Abstract
During larval development in Drosophila melanogaster, transcriptional activation of target genes by sterol regulatory element-binding protein (dSREBP) is essential for survival. In all cases studied to date, activation of SREBPs requires sequential proteolysis of the membrane-bound precursor by site-1 protease (S1P) and site-2 protease (S2P). Cleavage by S2P, within the first membrane-spanning helix of SREBP, releases the transcription factor. In contrast to flies lacking dSREBP, flies lacking dS2P are viable. The Drosophila effector caspase Drice cleaves dSREBP, and cleavage requires an Asp residue at position 386, in the cytoplasmic juxtamembrane stalk. The initiator caspase Dronc does not cleave dSREBP, but animals lacking dS2P require both drice and dronc to complete development. They do not require Dcp1, although this effector caspase also can cleave dSREBP in vitro. Cleavage of dSREBP by Drice releases the amino-terminal transcription factor domain of dSREBP to travel to the nucleus where it mediates the increased transcription of target genes needed for lipid synthesis and uptake. Drice-dependent activation of dSREBP explains why flies lacking dS2P are viable, and flies lacking dSREBP itself are not.
Genetic systems offer powerful tools for understanding the role of proteolysis in normal physiology. Studies of mutants lacking one or more proteases can reveal physiologically relevant details not accessible from biochemical approaches alone. One proteolytic signaling pathway that has yielded to the combination of genetics and biochemistry is the sterol regulatory element-binding protein (SREBP)3 pathway that plays a central role in the regulation of lipid metabolism (1). SREBPs are membrane-bound transcription factors found in all animals, from placozoans and cnidarians (2, 3) to mammals (4). Two membrane-spanning helices anchor the ∼120-kDa precursor form to the membranes of the endoplasmic reticulum (ER). Its large amino- and carboxyl-terminal domains reside in the cytoplasm, whereas a short loop projects into the ER lumen. In the ER membrane, SREBP forms a complex with Scap, a polytopic membrane protein harboring a sterol sensing domain.
When cellular demand for lipid rises, SREBP-Scap complexes exit the ER via COPII-coated vesicles and travel to the Golgi apparatus. Once there, SREBP is cleaved at two sites to release the soluble, active amino-terminal transcription factor domain. The site-1 protease (S1P) cuts within the luminal loop separating the two membrane-spanning helices. The membrane-bound amino-terminal fragment, which harbors the transcription factor domain, is the substrate for the site-2 protease (S2P). S2P cleaves SREBP within the first membrane-spanning helix, between Leu and Cys residues, and the intermediate form of SREBP accumulates only in the absence of cleavage by S2P (5).
Drosophila melanogaster has a single SREBP gene, dSREBP (also called HLH-106 (6)), as well as orthologues of S1P, S2P, and Scap (7). In mammalian cells and in flies, transcriptional up-regulation of the genes of lipid metabolism by SREBP is essential for survival. Mammalian cells lacking S1P, S2P, or Scap cannot activate SREBP and do not survive unless their culture medium is supplemented with free cholesterol and unsaturated fatty acids (1). Similarly, Drosophila lacking dSREBP die at the end of second instar but can be rescued by supplementing their diet with the ultimate end products of dSREBP activation, fatty acids (8). Unlike vertebrates, insects cannot synthesize cholesterol and therefore always have a requirement for sterols in their diet (9, 10).
We have recently shown that, in striking contrast to mammalian cells, for which loss of S2P is lethal, flies lacking dS2P survive rather well. In dS2P mutants, dSREBP continues to be activated. Thus, they exhibit a less severe deficit in the transcription of genes involved in lipid metabolism than larvae lacking dSREBP (8, 11). This explains why they survive and larvae lacking dSREBP do not.
In mammalian cells, SREBP can be cleaved also during apoptosis by caspases-3 and -7 (12, 13). Caspases nearly always cleave following an Asp residue. Caspase cleavage of mammalian SREBP occurs at a cytoplasmic site that lies in the juxtamembrane stalk between the DNA binding domain and the first membrane-spanning helix (14). The transcription factor domain released by caspase cleavage can activate a reporter gene under control of a synthetic SREBP target promoter (15), but the physiological significance of cleavage of SREBPs during apoptosis is unknown.
The genome of D. melanogaster encodes seven caspases as follows: damm (48C5), dcp1 (59E3), decay (89B18), dream (or strica, 42A8), dredd (1B12–13), drice (99C1), and dronc (or Nc, 67D2). Dronc, Dredd, and Dream are thought to be initiator (or apical) caspases that activate other caspases by cleaving them, whereas Damm, Dcp1, Decay, and Drice are effector (or executioner) caspases that cleave various target proteins. Here we combine biochemical and genetic techniques to investigate whether caspases are responsible for the activation of dSREBP in dS2P mutants.
We find that two Drosophila effector caspases, Drice and Dcp1, can cleave dSREBP in vitro and in cultured Drosophila S2 cells undergoing apoptosis. Flies lacking dS2P and drice (but not dcp1) phenocopy loss of dSREBP itself, dying during the second larval instar. Importantly, this synthetic lethality is rescued by supplementing the culture medium with fatty acids, just as seen for dSREBP mutants. The initiator caspase Dronc, which activates Drice, is not able to cleave dSREBP in vitro but is also required by dS2P mutants. Thus Drice can cleave dSREBP and is necessary for its activation in the absence of dS2P. This activation enables the survival of dS2P mutants.
EXPERIMENTAL PROCEDURES
Expression Plasmids—The plasmid Mtal-Grim, and plasmids harboring cDNAs of Dcp1, Drice, Dronc, Damm, Decay, Dream, and Dredd, were gifts from John Abrams (University of Texas Southwestern) and were used as a template to synthesize DNA fragments for RNAi synthesis. A genomic construct of dSREBP tagged at its amino terminus with enhanced YFP (Clontech) and also tagged with the HSV epitope was engineered such that tags are inserted in-frame at the unique AscI restriction site described (8). This construct, designated YFP-dSREBP, was originally designed for germ line transformation. It proved to be more efficiently expressed in S2 cells than versions tagged with HSV alone and was therefore chosen for many of the studies presented here. Site-directed mutagenesis using PCR and/or the QuickChange site-directed mutagenesis kit (Stratagene, Cedar Creek, TX) was employed to construct desired mutations, and the mutagenesis was confirmed by multiple sequencing runs for each strand. Germ line transformation of Drosophila was performed by BESTGENE (Chino Hills, CA).
Cell Culture—Transfection of S2 cells was performed as described (7). For RNAi studies, S2 cells transfected with Mtal-Grim using Cellfectin (Invitrogen) on day 0 were treated with dsRNA on day 1 for 5 h (16).
Apoptotic Induction—Cells were treated with 0.7 mm CuSO4 in IPL41 (Invitrogen) medium for 5 h prior to harvest. SDS-PAGE, and immunoblot analysis was performed as described (7), using anti-HSV antibody (Novagen) at 1:10,000 dilution.
Caspase in Vitro Assays—Recombinant Dcp1 and Drice were the generous gift from Dr. Xiaodong Wang (University of Texas Southwestern). Membrane fractions of HSV and enhanced YFP-tagged dSREBPs were purified from transfected S2 cells as described (7). The assays were performed in caspase reaction buffer (0.15 ml of 25 mm Tris, 25 mm NaCl, 2 mm dithiothreitol, pH 7.4) containing 100 μg (protein) of membrane fractions and 200 nm caspase. The reaction was incubated up to 60 min at 25 °C and was stopped by addition of sample loading buffer and boiling in preparation for SDS-PAGE.
Genetic Strains—All marker mutations and balancer chromosomes are described in and referenced by FlyBase (17). Crosses were maintained at 25 °C in vials containing freshly yeasted cornmeal/molasses/agar (8) except where noted. OreR flies served as wild type. dS2P1 is a deletion encompassing the dS2P locus, and dS2P2 is a transposon insertion within the dS2P open reading frame as described previously (11). dSREBP189 is a deletion extending into the open reading frame of dSREBP isolated in a screen for imprecise excisants of a nearby P element (8). Caspase mutants dcp1Prev1 (18), driceΔ1 (19), and dronc51 (20), were from Kimberly McCall, Bruce Hay, and John Abrams, via the Abrams Laboratory. The P{dSREBPg} and P{dSREBPg(D386A)} transgenes used are inserted on the 2nd chromosome.
dS2P, Caspase Double Mutant Assays—Drosophila S2P and caspase double mutant lines were maintained as heterozygous stocks as follows: dS2P/CyO, twist-GFP; driceΔ1/TM3, Ser, actin-GFP, and dS2P/CyO, twist-GFP; dronc51/TM3, Ser, actin-GFP. All crosses were set up using virgin females homozygous for dS2P and heterozygous for drice or dronc. These animals survived poorly on unsupplemented medium (see below). Thus, to collect a sufficient numbers of females, embryos from the heterozygous stocks were plated onto dishes containing 14:0 + 18:1 medium (semi-defined medium supplemented with 0.075% myristate and 0.15% oleate (w/v)). Third instar larvae homozygous for dS2P and heterozygous for drice or dronc were scored using the twist- and actin-GFP markers and transferred to vials containing regular/cornmeal/molasses agar medium. Adult females were collected as they emerged. Virgin dS2P2/dS2P2; driceΔ1 (or dronc51)/TM3, Ser, actin-GFP females were crossed to dS2P1/CyO, twist-GFP; driceΔ1 (or dronc51)/TM3, Ser, actin-GFP males. On day 0, embryos from an overnight collection were plated at 10 mg of embryos/dish onto duplicate 60-mm dishes containing semi-defined medium with no additions (“No Addition”) and 60-mm dishes containing 14:0 + 18:1 medium. Two days later, larvae from one “No Addition” and one “14:0 + 18:1” plate were separated from the food by floatation on a salt cushion and scored using the twist- and actin-GFP markers. On day 4, this procedure was repeated for the remaining two dishes. To calculate the percent expected, the observed ratio (calculated by dividing the number of larvae for each genotype by the expected total) was divided by the predicted Mendelian ratio. The total number of larvae expected was calculated based on the emergence of the doubly heterozygous mutants, which are expected to comprise 1/3 of the larvae because of embryonic lethality of animals homozygous for the third chromosome balancer. Thus, the expected total is (number of doubly heterozygous larvae/0.33).
Rescue of dSREBP189 Lethality by Genomic dSREBP Transgenes—Independent insertions of P{dSREBPg(D386A)} on the second chromosome were recombined onto both dS2P1 and dS2P2 alleles and then crossed into a dSREBP189 background. Recombinants were genotyped by PCR analysis and sequencing of the P{dSREBPg(D386A)} transgene. Lines homozygous or heterozygous for the second chromosome were generated as follows: dS2P, P{dSREBPg(D386A)}/dS2P, P{dSREBPg(D386A)}; dSREBP189/TM6B, Tb Hu e and dS2P, P{dSREBPg(D386A)}/CyO, twist-GFP; dSREBP189/TM6B, Tb Hu e. Virgin dS2P2, P{dSREBPg(D386A)}/dS2P2, P{dSREBPg-(D386A)}; dSREBP189/TM6B, Tb Hu e females were crossed to dS2P1, P{dSREBPg(D386A)}/CyO, twist-GFP; dSREBP189/TM6B, Tb Hu e males. On day 0, embryos from an overnight collection were seeded into 10 vials (at 1 mg embryos/vial) containing regular/cornmeal/molasses agar (No Addition) and 10 vials containing regular/cornmeal/molasses agar supplemented with 0.075% myristate and 0.15% oleate. Emerging adults were scored using twist-GFP and Hu markers until no further adults emerged (approximately day 19 AEL). The data are presented as percent of the expected calculated as described above.
Statistical Procedures—Data were analyzed using χ2 tests for independence and goodness of fit. The Sequential Bonferroni Correction of Holms was applied to pairwise comparisons.
RESULTS
dSREBP Is Cleaved during Apoptosis in Drosophila S2 Cells—Mammalian SREBPs were among the first experimentally verified substrates for cleavage by caspases (14). Therefore, to identify proteases other than dS1P and dS2P that can cleave dSREBP in dS2P– larvae, we first tested the hypothesis that dSREBP can be cleaved by Drosophila caspases.
To increase caspase activity in Drosophila S2 cells, we induced apoptosis by expression of the apoptotic activator Grim under control of the inducible metallothionine promoter (21). Following addition of copper sulfate (CuSO4), caspase activity in Grim-transfected cells increased dramatically as monitored by cleavage of the fluorescent substrate Ac-DEVD-aminomethylcoumarin (data not shown).
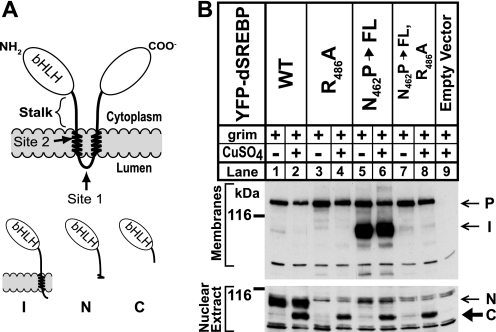
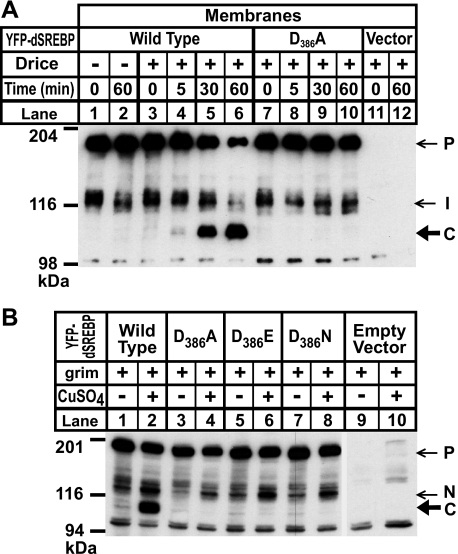
Fig. 1A shows a schematic of SREBP topology and the relationship of the various cleavage fragments. We cotransfected S2 cells with Grim and with tagged versions of dSREBP (YFP-dSREBP), either wild type or harboring specific mutations that block cleavage by dS1P and dS2P (7). The YFP-dSREBP constructs show similar levels of the precursor form (“P”) in the membrane fraction (Fig. 1B, upper panel, lanes 1–8). For dSREBP with wild type sequences at site-1 and site-2 (YFP-dSREBP WT), the nuclear form of dSREBP (“N”) is detected in both the absence and presence of CuSO4 (Fig. 1B, lower panel, lanes 1 and 2). A novel, more rapidly migrating band (hereafter designated “fragment C”) also appears in the nuclear extract of the CuSO4-treated sample (lane 2) but not the untreated one (lane 1).
FIGURE 1.
Cleavage of dSREBP during apoptosis in S2 cells. A, this schematic shows SREBP topology. bHLH designates the transcription factor domain. The gray bar represents the lipid bilayer. Arrows indicate the site-1 protease and site-2 protease cleavage sites. The cytoplasmic juxtamembrane stalk region is indicated by the brace. NH2 and COO– designate the amino and carboxyl termini. The various cleavage products are illustrated below. I, intermediate form; N, normal nuclear form; C, caspase-dependent band. B, cells were cotransfected with the indicated YFP-dSREBP constructs and with Mtal-Grim as described under “Experimental Procedures.” We added 0.7 mm CuSO4 to induce expression of Grim. Cells were fractionated, and the membrane pellet was washed with 0.1 m sodium carbonate prior to immunoblot analysis using an anti-HSV antibody as described under “Experimental Procedures.” P, precursor; I, intermediate form; N, normal nuclear form; C, caspase-dependent band; WT, wild type.
Mutating the crucial Arg at position 486 in site-1 to Ala blocks cleavage by dS1P (7), and no nuclear dSREBP is observed in the absence of CuSO4 (Fig. 1B, lower panel, lane 3). The smaller, novel fragment C appears when apoptosis is induced (Fig. 1B, lower panel, lane 4). Mutating an Asp-Pro motif within the first membrane-spanning helix of dSREBP blocks cleavage by dS2P but leaves cleavage at site-1 unaffected (7). The intermediate form (“I”) of dSREBP is the product of dS1P cleavage and remains attached to the membrane because it retains a single membrane-spanning helix. The intermediate form appears in both the presence and absence of CuSO4 (Fig. 1B, upper panel, lanes 5 and 6). The normal nuclear form is absent from the nuclear extract (Fig. 1B, lower panel, lanes 5 and 6), but fragment C appears when apoptosis is induced (Fig. 1B, lower panel, lane 6). The same pattern holds true when both site-1 and -2 are mutated (Fig. 1B, lower panel, lanes 7 and 8). These results parallel results using versions of dSREBP tagged with the HSV epitope alone (data not shown). Thus, dSREBP can be cleaved when caspase activity is up-regulated in S2 cells, and this cleavage does not require prior cleavage by dS1P or dS2P. Consistent with this finding, caspase cleavage of dSREBP during apoptosis is unaffected by treating S2 cells with palmitate and ethanolamine (data not shown), which suppresses cleavage of dSREBP by dS1P and dS2P (16).
dSREBP Is Cleaved by Drice and Dcp1—To investigate further the role of caspases in the production of dSREBP fragment C, we performed RNAi experiments in Drosophila S2 cells targeting dronc, dredd, decay, drice, dream, damm, and dcp1 and determined whether fragment C still was produced upon induction of apoptosis (supplemental Fig. 1). When apoptosis was induced by expression of Grim, RNAi against Drice blocked the appearance of fragment C (supplemental Fig. 1, lane 7), whereas RNAi against Dcp1 partially reduced the accumulation of fragment C (supplemental Fig. 1, lane 10). We consistently observed the partial effect of RNAi against Dcp1 and the full effect of Drice RNAi. The evident effect of RNAi treatment against Drice and Dcp1 indicates that at least these two effector caspases can mediate cleavage of dSREBP during Grim-induced apoptosis in S2 cells.
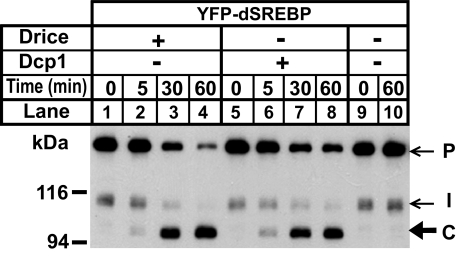
To test whether Drice and Dcp1 can cleave dSREBP directly, we prepared membrane fractions from S2 cells transfected with YFP-dSREBP and incubated these membranes in vitro with purified recombinant Drice or Dcp1 (Fig. 2). At 0 min, no caspase cleavage product is observed with either protease (Fig. 2, lanes 1 and 5). Increasing accumulation of fragment C is observed over time (Fig. 2, lanes 2–4 and 6–8). No product is observed at 60 min in the absence of added protease (Fig. 2, lane 10). These data demonstrate that Drice and Dcp1 can cleave membrane-bound dSREBP in vitro.
FIGURE 2.
Cleavage of dSREBP by Drice and Dcp1 in vitro. Membrane fractions were purified from S2 cells transfected with YFP-dSREBP as described. The membranes were incubated along with the indicated purified recombinant caspase in caspase reaction buffer. The reaction was stopped at the indicated times as described. The membranes were then subject to immunoblot analysis using an anti-HSV antibody. P, precursor; C, caspase-dependent band; I, intermediate form.
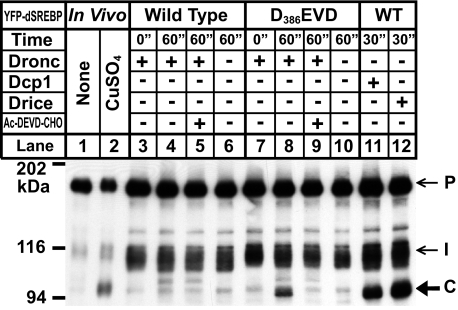
Dronc Does Not Cleave dSREBP—We also tested the ability of the initiator caspase Dronc to cleave dSREBP, using purified recombinant Dronc. These results are shown in Fig. 3. Incubation of membranes containing wild type dSREBP with either Drice or Dcp1 resulted in substantial accumulation of fragment C (Fig. 3, lanes 11 and 12). No accumulation of fragment C was observed with purified Dronc (Fig. 3, lane 4). Dronc can cleave Ac-DEVD-amidotrifluoromethylcoumarin (22, 23). When we introduced that cleavage site motif into the juxtamembrane region of dSREBP (383FTTDAGL was mutated to 383FTTDEVD), incubation with Dronc resulted in accumulation of fragment C (Fig. 3, lane 8), confirming that the enzyme had activity in this assay. With the mutant Dronc-cleavable substrate, accumulation of fragment C was blocked by the addition of the caspase inhibitor Ac-DEVD-CHO (Fig. 3, lane 9). Recombinant Dronc can cleave a version of dSREBP containing a motif that Dronc has been shown to cleave but does not cleave wild type dSREBP. Thus, the effector caspases Drice and Dcp1 cleave dSREBP, but the initiator caspase Dronc does not.
FIGURE 3.
The effector caspase Dronc does not cleave dSREBP. Membrane fractions were prepared from S2 cells transfected with the indicated plasmids and incubated with the indicated enzyme in caspase buffer for the times indicated. Samples shown in lanes 5 and 9 were treated with the caspase inhibitor Ac-DEVD-CHO (25 μm, Cayman Chemicals, Ann Arbor, MI) at the time of enzyme addition. Whole cell lysates from apoptotic S2 cells transfected with YFP-dSREBP and MTAL-grim (lanes 1 and 2) are shown for comparison of cleaved fragments. Samples were subjected to immunoblot analysis using anti-HSV antibody as described under “Experimental Procedures.” P, precursor; C, caspase-dependent band; I, intermediate form; WT, wild type.
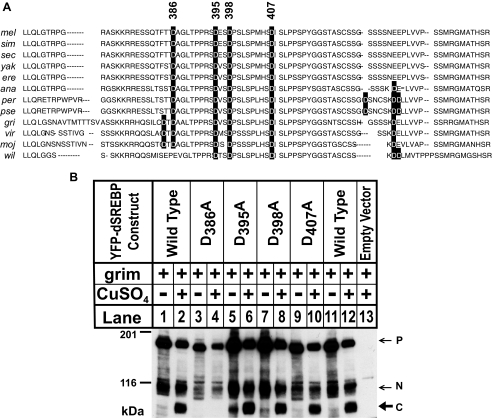
Cleavage of dSREBP by Caspases Requires an Asp Residue at Position 386—As their name indicates (24), caspases have a decided preference for an Asp residue in the P1 position. Drice and Dcp1 have cleavage specificities similar to mammalian caspase 3, with the sequence DEVD reported to be optimal for cleavage by Drice in vitro (25). Fig. 4A shows sequence alignment of the juxtamembrane stalk region (between the transcription factor domain and the first membrane-spanning helix) of SREBPs from each of the 12 SREBP sequences available from the genus Drosophila. There are four Asp residues within this region of dSREBP (Fig. 4A, black boxes), and these four are conserved among all species, save Drosophila willistoni. We note that many other attributes of the genome sequence of this species are also exceptional among the 12 species sequenced (26, 27). A multiple phylum alignment of this region is shown in supplemental Fig. 2.
FIGURE 4.
Caspase cleavage of dSREBP in cultured cells requires Asp-386. A, sequence alignment of SREBP homologues from 12 Drosophila species. In the abbreviated names, “mel” indicates melanogaster (CG8522-PA); sim, simulans (GD14825-PA); sec, sechellia (GM19644-PA), yak, yakuba (GE19622-PA), ere, erecta (GG16056-PA); ana, anassae (GF23590-PA); per, persimilis (GL15732-PA); pse, pseudoobscura (GA21134-PA); gri, grimshawii (GH14653-PA), wil, willistoni (GK17496-PA); vir, virilis (GJ11320-PA); and moj, mojavensis (GI11638-PA). Sequences were aligned using the ClustalW algorithm. Asp residues are shaded black. B, Drosophila S2 cells were transfected with constructs encoding wild type YFP-dSREBP or YFP-dSREBP harboring an Ala in place of Asp residues at, respectively, positions 386, 395, 398, or 407 in the juxtamembrane stalk. Expression of Grim was induced with CuSO4, and whole cell lysates were subjected to immunoblot analysis using anti-HSV antibody. P, precursor; N, normal nuclear form; C, caspase-dependent band.
We individually mutated each of these Asp residues to Ala in the context of YFP-dSREBP, and we assessed the ability of each construct to be cleaved during apoptosis (Fig. 4B). The apoptosis-dependent fragment C appears with wild type dSREBP (Fig. 4B, lanes 2 and 12) and with Asp to Ala mutants at positions 395, 398, and 407 (lanes 6, 8 and 10). No fragment is observed when the Asp at 386 is mutated to Ala (Fig. 4B, lane 4). The dependence of cleavage on an Asp at 386 (but not on any other Asp residue in this region) indicates that cleavage during apoptosis occurs at the sequence 383FTTD↓A387 in dSREBP, where the down arrow indicates the scissile bond. Consistent with this conclusion, in transfected S2 cells, fragment C comigrates with a truncated version of dSREBP that has a stop codon in place of residue 387 (data not shown).
This was somewhat surprising as Drice and Dcp1 are thought to prefer the sequence DXXD as is present at 395. We therefore further tested the requirement of Asp-386 for in vitro cleavage by Drice by incubating membrane fractions from S2 cells transfected with YFP-dSREBP, either wild type or harboring the D386A mutation (Fig. 5A). Accumulation of fragment C proceeded in a time-dependent fashion with the wild type substrate (Fig. 5A, lanes 3–6), but no fragment C was produced from the D386A mutant (lanes 7–10). In S2 cells, cleavage during apoptosis was also abolished when Asp-386 was substituted by Glu or Asn (Fig. 5B, lanes 6 and 8).
FIGURE 5.
Cleavage by Drice in vitro requires an Asp at position 386. A, in vitro cleavage of dSREBP requires Asp-386. Membrane fractions were purified from S2 cells transfected with YFP-dSREBP or YFP-dSREBP(D386A). Membranes were incubated for the indicated times in caspase reaction buffer in the presence or absence of purified, recombinant Drice. The reaction was stopped at the indicated times. The membranes were then subject to immunoblot analysis using an anti-HSV antibody. B, in vivo cleavage of dSREBP requires Asp-386. S2 cells were cotransfected with Mtal-grim and the indicated YFP-dSREBP construct, and the experiment was conducted as described in the legend to Fig. 1. Note that two intervening lanes between lanes 8 and 9 have been omitted for clarity. P, precursor; N, normal nuclear form; C, caspase-dependent band; I, intermediate form.
The supplemental Fig. 3 shows that the threonine residue at position 385 is also required for cleavage (lanes 4 and 6). Even when a serine is introduced at this position, cleavage is abolished (supplemental Fig. 3, lane 4). This Thr residue is as well conserved among Drosophila as Asp-386, whereas residues in the P3 and P4 positions are not highly conserved (Fig. 4A). Consistent with this evolutionarily acceptable variation, we find that a T384A mutant is cleaved as readily as wild type (data not shown).
Drice Is Required for the Survival of dS2P Mutant Larvae—In S2 cells and using purified enzyme in vitro, Drice and Dcp1 can cleave dSREBP, and cleavage is blocked by substituting Asp-386 with Ala. We hypothesized that this cleavage of dSREBP by caspases is responsible for the survival of flies lacking dS2P. To test this, we constructed stocks harboring null mutations both in dS2P as well as in one each of the two caspases that were shown to cleave dSREBP, using the alleles dcp1Prev1 and driceΔ1 (18, 19). If cleavage of dSREBP by one of these caspases were responsible for the survival of dS2P mutants, then flies lacking both that caspase and dS2P would be unable to activate dSREBP. The doubly mutant animals should evince the phenotype observed in flies completely lacking dSREBP (dSREBP189) and die at the end of second larval instar because of deficient transcription of dSREBP target genes. Importantly, if this “synthetic” lethality were indeed because of deficient dSREBP activation, then supplementing the larval diet with free fatty acids should afford substantial rescue of the dS2P, drice double mutants, just as seen for dSREBP mutants (8).
Flies homozygous for dcp1Prev1 are viable (18). We find that the dS2P dcp1Prev1 doubly mutant animals survive as well as dS2P mutants alone (data not shown). Dcp1 is not required by larvae lacking dS2P and is therefore not required to activate dSREBP.
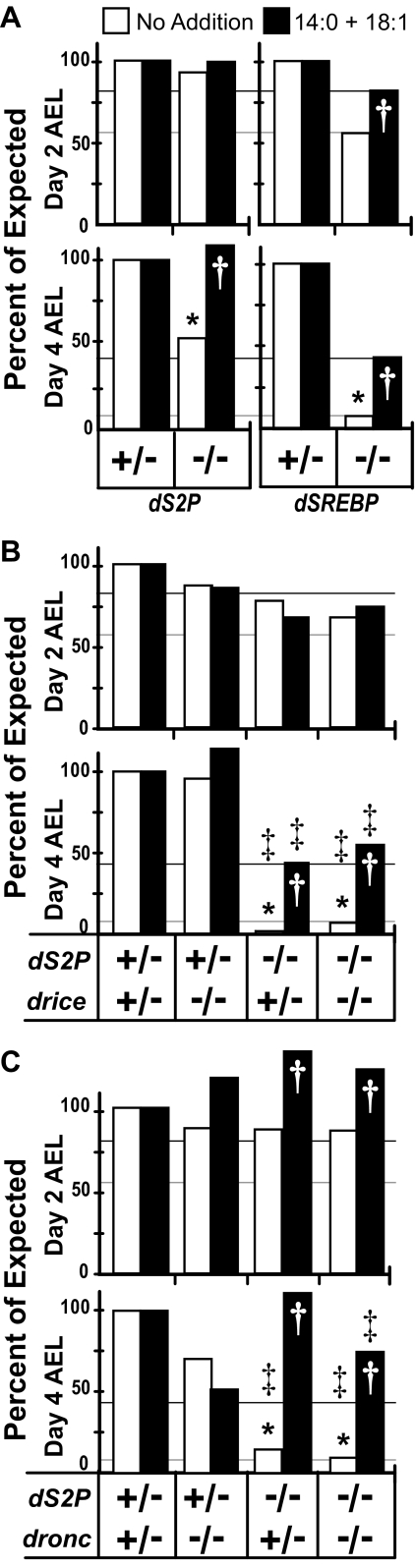
Larvae homozygous for dSREBP189 die at the end of second instar whereas larvae homozygous for driceΔ1 mostly die during pupation (19). This leaves a window of 2–3 days (between the middle of second instar, when larvae die because of insufficient dSREBP activity, and the onset of pupariation, when larvae mostly die if they lack Drice) in which to assess synthetic lethality in the dS2P; drice double mutants. For the experiment shown in Fig. 6, we collected embryos overnight from each of the indicated crosses and plated them onto duplicate, parallel cultures (two plates of unsupplemented medium and two plates of medium supplemented with fatty acids per cross). We genotyped all larvae from each cross from one plate of each medium at day 2 after egg laying (AEL), before dSREBP-dependent lethality, and again at day 4 AEL, after dSREBP-dependent lethality but before pupariation.
FIGURE 6.
Drice and Dronc are essential for the survival of larvae lacking dS2P. A, virgin females homozygous for dS2P2 were crossed to males heterozygous for dS2P1. Parallel cultures were inoculated with 10 mg/plate of embryos on regular or supplemented medium (14:0 + 18:1). Larvae were scored on day 2 and day 4 AEL. A cross of dSREBP189 heterozygotes served as a control for the efficacy of rescue. B, fly lines harboring mutations in both dS2P1 or dS2P2 and in driceΔ1 were constructed as described under “Experimental Procedures.” Virgin females homozygous for dS2P2 and heterozygous for driceΔ1 were crossed to males heterozygous for dS2P1 and driceΔ1. The dS2P homozygous, driceΔ1 heterozygous females were raised on medium supplemented with fatty acids to enable efficient recovery of these flies. Larvae homozygous or heterozygous for driceΔ1 and wild type for dS2P survived equally well under all conditions tested in this experiment. C, crosses of flies doubly mutant for dS2P and dronc51 were conducted as described in B. Horizontal lines corresponding to the value for dSREBP189 larvae under each condition are shown to facilitate comparison (black, supplemented medium; gray, unsupplemented medium). “+” indicates wild type, and “–” indicates null alleles as described above. Note that the values displayed are for whole populations rather than samples. * indicates p < 0.005 for day 2 compared with day 4. † indicates p < 0.005 for supplemented compared with unsupplemented medium. ‡ indicates p < 0.005 for dS2P transheterozygotes compared with dS2P; caspase larvae. A mean of 1057 larvae were scored for each cross and condition (range = 1446 to 751). The results shown are from a single experiment and are representative of three independent replications.
The dSREBP–/– mutants survive extremely poorly on unsupplemented medium, and as reported previously (8), their survival is significantly improved on medium supplemented with fatty acids (Fig. 6A, right panels). By day 4 AEL, the effect of fatty acid supplementation on promoting survival of dSREBP mutants is pronounced (Fig. 6A, lower right). This cross controls for the ability of fatty acid supplementation to rescue larval lethality in the absence of dSREBP.
Flies lacking dS2P are viable and may be maintained as homozygous stocks for hundreds of generations (11). As seen previously, on unsupplemented medium, the dS2P1/2 offspring of dS2P2/2 mothers survive at half the expected frequency (50.1%; Fig. 6A, day 4 AEL, open bars). Survival of dS2P1/2 larvae is fully restored on medium supplemented with fatty acids, indicating that the reduced viability of dS2P mutants is because of deficient activation of dSREBP. These animals are wild type for drice and dronc, and this cross controls for the reduced survival of larvae lacking dS2P but having the diploid complement of those proteases.
To determine whether the dS2P1/2 larvae that survive do so because of the action of Drice (which cleaves dSREBP in the juxtamembrane stalk region), we tested the survival of larvae lacking both dS2P and Drice. Like the dSREBP mutants, the dS2P; drice double mutants exhibit lethality between the second and third instars (Fig. 6B, right). At day 2 AEL on unsupplemented medium, the dS2P1/dS2P2; driceΔ1/driceΔ1 larvae are present in the population at about 2/3 of their expected frequency (Fig. 6B, right open bars) and survive about as well on medium supplemented with fatty acids (Fig. 6B, solid bars). Their survival is similar to dSREBP189 larvae (Fig. 6A, right). By day 4 AEL, on unsupplemented medium, dS2P1/dS2P2; driceΔ1/+ and dS2P1/dS2P2; driceΔ1/driceΔ1 larvae are almost undetectable in the population (Fig. 6B, lower right), whereas dS2P2/+; driceΔ1/driceΔ1 larvae survive about as well as dS2P2/+; driceΔ1/+ larvae (Fig. 6B, lower, left). This contrasts sharply and significantly with the dS2P1/2 mutants harboring two wild type copies of drice (Fig. 6A, day 4 AEL, left).
On medium supplemented with fatty acids, many more dS2P1/dS2P2; driceΔ1/+ and dS2P1/dS2P2; driceΔ1/driceΔ1 larvae survive, comparable with the survival of the dSREBP189 homozygotes in this experiment (Fig. 6B, day 4 AEL, right; Fig. 6A, day 4 AEL, right). The greatly reduced survival of dS2P1/ dS2P2; driceΔ1/+ and dS2P1/dS2P2; driceΔ1/driceΔ1 larvae on unsupplemented medium is not because of uncharacterized effects of the genetic background. Rather, their greatly increased survival on supplemented medium indicates that reduced survival of dS2P1/dS2P2; driceΔ1/+ and dS2P1/dS2P2; driceΔ1/driceΔ1 larvae results from a deficit in fatty acid metabolism subsequent to reduced dSREBP activity, just as observed for larvae lacking dSREBP itself (Fig. 6A, right).
Dronc Is Also Required for the Survival of dS2P Mutant Larvae—Drice is produced as a zymogen that can be activated by cleavage by the initiator caspase Dronc (22, 28, 29). Although Dronc cannot cleave dSREBP directly (Fig. 3), if Dronc is required in larvae to activate Drice, then larvae lacking both dS2P and Dronc should exhibit a phenotype similar to the dS2P; drice double mutants. At day 2 AEL, larvae of each genotype survive equally well (Fig. 6C). However, by day 4 AEL, very few dS2P1/dS2P2; dronc51/+ and dS2P1/dS2P2; dronc51/dronc51 larvae survive. Like the larvae lacking dSREBP and like the larvae lacking both dS2P and Drice, the dS2P; dronc– larvae survive dramatically better on supplemented medium (Fig. 6C, day 4 AEL).
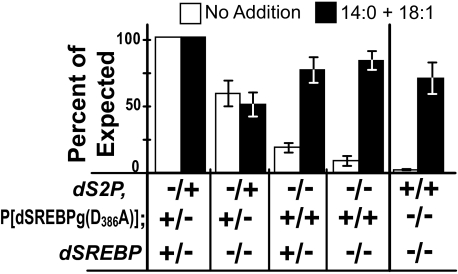
Asp-386 Is Required for Rescue in the Absence of dS2P—Drice cannot cleave dSREBP harboring the D386A mutation (Figs. 4 and 5). If the survival of flies lacking dS2P is because of caspase cleavage of dSREBP, then dSREBP harboring the D386A mutation should not rescue larvae lacking both endogenous dSREBP and dS2P. To test this hypothesis, we prepared animals transgenic for P{dSREBPg(D386A)}. This transposon carries a genomic DNA fragment encompassing the dSREBP locus and its upstream sequences (8) and harbors the D386A mutation that blocks cleavage of dSREBP by caspases (Fig. 4). This transgene was recombined onto second chromosomes harboring dS2P1 or dS2P2 alleles. The recombinant chromosomes were then moved into a dSREBP189/TM6B background. This permitted us to test the hypothesis that, in flies lacking dS2P, Asp-386-dependent cleavage of dSREBP is crucial for survival.
Fig. 7 shows that animals completely lacking endogenous dSREBP but harboring P{dSREBPg(D386A)} and heterozygous for dS2P survive at 60% the rate of their doubly heterozygous siblings. Thus, one copy of dSREBP harboring the D386A mutation is sufficient to afford substantial survival in the presence of endogenous dS2P. On unsupplemented medium, in the complete absence of dS2P and in the presence of two copies of P{dSREBPg(D386A)} and one endogenous, wild type copy of dSREBP, the animals survive at less than a quarter of the expected rate (Fig. 7, open bars). This may reflect haploinsufficiency for caspase-cleavable dSREBP in these animals, i.e. if a larva cannot cleave dSREBP at site-2 because of lack of dS2P, then a single caspase-cleavable copy of dSREBP (the endogenous wild type copy) is insufficient to support full survival.
FIGURE 7.
Survival of dS2P– mutants requires caspase-cleavable dSREBP. The loci considered are shown at left. For dS2P and dSREBP genotypes, + indicates wild type, and – indicates the null alleles. D386A designates the P{dSREBPg(D386A)} transgene. + indicates its presence, and – indicates its absence. dSREBP189 homozygotes served as a control for the efficacy of dietary rescue in this experiment (far right). Error bars represent the S.E.
In the complete absence of dS2P and dSREBP, animals carrying two copies of P{dSREBPg(D386A)} fare even more poorly on unsupplemented medium, surviving at only 10% of the expected rate (Fig. 7, open bars). In each of these latter cases, survival is substantially restored by supplementing the culture medium with free fatty acids (Fig. 7, solid bars). The dS2P1, dSREBPg(D386A)/dS2P2, dSREBPg(D386A); dSREBP189/dSREBP189 larvae survive nearly as well as expected on medium supplemented with fatty acids. Their greatly reduced survival observed on unsupplemented medium does not result from uncharacterized effects of the genetic background. Rather, rescue by dietary fatty acids indicates that reduced survival of dS2P1, dSREBPg(D386A)/dS2P2, dSREBPg(D386A); dSREBP189/dSREBP189 larvae results from deficient fatty acid metabolism. This deficit results from reduced dSREBP activity when dSREBP cannot be cleaved at site-2 (because of the absence of dS2P) or by Drice (because of the D386A mutation).
DISCUSSION
Cleavage of SREBP by caspases was first reported in 1995 (12–14), but the significance of those observations has remained unclear. Both in transfected S2 cells and in vitro, with purified enzyme, Drice cleaves dSREBP (Figs. 1 and 2), and this cleavage requires an Asp residue at position 386 in the juxtamembrane stalk (Figs. 4 and 5). Other Asp residues in this region are not required for cleavage. As caspases typically cleave following an Asp residue, the parsimonious interpretation of these data is that Drice cleaves dSREBP following Asp-386.
Cleavage of dSREBP by Drice releases the amino terminus. The present data demonstrate that dS2P mutants require drice for survival. Animals doubly mutant for dS2P and drice phenocopy dSREBP null mutants; they survive extremely poorly on unsupplemented medium and show greatly improved survival on medium supplemented with fatty acids (Fig. 6). Conversely, larvae whose only source for dSREBP is a dSREBP transgene, P{dSREBPg(D386A)}, which harbors a substitution of Asp-386 for Ala at the putative cleavage site, are viable in the presence of wild type dS2P. If these transgenic animals lack dS2P, the mutant transgenic larvae die at the end of second instar (Fig. 7). Just as observed for the double mutant dS2P, drice larvae (Fig. 6B), the P{dSREBPg(D386A)} transgenic larvae are substantially rescued by dietary supplementation with fatty acids (Fig. 7). The extent of rescue is similar to the rescue of dSREBP189 homozygotes in the same experiment, confirming that lethality results from deficits in fatty acid metabolism.
Larvae Lacking dS2P Also Require Dronc—The results with the dS2P1/dS2P2; dronc51/dronc51 double mutants are very similar to the results of experiments with driceΔ1 (Fig. 6, B and C), even though purified Dronc cannot cleave dSREBP directly (Fig. 3). This likely reflects the requirement for the initiator caspase Dronc to cleave the effector caspase Drice, such that in the absence of Dronc, Drice is not activated and cannot cleave dSREBP. Cleavage of Drice by Dronc, for example, is required for activation of Drice during apoptosis (22, 28, 29). These data suggest that Drice, activated by Dronc, cleaves dSREBP in larvae lacking dS2P.
Little apoptosis is observed in Drosophila between embryogenesis and pupariation. Cleavage of dSREBP by Drice during larval growth therefore does not appear to be related to apoptosis. Even in the absence of substantial apoptosis, however, mRNAs for Drice and Dronc are detected at low levels in larvae (30, 31). Our genetic data indicate that at least some of that message is translated to yield enzyme that is active during larval life when apoptosis is not observed. Constitutive activation of effector caspases by the apoptosome in the absence of apoptosis is seen elsewhere during Drosophila growth and development. For example, in embryos, there is a basal level of effector caspase activity several hours before the onset of programmed cell death (32). Caspase activity is also detected in hemocytes from 3rd instar larvae that are likewise not undergoing apoptosis (20).
Role of Caspase Cleavage of dSREBP—Does caspase cleavage of dSREBP play a role in normal larval physiology or is this phenomenon seen only when dS2P is absent? In larvae lacking dS2P, activation of dSREBP is readily detected in the fat body (11). The cleavage of dSREBP by Drice in the larval fat body could be a fortuitous consequence of the presence of a caspase site in the juxtamembrane stalk and the coincidental expression of Drice in the larval fat body. Alternatively, caspase cleavage may represent an important and pervasive physiologically relevant means of activating SREBP independently of the previously described machinery (Scap, S1P, and S2P). The present data do not allow firm distinction between these possibilities but an inference may be drawn from sequence data.
The site of caspase cleavage in SREBP-1 is conserved among its vertebrate homologues, and the site of caspase cleavage in SREBP-2, which is not homologous to the caspase site in SREBP-1, is likewise highly conserved among vertebrate SREBP-2s (supplemental Fig. 2). Similarly, the caspase site Asp in dSREBP is well conserved among Drosophila SREBPs (Fig. 4) as is the preceding Thr residue (supplemental Fig. 3). In the case of mammalian SREBP-1 and -2, and now for dSREBP, these putative sites have been validated experimentally. The differing positions of these confirmed caspase sites indicate that the absolute position of caspase cleavage within the juxtamembrane stalk is not crucial, whereas the presence of a caspase cleavage site is. Cleavage almost anywhere within this region may offer suitable means of releasing active SREBP and thus bypassing the normal processing machinery.
Cleavage of dSREBP is usually tightly controlled by end product feedback regulation. If SREBP in the ER membrane is the substrate for caspase cleavage, this would bypass feedback regulation, which relies of the control of ER-to-Golgi transport of SREBP (5).
Under what circumstances might a cell or organism need to bypass end product-mediated feedback suppression of the transcription of the genes of lipid synthesis? It may be desirable during periods of rapid membrane synthesis, such as fetal development in mammals. Rapid deposition of large stores of lipid may be another such a case. The mass of the Drosophila larvae increases roughly 200-fold between the time it emerges from the egg and the onset of pupariation about 5 days later. The majority of this increase in mass results from the storage of lipid in the fat body, which is needed to fuel metamorphosis (33). End product-mediated suppression of the transcription of the genes of lipid synthesis may be incompatible with the need for continued high levels of lipid accumulation and synthesis in the presence of large amounts of lipid already stored.
In dS2P mutant larvae, transcript abundance of dSREBP target genes is much greater than in larvae lacking dSREBP itself, and activation of dSREBP is readily detected in the fat body (11). This activation permits the survival of the mutant animals. However, in the complete absence of dS2P, the mutant offspring of mutant mothers survive only half as well as their heterozygous siblings (11). Their reduced survival results from a deficit in lipid metabolism; they survive at nearly the expected rate on medium supplemented with fatty acids. Therefore, cleavage of dSREBP by Drice is not fully redundant with the usual processing mechanism. Instead, it may serve to augment dSREBP activation in specific tissues to support the rapid deposition of lipid stores during larval life.
Supplementary Material
Acknowledgments
We are grateful to Indhumathy Subramaniyan, Edward Tambe-Ebot, Joseph Lockridge, Kaori Tanaka, Denise Parker, and Phuong Pham for excellent technical support and to Jeff Cormier for sequencing and real time PCR analysis. We thank Dr. John Abrams for providing plasmids carrying cDNAs for Drosophila caspases, for the Mtal-Grim vector, and for caspase mutant stocks. We also thank Dr. Xiaodong Wang for providing purified recombinant Drice and Dcp1 as well as the pFastBac1 vector carrying the Dronc cDNA.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM07145701A1. This work was also supported by The Perot Family Foundation.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
Footnotes
The abbreviations used are: SREBP, sterol regulatory element-binding protein; dSREBP, Drosophila SREBP; S1P, site-1 protease; S2P, site-2 protease; ER, endoplasmic reticulum; HSV, herpes simplex virus; YFP, yellow fluorescent protein; GFP, green fluorescent protein; RNAi, RNA interference; AEL, after egg laying.
References
- 1.Goldstein, J. L., Rawson, R. B., and Brown, M. S. (2002) Arch. Biochem. Biophys. 397 139–148 [DOI] [PubMed] [Google Scholar]
- 2.Srivastava, M., Begovic, E., Chapman, J., Putnam, N. H., Hellsten, U., Kawashima, T., Kuo, A., Mitros, T., Salamov, A., Carpenter, M. L., Signorovitch, A. Y., Moreno, M. A., Kamm, K., Grimwood, J., Schmutz, J., Shapiro, H., Grigoriev, I. V., Buss, L. W., Schierwater, B., Dellaporta, S. L., and Rokhsar, D. S. (2008) Nature 454 955–960 [DOI] [PubMed] [Google Scholar]
- 3.Putnam, N. H., Srivastava, M., Hellsten, U., Dirks, B., Chapman, J., Salamov, A., Terry, A., Shapiro, H., Lindquist, E., Kapitonov, V. V., Jurka, J., Genikhovich, G., Grigoriev, I. V., Lucas, S. M., Steele, R. E., Finnerty, J. R., Technau, U., Martindale, M. Q., and Rokhsar, D. S. (2007) Science 317 86–94 [DOI] [PubMed] [Google Scholar]
- 4.Wang, X., Briggs, M. R., Hua, X., Yokoyama, C., Goldstein, J. L., and Brown, M. S. (1993) J. Biol. Chem. 268 14497–14504 [PubMed] [Google Scholar]
- 5.Espenshade, P. J., and Hughes, A. L. (2007) Annu. Rev. Genet. 41 401–427 [DOI] [PubMed] [Google Scholar]
- 6.Theopold, U., Ekengren, S., and Hultmark, D. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 1195–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seegmiller, A. C., Dobrosotskaya, I., Goldstein, J. L., Ho, Y. K., Brown, M. S., and Rawson, R. B. (2002) Dev. Cell 2 229–238 [DOI] [PubMed] [Google Scholar]
- 8.Kunte, A. S., Matthews, K. A., and Rawson, R. B. (2006) Cell Metab. 3 439–448 [DOI] [PubMed] [Google Scholar]
- 9.Clark, A. J., and Bloch, K. (1959) J. Biol. Chem. 234 2578–2588 [PubMed] [Google Scholar]
- 10.Sang, J. H. (1956) J. Exp. Biol. 33 45–72 [Google Scholar]
- 11.Matthews, K. A., Kunte, A. S., Tambe-Ebot, E., and Rawson, R. B. (2009) Genetics 181 119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai, J. T., Brown, M. S., and Goldstein, J. L. (1996) Proc. Natl. Acad. Sci. U. S. A. 93 5437–5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang, X., Zelenski, N. G., Yang, J., Sakai, J., Brown, M. S., and Goldstein, J. L. (1996) EMBO J. 15 1012–1020 [PMC free article] [PubMed] [Google Scholar]
- 14.Wang, X., Pai, J. T., Wiedenfeld, E. A., Medina, J. C., Slaughter, C. A., Goldstein, J. L., and Brown, M. S. (1995) J. Biol. Chem. 270 18044–18050 [DOI] [PubMed] [Google Scholar]
- 15.Higgins, M. E., and Ioannou, Y. A. (2001) J. Lipid Res. 42 1939–1946 [PubMed] [Google Scholar]
- 16.Dobrosotskaya, I. Y., Seegmiller, A. C., Brown, M. S., Goldstein, J. L., and Rawson, R. B. (2002) Science 296 879–883 [DOI] [PubMed] [Google Scholar]
- 17.FlyBase Consortium (2003) Nucleic Acids Res. 31 172–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laundrie, B., Peterson, J. S., Baum, J. S., Chang, J. C., Fileppo, D., Thompson, S. R., and McCall, K. (2003) Genetics 165 1881–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muro, I., Berry, D. L., Huh, J. R., Chen, C. H., Huang, H., Yoo, S. J., Guo, M., Baehrecke, E. H., and Hay, B. A. (2006) Development (Camb.) 133 3305–3315 [DOI] [PubMed] [Google Scholar]
- 20.Chew, S. K., Akdemir, F., Chen, P., Lu, W. J., Mills, K., Daish, T., Kumar, S., Rodriguez, A., and Abrams, J. M. (2004) Dev. Cell 7 897–907 [DOI] [PubMed] [Google Scholar]
- 21.Chen, P., Nordstrom, W., Gish, B., and Abrams, J. M. (1996) Genes Dev. 10 1773–1782 [DOI] [PubMed] [Google Scholar]
- 22.Hawkins, C. J., Yoo, S. J., Peterson, E. P., Wang, S. L., Vernooy, S. Y., and Hay, B. A. (2000) J. Biol. Chem. 275 27084–27093 [DOI] [PubMed] [Google Scholar]
- 23.Dorstyn, L., and Kumar, S. (2008) Cell Death Differ. 15 461–470 [DOI] [PubMed] [Google Scholar]
- 24.Alnemri, E. S., Livingston, D. J., Nicholson, D. W., Salvesen, G., Thornberry, N. A., Wong, W. W., and Yuan, J. (1996) Cell 87 171. [DOI] [PubMed] [Google Scholar]
- 25.Fraser, A. G., McCarthy, N. J., and Evan, G. I. (1997) EMBO J. 16 6192–6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark, A. G., Eisen, M. B., Smith, D. R., Bergman, C. M., Oliver, B., Markow, T. A., Kaufman, T. C., Kellis, M., Gelbart, W., Iyer, V. N., Pollard, D. A., Sackton, T. B., Larracuente, A. M., Singh, N. D., Abad, J. P., et al. (2007) Nature 450 203–21817994087 [Google Scholar]
- 27.Vicario, S., Moriyama, E. N., and Powell, J. R. (2007) BMC Evol. Biol. 7 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meier, P., Silke, J., Leevers, S. J., and Evan, G. I. (2000) EMBO J. 19 598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muro, I., Hay, B. A., and Clem, R. J. (2002) J. Biol. Chem. 277 49644–49650 [DOI] [PubMed] [Google Scholar]
- 30.Fraser, A. G., and Evan, G. I. (1997) EMBO J. 16 2805–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorstyn, L., Colussi, P. A., Quinn, L. M., Richardson, H., and Kumar, S. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 4307–4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez, A., Chen, P., Oliver, H., and Abrams, J. M. (2002) EMBO J. 21 2189–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Church, R. B., and Robertson, F. W. (1966) Genet. Res. 7 383–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.