Abstract
Glycoside hydrolase family 55 consists of β-1,3-glucanases mainly from filamentous fungi. A β-1,3-glucanase (Lam55A) from the Basidiomycete Phanerochaete chrysosporium hydrolyzes β-1,3-glucans in the exo-mode with inversion of anomeric configuration and produces gentiobiose in addition to glucose from β-1,3/1,6-glucans. Here we report the crystal structure of Lam55A, establishing the three-dimensional structure of a member of glycoside hydrolase 55 for the first time. Lam55A has two β-helical domains in a single polypeptide chain. These two domains are separated by a long linker region but are positioned side by side, and the overall structure resembles a rib cage. In the complex, a gluconolactone molecule is bound at the bottom of a pocket between the two β-helical domains. Based on the position of the gluconolactone molecule, Glu-633 appears to be the catalytic acid, whereas the catalytic base residue could not be identified. The substrate binding pocket appears to be able to accept a gentiobiose unit near the cleavage site, and a long cleft runs from the pocket, in accordance with the activity of this enzyme toward various β-1,3-glucan oligosaccharides. In conclusion, we provide important features of the substrate-binding site at the interface of the two β-helical domains, demonstrating an unexpected variety of carbohydrate binding modes.
Many fungi produce β-1,3-glucans as the main components of the cell wall. The primary role of fungal β-1,3-glucans is structural; that is, to maintain cell wall rigidity and, thus, to protect the cell. The cell wall β-1,3-glucans are also suggested to be degraded for nutritional purposes after exhaustion of external nutrition (1). β-1,3-Glucans on the cell surface are thought to be involved in morphogenetic changes, i.e. aggregation and mycelial strand formation (2). Moreover, the hyphal sheath of pathogenic fungi contains extracellular β-1,3-glucans, which play an active role in wood cell wall degradation (3). Fungal β-1,3-glucans often contain some branches with β-1,6 glycosidic linkages, and these molecules are called β-1,3/1,6-glucans. The pattern of branching in β-1,3/1,6-glucans, e.g. linkage ratio and branch length, varies depending on fungal species, localization in the cell wall, and the growth phase of the cells.
Fungi are prominent producers of β-1,3-glucanases, possibly due to the wide availability of the substrate in their cell wall (4). β-1,3-Glucanases are often termed laminarinases, as one of the most widely studied natural β-1,3-glucan-containing polymers is laminarin. Degradation of β-1,3-glucans by fungi often involves the cooperative action of multiple β-1,3-glucanases rather than a single enzyme. Although some fungal β-1,3-glucanases are constitutively produced, their expression levels are often controlled by culture conditions (5). Many β-1,3-glucanases have been characterized, and they exhibit a wide variety of substrate specificities and modes of actions (6). These facts suggest the involvement of β-1,3-glucanases in mobilization of cell wall β-glucans, especially in the case of yeast enzymes (7). β-1,3-Glucanases have been classified mainly based on the catalytic properties, e.g. endo and exo modes of action (5, 6). In the carbohydrate-active enzymes (CAZy) data base (8-13) enzymes having β-1,3-glucanase activity are found in the glycoside hydrolase (GH)3 families 5, 16, 17, 55, 64, 72, and 81. All of the biochemically characterized enzymes in GH55 exhibit exo- or endo-β-1,3-glucanase activities, and they are found only in filamentous fungi (14-21). Lam55A is the exo-β-1,3-glucanase (or laminarinase A) from the Basidiomycete Phanerochaete chrysosporium, which belongs to GH55. The enzyme has no hydrolytic activity toward β-1,6- or β-1,3/1,4-glucan but shows high activity toward laminarin from Laminaria digitata (22), which is a β-1,3/1,6-glucan with an average degree of polymerization (DP) of ∼25. Here we report the crystal structure of the enzyme, thereby providing the first three-dimensional view of a GH55 family member. We also present a detailed characterization of its hydrolytic activity.
EXPERIMENTAL PROCEDURES
Materials—Laminarioligosaccharides (β-1,3-linked oligomers of d-glucopyranose) with DPs of 2-7 (laminaribiose, L2; laminaritriose, L3; laminaritetraose, L4; laminaripentaose, L5; laminarihexaose, L6; laminariheptaose, L7) were purchased from Seikagaku Corp. (Tokyo, Japan). 6-O-Glucosyl-laminaritriose (LG4, β-d-Glcp-(1→6)-β-d-Glcp-(1→3)-β-d-Glcp-(1→3)-d-Glcp) was prepared using β-1,3-glucanase Lam16A according to the method reported previously (23).
Protein Preparation—Native Lam55A protein was heterologously expressed in Pichia pastoris and purified as described previously (22). Selenomethionine-labeled Lam55A was expressed in buffered minimal methanol media (100 mm potassium phosphate, pH 6.0, 1.34% yeast nitrogen base without amino acids (Wako Pure Chemical Industries Ltd., Osaka, Japan), 4 × 10-5% biotin, 1% methanol) containing 0.1 mg·ml-1 l-selenomethionine, 0.09 mg·ml-1 l-isoleucine, 0.09 mg·ml-1 l-lysine, and 0.6 mg·ml-1 l-threonine using the same transformant of P. pastoris as that for native Lam55A. The purification procedures for selenomethionine-labeled Lam55A were the same as those for non-labeled Lam55A. For the crystallization experiments, the enzymes were treated with endoglycosidase H (New England Biolabs, Beverly, MA) as described previously (24) and further purified by gel permeation chromatography.
Kinetic Parameters—Lam55A was incubated with various concentrations of laminarioligosaccharides with DPs of 2-7 in 100 mm sodium acetate buffer, pH 4.5, at 30 °C, and the hydrolytic rate was estimated by monitoring the release of glucose, using Glucose CII-Test Wako (Wako Pure Chemical Industries Ltd.). To determine the Michaelis constant (Km) and catalytic rate (kcat), experimental data were regressed with the Michaelis-Menten equation using Kaleidagraph™ 3.6.4 (Synergy, Reading, PA).
Analysis of Products from Hydrolysis by Lam55A—High performance anion-exchange chromatography (model d-300 BioLC; Dionex, Sunnyvale, CA) with a CarboPac PA1 column (4 × 250 mm) was used to estimate the amount of hydrolytic products generated from L7. The column was equilibrated with 100 mm NaOH, and the reaction products were eluted with a linear gradient of 0-500 mm sodium acetate in 100 mm NaOH at a flow rate of 1 ml·min-1 over 30 min. A solution of 1 mm L7 was incubated with 12 nm Lam55A for various times, and the reaction mixtures were boiled and applied to the column equilibrated with 100 mm NaOH. The reaction products were eluted with a linear gradient of 0-500 mm sodium acetate in 100 mm NaOH at a flow rate of 1 ml·min-1 over 30 min as described previously (25). The anomeric configurations of the products from the first hydrolysis of LG4 and L3 were determined using an isocratic HPLC method (26, 27). Each substrate (20 mm) was incubated with 1.2 μm Lam55A for 1 min, and the reaction mixture was applied to a TSK-GEL Amide-80 column (4.6 × 250 mm; Tosoh Co., Tokyo, Japan). The hydrolysate from L3 was eluted with 80% acetonitrile at the flow rate of 2 ml·min-1 and from LG4 with 70% acetonitrile at the flow rate of 1.5 ml·min-1. Sugars were detected using a refractive index monitor (RI Model 504, GL Science, Tokyo, Japan). The retention times of α and β anomers of the compounds were determined based on the proportion of the equilibrated anomers (α:β = ∼4:6) using equilibrated solutions.
Crystallography—Crystals of native and selenomethionine-labeled Lam55A were obtained by means of the hanging-drop vapor-diffusion method with microseeding. The drops were formed by mixing 2 μl of protein solution (10 mg·ml-1) and 2 μl of the reservoir solution composed of 100 mm zinc acetate, 15% (w/v) polyethylene glycol 3350, 50 mm MES, pH 6.4, 10% (w/v) glycerol, and 2% (v/v) ethanol. The drops were microseeded after equilibration for 48 h at 25 °C. The microseeds were prepared by crushing native Lam55A crystals. The crystals were transferred to reservoir solutions containing 25% glycerol and then flash-cooled in a stream of nitrogen gas at 100 K. To obtain crystals of the complex with gluconolactone, the reservoir solution containing 50 mm gluconolactone was used for co-crystallization, and then 30% gluconolactone instead of glycerol was used as a cryoprotectant for the flash-cooling step. X-ray diffraction data sets were collected using synchrotron radiation at beam lines BL5A and NW12A of the Photon Factory, High Energy Accelerator Research Organization (KEK), Tsukuba, Japan, and data were processed and scaled using the HKL2000 program suite (28). The programs SHARP (29) and RESOLVE (30) were used for initial phase calculation and density modification, respectively. Automated model building was performed using the program ARP/wARP (31). Manual model rebuilding and refinement were achieved using Coot (32) and Refmac5 (33). The gluconolactone complex structure was solved starting from the refined native structure. Data collection and refinement statistics are shown in Tables 1 and 2, respectively. Figs. 1, 2B, 2C, 3, and 7 were prepared using PyMol (34), and ESPript (35) was used for Fig. 2A.
TABLE 1.
Data collection statistics
|
Data set
|
Unliganded
|
Gluconolactone complex
|
Selenomethionine
|
||
|---|---|---|---|---|---|
| Peak | Edge | Remote | |||
| Beamline | PF BL-5A | PF BL-5A | PF-AR NW12A | PF-AR NW12A | PF-AR NW12A |
| Wavelength (Å) | 1.000 | 1.000 | 0.97898 | 0.97917 | 0.96395 |
| Space group | P1 | P1 | P1 | ||
| Cell dimensions | |||||
| a (Å) | 66.3 | 66.5 | 66.1 | ||
| b (Å) | 67.1 | 67.1 | 67.2 | ||
| c (Å) | 105.2 | 109.8 | 105.0 | ||
| α (degree) | 81.0 | 93.9 | 81.1 | ||
| β (degree) | 76.3 | 106.8 | 76.5 | ||
| γ (degree) | 61.4 | 97.1 | 61.4 | ||
| Resolutiona(Å) | 50.00-1.70 (1.76-1.70) | 50.00-2.25 (2.33-2.25) | 50.00-2.18 (2.26-2.18) | ||
| Total reflections | 502,749 | 262,450 | 604,805 | 589,541 | 564,876 |
| Unique reflections | 149,947 | 77,511 | 78,414 | 77,974 | 76,496 |
| Completeness (%)a | 88.8 (64.5) | 91.9 (74.5) | 97.8 (91.7) | 96.8 (85.0) | 94.7 (73.9) |
| Average I/σ(I)a | 19.1 (3.0) | 10.9 (2.7) | 21.0 (4.92) | 20.6 (4.1) | 17.9 (3.2) |
| Rsym (%)a | 6.5 (24.7) | 9.7 (25.7) | 9.0 (27.0) | 9.5 (30.3) | 9.7 (33.6) |
Values in parentheses are for the highest resolution shell.
TABLE 2.
Refinement statistics
|
Data set
|
PDB accession code
|
|
|---|---|---|
| 3EQN | 3EQO | |
| Resolution (Å) | 27.2-1.70 | 31.5-2.3 |
| R-factor/R-free (%) | 14.9/18.4 | 14.2/20.0 |
| No. of reflections | 142399 | 73632 |
| No. of atoms | 13,016 | 12,519 |
| r.m.s.d. from ideal values | ||
| Bond lengths (Å) | 0.01 | 0.02 |
| Bond angles (degree) | 1.375 | 1.797 |
| Average B-factor (Å2) | ||
| Protein (chain A/B) | 14.5/14.3 | 18.9/21.1 |
| Sugar chain (chain A/B) | 32.9/25.4 | 44.0/44.8 |
| Glycerol | 22.3 | |
| Acetate ion | 25.4 | |
| Zinc ion | 20.7 | 39.1 |
| Sodium ion | 25.0 | |
| Gluconolactone (chain A/B) | 24.6/26.3 | |
| Water | 29.7 | 28.8 |
| Ramachandran plot (%) | ||
| Favored (chain A/B) | 87.4/87.9 | 84.5/84.4 |
| Allowed (chain A/B) | 12.4/11.9 | 15.3/15.3 |
| Disallowed (chain A/B) | 0.2/0.2 | 0.2/0.3 |
FIGURE 1.
Overall structure of Lam55A. A, ribbon representation of Lam55A monomer in the complex with gluconolactone. B, the view rotated 90° around the horizontal axis from that in A. The N domain, C domain, and linker region are colored blue, green, and magenta, respectively. The disulfide bonds (Cys-5—Cys-424, Cys-73—Cys-77, Cys-539—Cys-549, and Cys-692—Cys-698) are shown in red stick form. The gluconolactone molecule is shown as spheres with carbon and oxygen atoms in white and red, respectively. The loops forming the substrate-binding pocket (T1-4, T1-5, T1-6, and T1-7 from N domain; T3-6, T3-7, T3-8, and T3-9 from C domain) are colored yellow. The T3-5 loop from C domain is colored orange. The zinc atom is shown as an orange sphere. The N-linked sugar chain is shown in cyan stick form. C, the view rotated 90° around the vertical axis from that in A. The torsion angle between the helical axes of the two β-helix domains is indicated.
FIGURE 2.
β-Strands in the two β-helix domains. A, sequence alignment of N and C domains. Arrows for PB1, PB2, and PB3 are colored black, gray, and white, respectively. Identical amino acid residues are boxed in gray. The loops forming the substrate binding pocket and T3-5 loop from C domain are indicated by yellow- and orange-shaded boxes, respectively. Dashed lines indicate regions involved in antiparallel interaction between β-strands of N and C domains. Residues possibly involved in catalysis, Glu-633, Gln-146, Gln-176, and Ser-204, are shown in white type boxed in black. B, superimposition of N domain (blue) and C domains (green). PB1, PB2, and PB3 are colored blue, lime green, and red, respectively. Gluconolactone molecules bound to each domain are shown as spheres. The side chains of residues in N and C domains are shown in blue and orange sticks, respectively. Coils are numbered from the N terminus to the C terminus of each domain. AP indicates a region where β-strands have antiparallel interactions. C, aromatic stack and asparagine ladder found in the N domain.
FIGURE 3.
Binding of gluconolactone to Lam55A. A, stereoview with the Fobs - Fcalc omit electron density map contoured at 1.5 σ at the active site. Gluconolactone (white) residues from the N domain (blue) and C domain (green) are shown as stick models. Water molecules are shown as red spheres. Hydrogen bonds are depicted by black dashed lines. Distances between O-1 of gluconolactone and Glu-633 and between C1 and proximal water are indicated by red lines. The main chain carbonyl oxygen of Glu-146 forms a hydrogen bond with the proximal water. B, molecular surface of the gluconolactone complex structure. Amino acid residues highly conserved in biochemically characterized GH55 enzymes, including both exo- and endo-β-1,3-glucanases, are successively colored from yellow (>60%) to orange (>80%) and red (100%). Gluconolactone and aromatic residues located along the groove are shown as stick models. A dashed line indicates a curved groove on the molecular surface, and a circle with green dotted lines indicates the small pocket near the O-6 of gluconolactone.
FIGURE 7.
Comparison of active site formation between GH55 Lam55A and PL19 (formerly GH91) BsIFTase. A, superimposition of the structures of Lam55A (blue) and BsIFTase (red and pink for two symmetry-related molecules). B, close-up stereoview of the active site. Selected residues in the active site of Lam55A (Gln-146, Gln-176, Ser-204, and Glu-633) and the catalytic residues of BsIFTase (Asp-233 and Glu-244) are shown as stick models. Gluconolactone bound to Lam55A (carbon atoms in blue) and β-2,1-linked difructosaccharide bound to subsites +1 and +2 of BsIFTase (carbon atoms in pink) are shown as ball-and-stick models. Top views of monomeric Lam55A (C) and homotrimeric BsIFTase (D) are shown. Schematic diagrams are also shown in these panels. The two domains and the linker region of Lam55A and the three symmetry-related chains of BsIFTase are colored differently. The ligands are shown as spheres with the carbon atoms in yellow. The active site is located at the interface between two domains (GH55) or between two adjacent chains (BsIFTase).
RESULTS
Structure Determination—For the initial phase determination by the multiwavelength anomalous dispersion method, we prepared selenomethionine-substituted crystals from recombinant Lam55A protein produced by P. pastoris cells cultured in selenomethionine-containing medium (36). We determined the crystal structures of Lam55A in unliganded and gluconolactone-complexed forms at 1.7 and 2.3 Å resolutions, and they were refined to R-values (R-free) of 14.9% (18.4%) and 14.2% (20.0%), respectively. Both crystal structures contained two molecules (A and B) of mature Lam55A in the asymmetric unit. The structures of the four chains determined here, two molecules from two crystals, are very similar, with the r.m.s.d. for Cα atoms less than 0.22 Å in all combinations of the four. We will describe chain A of each crystal structure unless otherwise noted.
The N-terminal residues from the expression vector (Glu-Ala-Glu-Ala-Glu-Phe) were highly disordered and were removed from the models. Six Zn2+ ions, 4 sodium ions, 4 acetate molecules, and 11 glycerol molecules were found in the ligand-free structure, whereas only 2 Zn2+ ions were found in the gluconolactone complex structure. At one of the four potential N-glycosylation sites in the Lam55A sequence, Asn-231 exhibited extra electron density arising from its side-chain amide nitrogen atom. The electron density of two N-acetyl-β-d-glucosamine (GlcNAc) residues and one β-d-mannose residue was clearly visible, indicating that this glycosylation site was not susceptible to deglycosylation by endoglycosidase H. Further substitution by mannose residues, possibly attached at the O-3 or O-6 position of the terminal mannose residue, was only partially observed, with unclear electron density.
Overall Structure—Lam55A consists of two domains with a right-handed parallel β-helix fold (N domain, residues 1-361; C domain, residues 391-752) connected by a linker region (28 residues, 362-390). The two β-helix domains are positioned side-by-side, forming an overall shape like a rib cage (Fig. 1). As shown in Fig. 1C, the torsion angle between the helical axes of the two β-helix domains is ∼25°. The two domains are bound tightly via many interactions that include the disulfide bond between Cys-5 and Cys-424. The other disulfide bonds, Cys-73-Cys-77, Cys-539-Cys-549, and Cys-692-Cys-698 exist in long loops between β-strands (Fig. 1, A and B). A helical repeating unit of the β-helix fold, which is formed by three β-strands (designated as PB1, PB2, and PB3) linked by three turns (T1, T2, and T3), is termed a “coil” (37). We will hereafter designate the PBm β-strand and Tm turn included in the nth coil as PBm-n and Tm-n, respectively. Both the N and C domains of Lam55A consist of 12 coils, but flanking (1st, 2nd, 11th, and 12th) coils are incomplete or irregular (Fig. 2A). Both domains lack PB1-1, PB1-2, and PB3-11, and the C domain lacks PB2-12 (Fig. 2, A and B). Two C-terminal β-strands in the N domain (PB2-11 and PB2-12) protrude to the adjacent C-terminal domain and exhibit antiparallel interactions (Fig. 1A). In addition there are several antiparallel interactions in the 11th and 12th coils in both domains (indicated as AP in Fig. 2B). “Aromatic stack” and “asparagine ladder” are commonly found features in β-helical proteins, in which a number of aromatic (tyrosine and phenylalanine) or asparagine residues are located at equivalent positions in coils (38). Both of these features are found in the N domain of Lam55A but not in the C domain. The aromatic stack and asparagine ladder in the N-terminal domain are formed by three phenylalanine residues (Phe-185, Phe-213, and Phe-234), and four asparagine residues (Asn-236, Asn-258, Asn-290, and Asn-318), respectively (Fig. 2C). As shown in Fig. 2, A and B, the topologies of secondary structures in the two β-helix domains are very similar, whereas the pattern of loop insertion into the turn segments is significantly different.
Gluconolactone Complex Structure—Gluconolactone complex structure was determined using a crystal prepared by co-crystallization followed by soaking in the presence of a higher concentration of the ligand (Fig. 3A). The gluconolactone molecule exists at the bottom of a deep depression (substrate binding pocket) between the N and C domains (Fig. 3B). The binding pocket is formed by T1 turns (between PB1 and PB2) in the fourth to seventh coils of the N domain, and T3 turns (between PB3 and PB1) in the sixth to ninth coils of the C domain (Fig. 1A). A curved groove starting from the gluconolactone binding pocket runs around the protrusion formed by T1-4 in the N domain. Several aromatic residues (Phe-684, Trp-245, Phe-199, and Tyr-135) are located along the groove (Fig. 3B).
The gluconolactone molecule forms direct hydrogen bonds with Asn-147, Trp-572, Asp-575, Glu-610, and Glu-633, and water-mediated hydrogen bonds are formed with Gln-146, Gln-176, Ser-204, Gln-225, and Tyr-636 (Fig. 3A). Three acidic amino acid residues, Glu-633, Glu-610, and Asp-575, are completely conserved in GH55. Among them, Glu-633 is appropriately positioned as the catalytic acid, forming a direct hydrogen bond with the O-1 hydroxyl group of gluconolactone. Glu-610 recognizes the O-2 and O-3 hydroxyl groups, and Asp-575 recognizes the O-4 hydroxyl group. A water molecule appears to be appropriately positioned as the nucleophilic water, and the distance to the C-1 carbon atom is 2.7 Å. However, we could not convincingly identify the carboxylic catalytic base around this water molecule. The water is held by side chains of Ser-204 and Gln-176, and the backbone carbonyl group of Gln-146. Ser-204 and Gln-176 are also highly conserved in GH55.
Catalytic Features of Lam55A—The kinetic parameters of Lam55A for laminarioligosaccharides of various DP (L2-L7) were determined to investigate the correlation between the activity of Lam55A and the DP of substrates. As summarized in Table 3, the Km values decreased and kcat values increased with increasing DP of the substrates, resulting in a rapid increase of catalytic efficiency (kcat/Km) for longer substrates. The catalytic efficiency value for L6 and L7 was 1.57 × 104 and 1.11 × 104 times higher than that for L2, suggesting that the enzyme has a long substrate binding cleft with at least five subsites. To investigate the mode of action of Lam55A toward linear β-1,3-glucans, the time course of formation of hydrolysates produced from L7 by the Lam55A was followed with the high performance anion-exchange chromatography system. As shown in Fig. 4, glucose was generated as the major product even at the initial stage of the reaction, indicating that this enzyme is a typical exo-1,3-β-glucosidase (EC 3.2.1.58). L6, L5, and L4 are not accumulated during the reaction, indicating possible multiple attack of the enzyme, i.e. hydrolysis might occur several times on a single β-1,3-glucan chain without dissociation of the enzyme-substrate complex. The accumulation of small amounts of L2 and L3 is due to the low affinity of Lam55A for these oligosaccharides (Table 3).
TABLE 3.
Kinetic parameters for laminarioligosaccharides
| Substrate | Km | kcat | kcat/Km |
|---|---|---|---|
| μm | s−1 | ×105·s−1·m−1 | |
| Laminaribiose | 1960 ± 309 | 3.18 ± 0.20 | 0.0162 |
| Laminaritriose | 284 ± 32 | 30.4 ± 0.5 | 1.07 |
| Laminaritetraose | 30.4 ± 3.4 | 53.4 ± 2.2 | 17.6 |
| Laminaripentaose | 8.01 ± 0.23 | 59.1 ± 0.2 | 73.8 |
| Laminarihexaose | 4.07 ± 0.19 | 73.4 ± 0.6 | 180 |
| Laminariheptaose | 3.28 ± 0.14 | 83.3 ± 0.6 | 254 |
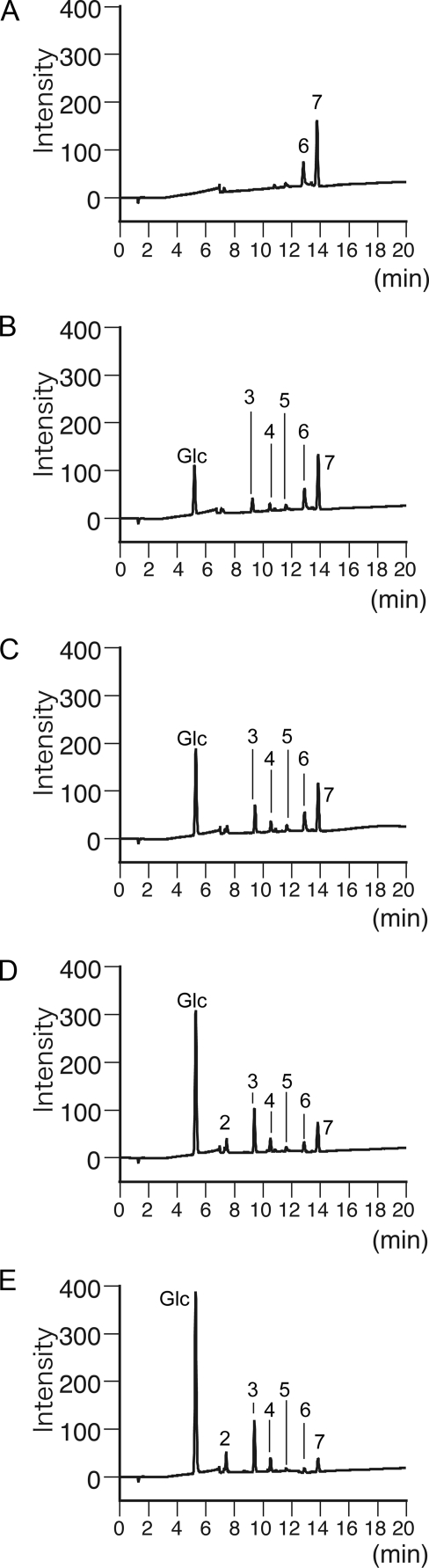
FIGURE 4.
High performance anion-exchange chromatography analysis of hydrolysis products of laminariheptaose (L7). L7 was incubated with Lam55A for 0 min (A), 1 min (B), 2 min (C), 4 min (D), and 8 min (E) in 100 mm sodium acetate buffer, pH 4.5, at 30 °C, and the reaction mixture was separated as described under “Experimental Procedures.” 2, laminaribiose; 3, laminaritriose; 4, laminaritetraose; 5, laminaripentaose; 6, laminarihexaose; 7, laminariheptaose.
The initial products of Lam55A hydrolysis against L3 and LG4 were analyzed with the HPLC system, which can separates the α- and β-anomers of oligosaccharides. As shown in Fig. 5, A and B, Lam55A produced glucose and L2 from L3 within 1 min; the generated glucose consisted only of the α-anomer, whereas both the substrate and generated L2 were mixtures of α and β anomers. This result clearly shows that the enzyme hydrolyzes the β-1,3-glucosidic linkage at the non-reducing end of L3 with net inversion of anomeric carbon. When LG4 was used as the substrate (Fig. 5, C and D), Lam55A produced gentiobiose (β-d-Glcp-(1→6)-d-Glcp) and L2 within 1 min. The generated gentiobiose, which is released from the non-reducing end of the substrate, was also composed of only the α-anomer, and the amount of L2 produced from L3 and LG4 was almost the same level, suggesting similar activities toward each substrate. These results clearly indicate that Lam55A hydrolyzes β-1,3-glucosidic linkages at the non-reducing end independently of substitution at the O-6 position with a single β-d-glucopyranose residue. Fig. 6 shows a schematic representation of the initial hydrolysis by Lam55A of the oligosaccharides examined in this study.
FIGURE 5.
HPLC analysis of hydrolysis products of laminaritriose (L3) and 6-O-glucosyllaminaritriose (LG4). Shown are L3 before (A) and after (B) incubation with Lam55A and LG4 before (C) and after (D) incubation with Lam55A. Each substrate (20 mm) was incubated with Lam55A for 1 min in 100 mm sodium acetate buffer, pH 4.5, at 30 °C, and the reaction mixture was separated as described under “Experimental Procedures.” L2, laminaribiose; Gen, gentiobiose.
FIGURE 6.
Schematic representation of hydrolysis of laminaritriose (A) and 6-O-glucosyl laminaritriose (B) by Lam55A. Lam55A hydrolyzes the glycosidic bond of the glucose residue at the non-reducing end independently of substitution at the O-6 position.
DISCUSSION
In this study we performed a detailed characterization of the enzymatic action of Lam55A. The mode of action of this enzyme was basically similar to those of several exo-β-1,3-glucanases from filamentous fungi characterized in earlier studies (39-41). Lam55A released α-glucose or α-gentiobiose from the non-reducing end of β-1,3-glucans or β-1,3/1,6-glucans (Fig. 5). This feature is notably similar to that of ExgS from Aspergillus saitoi (18). Therefore, our results clearly demonstrate that a GH55 enzyme has an inverting mechanism. In the general mechanism of inverting GHs, two acidic residues separated by about 10 Å serve as general acid and base catalysts (10, 42). The reaction proceeds by proton donation from the catalytic acid to the glycosidic bond oxygen, concurrently with nucleophilic attack by water at the anomeric carbon. A catalytic base is required to activate the nucleophilic water. However, there are several exceptional cases among inverting GHs. For example, the catalytic base of a GH6 enzyme, cellobiohydrolase Cel6A, indirectly interacts with nucleophilic water via another water, and the nucleophilic water is held by a serine residue and a main chain carbonyl oxygen (43, 44). Furthermore, the identity of the catalytic base of GH48 enzymes is still unclear even though the crystal structures of a number of enzyme complexes with substrates have been determined (45-48). In the reaction mechanism proposed for GH95 1,2-α-fucosidase, two asparagine residues are thought to play critical roles in withdrawing a proton from the nucleophilic water, and two neighboring acidic residues (Glu and Asp) are involved in the enhancement of water nucleophilicity (49). In the present study Glu-633 of Lam55A is suggested to be the catalytic acid, and we found a candidate for the nucleophilic water which seems to be positioned appropriately near the C-1 carbon atom of gluconolactone. However, there are no acidic residues that can interact with this water molecule. The nucleophilic water candidate is held by the side chains of Ser-204 and Gln-176 and the main chain carbonyl oxygen of Gln-146. The situation is similar in some respects to the case of the nucleophilic water in Cel6A, but we could not find a carboxylic acid group around the elements holding the nucleophilic water candidate. Although the catalytic mechanism of Lam55A cannot yet be defined due to the uncertainty in the identity of the catalytic base, GH55 enzymes may have a different mechanism from the normal inverting GHs. Alternatively, the orientation of gluconolactone observed here may not mimic the Michaelis complex. There are a number of highly conserved residues around this site, but only three of them (Asp-575, Glu-610, and Glu-633) have the carboxyl side chain (see supplemental Fig. S1.) These residues hold the hydroxyl groups of gluconolactone in the current complex structure, but two of them might act as the general acid and base catalysts in an alternative substrate binding mode.
Fig. 3B shows the molecular surface of Lam55A colored to show residue conservation within GH55 enzymes (the degree of conservation increases in the order of white, yellow, orange, and red), including both exo- and endo-β-1,3-glucanases. The high level of conservation around the gluconolactone binding pocket strongly indicates that this area is the catalytic cleavage site of GH55 enzymes. A long cleft forming an arc from the gluconolactone binding pocket appears to be available to bind a curved β-1,3-glucan chain. There are some additional aromatic residues lining the cleft, and the cleft exhibits a relatively high level of amino acid residue conservation in the region stretching up to Trp-245. Kinetic analysis showed that Lam55A prefers longer β-1,3-glucan chains with DP up to 6∼7 as substrates. Therefore, this long cleft is suggested to be the location of subsites +1 to +5 and perhaps more. In addition, one side of the substrate binding pocket of Lam55A is blocked by a long loop region, which is consistent with the exo mode of hydrolysis of the enzyme demonstrated by HPLC analysis (Fig. 4). This loop region corresponds to T3-6 between PB3-6 and PB1-7 in the C domain (residues 573-593), and the neighboring T3-5 loop region supports the T3-6 loop from the back (Fig. 1A, orange). These loop regions exhibit great sequence variation among GH55 exo- and endo-β-1,3-glucanases. Interestingly, a small pocket, about the size of a single glucose unit, is present beyond the O-6 hydroxyl group of gluconolactone (circled by a green broken line in Fig. 3B). Therefore, the substrate-binding site of Lam55A appears to accept a gentiobiose unit. The putative binding pocket for a single β-1,6-branched glucose unit shows a high degree of conservation, in accordance with the fact that many GH55 exo-β-1,3-glucanases (often designated as exo-β-1,3/1,6-glucanases) purified from filamentous fungi release gentiobiose units from laminarin-containing β-1,6-glucosidic branches (50, 51). To clarify the identity of the catalytic residues and the substrate binding mode of Lam55A, further studies such as construction of mutant enzymes and determination of the structures of the complexes with gentiobiose or longer substrates will be required.
A structural similarity search using the DALI server (52) revealed that both the N and C domains of Lam55A show similarity to those of various carbohydrate-active β-helical enzymes such as GH, polysaccharide lyase (PL), and carbohydrate esterase family enzymes in the CAZy data base. Close structural neighbors (Z scores > 15) include GH28, GH49, GH82, GH90, PL1, PL3, PL6, PL9, PL19 (formerly GH91), carbohydrate esterase 8, endorhamnosidase from Shigella flexneri Phage Sf6 (Sf6 TSP) (53), and A-module of mannuronan C-5-epimerase from Azotobacter vinelandii (AlgE4A) (54). Sf6 TSP has a glycoside hydrolase activity but has not yet been assigned to a CAZy family. The N domain of Lam55A is most similar to that of AlgE4A (PDB code 2PYH chain B; Z score = 25.2, and r.m.s.d. for 249 C-α atoms = 2.3 Å), and GH82, Sf6 TSP, and GH28 follow in this order (Z scores > 20). On the other hand, the C domain is most similar to that of Sf6 TSP (PDB code 2VBE chain A; Z score = 22.0, and r.m.s.d. for 246 C-α atoms = 2.5 Å), and GH28, GH82, and PL19 follow (Z scores > 20). A pairwise structural comparison between the N and C domains of Lam55A revealed that they are strikingly similar (Z score = 24.8, and r.m.s.d. for 253 C-α atoms = 2.2 Å) as compared with the other enzymes. This result provides strong support for the hypothesis that the two tandem β-helical domains of GH55 derive from a gene duplication event (16).
Although the presence of two β-helix folding motifs was suggested from the amino acid sequences of GH55 enzymes (55), the orientation of the two β-helix domains and the active site structure were unknown. The structure we present here reveals that the two β-helix domains (N and C domains) are located side by side and bound tightly via many interactions. Moreover, there is a long linker region between the two domains, having many interactions with both the N and C domains; therefore, the linker region may stabilize the association of the two β-helix domains. Such a role of the linker region of Lam55A is quite different from that of the linker in many other GHs, in which it serves to separate the catalytic domain and carbohydrate binding module and to introduce flexibility between the two domains (55).
Most of the known β-helical enzymes, including polysaccharide-degrading enzymes, have their active sites in the groove parallel to the helical axis within a monomer (37). The long concave surface formed by T3 and PB1 has been considered to be suitable for binding of a long and straight polysaccharide. Recently, two unusual examples, whose active sites are located at the monomer-monomer interface of two identical right-handed parallel β-helical domains, have been reported; they are PL19 inuline fructotransferase from Bacillus sp. snu-7 (BsIFTase) (57) (Fig. 7A) and Sf6 TSP (53). Both are homotrimeric enzymes, and each monomer consists of a β-helical fold similar to those of the Lam55A domains. The structure of Lam55A provides another example of an active site located at the interface of two β-helical domains, but it is formed by two domains in a single polypeptide (Fig. 7B). When the C-α atoms of the N and C domains of Lam55A and two monomers of BsIFTase are superimposed, the active sites of these enzymes almost overlapped (Fig. 7, A and B). BsIFTase catalyzes inverting intramolecular fructosyl transfer to release the terminal difructosaccharide unit from β-2,1-fructans, such as inulin (58). Glu-244 and Asp-233 are suggested to play crucial roles in the catalysis. BsIFTase was once classified into GH91 in the CAZy data base but afterward reclassified to PL19 according to the International Union of Biochemistry and Molecular Biology Enzyme Nomenclature because this enzyme actually catalyzes an elimination reaction (EC 4.2.2.18). As illustrated in Fig. 7B, the possible catalytic residues of Lam55A and BsIFTase do not overlap, indicating that the positions of catalytic residues are not conserved between GH55 and PL19 enzymes. Sf6 TSP is a retaining enzyme, and the two catalytic residues, Asp-399 and Glu-366, are located in different monomers. Although the identity of the catalytic residues of Lam55A is still unclear, the putative catalytic acid residue (Glu-633, colored green in Fig. 3A) and the residues holding the nucleophilic water candidate (colored blue in Fig. 3A) are located in different domains. The possible catalytic residues of Lam55A and Sf6 TSP do not overlap after structural alignment (data not shown).
β-Helix is one of the basic scaffolds for binding polysaccharides, as a β-helix domain called CASH (carbohydrate-binding proteins and sugar hydrolases) is widespread among carbohydrate-interacting proteins (59). To date, five GH families (GH28, -49, -55, -82, and -90), six PL families (PL1, -3, -6, -9, -16, -19), and one carbohydrate esterase family (CE8) are confirmed to have the right-handed parallel β-helix fold, and two of them (GH28 and -49) are further grouped into the clan GH-N. Rigden and Franco (55) found possible evolutional relationships among GH28, -49, -82, and -87. However, they suggested that GH55 is distinct from other β-helical GH families because of the inconsistency of the positions of catalytic residues. Here we provide an example of carbohydrate binding at the interface of two β-helical domains within a single polypeptide, demonstrating an unexpected variety of carbohydrate-binding modes of β-helical proteins.
Supplementary Material
Acknowledgments
We thank the staff of the Photon Factory for the X-ray data collection.
The atomic coordinates and structure factors (codes 3EQN and 3EQO) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
This work was supported by Grant-in-Aid for Scientific Research 17380102 (to M. S.) from the Japanese Ministry of Education, Culture, Sports, and Technology and by a Grant for the Research Collaboration of Assistant Professors (to K. I. and S. F.) from Graduate School of Agricultural and Life Sciences, the University of Tokyo.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: GH, glycoside hydrolase; Lam55A, exo-β-1,3-glucanase from P. chrysosporium; DP, degree of polymerization; L2, laminaribiose; L3, laminaritriose; L4, laminaritetraose; L5, laminaripentaose; L6, laminarihexaose; L7, laminariheptaose; LG4, 6-O-glucosyl-laminaritriose; PL, polysaccharide lyase; Sf6 TSP, endorhamnosidase from S. flexneri Phage Sf6; AlgE4A, A-module of mannuronan C-5-epimerase from A. vinelandii; BsIFTase, inulin fructotransferase from Bacillus sp. snu-7; HPLC, high performance liquid chromatography; MES, 4-morpholineethanesulfonic acid; r.m.s.d., root mean square deviation.
References
- 1.Zevenhuizen, L. P., and Bartnicki-Garcia, S. (1970) J. Gen. Microbiol. 61 183-188 [DOI] [PubMed] [Google Scholar]
- 2.Stone, B. A., and Clarke, A. E. (1992) Chemistry and Biology of (1,3)-β-glucans, pp. 283-364, La Trobe University Press, Bundoora, Australia
- 3.Ruel, K., and Joseleau, J. P. (1991) Appl. Environ. Microbiol. 57 374-384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielecki, S., and Galas, E. (1991) Crit. Rev. Biotechnol. 10 275-304 [DOI] [PubMed] [Google Scholar]
- 5.Pitson, S. M., Seviour, R. J., and McDougall, B. M. (1993) Enzyme Microb. Technol. 15 178-192 [DOI] [PubMed] [Google Scholar]
- 6.Martin, K., McDougall, B. M., McIlroy, S., Chen, J., and Seviour, R. J. (2007) FEMS Microbiol. Rev. 31 168-192 [DOI] [PubMed] [Google Scholar]
- 7.Adams, D. J. (2004) Microbiology 150 2029-2035 [DOI] [PubMed] [Google Scholar]
- 8.Bourne, Y., and Henrissat, B. (2001) Curr. Opin. Struct. Biol. 11 593-600 [DOI] [PubMed] [Google Scholar]
- 9.Henrissat, B., and Bairoch, A. (1996) Biochem. J. 316 695-696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies, G., and Henrissat, B. (1995) Structure 3 853-859 [DOI] [PubMed] [Google Scholar]
- 11.Henrissat, B., and Bairoch, A. (1993) Biochem. J. 293 781-788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henrissat, B. (1991) Biochem. J. 280 309-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantarel, B. L., Coutinho, P. M., Rancurel, C., Bernard, T., Lombard, V., and Henrissat, B. (2008) Nucleic Acids Res. 37 233-238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen-Kupiec, R., Broglie, K. E., Friesem, D., Broglie, R. M., and Chet, I. (1999) Gene (Amst.) 226 147-154 [DOI] [PubMed] [Google Scholar]
- 15.de la Cruz, J., Pintor-Toro, J. A., Benitez, T., Llobell, A., and Romero, L. C. (1995) J. Bacteriol. 177 6937-6945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donzelli, B. G., Lorito, M., Scala, F., and Harman, G. E. (2001) Gene (Amst.) 277 199-208 [DOI] [PubMed] [Google Scholar]
- 17.Giczey, G., Kerenyi, Z., Fulop, L., and Hornok, L. (2001) Appl. Environ. Microbiol. 67 865-871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasahara, S., Nakajima, T., Miyamoto, C., Wada, K., Furuichi, Y., and Ichishima, E. (1992) J. Ferment. Bioeng. 74 238-240 [Google Scholar]
- 19.Nobe, R., Sakakibara, Y., Fukuda, N., Yoshida, N., Ogawa, K., and Suiko, M. (2003) Biosci. Biotechnol. Biochem. 67 1349-1357 [DOI] [PubMed] [Google Scholar]
- 20.Nobe, R., Sakakibara, Y., Ogawa, K., and Suiko, M. (2004) Biosci. Biotechnol. Biochem. 68 2111-2119 [DOI] [PubMed] [Google Scholar]
- 21.Schaeffer, H. J., Leykam, J., and Walton, J. D. (1994) Appl. Environ. Microbiol. 60 594-598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai, R., Igarashi, K., and Samejima, M. (2006) Biotechnol. Lett. 28 365-371 [DOI] [PubMed] [Google Scholar]
- 23.Kawai, R., Igarashi, K., Yoshida, M., Kitaoka, M., and Samejima, M. (2006) Appl. Microbiol. Biotechnol. 71 898-906 [DOI] [PubMed] [Google Scholar]
- 24.Yoshida, M., Ohira, T., Igarashi, K., Nagasawa, H., Aida, K., Hallberg, B. M., Divne, C., Nishino, T., and Samejima, M. (2001) Biosci. Biotechnol. Biochem. 65 2050-2057 [DOI] [PubMed] [Google Scholar]
- 25.Kawai, R., Igarashi, K., Kitaoka, M., Ishii, T., and Samejima, M. (2004) Carbohydr. Res. 339 2851-2857 [DOI] [PubMed] [Google Scholar]
- 26.Honda, Y., and Kitaoka, M. (2004) J. Biol. Chem. 279 55097-55103 [DOI] [PubMed] [Google Scholar]
- 27.Koga, D., Yoshioka, K., and Arakane, Y. (1998) Biosci. Biotechnol. Biochem. 62 1643-1646 [DOI] [PubMed] [Google Scholar]
- 28.Otwinowski, Z., and Minor, W. (1997) Methods Enzymol. 276 307-326 [DOI] [PubMed] [Google Scholar]
- 29.Vonrhein, C., Blanc, E., Roversi, P., and Bricogne, G. (2007) Methods Mol. Biol. 364 215-230 [DOI] [PubMed] [Google Scholar]
- 30.Terwilliger, T. C. (2000) Acta Crystallogr. D Biol. Crystallogr. 56 965-972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrakis, A., Morris, R., and Lamzin, V. S. (1999) Nat. Struct. Biol. 6 458-463 [DOI] [PubMed] [Google Scholar]
- 32.Emsley, P., and Cowtan, K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60 2126-2132 [DOI] [PubMed] [Google Scholar]
- 33.Murshudov, G. N., Vagin, A. A., and Dodson, E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53 240-255 [DOI] [PubMed] [Google Scholar]
- 34.DeLano, W. L. (2002) Curr. Opin. Struct. Biol. 12 14-20 [DOI] [PubMed] [Google Scholar]
- 35.Gouet, P., Courcelle, E., Stuart, D. I., and Metoz, F. (1999) Bioinformatics 15 305-308 [DOI] [PubMed] [Google Scholar]
- 36.Larsson, A. M., Andersson, R., Stahlberg, J., Kenne, L., and Jones, T. A. (2003) Structure 11 1111-1121 [DOI] [PubMed] [Google Scholar]
- 37.Jenkins, J., and Pickersgill, R. (2001) Prog. Biophys. Mol. Biol. 77 111-175 [DOI] [PubMed] [Google Scholar]
- 38.Yoder, M. D., Lietzke, S. E., and Jurnak, F. (1993) Structure 1 241-251 [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto, R., and Nevins, D. (1983) Carbohydr. Res. 122 217-226 [Google Scholar]
- 40.Tsujihara, Y., Hamada, N., and Kobayashi, R. (1981) Agric Biol. Chem. 45 1201-1208 [Google Scholar]
- 41.Pitson, S. M., Seviour, R. J., McDougall, B. M., Woodward, J. R., and Stone, B. A. (1995) Biochem. J. 308 733-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCarter, J. D., and Withers, S. G. (1994) Curr. Opin. Struct. Biol. 4 885-892 [DOI] [PubMed] [Google Scholar]
- 43.Koivula, A., Ruohonen, L., Wohlfahrt, G., Reinikainen, T., Teeri, T. T., Piens, K., Claeyssens, M., Weber, M., Vasella, A., Becker, D., Sinnott, M. L., Zou, J. Y., Kleywegt, G. J., Szardenings, M., Stahlberg, J., and Jones, T. A. (2002) J. Am. Chem. Soc. 124 10015-10024 [DOI] [PubMed] [Google Scholar]
- 44.Varrot, A., Macdonald, J., Stick, R. V., Pell, G., Gilbert, H. J., and Davies, G. J. (2003) Chem. Commun. 946-947 [DOI] [PubMed]
- 45.Guimaraes, B. G., Souchon, H., Lytle, B. L., David Wu, J. H., and Alzari, P. M. (2002) J. Mol. Biol. 320 587-596 [DOI] [PubMed] [Google Scholar]
- 46.Parsiegla, G., Juy, M., Reverbel-Leroy, C., Tardif, C., Belaich, J. P., Driguez, H., and Haser, R. (1998) EMBO J. 17 5551-5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parsiegla, G., Reverbel, C., Tardif, C., Driguez, H., and Haser, R. (2008) J. Mol. Biol. 375 499-510 [DOI] [PubMed] [Google Scholar]
- 48.Parsiegla, G., Reverbel-Leroy, C., Tardif, C., Belaich, J. P., Driguez, H., and Haser, R. (2000) Biochemistry 39 11238-11246 [DOI] [PubMed] [Google Scholar]
- 49.Nagae, M., Tsuchiya, A., Katayama, T., Yamamoto, K., Wakatsuki, S., and Kato, R. (2007) J. Biol. Chem. 282 18497-18509 [DOI] [PubMed] [Google Scholar]
- 50.Ohno, N., Nono, I., and Yadomae, T. (1989) Carbohydr. Res. 194 261-271 [Google Scholar]
- 51.Ohno, N., Hashimoto, Y., and Yadomae, T. (1986) Carbohydr. Res. 158 217-226 [Google Scholar]
- 52.Holm, L., and Sander, C. (1995) Trends Biochem. Sci. 20 478-480 [DOI] [PubMed] [Google Scholar]
- 53.Muller, J. J., Barbirz, S., Heinle, K., Freiberg, A., Seckler, R., and Heinemann, U. (2008) Structure 16 766-775 [DOI] [PubMed] [Google Scholar]
- 54.Rozeboom, H. J., Bjerkan, T. M., Kalk, K. H., Ertesvag, H., Holtan, S., Aachmann, F. L., Valla, S., and Dijkstra, B. W. (2008) J. Biol. Chem. 283 23819-23828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rigden, D. J., and Franco, O. L. (2002) FEBS Lett. 530 225-232 [DOI] [PubMed] [Google Scholar]
- 56.Henrissat, B., and Davies, G. J. (2000) Plant Physiol. 124 1515-1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jung, W. S., Hong, C. K., Lee, S., Kim, C. S., Kim, S. J., Kim, S. I., and Rhee, S. (2007) J. Biol. Chem. 282 8414-8423 [DOI] [PubMed] [Google Scholar]
- 58.Kim, C. S., Hong, C. K., Kim, K. Y., Wang, X. L., Kang, S. I., and Kim, S. I. (2007) J. Microbiol. Biotechnol. 17 37-43 [PubMed] [Google Scholar]
- 59.Ciccarelli, F. D., Copley, R. R., Doerks, T., Russell, R. B., and Bork, P. (2002) Trends Biochem. Sci. 27 59-62 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.