Abstract
Cell transformation by v-Src involves rearrangement of the actin cytoskeleton, disassembly of focal adhesions, and the development of anchorage-independent growth. Here, we report that this is dependent on annexin 2, a v-Src substrate and calcium-dependent regulator of actin dynamics. Using a thermoactivatable mutant of v-Src, we show that at the permissive temperature, annexin 2 becomes phosphorylated and colocalizes with activated v-Src and focal adhesion kinase both at the plasma membrane and in a Rab11-positive compartment of the endosomal pathway. In cells depleted of annexin 2 by small interfering RNA, v-Src becomes activated at the permissive temperature but does not target to the plasma membrane or to perinuclear vesicles, and cell transformation does not occur. Our findings reveal a dual role for annexin 2, first as a regulator of v-Src trafficking and targeting and second as a v-Src effector in the reorganization of actin.
Annexin 2 was first identified as a major substrate of v-Src, the transforming gene product of the Rous sarcoma virus (1-3). It belongs to a large family of highly conserved proteins, members of which are present in all eukaryotic phyla and at least one prokaryote (4, 5). There are 12 annexin genes in humans, and they are implicated in the pathology of numerous diseases, including hematological disorders and cancer (6). The proteins are characterized by their ability to bind and order membrane phospholipids, particularly membranes enriched in cholesterol, with binding being most commonly but not invariably regulated by Ca2+ (7). Some annexins have been shown to interact with actin (8-11), and we recently demonstrated that annexin 2 regulates actin dynamics in vitro and in vivo by inhibiting actin polymerization at the rapidly growing barbed ends of actin filaments (12). Annexin 2 is associated with early endosomes (13), recycling endosomes (14), and phagosomes and enlargeosomes (15) and is critical for the actin-dependent rocketing of macropinosomes (16). In these contexts, annexin 2 has the capacity to bind the negatively charged lipid components of the vesicles while also potentially regulating the actin-based structures that promote vesicle formation, budding, and transport. Spatial and temporal control of annexin 2 activity may occur through binding to phosphatidylinositol 4,5-bisphosphate on the vesicles (17, 18), which is known to regulate the activity of many proteins involved in actin remodeling at these sites (19, 20).
Annexin 2 forms a heterotetrameric complex with S100A10 in vivo, and it was this complex that was first reported to bundle actin filaments in vitro. Phosphorylation of annexin 2 by v-Src was shown to completely inhibit this activity (21), suggesting that v-Src or c-Src could play a role in regulating annexin-actin interactions in vivo. Such a role for annexin 2 would be consistent with some of the downstream consequences of v-Src activation, which include remodeling of the cytoskeleton, extensive cellular ruffling, stress-fiber breakdown, and ultimately, focal adhesion turnover (22, 23). c-Src and v-Src phosphorylate a broad and largely overlapping range of substrates, many of which are implicated in cell motility and cell transformation (24-28) and among which focal adhesion kinase (FAK)2 is a prime target. FAK is a key regulator of focal adhesion dynamics and becomes phosphorylated on a number of tyrosines, some of which have been identified as targets of Src kinases (29-31). Indeed, Src and FAK form a complex that regulates focal adhesion turnover by phosphorylating members of the Rho family of small GTPases, the large scaffold protein paxillin, myosin light chain kinase, and ERK (32).
Recent publications have demonstrated the importance of the phosphorylation of annexin 2 in the epithelial-to-mesenchymal transition induced by v-Src overexpression or treatment with hepatocyte growth factor (33) and in the insulin-mediated transformation of baby hamster kidney cells (BHK-IR cells) (34). In both studies, expression of a pseudo-phosphorylated mutant of annexin 2 was sufficient to partially phenocopy the transformed phenotype. In this study, we used small interfering RNA (siRNA) to deplete fibroblasts of annexin 2 and then examined the effects of activation of a temperature-sensitive mutant of v-Src on cell morphology, F-actin, the localization of activated Src, and the phosphorylation of FAK. Consistent with previous reports, we observed that annexin 2 becomes phosphorylated when v-Src is active (3, 33). We demonstrate that cells lacking annexin 2 fail to undergo the phenotypic changes and anchorage-independent growth that characterize transformation and show that phosphorylation of FAK on tyrosine 576 is defective. We also show that the targeting of activated Src to the plasma membrane and its subsequent internalization and sorting onto Rab11-positive endosomes are compromised. Thus, our data reveal a major role for phosphorylated annexin 2 as a downstream effector of Src, inducing dynamic restructuring of the actin cytoskeleton, and also show that it has an essential upstream activity, regulating Src targeting and subsequent recycling.
EXPERIMENTAL PROCEDURES
Cell Lines and Antibodies—Rat-1 fibroblasts containing the L29A temperature-sensitive, constitutively active mutant of v-Src were a kind gift from Margaret Frame. MIO cells are a spontaneously immortalized human Müller (retinal glial) cell line described elsewhere (12). The anti-annexin 2 monoclonal antibody HH7 was a kind gift from Volker Gerke, and the antibody to total Src was a kind gift from Michael Way. Anti-FAK antibodies came from Santa Cruz Biotechnology, and FAK-pY576, FAK-pY925, Src family-pY416, and paxillin-pY118 came from BIOSOURCE. Alexa Fluor-conjugated phalloidins were obtained from Molecular Probes.
Indirect Immunofluorescence—Cells were fixed in 3.7% paraformaldehyde in phosphate-buffered saline (PBS) for 20 min, washed twice with PBS, and then stained with primary antibodies in PBS containing 0.2% Triton X-100 overnight at 4 °C. Cells were washed three times for 10 min with PBS and then incubated with secondary antibodies and/or Alexa Fluor-conjugated phalloidin (to observe the F-actin cytoskeleton) for 45 min at 37 °C. They were then washed a further three times and mounted in VECTASHIELD. Images were collected on a Leica SP2 AOBS confocal microscope.
Annexin 2 Depletion with siRNA—Annexin 2-specific siRNA oligonucleotides (target sequence AAGTGCCTATGGGTCAA) were obtained from Qiagen. R1LA29 cells were plated on Matek dishes or in 24-well plates in Dulbecco's modified Eagle's medium + 10% fetal calf serum overnight to provide a 40% confluent population. Complexes were made according to the manufacturer's instructions in Opti-MEM. Cells were transfected for 2 days and then treated with a second dose of siRNA complexes for a further 2 days. In most experiments, an siRNA duplex designed against green fluorescent protein (GFP) was used as a control. In the experiments in which the cells were subsequently to be transiently transfected with GFP constructs, an siRNA duplex designed against human annexin 11 (which had been shown not to work in these cells) was used as a control. The efficiency of protein depletion was assessed by Western blotting.
Rescue of Knockdown Phenotype Using hdanx2-GFP—Cells were depleted of annexin 2 for 3 days as described above and then were transiently transfected with either a GFP empty vector (Clontech) or a vector encoding GFP fused to the C terminus of annexin 2 (referred to as hdanx2-GFP) into which three silent mutations had been introduced (12). These variations from the normal sequence render transcripts from the construct resistant to hybridization with the siRNA complexes and are therefore not subject to degradation. Cells were left for a further 2 days in the presence of siRNA to allow endogenous annexin 2 to be robustly depleted in siRNA-transfected cells and to give the introduced hdanx2-GFP construct time to be expressed. After the first 24 h, some cells were placed at 35 °C to activate v-Src. Cells were then fixed and stained for F-actin with Cy5-phalloidin and Src-pY416 (to allow identification of the knockdown phenotype) and were imaged by confocal microscopy. Green cells expressing either hdanx2-GFP or GFP alone were counted and scored as being either transformed or untransformed. At least 300 GFP- or hdanx2-GFP-expressing cells were analyzed on three separate plates in three independent experiments.
Immunoprecipitation, SDS-PAGE, and Western Blotting—1 × 108 R1LA29 cells were washed twice with cold PBS, scraped off the flask with a rubber policeman, and resuspended in lysis buffer (150 mm NaCl, 20 mm Tris-HCl, pH 7.4, 1 mm EGTA, 2% Triton X-100, 1 mm Na3VO4). They were passaged 10 times though a 22-gauge needle and then spun on a microcentrifuge at 13,000 rpm for 10 min. The supernatant was carefully removed so as not to disturb the pellet and was spun again for a further 10 min. 400 μl of supernatant was then incubated for 90 min at 4 °C with 8 μg of the anti-annexin 2 monoclonal antibody or total mouse IgG control (Caltag Laboratories) and 35 μl of 50% Protein G-Sepharose 4B (Sigma). The samples were washed four times with 1.5 ml of lysis buffer and once with 1.5 ml of Tris-buffered saline (150 mm NaCl, 20 mm Tris-HCl, pH 7.4). Following SDS-PAGE and Western blotting, tyrosine-phosphorylated annexin 2 was identified using the pTyr-100 monoclonal antibody (Cell Signaling).
Anchorage-independent Growth—Cells were initially depleted of annexin 2 for 3 days with siRNA or treated with a GFP-specific control siRNA. They were then grown in soft agarose (0.4% agarose matrix in Dulbecco's modified Eagle's medium with 5% fetal bovine serum on a lower layer of 0.6% agarose in Dulbecco's modified Eagle's medium with 5% fetal bovine serum) at 41 or 35 °C for the final 48 h to activate v-Src and to allow colonies to form. Colonies were observed by low power phase microscopy and were classified according to how many cells were present in each clump of cells. The data were calculated from the mean of three plates.
Statistical Methods—Student's t test was carried out to test the significance of all quantified observations. Two-tailed tests were carried out assuming that control and test samples were of equal variance.
RESULTS
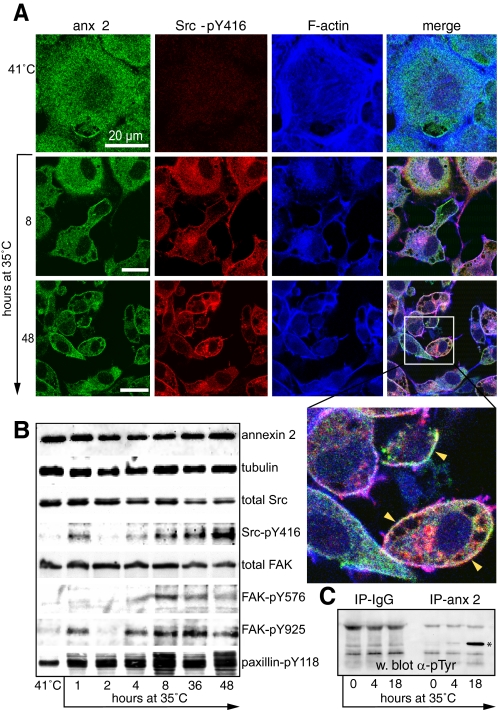
Redistribution of Annexin 2, v-Src, and FAK-pY576 during Transformation—To test the hypothesis that a functional relationship may exist between annexin 2, Src, and FAK, we used the well characterized Rat-1 embryonic fibroblast model of v-Src transformation that expresses the thermoactivatable LA29 mutant of Src (R1LA29 cells). In agreement with other studies, we found that when cells were cultured at 41 °C (the nonpermissive temperature), the F-actin cytoskeleton appeared to be predominantly organized into basal stress fibers, with the cells flattened and apparently contact-inhibited (27). Annexin 2 is distributed throughout the cytoplasm in such cells but is excluded from the nucleus, and there is little detectable staining of Src-pY416 (Fig. 1A). Phosphorylation of Src at tyrosine 416 (Src-pY416) stabilizes the protein in an open conformation (25) and is indicative of its activity. Over a 48-h time course following a switch to the permissive temperature (35 °C), annexin 2 became enriched at the cell cortex, and Src-pY416 was observed at the focal adhesions (not visible in Fig. 1, which shows a confocal section through the middle of the cell, but clear in Fig. 3A) and then also the plasma membrane. Transformation was also accompanied by dramatic rearrangement of the F-actin cytoskeleton and a change in cell shape. Thus, cells switched from a flattened, stress fiber-dominated phenotype to become rounder, elongated, or fusiform with cortical F-actin. Western blot analysis revealed rapid appearance of Src-pY416, FAK-pY925, and paxillin-pY118 at 1 h (Fig. 1B). Phosphorylation of FAK on tyrosine 576 (in the kinase domain activation loop) appeared somewhat later. In some experiments, we observed an apparent biphasic peak of phosphorylation of Src and FAK-pY925 (as shown in these blots), but this was not seen consistently. We did not observe tyrosine phosphorylation of annexin 2 in cells at 41 °C, but after 4 h at 35 °C, it became detectable and was strongly phosphorylated by 18 h (Fig. 1C). These findings are consistent with published reports of annexin 2 tyrosine phosphorylation by the temperature-sensitive NY68 Src mutant (35, 36). Interestingly, the relocalization of annexin 2 to the cortex is evident even after 1 h at the permissive temperature (see example in Fig. 3), suggesting that annexin 2 translocates before it becomes tyrosine-phosphorylated. By 48 h, many of the cells showed marked enrichment of Src-pY416 and annexin 2 in the cortex, in ruffles, on large macropinosomes, and on perinuclear vesicles (Fig. 1A, inset). When we examined R1LA29 cells transiently transfected with a GFP fusion of the temperature-sensitive LA29 Src mutant, we observed migration of v-Src-GFP from what appeared to be focal adhesions (short, linear, and basal structures at the periphery of the cell) to large macropinosomes when they were grown at the permissive temperature (supplemental Fig. 1 and movie). The protein appeared to spool off the focal adhesion onto the nascent macropinosome. Similar results were observed for c-Src-GFP (data not shown).
FIGURE 1.
Annexin 2 and Src-pY416 relocalize during v-Src transformation of R1LA29 cells. A, R1LA29 cells were grown at the nonpermissive temperature (41 °C) and then switched to the permissive temperature (35 °C) for 8 and 48 h. Cells were stained for annexin 2 (anx 2), Src-pY416, and F-actin. The inset on the 48-h merged image shows the colocalization of annexin 2 and Src-pY416 at the plasma membrane (arrowheads). Scale bars, 20 μm. B, cell extracts were prepared from cells grown at 41 and 35 °C for the times indicated. Samples were immunoblotted for annexin 2, tubulin (as a loading control), total Src, total FAK, and also for tyrosine-phosphorylated forms of Src, FAK, and paxillin. Protein bands were visualized by enhanced chemiluminescence. C, annexin 2 is phosphorylated on tyrosine when v-Src is active. Annexin 2 was immunoprecipitated (IP-anx 2) from extracts of cells grown at 41 or 35 °C for 4 or 18 h. The samples were resolved by SDS-PAGE, Western-blotted (w. blot), and probed with antibodies to phosphotyrosine. No phosphorylated annexin 2 was immunoprecipitated by control mouse IgG (IP-IgG), and it was virtually undetectable in cells grown at 41 °C. However, a phosphoannexin 2 band was clearly visible after 4 h at 35 °C, which became prominent by 18 h (asterisk). All blots are representative of three independent experiments.
FIGURE 3.
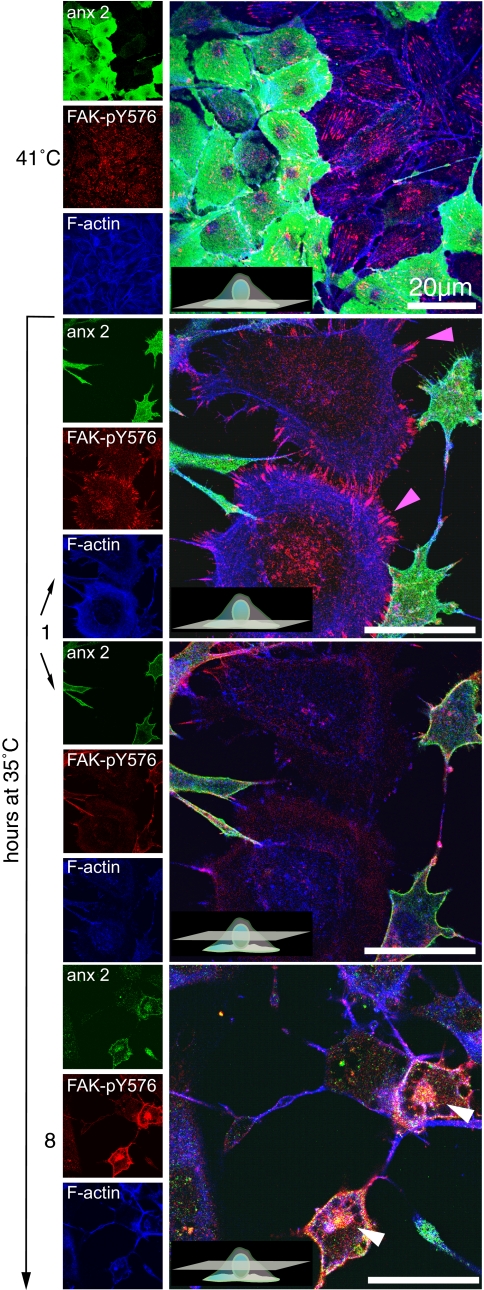
Relocalization of FAK-pY576 in response to v-Src-mediated cell transformation is dependent upon annexin 2. R1LA29 cells were partially depleted of annexin 2 as described in the legend to Fig. 5, fixed, and immunostained for annexin 2 (anx 2), FAK-pY576, and F-actin in both the untransformed and transformed states. Following the switch from restrictive to permissive temperature for 1 h, annexin 2-depleted cells (pink arrowheads) remained large and flat with numerous focal adhesions enriched in FAK-pY576. In contrast, annexin 2-positive cells acquired a transformed phenotype in which annexin 2 relocalized to the cell cortex. By 8 h, there was a dramatic increase in the cellular content of FAK-pY576 in annexin 2-positive cells, in which it relocalized to the annexin 2-rich cortex and to the perinuclear annexin 2-rich and actin-rich vesicles (white arrowheads). Relocalization of FAK-pY576 did not occur in annexin 2-depleted cells. Scale bars, 20 μm.
In agreement with other studies (33, 34), we were able to partially phenocopy cellular transformation by overexpression of an annexin 2Y23E-GFP (phosphomimetic) fusion protein. Cells overexpressing this construct showed varying degrees of actin remodeling, including the restructuring of stress fibers, cellular ruffling, and macropinosome formation (data not shown). Taken together, these observations support the notion that annexin 2, when phosphorylated by v-Src on tyrosine 23, is an important effector of actin remodeling.
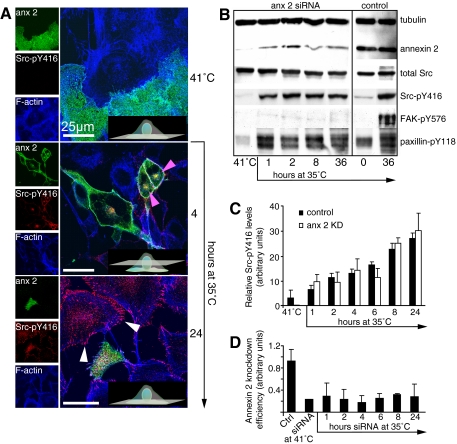
Annexin 2 Is Required for Plasma Membrane Targeting of v-Src—To determine whether annexin 2 is required for the relocalization of temperature-sensitive v-Src, we used siRNA to deplete R1LA29 cells of annexin 2 prior to switching the cells to the permissive temperature. At the restrictive temperature, annexin 2-depleted cells were not morphologically different from wild-type cells, and there was no obvious difference in the staining intensity or localization of active Src, F-actin, or FAK-pY576 (Figs. 2A and 3). At the permissive temperature, however, cells lacking annexin 2 failed to exhibit the relocalization of active Src, FAK-pY576, paxillin-pY118, and actin to the perinuclear endosomal compartment, membrane ruffles, or macropinosomes, and the morphological changes associated with transformation did not occur. The cells remained large and flat with abundant stress fibers. Cells depleted of annexin 2 still contained active Src, as observed on Western blotting (Fig. 2B), but this was almost entirely localized at focal adhesions (see white arrowheads at the 24-h time point in Fig. 2A). Fig. 2 (C and D) shows histograms representing the proportion of Src phosphorylation on tyrosine 416 observed at various time points and the efficiency of annexin 2 knockdown achieved at each time point. There was no statistically significant difference in the kinetics of v-Src phosphorylation (believed to be an auto-catalytic event) between control and annexin 2-depleted cells, suggesting that v-Src activation alone is insufficient to elicit the morphological changes associated with transformation.
FIGURE 2.
Membrane targeting of activated Src requires annexin 2. A, R1LA29 cells were depleted of annexin 2 by siRNA treatment for 4 days at 41 °C. Cultures were then moved to 35 °C for the times indicated prior to fixation and immunostaining for annexin 2 (anx 2), Src-pY416, and F-actin. The top panel shows confocal sections at the base of cells grown at 41 °C, with both annexin 2-positive and annexin 2-negative cells in the field of view. Annexin 2 is present throughout the cytoplasm, the actin cytoskeleton is dominated by stress fibers, and Src-pY416 is undetectable. After 4 h at 35 °C, annexin 2, actin, and Src-pY416 appear at the actin-rich cortex and in a perinuclear vesicular domain (pink arrowheads), whereas there is no appearance of Src-pY416 at these sites and no change in cell shape in annexin 2-depleted cells. After 24 h, Src-pY416 is clearly enriched at the focal adhesions (white arrowheads) in annexin 2-depleted cells, but there is no morphological transformation. Insets show diagrammatic representations of the confocal plane. Scale bars, 25 μm. B, whole cell extracts were prepared from R1LA29 cells following a 4-day culture at 41 °C in the presence of control or annexin 2 siRNA and from cells that had been switched to the permissive temperature for the times indicated and immunoblotted as described in the legend to Fig. 1B. The right panel shows extracts from the control siRNA-treated cells at the restrictive temperature and after 36 h at the permissive temperature. There is an increase in phosphorylation of Src on phospho-Tyr416 and of paxillin on phospho-Tyr118, but phosphorylation on FAK at phospho-Tyr576 is impaired. C, densitometric analysis of Src-pY416 Western blots in control and annexin 2-depleted cells shows that the band intensity, indicative of Src activation, increases in a similar manner under both experimental conditions. KD, knockdown. D, a densitometric analysis shows that annexin 2 levels are maintained at least 50% below the control (Ctrl; when normalized to tubulin) for the duration of the experiment.
Phosphorylation of FAK on tyrosine 576 was visualized by immunostaining both control and annexin 2-depleted cells (Fig. 3). Levels of FAK-pY576 were almost undetectable by Western blot analysis following siRNA treatment for annexin 2 (Fig. 2B), whereas total FAK in the cells remained constant (data not shown). In annexin 2-depleted cells, FAK-pY576 remained tightly associated with the focal adhesions (Fig. 3, pink arrowheads), whereas in annexin 2-positive cells, FAK-pY576 levels increased, and the protein relocated, together with annexin 2, to the cortex and to perinuclear vesicles (white arrowheads). The kinetics of phosphorylation of other potential targets of activated Src such as paxillin-pY118 and FAK-pY925 (data not shown) were not affected by the depletion of annexin 2 (Fig. 2B).
We also investigated annexin 2 involvement in the activation of endogenous c-Src by performing siRNA experiments to deplete annexin 2 in MIO cells, an untransformed, spontaneously motile glial cell line described in previous studies (12). In these cells, Src-pY416 is normally enriched at dynamic ruffles and at the cell cortex, whereas in cells depleted of annexin 2, Src-pY416 was mislocalized to the ends of F-actin stress fibers (supplemental Fig. 2). We showed previously that the abundant F-actin stress fibers in annexin 2-depleted MIO cells are tethered at focal adhesions (12); thus, annexin 2 depletion in both MIO and R1LA29 cells results in the accumulation of active Src at focal adhesions.
To confirm that the resistance of annexin 2-depleted cells to transformation by Src was a direct result of the loss of annexin 2 and not due to some other epiphenomenon, we performed a “reversal of phenotype” experiment in which an siRNA-resistant “hardened” annexin 2-GFP fusion protein (hdanx2-GFP) was expressed in knockdown R1LA29 cells (12). The proportion of cells that appeared to be flat and untransformed (by examination of the actin phenotype, cell shape, and localization of active Src to the plasma membrane) was established at both the permissive and nonpermissive temperatures. As expected, a higher proportion of cells remained morphologically untransformed when annexin 2 was depleted. However, more of these cells subsequently adopted a transformed phenotype when the hdanx2-GFP construct was expressed compared with those transformed with GFP alone, indicating that ectopic overexpression of annexin 2 can indeed compensate for loss of the endogenous protein (supplemental Fig. 3).
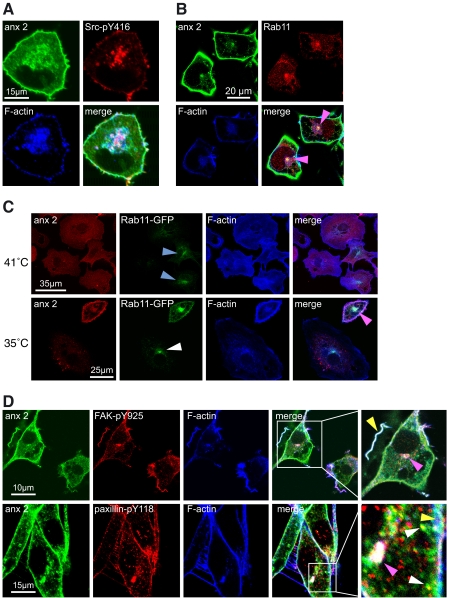
Targeting of Annexin 2 and Src to Rab11-positive Vesicles—In cells in which v-Src is fully active, we observed relocalization of annexin 2, Src-pY416, FAK-pY576, FAK-pY925, paxillin-pY118, PAK1 (data not shown), and F-actin not only to dynamic actin-rich ruffles at the cell cortex but also to cytoplasmic and perinuclear vesicles (Fig. 4, A and D). A subset of the annexin 2-positive vesicles, most notably those at the perinuclear compartment, also labeled positively for the recycling endosomal marker Rab11 (Fig. 4B, pink arrowheads). To confirm this result and to examine the role of annexin 2 in the formation of this endosomal compartment, cells were transiently transfected with Rab11-GFP (Fig. 4C). At the nonpermissive temperature and despite forced expression, Rab11-GFP was very faint in all cells, exhibiting some perinuclear enrichment with vesicles throughout the cytoplasm. In contrast, following a period of growth at the permissive temperature, Rab11-GFP-positive vesicles formed a more prominent perinuclear aggregation, in addition to a marked increase in Rab11-GFP at the plasma membrane. The Rab11-GFP-positive perinuclear compartment also stained for annexin 2 (Fig. 4C), which, taken with the data in Fig. 4A, shows that annexin 2, Src-pY416, and Rab11 all accumulate in this compartment. In cells switched to the permissive temperature and depleted of annexin 2 (Fig. 4C, white arrowhead), the localization of Rab11-GFP resembled that observed in normal cells at the nonpermissive temperature (blue arrowheads). These observations show that although annexin 2 is not required for the targeting of Rab11 to the perinuclear endosomal compartment, annexin 2 is absolutely required for the association of activated v-Src with this compartment.
FIGURE 4.
Annexin 2, actin, and phosphorylated components of the focal adhesion colocalize on Rab11-positive perinuclear vesicles when v-Src is active. A, R1LA29 cells were grown at 35 °C for 8 h and stained for annexin 2 (anx 2), Src-pY416, and F-actin. Annexin 2 and a small amount of Src-pY416 are present at the actin-rich cortex of the cell, and there is an accumulation of annexin 2, Src-pY416, and F-actin on vesicular structures in the perinuclear region of the cell. B, cells were prepared as in A and immunostained for annexin 2, Rab11, and F-actin. The merged image shows intense colocalization of annexin 2 at the perinuclear endosomal compartment (pink arrowheads). C, R1LA29 cells were transfected with Rab11-GFP and immunostained for annexin 2 and F-actin. At 41 °C and in cells expressing annexin 2, Rab11-GFP staining was faint, although mainly targeted to the perinuclear endosomal compartment (blue arrowheads). In cells switched to the permissive temperature and partially depleted of annexin 2, the localization and intensity of Rab11-GFP appeared similar to those observed in control cells at 41 °C (white arrowhead). However, as in B, control cells exhibited strong enrichment of annexin 2 and Rab11-GFP at the perinuclear endosomal compartment (pink arrowhead). D, in cells prepared as in A, annexin 2 colocalizes with FAK-pY925 and paxillin-pY118 at the cell cortex, on ruffling membranes (yellow arrowheads), on vesicles in the cytoplasm (white arrowheads), and in the perinuclear recycling compartment (pink arrowheads). Scale bars, 10 and 15 μm, as indicated.
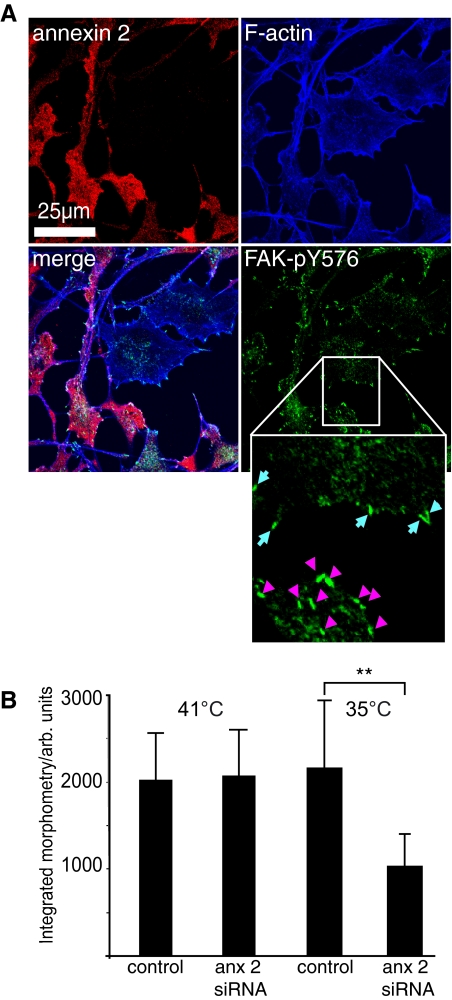
Remodeling of Focal Adhesions and Cell Transformation—Although the relative importance of phosphorylation on the various tyrosines in FAK is not fully understood, the current consensus is that phosphorylation of Tyr576 and Tyr577, which are in the kinase activation loop, is indicative of enhanced kinase activity (31). We therefore hypothesized that if annexin 2 has an essential role in Src targeting and activation, then phosphorylation of FAK, remodeling of focal adhesions, and cell transformation would be defective in cells lacking annexin 2. To address these questions, we performed a semiquantitative analysis in which R1LA29 cells were depleted of annexin 2 with siRNA, and the fluorescence intensity of FAK-pY576 staining was determined at individual focal adhesions or invadopodia (actin-rich structures analogous to focal adhesions that form in the transformed cells) (Fig. 5A). Although there was no difference in the integrated fluorescence signal from untransformed control and knockdown cells at 41 °C, at the permissive temperature, there was ∼50% less FAK-pY576 staining at the focal adhesions of annexin 2-depleted cells compared with the invadopodia of the morphologically transformed annexin 2-positive cells (Fig. 5B). The quantitative reduction in immunostaining of FAK-pY576 in the annexin 2-depleted cells is consistent with the results in Figs. 2B and 3 and reinforces the idea that annexin 2 is required for the tyrosine phosphorylation of FAK on tyrosine 576 by activated v-Src, a key step promoting the dynamic remodeling of both the actin cytoskeleton and focal adhesions that are necessary for cell transformation.
FIGURE 5.
Phosphorylation of FAK on tyrosine 576 is increased in v-Src-transformed cells in an annexin 2-dependent manner. A, R1LA29 cells were partially depleted of annexin 2 using siRNA, and v-Src transformation was induced by growing the cells at the permissive temperature for 24 h. Cells were then fixed and stained for annexin 2, F-actin, and FAK-pY576. FAK-pY576 fluorescence levels in focal adhesions were measured in annexin 2-positive and annexin 2-depleted cells on the same plate at 41 °C and also in the invadopodia of annexin 2-positive transformed cells and focal adhesions of untransformed annexin 2-depleted cells at 35 °C. The inset shows focal adhesions in untransformed (annexin 2-depleted) cells (blue arrows) and invadopodia (pink arrowheads). Scale bar, 25 μm. B, at 41 °C, there was no statistical difference between the amount of FAK-pY576 in annexin 2-positive and annexin 2-depleted cells, whereas at 35 °C, there was more FAK-pY576 present in the invadopodia of transformed cells than in the focal adhesions of annexin 2-depleted (anx 2) untransformed cells. Data represent the total integrated signal intensity per focal adhesion/invadopodium and are the mean of three independent experiments. **, p < 1 × 10-22. arb. units, arbitrary units.
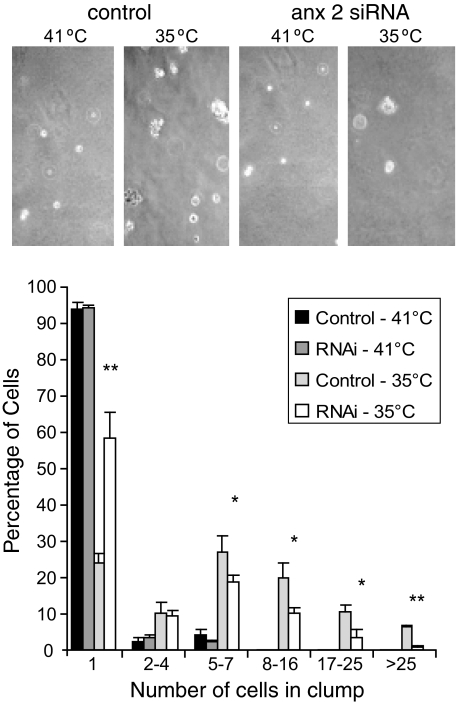
Finally, to examine whether the changes in Src signaling and cell morphology observed upon siRNA-mediated annexin 2 depletion have consequences for anchorage-independent growth, we performed an assay in which proliferation of both control and annexin 2-depleted R1LA29 cells was examined at the nonpermissive and permissive temperatures in soft agar. At 41 °C, as expected, ∼95% of both control and annexin 2-depleted cells remained as single cells, indicating that the cells were unable to proliferate when Src was inactive. However, at 35 °C, when Src is active, only 25% of control cells failed to divide, whereas nearly 60% of cells in the annexin 2-depleted culture remained as single undivided cells (Fig. 6). These findings confirm the supposition implied by the morphological consequences of annexin 2 depletion, namely that annexin 2 is necessary for v-Src-mediated cell transformation.
FIGURE 6.
Transformation by v-Src requires annexin 2. Following exposure to either control or annexin 2 (anx 2) siRNA, R1LA29 cells were cultured in soft agar at both restrictive and permissive temperatures. The histogram shows the range of clump sizes according to the number of constituent cells. *, p = 0.023 (5-7 cells), 0.032 (8-16 cells), and 0.017 (17-25 cells); **, p = 0.0025 (1 cell) and 0.0003 (>25 cells). Error bars represent the S.E. of three different plates. RNAi, RNA interference.
DISCUSSION
In this study, we have used a cell line expressing a temperature-sensitive mutant of v-Src to investigate the role of annexin 2 in v-Src-mediated cell transformation. Although in vitro experiments have shown that tyrosine phosphorylation of annexin 2 by Src leads to reduced affinity for phospholipid bilayers (37) and impaired actin-bundling activity (21), the effects of tyrosine phosphorylation on annexin 2 in vivo are less clear. Here, our data suggest that v-Src activation stimulates annexin 2-dependent remodeling of the actin cytoskeleton. This is consistent with our previous studies in human Müller cells in which we showed that annexin 2 is involved in the remodeling of actin filaments by barbed end capping (12) and with recent reports that tyrosine phosphorylation of annexin 2 is sufficient to induce the changes in actin dynamics that accompany transformation and epithelial-to-mesenchymal transition (33, 34).
Interestingly, in the study of de Graauw et al. (33), pseudo-phosphorylated annexin 2 also induced hepatocyte growth factor-independent branching and restructuring of Madin-Darby canine kidney cells grown as cysts in a three-dimensional collagen matrix. When cells were depleted of annexin 2 in this system, the cysts failed to form a proper lumen, underlining the importance of annexin 2 in maintaining cellular junctions and polarity, a process known to be dependent upon c-Src (38-40). In their study, annexin 2 was shown to exert its effects on actin dynamics by regulating ROCK- and LIM kinase-dependent phosphorylation of cofilin on serine 3 (33), which is an established step in v-Src transformation of fibroblasts (41). A further possibility is that phosphorylation of annexin 2 brings about actin remodeling as a direct consequence of its association with actin, without the necessity of signaling through ROCK. This suggestion is not ruled out by published observations because the molecular link between annexin 2 and the ROCK/LIM kinase/cofilin pathway is not fully understood. Indeed, it is possible that both direct and indirect mechanisms could exist.
Unlike other members of the Src family that are both myristoylated and palmitoylated and therefore constitutively associated with membranes, c-Src itself is only myristoylated. Its membrane binding is therefore partially dependent upon a number of protein-protein interactions mediated by three “anchorage domains” (42). Using the LA31 thermoactivatable v-Src protein, it was shown that membrane recruitment of v-Src is synchronous with its thermoactivation. Membrane association is weak, dependent upon cholesterol, and represents incorporation into “lipid rafts” (in this case enriched in GM1 ganglioside but lacking flotillin-2 or caveolin) as well as nonraft components on endosomes (43). Differential localization of v-Src was shown to have consequences for its downstream signaling. Cholesterol depletion impaired Src recruitment to the plasma membrane and its subsequent activation of phosphatidylinositol 3-kinase/Akt while preserving its association with internal endosomes and mitogen-activated protein kinase (MAPK)-ERK activation. The kinetics of activation of these pathways suggest that localization to and signaling from the endosomal compartment precedes that at the plasma membrane.
The colocalization we observed between annexin 2 and active Src, FAK-Y576, and paxillin-Y118 on internal membranes during transformation is consistent with the idea that trafficking and targeting of v-Src to the plasma membrane are coupled to the retrieval and recycling of v-Src and other focal adhesion components via macropinosomes or endosomes. This is significant in light of previous studies showing that the cellular localization and organization of recycling endosomes are dependent upon annexin 2 (44). These observations show that annexin 2 is required for the intracellular targeting of activated Src to the appropriate vesicular compartments and plasma membrane. When the failure of v-Src to accumulate on the perinuclear Rab11-positive endosomal recycling compartment in annexin 2-depleted cells is also taken into account, these results suggest that without annexin 2, there is neither endosomal traffic of v-Src to nor macropinocytic/endocytic cycling from the plasma membrane. This result thus identifies these perinuclear vesicular structures as the previously reported endosomal recycling compartment through which Src has been shown to traffic en route to the plasma membrane (45). Trafficking through this compartment was shown to be essential for full activation of v-Src and was blocked in cells expressing a dominant-negative mutant of Rab11. Instead, activated Src remains on the focal adhesions, but even at this site, it fails to phosphorylate FAK on tyrosine 576, and so there is no turnover of the focal adhesion, and cell transformation fails to occur.
Given that annexin 2 is essential for the formation of actin tails that propel endosomes and macropinosomes from the plasma membrane to the interior of the cell (16, 18, 46), we propose that annexin 2 forms part of the actin regulatory machinery that regulates the endosomal trafficking and activation of Src. In a recent study, tyrosine phosphorylation of annexin 2 was shown to promote its association with endosomal membranes (47). Thus, v-Src activation and its subsequent phosphorylation of annexin 2 could be the trigger to promote association of both proteins with membranes and to initiate actin-dependent dynamic cycling through the endosomal pathway.
In conclusion, our observations identify a functional link between v-Src and annexin 2 in cell transformation, a connection that was first hinted at more than 20 years ago when annexin 2 was discovered and cloned as a major cellular substrate of v-Src (3). Our data suggest a role for annexin 2 not only in the trafficking of Src to the plasma membrane that is essential for its activation but also in the stimulation of actin dynamics and the remodeling of focal adhesions that are required for transformation. Given the correlation between altered patterns of expression, phosphorylation, and localization of Src and annexin 2 in many high-grade metastatic cancers (6, 24-28, 48-52), an improved understanding of the cross-talk between these ubiquitous proteins is imperative.
Supplementary Material
Acknowledgments
We thank Margaret Frame for providing the R1LA29 cell line, Volker Gerke and Ursula Rescher for anti-annexin 2 antibodies, Miguel Seabra for the Rab11-GFP construct, and Mark Evans for technical assistance.
This work was supported by Wellcome Trust Program Grant GR072694MF.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1-3 and a movie.
Author's Choice—Final version full access.
Footnotes
The abbreviations used are: FAK, focal adhesion kinase; ERK, extracellular signal-regulated kinase; siRNA, small interfering RNA; GFP, green fluorescent protein; PBS, phosphate-buffered saline.
References
- 1.Glenney, J. R., Jr. (1985) FEBS Lett. 192 79-82 [DOI] [PubMed] [Google Scholar]
- 2.Glenney, J. R., Jr., and Tack, B. F. (1985) Proc. Natl. Acad. Sci. U. S. A. 82 7884-7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saris, C. J., Tack, B. F., Kristensen, T., Glenney, J. R., Jr., and Hunter, T. (1986) Cell 46 201-212 [DOI] [PubMed] [Google Scholar]
- 4.Gerke, V., and Moss, S. E. (2002) Physiol. Rev. 82 331-371 [DOI] [PubMed] [Google Scholar]
- 5.Gerke, V., Creutz, C. E., and Moss, S. E. (2005) Nat. Rev. Mol. Cell Biol. 6 449-461 [DOI] [PubMed] [Google Scholar]
- 6.Hayes, M. J., and Moss, S. E. (2004) Biochem. Biophys. Res. Commun. 322 1166-1170 [DOI] [PubMed] [Google Scholar]
- 7.Babiychuk, E. B., and Draeger, A. (2000) J. Cell Biol. 150 1113-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glenney, J. (1986) Proc. Natl. Acad. Sci. U. S. A. 83 4258-4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin, F., Derancourt, J., Capony, J. P., Watrin, A., and Cavadore, J. C. (1988) Biochem. J. 251 777-859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filipenko, N. R., and Waisman, D. M. (2001) J. Biol. Chem. 276 5310-5316 [DOI] [PubMed] [Google Scholar]
- 11.Hayes, M. J., Rescher, U., Gerke, V., and Moss, S. E. (2004) Traffic 5 571-576 [DOI] [PubMed] [Google Scholar]
- 12.Hayes, M. J., Shao, D., Bailly, M., and Moss, S. E. (2006) EMBO J. 25 1816-1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emans, N., Gorvel, J. P., Walter, C., Gerke, V., Kellner, R., Griffiths, G., and Gruenberg, J. (1993) J. Cell Biol. 120 1357-1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trischler, M., Stoorvogel, W., and Ullrich, O. (1999) J. Cell Sci. 112 4773-4783 [DOI] [PubMed] [Google Scholar]
- 15.Lorusso, A., Covino, C., Priori, G., Bachi, A., Meldolesi, J., and Chieregatti, E. (2006) EMBO J. 29 5443-5456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merrifield, C. J., Rescher, U., Almers, W., Proust, J., Gerke, V., Sechi, A. S., and Moss, S. E. (2001) Curr. Biol. 11 1136-1141 [DOI] [PubMed] [Google Scholar]
- 17.Hayes, M. J., Merrifield, C. J., Shao, D., Ayala-Sanmartin, J., Schorey, C. D., Levine, T. P., Proust, J., Curran, J., Bailly, M., and Moss, S. E. (2004) J. Biol. Chem. 279 14157-14164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes, M. J., Shao, D. M., Grieve, A., Levine, T., Bailly, M., Moss, S. E. (2008) Biochim. Biophys. Acta, in press [DOI] [PMC free article] [PubMed]
- 19.Araki, N., Egami, Y., Watanabe, Y., and Hatae, T. (2007) Exp. Cell Res. 313 1496-1507 [DOI] [PubMed] [Google Scholar]
- 20.Scott, C. C., Dobson, W., Botelho, R. J., Coady-Osberg, N., Chavrier, P., Knecht, D. A., Heath, C., Stahl, P., and Grinstein, S. (2005) J. Cell Biol. 169 139-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubaishy, I., Jones, P. G., Bjorge, J., Bellagamba, C., Fitzpatrick, S., Fujita, D. J., and Waisman, D. (1995) Biochemistry 34 14527-14534 [DOI] [PubMed] [Google Scholar]
- 22.Boschek, C. B., Jockusc, B. M., Friis, R. R., Back, R., Grundman, E., and Bauer, H. (1981) Cell 24 175-184 [DOI] [PubMed] [Google Scholar]
- 23.Meijne, A. M. L., Ruuls-Van Stalle, L., Feltkamp, C. A., McCarthy, J. B., and Roos, E. (1997) Exp. Cell Res. 234 477-485 [DOI] [PubMed] [Google Scholar]
- 24.Frame, M. C., and Brunton, V. G. (2002) Curr. Opin. Genet. Dev. 12 36-43 [DOI] [PubMed] [Google Scholar]
- 25.Frame, M. C. (2002) Biochim. Biophys. Acta 1602 114-130 [DOI] [PubMed] [Google Scholar]
- 26.Carragher, N. O., and Frame, M. C. (2002) Int. J. Biochem. Cell Biol. 34 1539-1543 [DOI] [PubMed] [Google Scholar]
- 27.Frame, M. C., Fincham, V. J., Carragher, N. O., and Wyke, J. A. (2002) Nat. Rev. Mol. Cell Biol. 3 233-245 [DOI] [PubMed] [Google Scholar]
- 28.Timpson, P., Jones, G. E., Frame, M. C., and Brunton, V. G. (2001) Curr. Biol. 11 1836-1846 [DOI] [PubMed] [Google Scholar]
- 29.Calalb, M. B., Polte, T. R., and Hanks, S. K. (1995) Mol. Cell. Biol. 15 954-963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calalb, M. B., Zhang, X., Polte, T. R., and Hanks, S. K. (1996) Biochem. Biophys. Res. Commun. 228 662-668 [DOI] [PubMed] [Google Scholar]
- 31.Mitra, S. K., and Schlaepfer, D. D. (2006) Curr. Opin. Cell Biol. 18 516-523 [DOI] [PubMed] [Google Scholar]
- 32.Webb, D. J., Donais, K., Whitmore, L. A., Thomas, S. M., Turner, C. E., Parsons, J. T., and Horwitz, A. F. (2004) Nat. Cell Biol. 6 154-161 [DOI] [PubMed] [Google Scholar]
- 33.de Graauw, M., Tijdens, I., Smeets, M. B., Hensbergen, P. J., Deelder, A. M., and van de Water, B. (2008) Mol. Cell. Biol. 28 1029-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rescher, U., Ludwig, C., Konietzko, V., Kharitonenkov, A., and Gerke, V. (2008) J. Cell Sci. 121 2177-2185 [DOI] [PubMed] [Google Scholar]
- 35.Garber, E. A., Krueger, J. G., Hanafusa, H., and Goldberg, A. R. (1983) Virology 126 73-86 [DOI] [PubMed] [Google Scholar]
- 36.Yamaoka, K., Imajoh-Ohmi, S., Fukuda, H., Akita, Y., Kurosawa, K., Yamamoto, Y., and Sanai, Y. (2006) Biochem. Biophys. Res. Commun. 345 1240-1246 [DOI] [PubMed] [Google Scholar]
- 37.Powell, M. A., and Glenney, J. R. (1987) Biochem. J. 247 321-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada, A., Irie, K., Hirota, T., Ooshio, T., Fukuhara, A., and Takai, Y. (2005) J. Biol. Chem. 280 6016-6027 [DOI] [PubMed] [Google Scholar]
- 39.Yamada, A., Fujita, N., Sato, T., Okamoto, R., Ooshio, T., Hirota, T., Morimoto, K., Irie, K., and Takai, Y. (2006) Oncogene 25 5085-5102 [DOI] [PubMed] [Google Scholar]
- 40.Martin-Belmonte, F., Gassama, A., Datta, A., Yu, W., Rescher, U., Gerke, V., and Mostov, K. (2007) Cell 128 383-397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pawlak, G., and Helfman, D. M. (2002) J. Biol. Chem. 277 26927-26933 [DOI] [PubMed] [Google Scholar]
- 42.Kaplan, K. B., Varmus, H. E., and Bishop, J. M. (1990) Mol. Cell. Biol. 10 1000-1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Diesbach, P., Medts, T., Carpentier, S., D'Auria, L., van der Smissen, P., Platek, A., Mettlen, M., Caplanusi, A., van den Hove, M. F., Tyteca, D., and Courtoy, P. J. (2008) Exp. Cell Res. 314 1465-1479 [DOI] [PubMed] [Google Scholar]
- 44.Zobiack, N., Rescher, U., Ludwig, C., Zeuschner, D., and Gerke, V. (2003) Mol. Biol. Cell 14 4896-4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandilands, E., Cans, C., Fincham, V. J., Brunton, V. G., Mellor, H., Prendergast, G. C., Norman, J. C., Superti-Furga, G., and Frame, M. C. (2004) Dev. Cell 7 855-869 [DOI] [PubMed] [Google Scholar]
- 46.Merrifield, C. J., Moss, S. E., Ballestrem, C., Imhof, B. A., Giese, G., Wunderlich, I., and Almers, W. (1999) Nat. Cell Biol. 1 72-74 [DOI] [PubMed] [Google Scholar]
- 47.Morel, E., and Gruenberg, J. (2009) J. Biol. Chem. 284 1604-1611 [DOI] [PubMed] [Google Scholar]
- 48.Mohammad, H. S., Kurokohchi, K., Yoneyama, H., Tokuda, M., Morishita, A., Jian, G., Shi, L., Murota, M., Tani, J., Kato, K., Miyoshi, H., Deguchi, A., Himoto, T., Usuki, H., Wakabayashi, H., Izuishi, K., Suzuki, Y., Iwama, H., Deguchi, K., Uchida, N., Sabet, E. A., Arafa, U. A., Hassan, A. T., El-Sayed, A. A., and Masaki, T. (2008) Int. J. Oncol. 33 1157-1163 [PubMed] [Google Scholar]
- 49.Yao, H., Zhang, Z., Xiao, Z., Chen, Y., Li, C., Zhang, P., Li, M., Liu, Y., Guan, Y., Yu, Y., and Chen, Z. (2008) Lung Cancer, in press [DOI] [PubMed]
- 50.Mussunoor, S., and Murray, G. I. (2008) J. Pathol. 216 131-140 [DOI] [PubMed] [Google Scholar]
- 51.Fizazi, K. (2007) Ann. Oncol. 18 1765-1773 [DOI] [PubMed] [Google Scholar]
- 52.Hilbig, A. (2008) Recent Res. Cancer Res. 177 179-185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.