune and neuroendocrine functioning (Buss et al., 2003, Davidson et al., 1999; Kang et al., 1991; Rosenkranz et al., 2003), the relationship found between the emotional expression component of EAC and frontal asymmetry suggests that the tendency to act on one’s emotions in response to stress may represent one out of many potential motivational and cognitive mechanisms involved in self-regulation and associated with activity in the prefrontal cortex. Thus the relationship between emotional expression and adaptive health outcomes may be due to its association with these underlying self-regulatory processes.
The stress manipulation (i.e., the TSST) caused significant increases in cortisol levels and in levels of the soluble receptor for the proinflammatory cytokine TNF-α (sTNFαRII), which reflects TNF-α activity. It also produced marginally significant increases in interleukin-6 (IL-6). As expected, significantly elevated cortisol levels were seen approximately 25 minutes following the onset of the stressor which returned to pre-stress levels by 55 minutes, whereas the increases in the proinflammatory cytokines persisted over the 55 minutes following the onset of the stress task. Although a growing body of research has similarly found increases in proinflammatory markers following acute laboratory-induced psychological stress (see Steptoe et al., 2007), cortisol, which is also released in response to acute laboratory stress (see Dickerson and Kemeny, 2004), is well-known for its suppressive effects on proinflammatory cytokine production and expression. This inverse relationship was somewhat evident in the present investigation, as baseline levels of cortisol were marginally negatively correlated with baseline levels of sTNFαRII and IL-6. Nonetheless, there are several biological mechanisms by which increases in proinflammatory cytokines could be expected in response to acute psychological stress (see Steptoe et al., 2007 for a review). For instance, the autonomic nervous system and neuroendocrine pathways are involved in the stimulation of TNF-α and IL-6 production (Sanders and Kavelaars, 2007). Additionally, Bierhaus and colleagues (2003) have demonstrated that NF-κB in peripheral blood mononuclear cells (PBMC), a transcription factor that is involved in setting in motion the inflammatory signaling cascade, is rapidly induced during acute stress exposure (i.e., the TSST) in parallel with catecholamine and cortisol responses. Bierhaus et al. (2003) additionally found that this increase in NF-κB activity returned to baseline levels within 60 minutes of the stressor, and that participants who did not show any stress-dependent increases in stress hormones did not induce NF-κB binding activity; this pattern suggests that the latter response depends on the response to psychological stress. Thus the inter-relationships among sympathetic, neuroendocrine, and immune responses to stress are complex, and, although the effect is well documented, at the current time the precise mechanisms through which proinflammatory cytokine levels increase in response to psychological stressors remain unclear.
The findings of this investigation also suggest that levels of emotional approach coping may be related to stress-related levels of sTNFαRII. Participants’ self-reported levels of emotional processing were significantly inversely related to their stress-related levels of sTNFαRII 25 minutes after the onset of the TSST (this relationship was marginally significant when controlling for health behaviors), and their levels of emotional expression were marginally related to their stress-related levels of sTNFαRII as well (although nonsignificant when controlling for health behaviors). For the most part, these data suggest that people who are more likely to cope with stressors by approaching their emotions, particularly through emotional processing, may evince a less-pronounced TNF-α stress response compared with those lower in EAC. Thus, if people who are less likely to approach their emotions surrounding stressors produce greater stress-induced amounts of proinflammatory cytokines, over repeated instances of stress this could eventually lead to the development of a chronic, low-grade inflammatory state, which, in turn, may accelerate the risk of them developing a number of age-related diseases. Indeed, elevated levels of proinflammatory cytokines, including TNF-α and IL-6, have been associated with numerous adverse health outcomes, such as coronary heart disease, type II diabetes, osteoporosis, arthritis, certain cancers, periodontal disease, and functional decline (e.g. Harris, et al., 1999; Kiecolt-Glaser et al., 2002; Papanicolaou et al., 1998; Ridker et al., 2000a, 2000b; Seeman et al., 1997).
This investigation also permitted exploration of the relationships between frontal EEG asymmetry and the biological parameters measured in the stress-reactivity study. A growing body of research shows that frontal EEG asymmetry is associated with certain immune parameters, and some similar relationships were seen in the present investigation. Specifically, greater left-sided frontal EEG asymmetry was significantly related to lower stress-related sTNFαRII levels, and was marginally related to lower stress-related IL-6 levels, approximately 55 minutes after the onset of the laboratory stressor. In addition, greater left-sided frontal EEG asymmetry was highly significantly related to lower baseline levels of IL-6. Whereas previous research linking frontal asymmetry to immune function has reported relationships between neural activity and NK cell activity (Davidson et al., 1999; Kang et al., 1991) and antibody response to vaccination (Rosenkranz et al., 2003), to our knowledge, the present investigation is the first to link frontal EEG asymmetry to baseline levels of IL-6 and to stress-related levels of sTNFαRII, and to a lesser extent, IL-6. Although the exact biological pathways underlying these relationships are yet to be identified, these findings suggest that individuals with greater dispositional approach orientations (measured with EEG) may be less reactive to acute stress situations such as the TSST, as indicated by these immune parameters. They also lend credence to the idea that individuals who are less approach oriented may have a relatively elevated baseline inflammatory state.
Finally, the present study also considered individual difference variables that could affect the main relationships under investigation. That is, BAS was assessed to measure general self-reported approach tendencies, and the BDI was administered to assess depressive symptoms that could have been influencing proinflammatory cytokine levels. EAC scores and BAS scores were not correlated, and controlling for BAS did not affect the relationships between EAC and frontal EEG asymmetry. Thus, it appears that the relationship between EAC and frontal asymmetry reflects something specific about emotional approach coping and not approach more generally. Also, BDI scores were not related to proinflammatory cytokine levels, and controlling for BDI scores in the analyses involving the proinflammatory cytokines did not considerably alter any of the results. One reason for the lack of a relationship between depressive symptoms and cytokines may be that there was a restricted range in the BDI scores, as depressed persons were screened out of the study. Nevertheless, how depression affects the relationships between EAC and immune parameters remains important to consider in the future, given the known relationships among depression, proinflammatory cytokines, emotions, and coping processes.
Limitations and Future Directions
This study is not without certain limitations. First, only a sub-sample of the participants underwent the laboratory stress protocol, and a larger sample would have yielded more power to detect significant relationships among variables. Thus, the conclusion that EAC is not related to IL-6 or cortisol may be premature. Furthermore, had the post-stress monitoring of proinflammatory cytokines been longer, relationships between stress-related levels of IL-6 and EAC and/or frontal asymmetry might have eventually been seen, as cytokine stress responses take some time to emerge and may thus be more apparent over longer periods. For example, Steptoe and colleagues (2001) found that stress-induced increases in plasma IL-6 were most apparent 2 hours following stress [although other studies have found increases sooner (e.g., von Känel et al., 2006)].
Although not necessarily a limitation, it should be noted that, because we were interested in whether dispositional EAC is associated with dispositional motivation orientations and typical responses to stress, we felt these measures need not be collected during the same session; thus, the stress reactivity data were collected during a different session than the resting frontal EEG asymmetry data and EAC data. Although it is possible that outside circumstances could have influenced participants in different ways during each session, we do not expect that this was a major factor in the results, as the measures obtained (i.e., dispositional EAC and resting frontal EEG asymmetry) are both trait measures and have been shown to have high test-retest reliability (Austenfeld and Stanton, 2004; Tomarken et al., 1992b). In actuality, the fact that some of the measures were collected in two separate sessions and that meaningful relationships were nonetheless found between them may, in fact, attest to the strength of these relationships.
Also due to the nature of the research questions involving dispositional orientations and responses, the data collected were correlational. Although the pattern of correlations found in this investigation may appear suggestive of a statistically-mediated relationship between the emotional expression component of EAC and stress-related sTNFαRII levels by frontal EEG asymmetry, a full mediational model was not put forth or tested, as there is not a clear theoretical reason to expect this pathway. That is, the measures of EAC and frontal asymmetry assessed in this investigation reflect “trait” assessments, and it is not clear theoretically whether there should be causal relations among these variables. Furthermore, although one may find statistical mediation among a set of variables, statistical effects alone cannot establish a causal model.
It would be interesting in future research, however, to more directly test the causal pathways underlying some of the relationships among the psychological, neural, and immunological variables assessed in this study. For instance, we have speculated that people lower in emotional processing may be less adept at coping with stressors than those higher in emotional processing, a consequence of which may be a tendency toward heightened TNF-α reactivity to stress; the possibility also exists, however, that having a heightened TNF-α response to stress could cause people to be less likely to approach their emotions surrounding stressors. In actuality, it is probably the case that reciprocal causal pathways exist, as, for example, administration of proinflammatory cytokines to animals has been shown to induce strong behavioral disengagement (Dantzer et al., 2001; Yirmiya et al., 1999).
It appears that the ways in which EAC and health may be related could be different for the two EAC components, as only emotional expression was related to frontal cortical asymmetry, and emotional processing was more strongly related to post-stress sTNFαRII levels than was emotional expression. Future experimental studies could thus help clarify some of these relationships. Despite these differences, one common mechanism of EAC’s effects may involve appraisal processes, as one’s appraisal of the stressfulness of a situation may be lessened by expressing one’s emotional reactions to the stressor and/or by gaining a better understanding of these reactions, thus reducing the stressor’s perceived significance and/or intensity and, in turn, one’s physiological responses to it (Lazarus and Folkman, 1984).
Conclusion
The present investigation considered relationships among biological, electrophysiological, and self-report data to identify the neurobiological correlates of emotional approach coping. As hypothesized, higher levels of EAC were related to greater left-sided frontal EEG asymmetry, indicative of greater trait approach motivation. Higher EAC scores were also associated with lower sTNFαRII levels in response to a laboratory stress task. Additionally, frontal EEG asymmetry was found to be related to baseline and stress-related levels of proinflammatory cytokines. The results shed light on the debate between the functionalist and more traditional views of emotions concerning the adaptive potential of emotion-focused coping. Our findings suggest that focusing coping efforts on emotions is potentially beneficial, consistent with the functionalist view that emotions are adaptive and organize self-regulatory responses. Importantly, no evidence was found in support of the idea that coping via focusing on one’s emotions is maladaptive, as the more traditional view of emotions would posit. Overall, the results are consistent with the growing body of research suggesting that emotional processes serve broad regulatory functions that promote adaptive responses to stress.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
sTNFαRII is the soluble receptor for the proinflammatory cytokine tumor necrosis factor-alpha (TNF-α) and reflects TNF-α activity; sTNFαRII was assessed in this study because it is more stable and reliably measured than TNF-α (see Dickerson et al., 2004).
Age data are not based on the full sample, as these data were not available for some of the participants.
T-tests revealed that participants who participated in both the EEG study and the stress-reactivity study were older, t (33) = 4.09, p < .01, and more likely to be female, t (44) = 2.35, p = .02, compared to those who only participated in the EEG study. This age difference is most likely superficial, however, as some of the age data were missing from the group of participants who participated only in the EEG study. The age data that were available for the EEG-only group were for participants in an introductory psychology course of 18 to 20 year olds, although older students were presumably included in this EEG-only group as well. The two groups of participants did not differ in EAC scores or frontal cortical asymmetry scores (all p’s > .12).
Shorter versions of the EAC scales also exist, in which emotional processing and emotional expression are each assessed with only 4 items (Stanton et al., 2000b). All findings reported herein are based on the expanded 8-item versions of the EAC scales; however, when the analyses were conducted with the 4-item versions of the scales, the findings were nearly identical.
There were no significant relationships found between EAC scores and EEG asymmetry at non-frontal scalp sites (i.e., the parietal, temporal, and occipital regions).
In this sub-sample of participants, the correlation between the two EAC scales was r (20) = .61, p < .01.
References
- Ackerman KD, Martino M, Heyman R, Moyna NM, Rabin BS. Stressor-induced alteration of cytokine production in multiple sclerosis patients and controls. Psychosom. Med. 1998;60:484–491. doi: 10.1097/00006842-199807000-00016. [DOI] [PubMed] [Google Scholar]
- Allen JJ, Harmon-Jones E, Cavender JH. Manipulation of frontal EEG asymmetry through biofeedback alters self-reported emotional responses and facial EMG. Psychophysiology. 2001;38:685–693. [PubMed] [Google Scholar]
- Amodio DM, Master SL, Yee CM, Taylor SE. Neurocognitive components of the behavioral inhibition and activation systems: implications for theories of self-regulation. Psychophysiology. 2008;45:11–19. doi: 10.1111/j.1469-8986.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Shah JY, Sigelman J, Brazy PC, Harmon-Jones E. Implicit regulatory focus associated with asymmetrical frontal cortical activity. J. Exp. Soc. Psychol. 2004;40:225–232. [Google Scholar]
- Austenfeld JA, Stanton AL. Coping through emotional approach: a new look at emotion, coping, and health-related outcomes. J. Pers. 2004;72:1335–1363. doi: 10.1111/j.1467-6494.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berghuis JP, Stanton AL. Adjustment to a dyadic stressor: a longitudinal study of coping and depressive symptoms in infertile couples over an insemination attempt. J. Consult. Clin. Psychol. 2002;70:433–438. doi: 10.1037//0022-006x.70.2.433. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychological stress into mononuclear cell activation. Proc. Natl. Acad. Sci. USA. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L, Edwards S, Mohamed-Ali V, Steptoe A. Socioeconomic status and stress-induced increases in interleukin-6. Brain Behav. Immun. 2004;18:281–290. doi: 10.1016/j.bbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Buss KA, Malmstadt Schumacher JR, Dolski I, Kalin NH, Hill Goldsmith H, Davidson RJ. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Behav. Neurosci. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The wisdom of the body. New York: W.W. Norton; 1932. [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J. Pers. Soc. Psychol. 1994;67:319–333. [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40:106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error: emotion, reason, and the human brain. New York: Putnam; 1994. [Google Scholar]
- Dantzer R, Bluthé R, Castanon N, Chauvet N, Capuron L, Goodall G, Kelley KW, Konsman JP, Layé S, Parnet P, Pousset F. Cytokine effects on behavior. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. New York: Academic Press; 2001. pp. 583–612. [Google Scholar]
- Das UN. Vagus nerve stimulation, depression, and inflammation. Neuropsychopharmacology. 2007;32:2053–2054. doi: 10.1038/sj.npp.1301286. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Emotion and affective style: hemispheric substrates. Psychol. Sci. 1992;3:39–43. [Google Scholar]
- Davidson RJ, Coe CC, Dolski I, Donzella B. Individual differences in prefrontal activation asymmetry predict natural killer cell activity at rest and in response to challenge. Brain Behav. Immun. 1999;13:93–108. doi: 10.1006/brbi.1999.0557. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Larson CL. Human electroencephalography. In: Cacioppo JT, Bernston GG, Tassinary LG, editors. Handbook of Psychophysiology. New York: Cambridge University Press; 2000. pp. 27–52. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME, Aziz N, Kim KH, Fahey JL. Immunological effects of induced shame and guilt. Psychosom. Med. 2004;66:124–131. doi: 10.1097/01.psy.0000097338.75454.29. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. Clarifying the emotive functions of asymmetrical fontal cortical activity. Psychophysiology. 2003;40:838–848. doi: 10.1111/1469-8986.00121. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. J. Pers. Soc. Psychol. 2001;80:797–803. [PubMed] [Google Scholar]
- Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr, Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. J. Abnorm. Psychol. 1990;99:22–31. doi: 10.1037//0021-843x.99.1.22. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J. Abnorm. Psychol. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Horowitz MJ. Stress Response Syndromes. New York: Jacob Aronson; 1976. [Google Scholar]
- Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behav. Neurosci. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Kang D-H, Davidson RJ, Coe CL, Wheeler RE, Tomarken AJ, Ershler WB. Frontal brain asymmetry and immune function. Behav. Neurosci. 1991;105:860–869. doi: 10.1037//0735-7044.105.6.860. [DOI] [PubMed] [Google Scholar]
- Keltner D, Gross JJ. Functional accounts of emotions. Cogn. Emot. 1999;13:467–480. [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu. Rev. Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivatory cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. New York: Springer; 1984. [Google Scholar]
- Lewis RS, Weekes NY, Wang TH. The effect of a naturalistic stressor on frontal EEG asymmetry, stress, and health. Biol. Psychol. 2007;75:239–247. doi: 10.1016/j.biopsycho.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Lindsley DB, Wicke JD. The electroencephalogram: autonomous electrical activity in man and animals. In: Thompson R, Patterson MN, editors. Bioelectric Recording Techniques. New York: Academic Press; 1974. pp. 3–83. [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a TH1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Martell CR, Addis ME, Jacobson NS. Depression in Context: Strategies for Guided Action. New York: W. W. Norton; 2001. [Google Scholar]
- Mendolia M, Kleck RE. Effects of talking about a stressful event on arousal: does what we talk about make a difference? J. Pers. Soc. Psychol. 1993;64:283–292. doi: 10.1037//0022-3514.64.2.283. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moulds ML, Kandris E, Starr S, Wong ACM. The relationship between rumination, avoidance and depression in a non-clinical sample. Behav. Res. Ther. 2007;45:251–261. doi: 10.1016/j.brat.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Nishanian P, Aziz N, Chung J, Detels R, Fahey JL. Oral fluids as an alternative to serum for measurement of markers of immune activation. Clin. Diagn. Lab. Immunol. 1998;5:507–512. doi: 10.1128/cdli.5.4.507-512.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP. The pathophysiologic roles of interleukin-6 in human disease. Ann. Intern. Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- Penley JA, Tomaka J, Wiebe JS. The association of coping to physical and psychological health outcomes: a meta-analytic review. J. Behav. Med. 2002;25:551–603. doi: 10.1023/a:1020641400589. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Kiecolt-Glaser JK, Glaser R. Disclosure of traumas and immune function: health implications for psychotherapy. J. Consult. Clin. Psychol. 1988;56:239–245. doi: 10.1037//0022-006x.56.2.239. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol. Sci. 2005;16:805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Pfeffer M, Sacks F, Lepage S, Braunwald E. Elevation of tumor necrosis factor-alpha and increased risk of recurrent coronary events after myocardial infarction. Circulation. 2000a;101:2149–2153. doi: 10.1161/01.cir.101.18.2149. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently health men. Circulation. 2000b;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- Rosenkranz MA, Jackson DC, Dalton KM, Dolski I, Ryff CD, Singer BH, Muller D, Kalin NH, Davidson RJ. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc. Natl. Acad. Sci. U.S.A. 2003;100:11148–11152. doi: 10.1073/pnas.1534743100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders VM, Kavelaars A. Adrenergic regulation of immunity. In: Ader R, editor. Psychoneuroimmunology. 4th ed. San Diego: Academic Press; 2007. pp. 63–83. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen B. Impact of social environment characteristics on neuroendocrine regulation. Psychosom. Med. 1996;58:459–471. doi: 10.1097/00006842-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Singer B, Rowe JW, Horwitz R, McEwen BS. The price of adaptation – allostatic load and its health consequences: MacArthur studies of successful aging. Arch. Intern. Med. 1997;157:2259–2268. [PubMed] [Google Scholar]
- Smith JA, Lumley MA, Longo DJ. Contrasting emotional approach coping with passive coping for chronic myofascial pain. Ann. Behav. Med. 2002;24:326–335. doi: 10.1207/S15324796ABM2404_09. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Danoff-Burg S, Cameron CL, Bishop MM, Collins CA, Kirk SB, Twillman R. Emotionally expressive coping predicts psychological and physical adjustment to breast cancer. J. Consult. Clin. Psychol. 2000a;68:875–882. [PubMed] [Google Scholar]
- Stanton AL, Danoff-Burg S, Cameron CL, Ellis AP. Coping through emotional approach: problems of conceptualization and confounding. J. Pers. Soc. Psychol. 1994;66:350–362. doi: 10.1037//0022-3514.66.2.350. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Kirk SB, Cameron CL, Danoff-Burg S. Coping through emotional approach: scale construction and validation. J. Pers. Soc. Psychol. 2000b;78:1150–1169. doi: 10.1037//0022-3514.78.6.1150. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Parsa A, Austenfeld JL. The adaptive potential of coping through emotional approach. In: Snyder CR, Lopez SJ, editors. Handbook of Positive Psychology. New York: Oxford University Press; 2002. pp. 148–158. [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Willemsen G, Owen N, Flower L, Mohamed-Ali V. Acute mental stress elicits delayed increases in circulating inflammatory cytokine levels. Clin. Sci. (Lond.) 2001;101:185–192. [PubMed] [Google Scholar]
- Taylor SE, Stanton AL. Coping resources, coping processes, and mental health. Annu. Rev. Clin. Psychol. 2007;3:377–401. doi: 10.1146/annurev.clinpsy.3.022806.091520. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. J. Pers. Soc. Psychol. 1990;59:791–801. doi: 10.1037//0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. J. Pers. Soc. Psychol. 1992a;62:676–687. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Kinney L. Psychometric properties of resting anterior EEG asymmetry: temporal stability and internal consistency. Psychophysiology. 1992b;29:576–592. doi: 10.1111/j.1469-8986.1992.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cacioppo J, Kiecolt-Glaser J. The relationship between social support and physiological processes: a review with emphasis on underlying mechanisms and implications for health. Psychol. Bull. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- von Känel R, Kudielka BM, Preckel D, Hanebuth D, Fischer JE. Delayed response and lack of habituation in plasma interleukin-6 to acute mental stress in men. Brain Behav. Immun. 2006;20:40–48. doi: 10.1016/j.bbi.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Wittling W. Brain asymmetry in the control of autonomic-physiologic activity. In: Davidson RJ, Hugdahl K, editors. Brain Asymmetry. Cambridge, MA: The MIT Press; 1995. pp. 305–357. [Google Scholar]
- Yirmiya R, Weidenfeld J, Pollak Y, Morag M, Morag A, Avitsur R, Barak O, Reichenberg A, Cohen E, Shavit Y, Ovadia H. Cytokines, “depression due to a general medical condition,” and antidepressant drugs. Adv. Exp. Med. Biol. 1999;461:238–316. doi: 10.1007/978-0-585-37970-8_16. [DOI] [PubMed] [Google Scholar]