Abstract
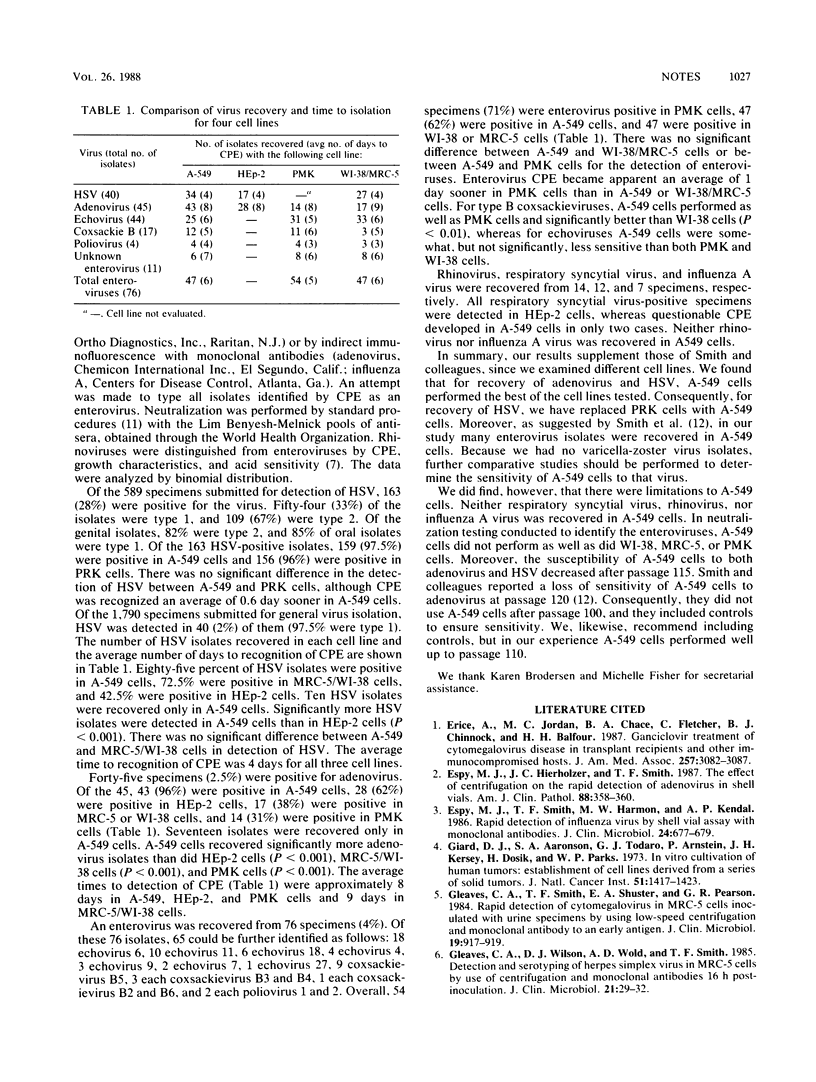
A-549 cells were compared with other cell lines for virus recovery, except from specimens submitted specifically for detection of cytomegalovirus. Of 589 specimens submitted specifically for detection of herpes simplex virus (HSV), 163 (28%) were positive for HSV--159 (97.5%) in A-549 cells and 156 (96%) in primary rabbit kidney cells. HSV cytopathic effect was identified an average of 0.6 day earlier in A-549 cells. Virus was recovered from 194 (11%) of 1,790 specimens submitted for general virus isolation. Of 40 HSV isolates, 85% were positive in A-549 cells, 72.5% were positive in MRC-5/WI-38 cells, and 42.5% were positive in HEp-2 cells. With adenovirus, 96% of 45 isolates were detected in A-549 cells, 62% were detected in HEp-2 cells, 38% were detected in MRC-5/WI-38 cells, and 31% were detected in PMK cells. Of the 76 enterovirus-positive specimens, 71% were positive in PMK cells, 62% were positive in A-549 cells, and 62% were positive in MRC-5/WI-38 cells. None of 12 respiratory syncytial virus, 14 rhinovirus, or 7 influenza A virus isolates were detected in A-549 cells. Of the cell lines examined, A-549 cells performed optimally for recovery of HSV and adenovirus, they allowed good growth of many of the enterovirus isolates, but they did not allow recovery of any of the respiratory syncytial virus, rhinovirus, or influenza A virus isolates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erice A., Jordan M. C., Chace B. A., Fletcher C., Chinnock B. J., Balfour H. H., Jr Ganciclovir treatment of cytomegalovirus disease in transplant recipients and other immunocompromised hosts. JAMA. 1987 Jun 12;257(22):3082–3087. [PubMed] [Google Scholar]
- Espy M. J., Hierholzer J. C., Smith T. F. The effect of centrifugation on the rapid detection of adenovirus in shell vials. Am J Clin Pathol. 1987 Sep;88(3):358–360. doi: 10.1093/ajcp/88.3.358. [DOI] [PubMed] [Google Scholar]
- Espy M. J., Smith T. F., Harmon M. W., Kendal A. P. Rapid detection of influenza virus by shell vial assay with monoclonal antibodies. J Clin Microbiol. 1986 Oct;24(4):677–679. doi: 10.1128/jcm.24.4.677-679.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Gleaves C. A., Smith T. F., Shuster E. A., Pearson G. R. Rapid detection of cytomegalovirus in MRC-5 cells inoculated with urine specimens by using low-speed centrifugation and monoclonal antibody to an early antigen. J Clin Microbiol. 1984 Jun;19(6):917–919. doi: 10.1128/jcm.19.6.917-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleaves C. A., Wilson D. J., Wold A. D., Smith T. F. Detection and serotyping of herpes simplex virus in MRC-5 cells by use of centrifugation and monoclonal antibodies 16 h postinoculation. J Clin Microbiol. 1985 Jan;21(1):29–32. doi: 10.1128/jcm.21.1.29-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisher K. K., Menegus M. A. Evaluation of three types of cell culture for recovery of adenovirus from clinical specimens. J Clin Microbiol. 1987 Jul;25(7):1323–1324. doi: 10.1128/jcm.25.7.1323-1324.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M., Smith B., Szakal A., Nelson-Rees W., Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976 Jan 15;17(1):62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Craft D. W., Shiromoto R. S., Yan P. O. Alternative cell line for virus isolation. J Clin Microbiol. 1986 Aug;24(2):265–268. doi: 10.1128/jcm.24.2.265-268.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods G. L., Mills R. D. Conventional tube cell culture compared with centrifugal inoculation of MRC-5 cells and staining with monoclonal antibodies for detection of herpes simplex virus in clinical specimens. J Clin Microbiol. 1988 Mar;26(3):570–572. doi: 10.1128/jcm.26.3.570-572.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



