Abstract
One of the major issues for modern neuroscience research concerns the molecular and cellular mechanisms that underlie the acquisition, storage, and recollection of memories by the brain. Regulation of the strength of individual synaptic inputs (synaptic plasticity) has, for decades, been the front-running candidate mechanism for cellular information storage, with some direct supporting evidence recently obtained. Research into the molecular mechanisms responsible for changing synaptic strength has, to date, primarily focused on trafficking and properties of the neurotransmitter receptors themselves (AMPARs and NMDARs). However, recent evidence indicates that, subsequent to receptor activation, synaptic inputs are subject to regulation by synaptically located K+ channels. It is therefore critical to understand the biophysical properties and subcellular localization (density and distribution) of these channels and how their properties are modulated. Here we will review recent findings showing that two different classes of K+ channels (A-type and small conductance, Ca2+-activated K+ channels), beyond their traditional role in regulating action potential firing, contribute to the regulation of synaptic strength in the hippocampus. In addition, we discuss how modulation of these channels' properties and expression might contribute to synaptic plasticity.
Keywords: Potassium channel, Kv4.2, SK, Trafficking, Synaptic plasticity
Potassium channels in excitable cells tend to dampen membrane excitability given the hyperpolarized reversal potential of K+ ion flux across the neuronal membrane. In neurons, K+ channels set the resting membrane potential, oppose depolarizations from rest, and repolarize action potentials (APs). The large diversity of K+ channels allows for a wide variety of firing patterns across neuronal types and within a single neuron type under different conditions. Activity-dependent modulation of K+ channel properties or distribution can generate a plasticity of intrinsic excitability, perhaps contributing to some forms of memory storage (Zhang and Linden 2003).
Historically, the effort to understand these channels has begun with their electrophysiological characterization combined with the biochemical identification of acceptors for neurotoxins and other pharmacological agents known to affect neuronal excitability. These approaches led to the cloning and expression of cDNAs encoding the principal, pore-forming α-subunits of K+ channels, with more than 100 genes identified to date. The array of K+ channel genes that together regulate the neuronal excitability are classified into four families according to their genetic homology, transmembrane topology, and functional activation: 1) voltage-gated K+ channels, 2) Ca2+-activated K+ channels, 3) inward rectifier K+ channels, and 4) leak K+ channels (Coetzee and others 1999). K+ channels are further subclassified based on their unique biophysical kinetics including time and voltage dependence of their activation, inactivation, and deactivation.
Heterologous expression and genetic manipulation of cloned K+ channel subunits have led to insights into the molecular identity of channels underlying distinct native current and into their essential roles in electrical signal processing. There are several recent reviews summarizing molecular diversity, biophysical properties, channel structure, subcellular localization, kinase modulation, and functional roles of various K+ channels (Coetzee and others 1999; Jerng and others 2004a; Lai and Jan 2006; Swartz 2004; Trimmer and Rhodes 2004; Yuan and Chen 2006). Here, we focus on the two types of K+ channels (voltage-gated A-type K+ channels and small conductance Ca2+-activated K+ channels) that have recently been found to affect synaptic signaling in CA1 pyramidal dendrites of the hippocampus. K+ channel activity is governed by its subcellular distribution, modulation by posttranslational modifications, and by associating with auxiliary subunits. Therefore we focus on the non-uniform localization and mechanisms of A- and SK-type K+ channel modulation including activity-dependent modulation and trafficking of K+ channels, which may link synaptic plasticity with the plasticity of intrinsic excitability.
A-Type K+ Channels
The transient or A-type K+ current (IA) is subthreshold activating and rapidly inactivating (within ~100 ms). The transient, A-type current was first described by Hagiwara and others in molluskan neurons (Hagiwara and others 1961). Connor and Stevens, using two electrodes to voltage-clamp gastropod somata, named this current IA (Connor and Stevens 1971). IA was distinguished from other molluskan voltage-dependent K+ currents by its rapid activation and inactivation. Typically, A-type currents are active at subthreshold potentials and completely inactive at -40 mV. Connor and Stevens hypothesized that IA regulates AP frequency with the hyperpolarization following an AP serving to remove inactivation. Thompson later showed molluskan A-type channels to be sensitive to 4-aminopyridine (4-AP) but relatively insensitive to tetraethylammonium (TEA) (Thompson 1977).
In heterologous expression systems, A-type currents are mediated by Kv1.4, Kv3.4, or the Kv4 family subunits (Kv4.1-Kv4.3) that show distinct subcellular distributions; that is, Kv1.4 and Kv3.4 are mainly detected in axons, whereas somatodendritic A-current is comprised mainly of subunits from the Kv4 family (Coetzee and others 1999; Rudy and McBain 2001; Song 2002). Handicapped by the technical limitations (e.g., antibody specificity), subcellular visualization of A-type K+ channel subunits has proven difficult, although there was an early suggestion that Kv4.2 channels opposed presynaptic terminals in the hippocampus (Alonso and Widmer 1997). Recently, direct evidence establishing Kv4.2 as the molecular identity of the transient A-current in CA1 pyramidal neurons has been shown using molecular techniques to decrease functional Kv4.2 activity followed by electrophysiological recordings to document decreases in A-currents (Chen and others 2006; Kim and others 2005; Lauver and others 2006).
Dendritic A-Type K+ Channels Control AP Back-Propagation
In hippocampal CA1 pyramidal dendrites, the density of IA increases with distance from the soma (Hoffman and others 1997). Moreover, in distal dendrites, A-type channels have a hyperpolarization-shifted conductancevoltage curve resulting in an increased probability of channel opening at physiological voltages. Block of IA with 4-AP was demonstrated to enhance the back-propagation of dendritic APs and increase the likelihood of AP initiation in dendrites (Hoffman and others 1997).
More recently, studies have shown that genetic downor up-regulation of Kv4.2 alters dendritic Ca2+ influx during back-propagation (Chen and others 2006; Kim and others 2005). As intracellular Ca2+ elevation is necessary for the induction of long-term potentiation (LTP, a long-lasting increase in synaptic strength), these results indicated that regulation of back-propagation by Kv4.2 might be an important factor in the induction of synaptic plasticity. Indeed, in physiological induction protocols, which rely on AP back-propagation, LTP threshold is reduced when the activity of dendritic A-type channels is reduced (Chen and others 2006; Ramakers and Storm 2002; Watanabe and others 2002).
Synaptic Localization and Activation of A-Type K+ Channels
After decades of research into the molecular mechanisms of information storage, N- methyl-D-aspartate receptor (NMDAR)-dependent changes in synaptic strength remain the best candidate for the cellular analogue of learning and memory (Barco and others 2006; Kandel 2001; Malenka and Nicoll 1993; Nicoll and Malenka 1999). Recent evidence suggests that the regulation of synaptic surface expression of glutamate receptors can, in part, determine synaptic strength (Collingridge and others 2004; Malinow and Malenka 2002). However, subsequent to synaptic transmission, postsynaptic potentials are subject to significant filtering in CA1 dendrites. Depending on the location and timing of synaptic input, voltage and Ca2+-gated channels located in dendritic shafts and/or spines may be activated to amplify or otherwise shape synaptic signals as they propagate toward the axon where AP threshold is lowest (Stuart and others 1997). It is possible then that activity-dependent regulation of ion channel properties and/or surface expression pattern could contribute to the expression of synaptic plasticity. Hoffman and colleagues in 1997 provided an early suggestion that A-type K+ channels might shape synaptic input. In this study, small dendritic current injections, aimed to mimic excitatory postsynaptic potentials (EPSPs), were enhanced by bath 4-AP application (Hoffman and others 1997). Later Ramakers and Storm confirmed a role of IA in synaptic integration in rat CA1 pyramidal cells, showing that heteropodatoxin-3 (HpTX3), a blocker of postsynaptic A-type currents, strongly enhanced the amplitude and summation of excitatory postsynaptic responses (Ramakers and Storm 2002).
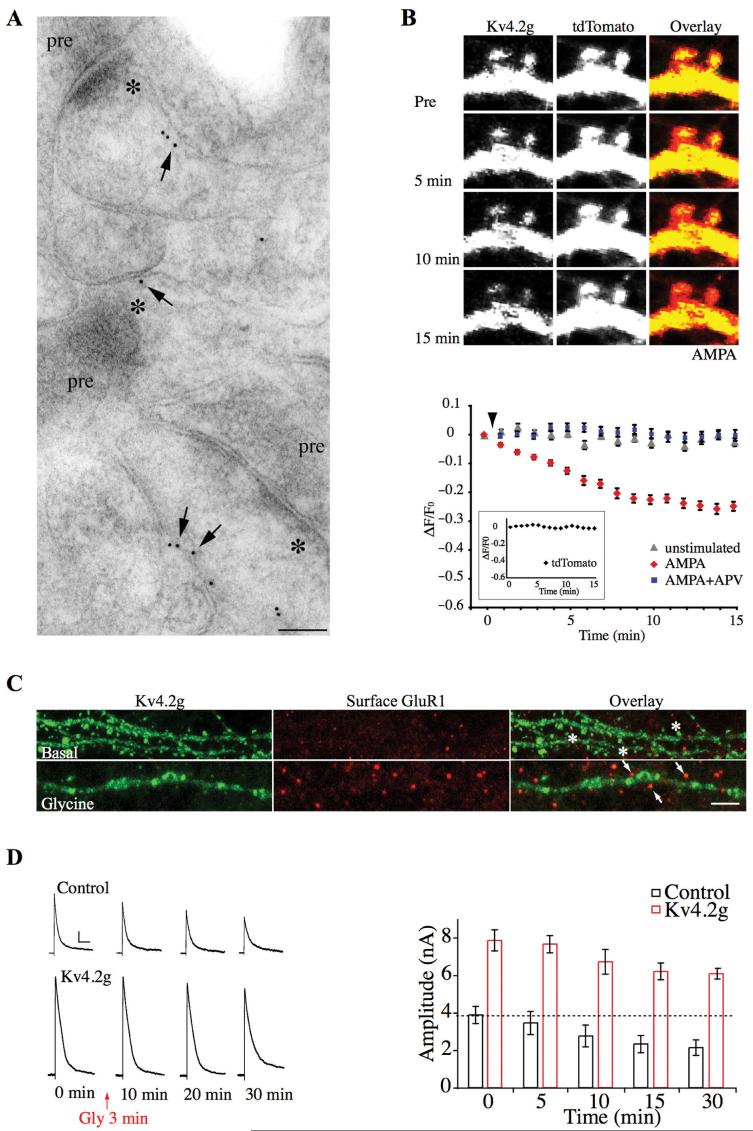
More recently, Kim and colleagues showed that Kv4.2 subunits are localized to hippocampal dendritic spines. Enhanced green fluorescence protein (EGFP)-tagged Kv4.2 (Kv4.2g) fluorescence was greater in cultured hippocampal neuron spines compared to dendritic shafts, suggesting Kv4.2 spine enrichment (Kim and others 2005). Electron microscopy confirmed that endogenous Kv4.2 is localized to spines in adult hippocampal CA1 pyramidal neurons (Kim and others 2007) (Fig. 1). Finally, altering the functional expression level of Kv4.2 led to changes in miniature excitatory postsynaptic current (mEPSC) amplitude (Kim and others 2007). Investigating the possibility that synaptically located Kv4.2 subunits are involved in synaptic plasticity, Kim and colleagues observed the activitydependent redistribution and internalization of Kv4.2 channels (Kim and others 2007) (Fig. 1). Excitatory stimulation led to a clathrin-mediated decrease in the surface expression of Kv4.2g channels. This finding was confirmed for endogenous Kv4.2 channels using a biotinylation assay in brain slices and by recording a decrease in IA in young dissociated hippocampal neurons. Live imaging experiments and electrophysiological recordings showed that, as with many forms of synaptic plasticity, activitydependent Kv4.2 internalization requires NMDA receptor activation. Furthermore, mEPSC amplitude was enhanced by stimuli that induced Kv4.2 internalization. Finally, a chemically induced LTP protocol resulted in synaptic insertion of GluR1-containing AMPA receptors along with Kv4.2 internalization.
Fig. 1.
Synaptic Kv4.2 channels contribute to long-term potentiation. (A) Immunogold labeling for endogenous Kv4.2 showing its expression in CA1 spines. For electron micrographs, stars (*) indicate the postsynaptic density and “pre” indicates the presynaptic terminal. Scale bar: 100 nm. (B) Time-lapse images showing Kv4.2g fluorescent intensity decrease upon AMPA (50 μM) stimulation in spines of hippocampal neurons coexpressing Kv4.2g and tdTomato (top). Time course of averaged fractional fluorescent changes (ΔF/F0) of Kv4.2g in spines (bottom). AMPA stimulation resulted in a progressive decrease of Kv4.2g- specific fluorescent intensity in spines, with no significant change in tdTomato fluorescent intensity (inset). AMPA-mediated decreases in spine Kv4.2g fluorescence intensity were blocked by APV. (C) ChemLTP induced with brief glycine exposure, results in synaptic insertion of GluR1 but also Kv4.2g internalization. Stars = Kv4.2g-positive clusters; arrows = Kv4.2g-negative clusters. Scale bar: 8 μm. (D) Examples of the decrease in transient K+ currents during chemLTP. A-type K+ currents are decreased by Kv4.2 internalization during chemLTP. Scale bar: 1 nA, 100 ms. (Modified from Kim and others 2007.)
In recordings from adult CA1 hippocampal dendrites, Frick and colleagues reported that the voltage dependence of steady-state A-channel inactivation is altered after LTP induction via a hyperpolarized shift. This shift decreases the proportion of channels available for activation at resting potentials, enhancing dendritic excitability (Frick and others 2004). Together these results present a novel mechanism to coordinate synaptic integration and plasticity through the activity-dependent regulation of Kv4.2 activity. The compartmentalized, non-uniform distribution of K+ channels can thus dramatically impact neuronal signaling through local regulation of the neuronal membrane excitability.
Subcellular Compartmentalization and Trafficking of Kv4.2 Channels
Given the potential importance of K+ channel trafficking to synaptic integration and plasticity, it is important to know the mechanisms involved in regulating membrane expression. In neurons, the functional expression of channels in the plasma membrane is regulated at a number of different levels: transcription, translation, posttranslational modification, and interaction with auxiliary proteins. Polarized sorting of K+ channel proteins to axon- or dendrite-directed cargo vesicles, targeted insertion at discrete sites within axonal or dendritic membrane, localized stabilization of K+ channels in the plasma membrane, and activity-dependent movement are all involved in determining which channels are expressed at which locations. Posttranslational modifications and auxiliary subunits, in addition to regulating a channel's biophysical properties, likely also contribute the channels' normal or activity-dependent distribution in the membrane.
The effort to elucidate the molecular mechanisms underlying subcellular localization of neuronal membrane proteins (dendritic or axonal) has been to identify targeting motifs within the primary structure of transmembrane proteins that are localized in distinct subcellular compartments. For Kv4.2, Riviera and colleagues demonstrated that a highly conserved 16-amino-acid dileucine-containing motif, located in the cytoplasmic C-terminus, is both necessary for dendritic targeting of the channel and sufficient to induce nonspecifically localized transmembrane proteins to target to dendrites (Rivera and others 2003). When the C-terminus of the dendritic A-type K+ channel subunit Kv4.2 is exchanged with that of the axonal A-type K+ channel subunit Kv1.4, these chimeric channels showed opposite polarity of their natural distribution. The dileucine motif of Kv4.2 in the C-terminus is thus critical for dendritic transport (Fig. 2).
Fig. 2.
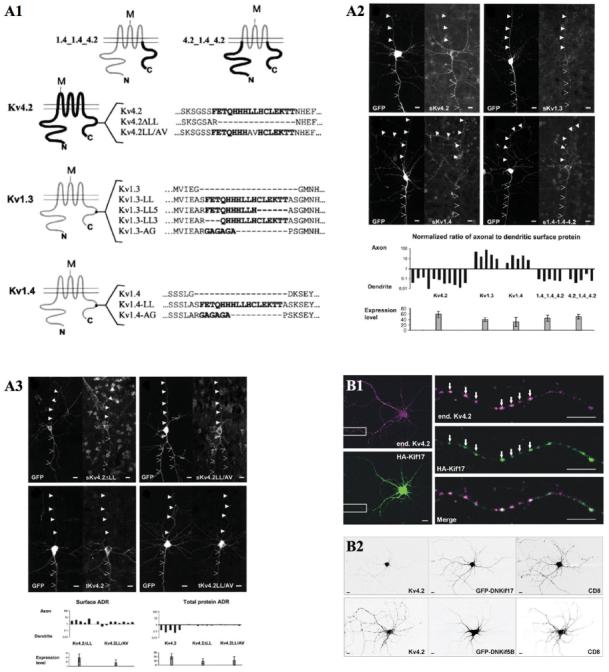
Subcellular compartmentalization and trafficking of A-type K+ channels. (A1) Schematics of voltage-gated K+ channels and chimertic channels. A 16-amino-acid, dileucine motif of C-termini is shown in bold. M represents extra-cellular 8 tandem Myc tags. (A2) Subcellular surface expression of K+ channel constructs in cortical pyramidal cells. Green fluorescent protein and each K+ channel construct were coexpressed in neurons for 40 hours. Anti-Myc surface staining (top) and the surface axon-to-dendrite ratio analysis (sADR; bottom) were performed. It revealed that the C-terminus of Kv4.2 contains a dendritic localization signal. In addition, the dileucine (LL)-containing motif is sufficient to mediate dendritic localization (A3). (B1) Kv4.2 colocalizes with Kif17 in dissociated cortical neurons. (B2) Specifically, a dominant negative mutant of Kif17 (DNKif17) blocks proper localization of endogenous Kv4.2, but not that of Kif5B. Scale bars: 10 μm. (Modified from Rivera and others 2003 and Chu and others 2006 with permission.)
Recently, it was demonstrated that Kv4.2 is transported to dendrites by binding with the kinesin isoform Kif17, which is also involved in dendritic transport of the NMDA receptors (Chu and others 2006; Setou and others 2000). Using immunostaining and a co-immunoprecipitation assay, endogenous Kv4.2 was shown to interact specifically with Kif17 and not with another kinesin isoform, Kif5B, which is responsible for dendritic transport of AMPA receptors (Setou and others 2002). The attachment of Kv4.2 to Kif17 is not through the dileucine motif of Kv4.2 but rather through its extreme C-terminal. Remarkably, a dominant negative Kif17 inhibits dendritic localization of Kv4.2 (Fig. 2). Because Kif17 does not interact with Kv4.2 via the dileucine motif, which is necessary for dendritic localization of this channel, Kif17 is speculated to be the specific motor that transports Kv4.2 to dendrites, but not the determinant that specifies Kv4.2 dendritic localization (Chu and others 2006).
Auxiliary Subunits and Posttranslational Modification of Kv4.2 Channels
As important regulators of dendritic signaling, A-type K+ channels are prime targets for modulation. Indeed, a number of protein kinases, known to participate in the induction and expression of synaptic plasticity (PKA, PKC, ERK), down-regulate the activity of these K+ channels resulting in increased excitability in distal dendrites (Hoffman and others 1997; Johnston and others 2000a, 2000b; Watanabe and others 2002). Kv4.2 subunits contain two consensus PKA phosphorylation sites (Anderson and others 2000), three C-terminal ERK sites (Adams and others 2000), two C-terminal PKC sites, and two C-terminal calcium-calmodulin-dependent kinase II (CaMKII) sites (Varga and others 2004). Phospho-specific antibodies for Kv4.2 subunits phosphorylated at either the PKA site or all three ERK sites have been developed, and immunohistochemical studies demonstrate the presence of each of these phosphorylated Kv4.2 subunits in the CA1 region of the hippocampus (Varga and others 2000). Activation of PKA and PKC in hippocampal dendrites can lead to phosphorylation of Kv4.2 via a downstream ERK-specific signaling pathway (Yuan and others 2002). Constitutively active CaMKII in hippocampal neurons up-regulated A-type K+ currents, suggesting that CaMKII phosphorylation of Kv4.2 contributes to Kv4.2 surface expression or stabilization (Varga and others 2004).
Membrane insertion of Kv4.2 channels in heterologous expression systems is enhanced by the presence of two families of accessory proteins that also alter the kinetics and voltage-dependent properties of Kv4.2: the Ca2+-binding Kv channel-interacting proteins (KChIPs; An and others 2000; Shibata and others 2003) and dipeptidyl-peptidase-like proteins (DPLs; Jerng and others 2005; Jerng and others 2004b; Nadal and others 2003). The formation of specific ternary Kv4.2-DPL-KChIP complexes results in currents with different kinetic properties of activation and inactivation, which allows for variations of IA in different cell types and within the same cell under different conditions. In hippocampal CA1 pyramidal neurons, DPPX is the prominent DPL family member whereas two different KChIPs are expressed (KChIP2 and KChIP4; Jerng and others 2005). KChIPs bind to the Kv4.2 N-terminal T1 domain with a 4:4 subunit stoichiometry (Kim and others 2004; Zhou and others 2004), whereas DPPX appears to bind to Kv4.2 extracellularly near the first transmembrane domain (Ren and others 2005).
Interestingly, PKA modulation of Kv4.2 currents in oocytes requires coexpression with KChIP auxiliary subunits (Schrader and others 2002), and Kv4.2 must be inserted into the plasma membrane for phosphorylation by PKA at S552 (Shibata and others 2003). In addition to demonstrating a role for PKA and KChIPs in Kv4.2 modulation and trafficking, these results provide an example of the complex interactions between posttranslational modifications and auxiliary subunits. Although posttranslational modifications and auxiliary subunit binding have been shown to alter functional properties and membrane expression of Kv4.2 channels in heterologous systems, a number of questions remain that must be addressed in native environments. Are certain combinations of auxiliary subunit complexes and/or phosphorylation states responsible for the Kv4.2 density gradient in CA1 dendrites? For Kv4.2 spine localization and stabilization? For activity-dependent trafficking of Kv4.2 channels? If so, how are these changes regulated and what is their effect on dendritic integration, synaptic plasticity, and the plasticity of intrinsic excitability?
Small-Conductance, Ca2+-Activated K+ Channels
Ca2+-activated K+ channels are activated by a rise in intracellular [Ca2+] during an action potential and the resultant current (IAHP; after-hyperpolarization current) influences firing frequency and affects neuronal integration. These channels are widely expressed in the CNS and different channel types contribute to distinct temporal components of the IAHP (fast, medium, and slow; Blatz and Magleby 1986; Storm 1989; Sah 1996). Based on biophysical and pharmacological properties, three subfamilies of Ca2+-activated K+ channels have been classified, namely small-conductance Ca2+-activated K+ channels (SK), large conductance Ca2+-activated K+ channels (BK), and IK (Faber and Sah 2003; Sah 1996; Vergara and others 1998).
Taking advantage of the specific blocker from bee venom (apamin), SK channels are well characterized at the molecular and physiological levels. Unlike the other classes of Ca2+-activated K+ channels, SK channels are not voltage-gated but purely Ca2+ activated (Schumacher and others 2001; Xia and others 1998). These channels are high-affinity Ca2+ sensors and their current exhibits Ca2+ dose responses. However, Ca2+ does not bind directly to the channel subunits. Yeast two-hybrid, biochemical, electrophysiological, and biophysical structural studies have demonstrated that SK channels directly interact with calmodulin (CaM) via their CaM-binding domain (CaMBD), which follows from the sixth transmembrane domain (S6) (Schumacher and others 2001; Xia and others 1998). This Ca2+/CaM/CaMBD complex accounts for rapid Ca2+-gating mechanism of SK channels. Three genes encoding SK channels in CNS have been identified: SK1, 2, and 3 (Bond and others 2005; Kohler and others 1996; Sailer and others 2002). SK1 and SK2 subunits are highly distributed in the hippocampus and cortex, whereas SK3 subunits are found in the thalamus, hyper-thalamus, and midbrain. Genetically knocking out the SK subunits has revealed that only SK2 channels are necessary for the medium component of IAHP but that none of the SK channels underlie the fast or slow components of the IAHP (Bond and others 2004; but see Gu and others 2005).
Additionally, pharmacological blockade of SK channels with apamin increases neuronal excitability and facilitates the induction of LTP in hippocampus and memory encoding (Stackman and others 2002; Tzounopoulos and Stackman 2003). This is supported by genetic manipulations showing SK channels modulate hippocampal synaptic plasticity and learning and memory (Blank and others 2003; Hammond and others 2006). SK2 overexpression attenuates hippocampal synaptic plasticity and impairs spatial learning in the Morris water maze as well as contextual and cured fear conditioning (Hammond and others 2006). Blank and colleagues in 2003 suggested the idea that age-dependent deficits of learning, as well as LTP, is correlated with the increased expression of hippocampal SK3 channels in development. SK3 channels are more highly expressed in aged mice and knockdown of SK3 rescues age-related impairments in LTP and trace fear conditioning (Blank and others 2003).
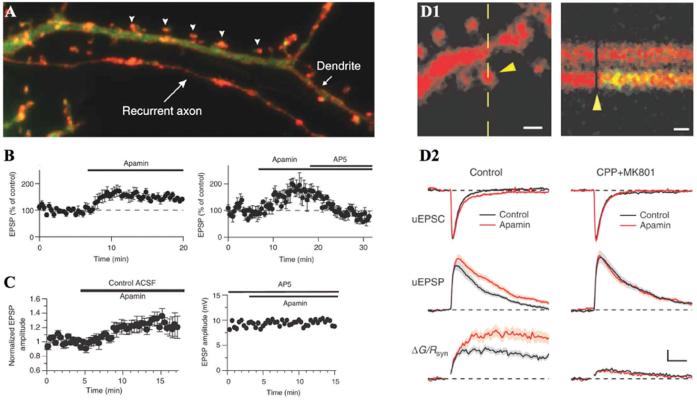
Recent studies have demonstrated that, like Kv4.2, SK2 channels are localized to dendritic spines, and play a role in shaping synaptic events in an NMDAR-dependent manner in both the hippocampus and the amygdala (Faber and others 2005; Ngo-Anh and others 2005; Fig. 3). Combined two-photon laser scanning microscopy and two-photon laser uncaging of glutamate revealed that SK channels regulate NMDAR-dependent Ca2+ influx within individual spines. Activation of NMDARs leads to a compartmentalized rise in spine [Ca2+] (Sabatini and others 2002) that was shown to activate apamin-sensitive SK channels to negatively affect the magnitude of EPSPs measured at the soma. This regulatory activity of SK channels within individual spines provides a local shunting current reducing EPSP amplitude. The resulting reduction in voltage acts to prevent the unblock of Mg2+ from NMDARs. Thus, SK channels act to determine the direction and extent of synaptic plasticity by regulating NMDAR-dependent Ca2+ signals within dendritic spines.
Fig. 3.
SK channels regulate the induction of long-term potentiation by N- methyl-D-aspartate receptor (NMDAR)-dependent Ca2+ feedback loop in dendritic spines. (A) Small-conductance Ca2+-activated K+ channels (SK) channels (red) are localized to hippocampal dendritic spines (arrowheads). (B) Blockade of SK channels with apamin facilitated excitatory postsynaptic potentials (EPSPs) in hippocampus. The apamin effect on EPSP potentiation was abolished by the NMDAR blocker, APV. These phenomena were observed in amygdala as well (C). (D1) Single synapse responses and NMDAR-dependent spine Ca2+ signal evoked with uncaging of glutamate. Scale bars: 10 μm (left), 25 ms (right). (D2) Blocking SK channels with apamin increases NMDAR-mediated spine Ca2+ transients. (Modified from Ngo-Anh and others 2005 and Faber and others 2005 with permission.)
In another study in the hippocampus, Kv4.2 and SK channels have been demonstrated to play unique, complementary roles in regulating local synaptic integration (Cai and others 2004). Apamin-sensitive SK channels were determined to be responsible for the duration of voltage-dependent Ca2+ channel-mediated local dendritic plateau potentials induced by photolyzed uncaging of glutamate. Manipulations that affected SK channels increased the duration of plateau potentials without affecting their amplitude or compartmentalization. Kv4.2 channels complemented SK channels, accounting for the amplitude of plateau potentials and their compartmentalization by limiting the forward propagation of depolarization from a daughter dendritic branch into the mother branch.
Taken together, recent investigations into the synaptic role of K+ channels suggest that actions of both SK and Kv4.2 channels are initiated by Ca2+ influx though NMDARs. SK channels, upon activation by NMDAR-mediated Ca2+, shape synaptic events, forming a feedback loop to the induction of synaptic plasticity. Kv4.2 channels also shape EPSPs but enhance synaptic integration through their NMDAR and Ca2+-dependent internalization. Given their novel and important roles in regulating synaptic integration and plasticity, both channels should continue to receive intense investigation. With more research we may find a tight functional link between glutamate receptors and both types of K+ channels at individual synapses.
Subcellular Compartmentalization and Trafficking of SK Channels
It is clear that subcellular localization is an important determinant of the physiological significance and role of SK channels. As described above, SK channels constitutively associate with CaM and Ca2+ binding to CaM is the mechanism of channel gating. Interestingly, a Ca2+-independent interaction between SK channels and CaM is necessary for appropriate membrane trafficking of SK channels (Decimo and others 2006; Lee and others 2003; Maylie and others 2004; Xia and others 1998). Point mutations in SK2 (SK2:64/67, R464E/K467E in CaMBD) have been designed to reduce the affinity CaM in Ca2+-free conditions. These mutations reverse the amino acid charges in the CaMBD of SK2. After heterologous expression of SK2:64/67, electrophysiological whole-cell recordings and surface immunocytochemistry failed to detect surface SK channels when Ca2+ was dialyzed into the cell through the patch pipette. However, cells cotransfected SK2:64/67 with wild-type CaM or mutated CaM (CaM1, 2, 3, 4- lacking the ability to bind Ca2+) yielded robust SK currents. Finally, coexpression of SK2:64/67 with another mutated CaM (CaM:84/87), containing the double charge reversal E84R/E87K, restored SK channel activity that was not abolished with exposure to Ca2+-free solution. These results suggest that Ca2+-independent interaction between SK channels and CaM is necessary for cell surface expression (Lee and others 2003).
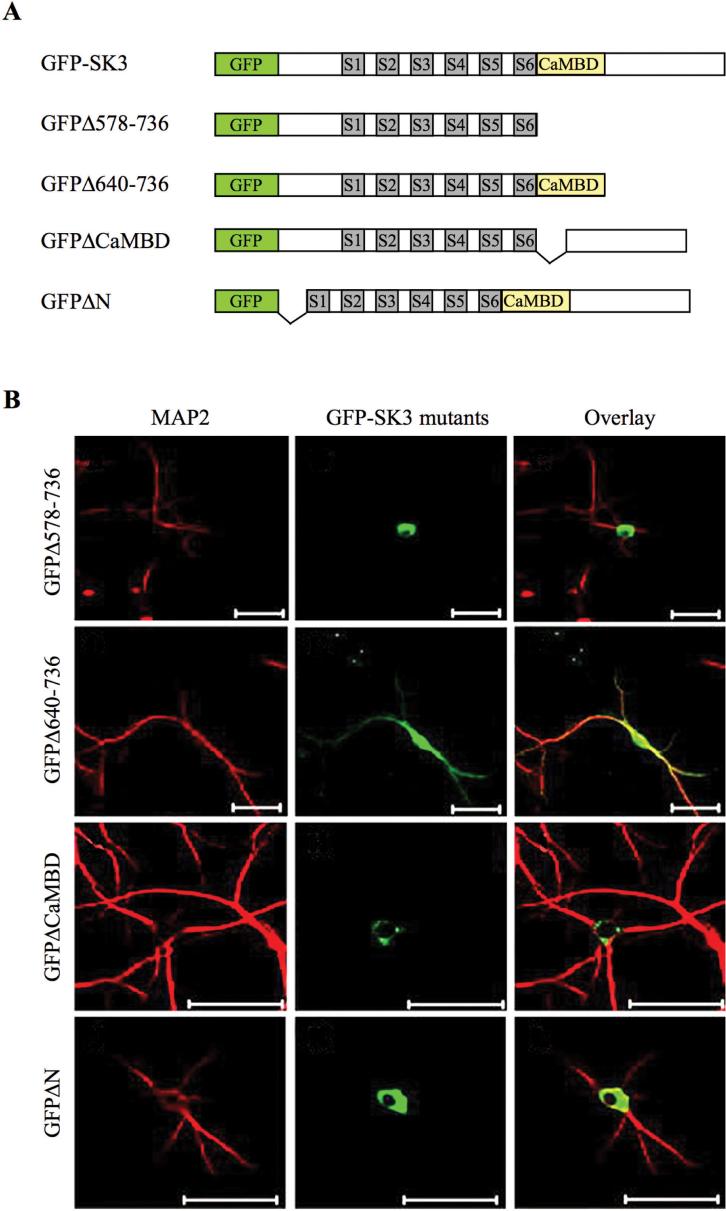
Decimo and colleagues provided evidence that both the CaMBD and the N-terminal domain of SK3 play a fundamental role in trafficking in neurites (Fig. 4; Decimo and others 2006). Using confocal imaging after transfecting different green fluorescence protein (GFP)-fused SK3 truncations into cultured hippocampal neurons, they show that neurons expressing N-terminal region (GFPΔN) or CaMBD-truncated SK3 (GFPΔCaMDB) have fluorescent protein accumulated in the soma and colocalized with the ER. However, a C-terminal mutant truncated after CaMBD (579-638), (GFPΔ640-736), showed a neurite expression pattern similar to the full-length SK3, demonstrating the necessity of CaMBD for neurite expression.
Fig. 4.
Subcellular compartmentalization and trafficking of SK channels. (A) Schematics of green fluorescence protein (GFP)-tagged SK3 and its truncated constructs. Transmembrane domains are indicated as gray boxes, S1-S6, and the calmodulin-binding domain (CaMBD) as a yellow box. (B) Expression and localization of truncated GFP-SK3s in cultured hippocampal neurons stained by anti-MAP2. Confocal images revealed that the N-terminal domain and CaMBD are necessary for proper SK channel transport. Scale bars: 50 μm. (Modified from Decimo and others 2006 with permission.)
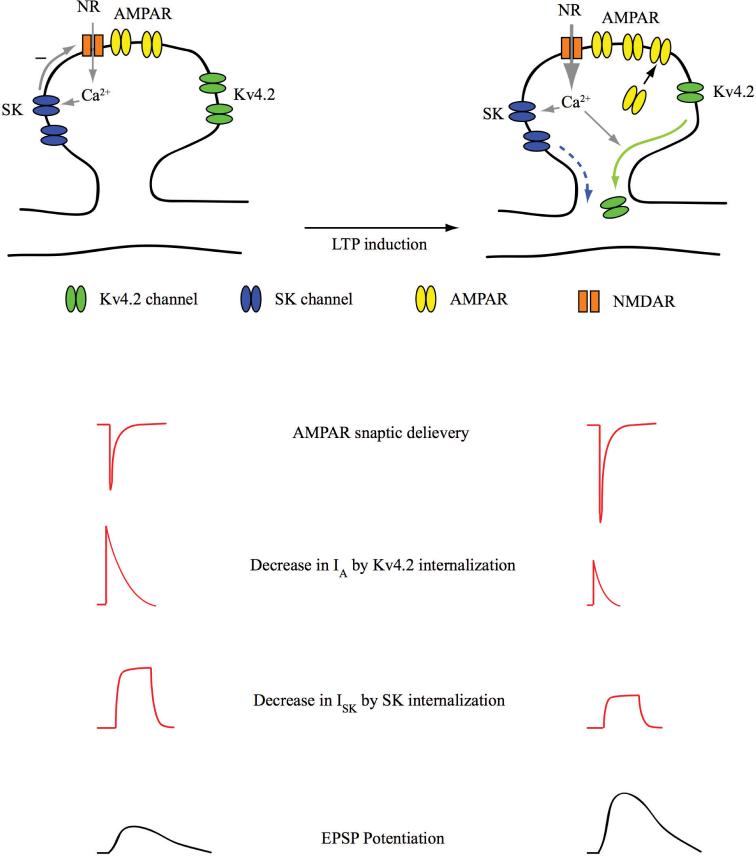
Surface expression of SK channels is regulated by cAMP-dependent protein kinase (PKA; Ren and others 2006). In a heterologous expression system, PKA activation with forskolin triggered a large decrease in SK2 channel surface expression. In contrast, SK2 channels with amino acid changes at three PKA phosphorylation sites of the C-terminal domain (S568A, S569A, and S570A) showed no decrease of SK2 surface expression by PKA activation. More recently, in hippocampal CA1 neurons, LTP induction by theta burst pairing triggers a decrease in the activity of SK channels (Lin and others 2008). Taken together, these recent studies suggest that, like Kv4.2, SK channels are likely internalized in an activity-dependent manner. Based on spine localization and activity-dependent internalization of SK channels, we can speculate that local synaptic excitability is regulated by activity-dependent movement of SK channels, along with that of Kv4.2 and glutamate receptors (Fig. 5). In an individual synapse undergoing plasticity, Mg2+ unblock of NMDARs, in addition to inducing the trafficking of AMPARs, results in decreased Kv4.2 and SK channel activity, through their internalization. In this way, glutamate receptors and K+ channels may work in concert to induce synaptic plasticity. Identifying specific components that are responsible for trafficking of each channel may lead us to a functional coordination between glutamate receptors and K+ channels (e.g., Kv4.2 and SK channels) in response to the pattern of local synaptic events.
Fig. 5.
Schematic model for functional link between glutamate receptors (AMPARs and NMDARs) and K+ channels (Kv4.2 and SK) in spine plasticity. In an individual synapse under basal conditions (left), EPSPs mediated by glutamate receptors are shaped by the activity of synaptic Kv4.2 and SK channels. In an individual synapse undergoing long-term potentiation (right), EPSPs are potentiated not only by the trafficking of AMPARs, but also by Kv4.2 and SK2 internalization. AMPAR = AMPA receptor; NMDAR = N-methyl-D-aspartate receptor; EPSP = excitatory postsynaptic potential.
Summary
Over the past few decades it has become clear that synaptic signal transmission is far from the simple adding up of excitatory and inhibitory inputs to determine neuronal output. Here we have reviewed recent evidence showing that the activity of K+ channels contribute to the computations performed in dendrites subsequent to postsynaptic receptor activation. K+ channel subcellular location, density, and voltage-dependent properties play critical roles in shaping electrical signaling in the neuron, which is the foundation for more complex computations performed at the network level. Knowledge of subcellular compartmentalization, trafficking, and modulation of dendritic ion channels is necessary for a full understanding of their contribution to synaptic signaling and intrinsic excitability. We have begun to uncover some of the various protein kinases, scaffold proteins, and auxiliary interaction proteins that regulate K+ channel activity. However, how and where these modulatory factors interact remains to be determined. Furthermore, it will be of interest to determine the specific mechanisms distinguishing and sorting distinct ion channels. For example, Kim and others (2007) have demonstrated that LTP induction results in not only synaptic AMPAR insertion but also Kv4.2 internalization. How does the neuron govern this two-way trafficking of Kv4.2 internalization and AMPAR insertion in synapses?
More sophisticated technical approaches may provide more information of how K+ channels are dynamically trafficked in and out of synaptic compartments in response to synaptic activities has fundamental implications for our understanding of the mechanisms of synaptic plasticity that underlies information encoding and storage. Studies employing single molecule labeling, electron microscopic ultrastructure, and two-photon uncaging will allow for a more precise correlation between channel localization, activity-dependent movement, and discrete functional compartments (e.g., active zones of terminals, postsynaptic membranes, dendritic branch points, etc.), which will provide additional information on the specialized roles of K+ channels.
Acknowledgments
We thank Dr. Rebecca Hammond for critical review of this manuscript. This work was supported by the National Institute of Child Health and Human Development Intramural Research Program.
References
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75(6):2277–87. doi: 10.1046/j.1471-4159.2000.0752277.x. and others. [DOI] [PubMed] [Google Scholar]
- Alonso G, Widmer H. Clustering of KV4.2 potassium channels in postsynaptic membrane of rat supraoptic neurons: an ultrastructural study. Neuroscience. 1997;77(3):617–21. doi: 10.1016/s0306-4522(96)00561-1. [DOI] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403(6769):553–6. doi: 10.1038/35000592. and others. [DOI] [PubMed] [Google Scholar]
- Anderson AE, Adams JP, Qian Y, Cook RG, Pfaffinger PJ, Sweatt JD. Kv4.2 phosphorylation by cyclic AMP-dependent protein kinase. J Biol Chem. 2000;275(8):5337–46. doi: 10.1074/jbc.275.8.5337. [DOI] [PubMed] [Google Scholar]
- Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97(6):1520–33. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Kye MJ, Radulovic J, Spiess J. Small-conductance, Ca2+-activated K+ channel SK3 generates age-related memory and LTP deficits. Nat Neurosci. 2003;6(9):911–2. doi: 10.1038/nn1101. [DOI] [PubMed] [Google Scholar]
- Blatz AL, Magleby KL. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323(6090):718–20. doi: 10.1038/323718a0. [DOI] [PubMed] [Google Scholar]
- Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24(23):5301–6. doi: 10.1523/JNEUROSCI.0182-04.2004. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15(3):305–11. doi: 10.1016/j.conb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44(2):351–64. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Chen X, Yuan LL, Zhao C, Birnbaum SG, Frick A, Jung WE. Deletion of Kv4.2 gene eliminates dendritic A-type K+ current and enhances induction of long-term potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 2006;26(47):12143–51. doi: 10.1523/JNEUROSCI.2667-06.2006. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PJ, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem. 2006;281(1):365–73. doi: 10.1074/jbc.M508897200. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–85. doi: 10.1111/j.1749-6632.1999.tb11293.x. and others. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5(12):952–62. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decimo I, Roncarati R, Grasso S, Clemens M, Chiamulera C, Fumagalli G. SK3 trafficking in hippocampal cells: the role of different molecular domains. Biosci Rep. 2006;26(6):399–412. doi: 10.1007/s10540-006-9029-5. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8(5):635–41. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Calcium-activated potassium channels: multiple contributions to neuronal function. Neuroscientist. 2003;9(3):181–94. doi: 10.1177/1073858403009003011. [DOI] [PubMed] [Google Scholar]
- Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7(2):126–35. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566(Pt 3):689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Kusano K, Saito N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961;155:470–89. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26(6):1844–53. doi: 10.1523/JNEUROSCI.4106-05.2006. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387(6636):869–75. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Kunjilwar K, Pfaffinger PJ. Multiprotein assembly of Kv4.2, KChIP3 and DPP10 produces ternary channel complexes with ISA-like properties. J Physiol. 2005;568(Pt 3):767–88. doi: 10.1113/jphysiol.2005.087858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004a;27(4):343–69. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Qian Y, Pfaffinger PJ. Modulation of Kv4.2 channel expression and gating by dipeptidyl peptidase 10 (DPP10) Biophys J. 2004b;87(4):2380–96. doi: 10.1529/biophysj.104.042358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Magee JC, Poolos NP, Watanabe S, Colbert CM. Dendritic potassium channels in hippocampal pyramidal neurons. J Physiol. 2000a;525(Pt 1):75–81. doi: 10.1111/j.1469-7793.2000.00075.x. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston D, Hoffman DA, Poolos NP. Potassium channels and dendritic function in hippocampal pyramidal neurons. Epilepsia. 2000b;41(8):1072–3. doi: 10.1111/j.1528-1157.2000.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294(5544):1030–8. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- Kim J, Jung SC, Clemens AM, Petralia RS, Hoffman DA. Regulation of dendritic excitability by activity-dependent trafficking of the a-type k(+) channel subunit kv4.2 in hippocampal neurons. Neuron. 2007;54(6):933–47. doi: 10.1016/j.neuron.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wei DS, Hoffman DA. Kv4 potassium channel subunits control action potential repolarization and frequency-dependent broadening in rat hippocampal CA1 pyramidal neurones. J Physiol. 2005;569(Pt 1):41–57. doi: 10.1113/jphysiol.2005.095042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim LA, Furst J, Butler MH, Xu S, Grigorieff N, Goldstein SA. Ito channels are octomeric complexes with four subunits of each Kv4.2 and K+ channel-interacting protein 2. J Biol Chem. 2004;279(7):5549–54. doi: 10.1074/jbc.M311332200. [DOI] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273(5282):1709–14. doi: 10.1126/science.273.5282.1709. and others. [DOI] [PubMed] [Google Scholar]
- Lai HC, Jan LY. The distribution and targeting of neuronal voltage-gated ion channels. Nat Rev Neurosci. 2006;7(7):548–62. doi: 10.1038/nrn1938. [DOI] [PubMed] [Google Scholar]
- Lauver A, Yuan LL, Jeromin A, Nadin BM, Rodriguez JJ, Davies HA. Manipulating Kv4.2 identifies a specific component of hippocampal pyramidal neuron A-current that depends upon Kv4.2 expression. J Neurochem. 2006;99(4):1207–23. doi: 10.1111/j.1471-4159.2006.04185.x. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Ngo-Anh TJ, Bruening-Wright A, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin: cell surface expression and gating. J Biol Chem. 2003;278(28):25940–6. doi: 10.1074/jbc.M302091200. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11(2):170–7. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci. 1993;16(12):521–7. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–26. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004;554(Pt 2):255–61. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de Miera E, Ma Y, Mo W. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37(3):449–61. doi: 10.1016/s0896-6273(02)01185-6. and others. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8(5):642–9. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–25. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Ramakers GM, Storm JF. A postsynaptic transient K(+) current modulated by arachidonic acid regulates synaptic integration and threshold for LTP induction in hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 2002;99(15):10144–9. doi: 10.1073/pnas.152620399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Hayashi Y, Yoshimura N, Takimoto K. Transmembrane interaction mediates complex formation between peptidase homologues and Kv4 channels. Mol Cell Neurosci. 2005;29(2):320–32. doi: 10.1016/j.mcn.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ren Y, Barnwell LF, Alexander JC, Lubin FD, Adelman JP, Pfaffinger PJ. Regulation of surface localization of the small conductance Ca2+-activated potassium channel, Sk2, through direct phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2006;281(17):11769–79. doi: 10.1074/jbc.M513125200. and others. [DOI] [PubMed] [Google Scholar]
- Rivera JF, Ahmad S, Quick MW, Liman ER, Arnold DB. An evolutionarily conserved dileucine motif in Shal K+ channels mediates dendritic targeting. Nat Neurosci. 2003;6(3):243–50. doi: 10.1038/nn1020. [DOI] [PubMed] [Google Scholar]
- Rudy B, McBain CJ. Kv3 channels: voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24(9):517–26. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Oertner TG, Svoboda K. The life cycle of Ca(2+) ions in dendritic spines. Neuron. 2002;33(3):439–52. doi: 10.1016/s0896-6273(02)00573-1. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19(4):150–4. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci. 2002;22(22):9698–707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader LA, Anderson AE, Mayne A, Pfaffinger PJ, Sweatt JD. PKA modulation of Kv4.2-encoded A-type potassium channels requires formation of a supramolecular complex. J Neurosci. 2002;22(23):10123–33. doi: 10.1523/JNEUROSCI.22-23-10123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410(6832):1120–4. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin super-family motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288(5472):1796–802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- Setou M, Seog DH, Tanaka Y, Kanai Y, Takei Y, Kawagishi M. Glutamate-receptor-interacting protein GRIP1 directly steers kinesin to dendrites. Nature. 2002;417(6884):83–7. doi: 10.1038/nature743. and others. [DOI] [PubMed] [Google Scholar]
- Shibata R, Misonou H, Campomanes CR, Anderson AE, Schrader LA, Doliveira LC. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J Biol Chem. 2003;278(38):36445–54. doi: 10.1074/jbc.M306142200. and others. [DOI] [PubMed] [Google Scholar]
- Song WJ. Genes responsible for native depolarization-activated K+ currents in neurons. Neurosci Res. 2002;42(1):7–14. doi: 10.1016/s0168-0102(01)00305-4. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22(23):10163–71. doi: 10.1523/JNEUROSCI.22-23-10163.2002. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989;409:171–90. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20(3):125–31. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Swartz KJ. Towards a structural view of gating in potassium channels. Nat Rev Neurosci. 2004;5(12):905–16. doi: 10.1038/nrn1559. [DOI] [PubMed] [Google Scholar]
- Thompson SH. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977;265(2):465–88. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Stackman R. Enhancing synaptic plasticity and memory: a role for small-conductance Ca(2+)-activated K+ channels. Neuroscientist. 2003;9(6):434–9. doi: 10.1177/1073858403259282. [DOI] [PubMed] [Google Scholar]
- Varga AW, Anderson AE, Adams JP, Vogel H, Sweatt JD. Input-specific immunolocalization of differentially phosphorylated Kv4.2 in the mouse brain. Learn Mem. 2000;7(5):321–32. doi: 10.1101/lm.35300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci. 2004;24(14):3643–54. doi: 10.1523/JNEUROSCI.0154-04.2004. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8(3):321–9. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 2002;99(12):8366–71. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395(6701):503–7. doi: 10.1038/26758. and others. [DOI] [PubMed] [Google Scholar]
- Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22(12):4860–8. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LL, Chen X. Diversity of potassium channels in neuronal dendrites. Prog Neurobiol. 2006;78(6):374–89. doi: 10.1016/j.pneurobio.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4(11):885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- Zhou W, Qian Y, Kunjilwar K, Pfaffinger PJ, Choe S. Structural insights into the functional interaction of KChIP1 with Shal-type K(+) channels. Neuron. 2004;41(4):573–86. doi: 10.1016/s0896-6273(04)00045-5. [DOI] [PubMed] [Google Scholar]